Abstract
Sulfate transport by tobacco cells (Nicotiana tabacum L. var. Xanthi) cultured in liquid medium was investigated.
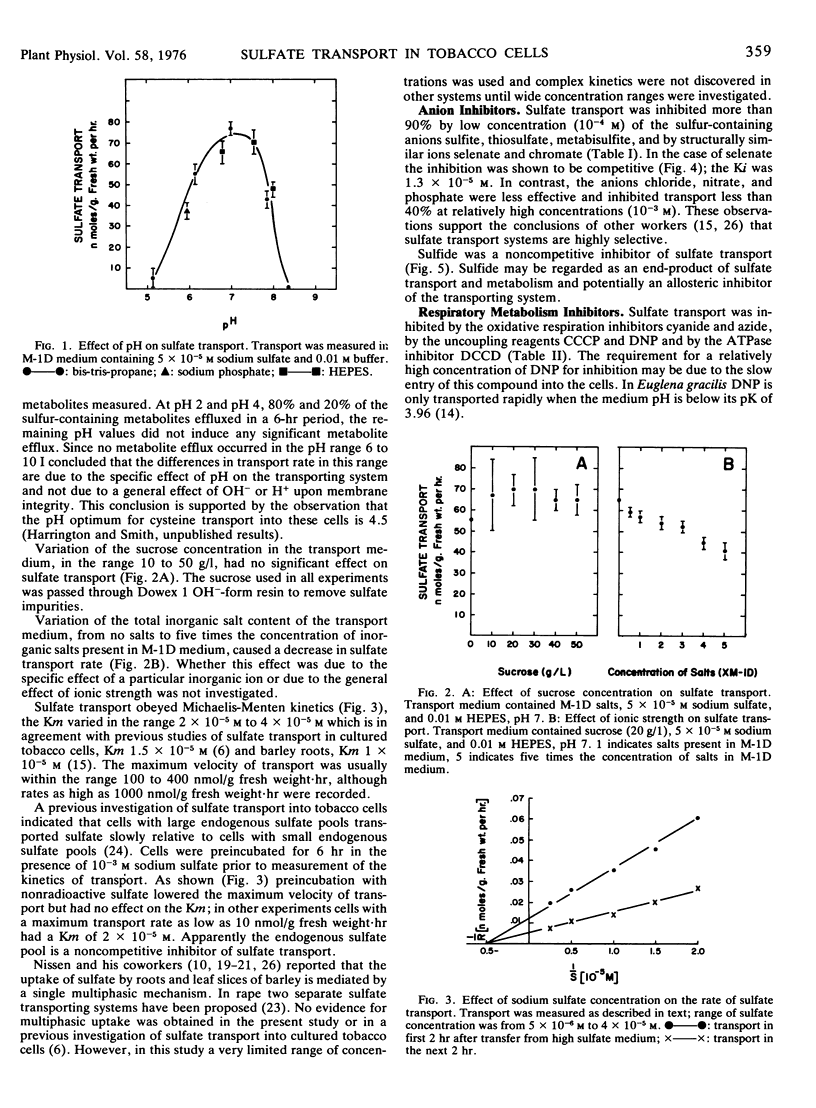
Transport was linear with time, had a sharp pH optimum between 6.5 and 7.5, and obeyed Michaelis-Menten kinetics. The Km varied within the range 2 × 10−5m and 4 × 10−5m and the maximum velocity was in the range 100 to 400 nanomoles per gram fresh weight·hour.
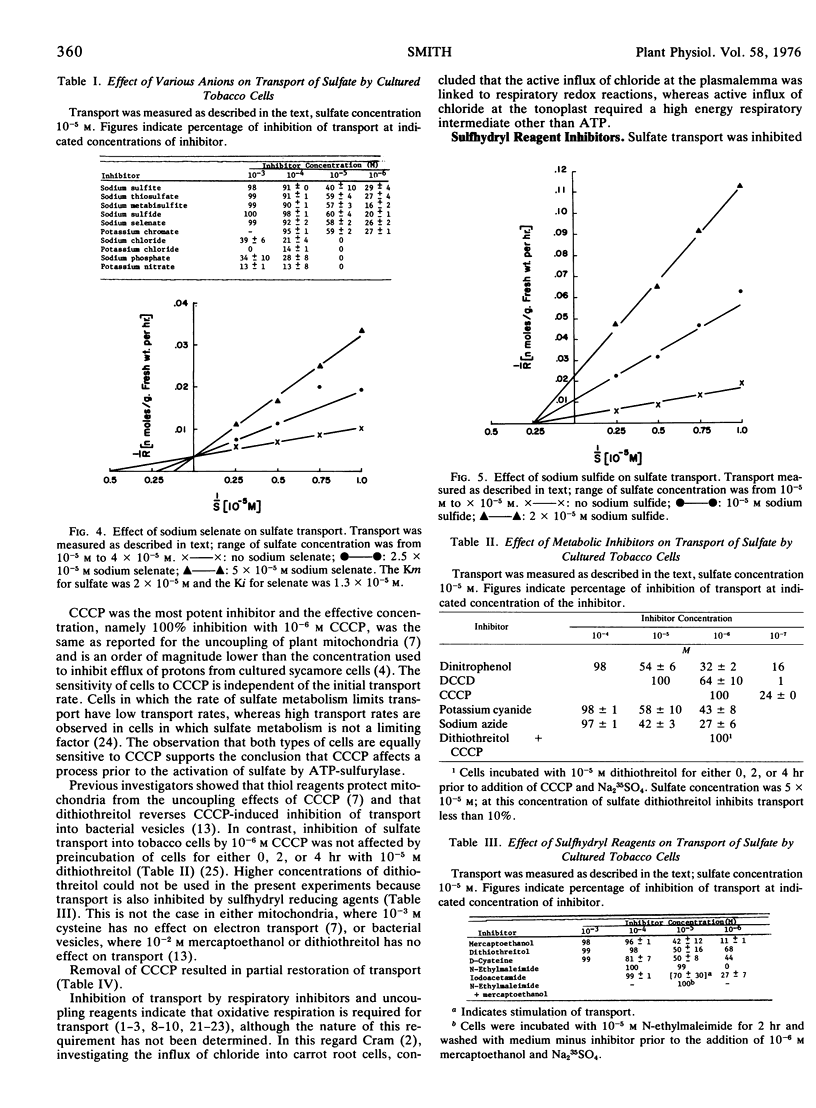
Transport was inhibited more than 90% by 10−4m sulfite, thiosulfate, metabisulfite, sulfide, selenate, and chromate, but was inhibited less than 40% by 10−3m chloride, nitrate, or phosphate. Selenate was a competitive and sulfide a noncompetitive inhibitor of sulfate transport.
The oxidative respiration inhibitors, azide and cyanide, uncoupling reagents, carbonylcyanide m-chlorophenylhydrazone (CCCP) and dinitrophenol, and the ATPase inhibitor N,N′-dicyclohexylcarbodiimide (DCCD) were all potent inhibitors of transport. Inhibition by CCCP was not prevented by preincubation of cells with dithiothreitol. Removal of CCCP from the transporting medium resulted in a partial resumption of transport, in contrast removal of DCCD had no effect.
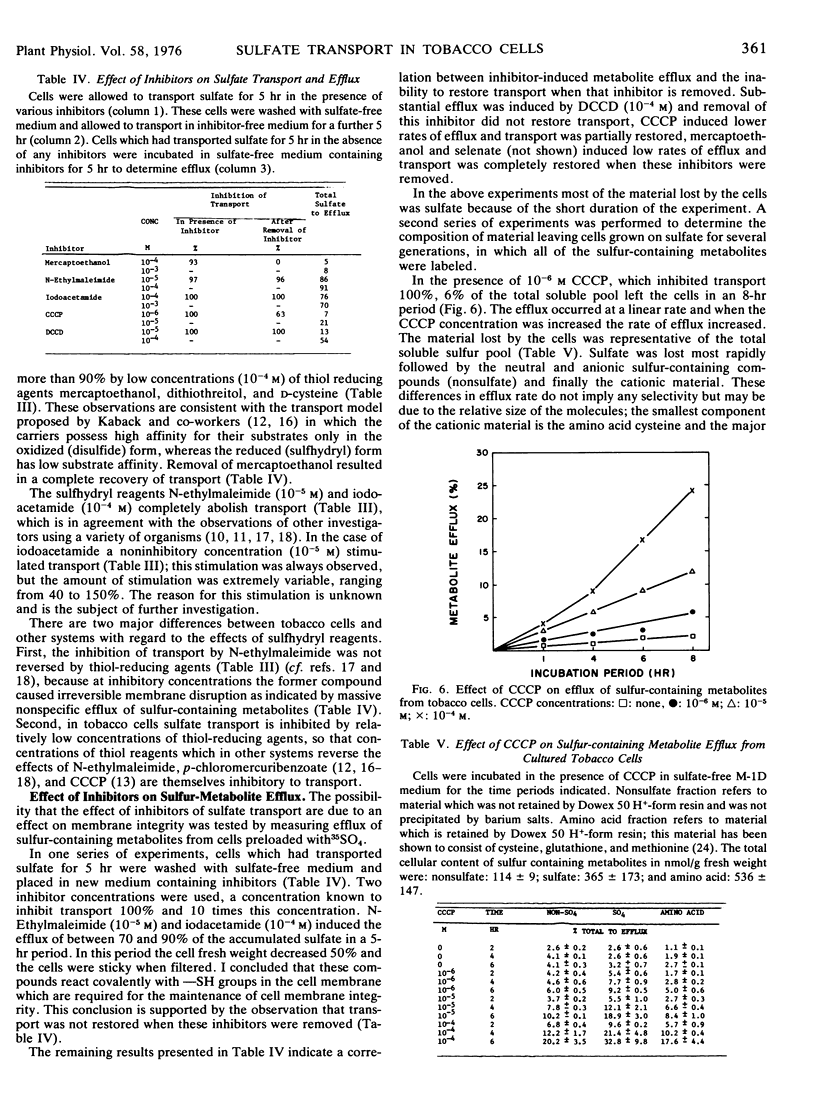
Sulfate transport was inhibited more than 90% by 10−4m mercaptoethanol, dithiothreitol, or d-cysteine and was abolished by either 10−5m N-ethylmaleimide or 10−4m iodoacetamide. Removal of mercaptoethanol from the transporting medium resulted in a return to maximal rates of transport whereas when either N-ethylmaleimide or iodoacetamide were removed transport remained inhibited.
N-ethylmaleimide (10−5m) and iodoacetamide (10−4m), which inhibited transport completely, induced the efflux of between 70 and 90% of the transported sulfate in 5 hours. Metabolite efflux was induced by the following compounds, which are listed according to their effectiveness, DCCD, CCCP, mercaptoethanol, and selenate. Increasing the concentration of an inhibitor, in excess of that required to inhibit transport 100%, increased the rate of nonspecific metabolite efflux from the cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. R., Eckermann G., Grant M., Robertson R. N. Salt accumulation and adenosine triphosphate in carrot xylem tissue. Proc Natl Acad Sci U S A. 1966 Mar;55(3):560–564. doi: 10.1073/pnas.55.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram W. J. Respiration and energy-dependent movements of chloride at plasmalemma and tonoplast of carrot root cells. Biochim Biophys Acta. 1969 Mar 11;173(2):213–222. doi: 10.1016/0005-2736(69)90105-9. [DOI] [PubMed] [Google Scholar]
- Fisher M. L., Albersheim P. Characterization of a H Efflux from Suspension-cultured Plant Cells. Plant Physiol. 1974 Mar;53(3):464–468. doi: 10.1104/pp.53.3.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L. The effects of amino acids and ammonium on the growth of plant cells in suspension culture. Plant Physiol. 1970 Apr;45(4):372–375. doi: 10.1104/pp.45.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEYTLER P. G. uncoupling of oxidative phosphorylation by carbonyl cyanide phenylhydrazones. I. Some characteristics of m-Cl-CCP action on mitochondria and chloroplasts. Biochemistry. 1963 Mar-Apr;2:357–361. doi: 10.1021/bi00902a031. [DOI] [PubMed] [Google Scholar]
- Hart J. W., Filner P. Regulation of sulfate uptake by amino acids in cultured tobacco cells. Plant Physiol. 1969 Sep;44(9):1253–1259. doi: 10.1104/pp.44.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Mineral ion contents and cell transmembrane electropotentials of pea and oat seedling tissue. Plant Physiol. 1967 Jan;42(1):37–46. doi: 10.1104/pp.42.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanjean R., Hourmant A., Ducet G. Effet des inhibiteurs de groupes SH sur le transport du phosphate chez Chlorella pyrenoidosa. Biochimie. 1975;57(3):383–390. doi: 10.1016/s0300-9084(75)80315-4. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Barnes E. M., Jr Mechanisms of active transport in isolated membrane vesicles. II. The mechanism of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in membrane preparations from Escherichia coli. J Biol Chem. 1971 Sep 10;246(17):5523–5531. [PubMed] [Google Scholar]
- Kaback H. R., Reeves J. P., Short S. A., Lombardi F. J. Mechanisms of active transport in isolated bacterial membrane vesicles. 18. The mechanism of action of carbonylcyanide m-chlorophenylhydrazone. Arch Biochem Biophys. 1974 Jan;160(1):215–222. doi: 10.1016/s0003-9861(74)80028-7. [DOI] [PubMed] [Google Scholar]
- Kahn J. S. Physiological adaptation of Euglena gracilis to uncouplers and inhibitors of oxidative phosphorylation. Arch Biochem Biophys. 1974 Sep;164(1):266–274. doi: 10.1016/0003-9861(74)90031-9. [DOI] [PubMed] [Google Scholar]
- Leggett J. E., Epstein E. Kinetics of Sulfate Absorption by Barley Roots. Plant Physiol. 1956 May;31(3):222–226. doi: 10.1104/pp.31.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi F. J., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. 8. The transport of amino acids by membranes prepared from Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):7844–7857. [PubMed] [Google Scholar]
- Marzluf G. A. Uptake and efflux of sulfate in Neurospora crassa. Biochim Biophys Acta. 1974 Mar 29;339(3):374–381. doi: 10.1016/0005-2736(74)90164-3. [DOI] [PubMed] [Google Scholar]
- Nelson S. O., Glover G. I., Magill C. W. The essentiality of sulfhydryl groups to transport in Neurospora crassa. Arch Biochem Biophys. 1975 Jun;168(2):483–489. doi: 10.1016/0003-9861(75)90278-7. [DOI] [PubMed] [Google Scholar]


