Abstract
Although older adults exhibit normal accuracy in performing word retrieval and generation (lexical production; e.g., object naming), they are generally slower in responding than younger adults. To maintain accuracy, older adults recruit compensatory mechanisms and strategies. We focused on two such possible compensatory mechanisms, one semantic and one executive. These mechanisms are reflected at inter- and intra-hemispheric levels by various patterns of reorganization of lexical production cerebral networks. Hemispheric reorganization (HR) changes were also evaluated in relation to increase naming latencies. Using functional magnetic resonance imaging (fMRI), we examined 27 healthy participants (from 30 years to 85 years) during an object naming task, exploring and identifying task-related patterns of cerebral reorganization. We report two main results. First, we observed a left intra-hemispheric pattern of reorganization, the left anterior-posterior aging (LAPA) effect, consisting of supplementary activation of left posterior (temporo-parietal) regions in older adults and asymmetric activation along the left fronto-temporal axis. This pattern suggests that older adults recruit posterior semantic regions to perform object naming. The second finding consisted of bilateral recruitment of frontal regions to maintain appropriate response times, especially in older adults who were faster performers. This pattern is discussed in terms of compensatory mechanism. We suggest that aging is associated with multiple, co-existing compensation and reorganization mechanisms and patterns associated with lexical production.
Keywords: object naming, aging, fMRI, hemispheric specialization, LAPA, HAROLD
Introduction
Anomia and tip-of-the-tongue phenomena are frequently reported by older adults in daily life (Perlmutter, 1978; Zelinski et al., 1980; Cavanaugh et al., 1983). They reflect difficulty in retrieving and generating words (Obler and Albert, 1980; Burke and Shafto, 2004; Salthouse and Mandell, 2013). However, despite these frequent subjective complaints, the objective evidence for lexical production and naming deficits in aging varies according to the task and material (Ska and Goulet, 1989; Goulet et al., 1994; Obler et al., 2010; Baciu et al., 2016; Votruba et al., 2016). For instance, object naming is preserved longer in aging, compared to naming famous people (Cohen and Faulkner, 1986; Evrard, 2002; James, 2004). Boudiaf et al. (2016) have observed that older adults name objects as accurately as younger ones, despite longer naming latencies. These results indicate possible dissociation between accuracy (i.e., number of correct responses) and latencies (i.e., time required to produce the appropriate word). At the cerebral level, aging modulates cerebral activity (Greenwood, 2007), reflected either by increased or decreased activity, or by additional recruitment of regions that are not usually engaged by similar tasks in younger adults (e.g., Cabeza, 2002; Greenwood, 2007; Park and Reuter-Lorenz, 2009; Lövdén et al., 2010; Grady, 2012). These modifications are reflected in multiple patterns of inter- and intra-hemispheric cerebral reorganization (e.g., Grady et al., 1994; Cabeza, 2002; Davis et al., 2008). In our study, we sought to determine whether the dissociation of naming performance in aging (i.e., preservation of naming accuracy despite longer latencies) is associated with intra- and inter-hemispheric cerebral reorganization mechanisms.
Two hypotheses may explain why older adults may show only limited decline in object naming accuracy: one semantic and the other executive. The semantic hypothesis is based on the fact that the experience that older people have acquired over the years, resulting in a larger vocabulary and semantic memory (Verhaeghen, 2003), is associated with an increased number of shared features and representations in semantic memory (Laver and Burke, 1993). Therefore, the relative preservation of object naming in older adults might be explained by a supplementary contribution of semantic processes that facilitate lexico-semantic retrieval during naming (Boudiaf et al., 2016). In line with the semantic hypothesis, using functional magnetic resonance imaging (fMRI), Baciu et al. (2016) reported higher supplementary bilateral posterior temporo-parietal activity for object naming in older adults compared to younger adults, suggesting greater involvement of lexico-semantic processes. This is in agreement with findings from Ansado et al. (2013) for lexical production, and Lacombe et al. (2015) for language comprehension. In addition, Lacombe et al. (2015) reported that increased posterior activity observed in older adults reflects age-related reorganization of networks responsible for the conceptual retrieval and semantic representations of words. Ansado et al. (2013) suggested that preserved lexical production in older adults could be explained by greater support from semantic processes in temporal regions (Verhaeghen, 2003; Patterson et al., 2007). In terms of inter- and intra-hemispheric reorganization (HR), the semantic hypothesis predicts increased temporo-parietal and decreased frontal activity. This HR mechanism can be put in perspective with the posterior-anterior shift in aging (PASA, Davis et al., 2008) that posits that older adults exhibit greater anterior frontal and decreased posterior occipital activation, reflecting executive-based compensation (frontal regions) in the context of a sensory deficit (occipital regions; Grady et al., 1994; Park et al., 2004; Davis et al., 2008). Other PASA variants reported decreased activity in regions other than the occipital lobe, such as medial temporal areas (Gutchess et al., 2005; see Morcom and Johnson, 2015). According to Ansado et al. (2013), age-related increases in temporo-parietal or frontal activity might depend on the particular cognitive processes involved. Along the same lines, the second hypothesis (i.e., executive hypothesis) posits a supplementary recruitment of executive functions in older adults to perform the task (Wingfield and Grossman, 2006; Helder et al., 2016). Using fMRI, Wierenga et al. (2008) revealed supplementary right hemispheric activity in older adults, variably correlated with the performance. Right pre-central activity was negatively correlated, whereas right inferior frontal activity was positively correlated with task accuracy. This reduced inter-hemispheric frontal asymmetry in aging was interpreted as a difficulty in retrieving words, resulting from a decline in executive function (Wierenga et al., 2008). In terms of inter- and intra-hemispheric HR, increased frontal activity was related to hemispheric asymmetry reduction in older adults (HAROLD; Cabeza, 2002) model. According to the HAROLD model, compared to younger adults, older adults exhibit a lower degree of hemispheric asymmetry, mainly in prefrontal regions (Cabeza, 2002; Rajah and D’Esposito, 2005; Park and Reuter-Lorenz, 2009). According to HAROLD, the supplementary involvement of right prefrontal regions reflects engagement of compensatory executive mechanisms to maintain performance. Furthermore, the HAROLD model suggests a dissociation between high- and low-performing older adults, with the latter showing less hemispheric asymmetry reduction than the former (Cabeza et al., 2002).
For naming latencies, Obler et al. (2010) observed that object naming latencies related to aging correlated to white matter density in both frontal and temporo-parietal regions using voxel-based morphometry (VBM). This suggests that the anatomical basis of naming processes with aging is organized along fronto-temporo-parietal or antero-posterior axes. Other authors have shown that the left antero-posterior axis was related to task demands (Wierenga et al., 2008; Galdo-Alvarez et al., 2009; Diaz et al., 2016). By comparing comprehension (low task-demand) vs. production (high task-demand) tasks, Diaz et al. (2016) observed that the higher the task demands, the stronger the older adults recruit frontal regions. This result suggests that older adults increase the recruitment of executive functioning to maintain lexical production, as during naming. However, older adult’s performance is highly variable (Hultsch et al., 2002). Whereas some older adults show decreased performance, others tend to show similar performance, as compared to younger adults. Such variability may be explained in terms of cognitive reserve (i.e., higher educational level or more flexible neural networks; Stern, 2002, 2009), allowing older adults to preserve high cognitive functioning. Indeed, Cabeza et al. (2002) have observed that in high-performing older adults, a memory task recruited both left and right frontal regions, whereas low-performing older adults recruited only left frontal regions. Thus, the aforementioned HAROLD pattern may be related to higher cognitive reserve, suggesting that differential frontal recruitment, related to executive functioning, can be expected in older adults. Wierenga et al. (2008) showed that in older adults correlations between BOLD-contrast signals and response latency were found in several frontal regions, suggesting supplementary selection of lexical representations by older adults. However, the distribution of latencies as a function of the BOLD-contrast response (see Wierenga et al., 2008) appeared to be driven by extreme values, weakening the statistical validity of their results. In this study, we evaluated the influence of age on naming latencies (i.e., between young and old adults) and cognitive reserve (i.e., within older adults only). The effect of cognitive reserve on naming latencies in older adults was explored by dividing the older group into two groups, slower (longer latencies or response times) or faster (shorter latencies) adults.
In summary, older adults show preserved naming accuracy but longer naming latencies compared to younger adults, suggesting a dissociation of neural substrates for accuracy and latencies. Our main goal was to determine HR mechanisms that may account for this dissociation, with two objectives. To explore the respective role of semantic and executive mechanisms in naming accuracy preservation with aging (Objective 1), we inferred these mechanisms using HR indices, with semantic mechanisms reflected by greater posterior (temporo-parietal) asymmetry, and executive mechanisms reflected by greater anterior (frontal) asymmetry. To determine which HR mechanisms are related to naming latencies (Objective 2), we compared the effect of naming latencies between younger and older adults, and between faster and slower older adults.
Materials and Methods
Participants
Among the 30 participants initially recruited, 27 (8 females) aged from 30 to 85 (M = 56.22 years old, SD = 17.53, variation coefficient = 0.31) were finally retained, divided into two groups: the Younger Group (YG; n = 13, M = 40.07 years old, SD = 8.33, 5 females) and the Older Group (OG; n = 14, M = 71.21 years old, SD = 6.93, 3 females). All participants were right-handed (Edinburgh Handedness Inventory; Oldfield, 1971) and had normal or corrected-to-normal vision. Participants were cognitively unimpaired (Mini Mental State Examination, MMSE; Folstein et al., 1975), had no psychiatric symptoms (Hospital Anxiety and Depression, HAD; Zigmond and Snaith, 1983) or episodic memory deficit (“5 words” test, Dubois et al., 2002). All participants were highly educated (Poitrenaud questionnaire; Kalafat et al., 2003) and were native French speakers. They gave their written informed consent for the study, which was approved by the local ethics committee (CPP no. 2014-A00569-38). Demographic information and inclusion criteria are mentioned in Table 1.
Table 1.
Demographic information and inclusion criteria for all participants.
| YG N = 13 5 females |
OG N = 14 3 females |
Mann-Whitney tests | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Z | p | |
| Age | 40.07 | 8.33 | 71.21 | 6.93 | −4.418 | <0.01 |
| Socio-cultural | 4.00 | 0.00 | 3.85 | 0.36 | −1.390 | 0.550 |
| Laterality | 85.00 | 16.99 | 90.00 | 11.87 | −0.817 | 0.430 |
| MMSE | 29.53 | 0.66 | 29.07 | 1.20 | −1.024 | 0.375 |
| HAD_A | 5.92 | 2.46 | 6.21 | 1.96 | −0.098 | 0.943 |
| HAD_D | 2.46 | 1.56 | 3.92 | 2.84 | −1.329 | 0.202 |
| Dubois | 10.00 | 0.00 | 9.92 | 0.26 | −0.964 | 0.756 |
Mann-Whitney tests were performed between younger and older participants. Analyses show that younger and older participants differ only on age. Significant results are in bold. Abbreviation: YG, Younger group; OG, Older group; MMSE, Mini-Mental State Evaluation; HAD_A, Hospital Anxiety and Depression scale_Anxiety; HAD_D, Hospital Anxiety and Depression scale_Depression.
Cognitive Assessment
Several neuropsychological tests were administered to evaluate cognitive domains, including language, memory, visual processing, and executive function, that are commonly used to measure cognitive function associated with naming (Duffau et al., 2014). Language was assessed using verbal fluency (generation of words that are related to the same semantic category; Cardebat et al., 1990), the verbal automatisms test (completion of overlearned French expressions by the participants; Beauregard, 1971), and Mill-Hill B vocabulary scale (explanation of words meaning and selection of the appropriate synonym for a word among a list; Deltour, 1993). Memory was assessed using forward and backward digit span tests (short-term and working memory; Wechsler, 1997) and the MacNair questionnaire to assess subjective memory complaints (McNair and Kahn, 1983). Visual processing was evaluated using the version A of the Trail Making Test (TMT-A; Tombaugh, 2004). Finally, executive functioning was assessed using Version B of the TMT (TMT-B; Tombaugh, 2004). We computed a difference score between the TMT-B and the TMT-A, referred as the “executive score”, as mentioned in the next sections of the manuscript (Corrigan and Hinkeldey, 1987). This executive score reflected cognitive flexibility, and removed simple sequencing, visual scanning and psychomotor functioning effects.
Object Naming: Stimuli and Procedure
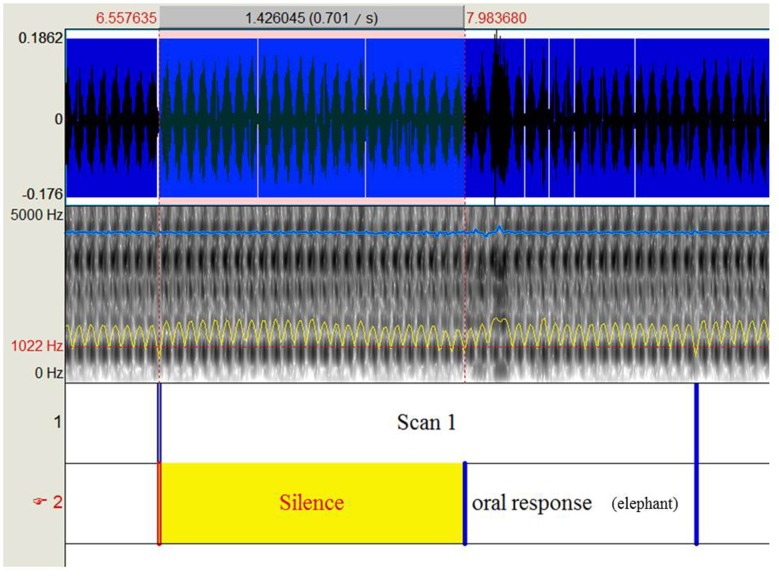
The participants performed an object naming task with 80 black and white drawings of objects and animals (DO-80 test; Metz-Lutz et al., 1991) using a blocked design alternating four task and control periods. Twenty images lasting 2 s each with an inter-stimulus interval of 500 ms were presented during each task period, and participants were instructed to name the images as accurately and rapidly as possible. During each control period, 20 simple images (circles and squares) were presented and participants were instructed to respond either “square” or “round.” Stimuli were displayed using E-prime (E-prime Psychology Software Tools Inc., Pittsburgh, PA, USA) and were projected onto a screen behind the magnet using a video projector. Oral responses were recorded via an MRI-compatible microphone (FORMI™ II, version 1.2) attached to the coil. We used Praat software (Boersma and Weenink, 2001) to measure the response times for each item and each participant. Specifically, we delimited 160 periods of 2.5 s from each audio file (one per participant) that correspond to the repetition time for scan acquisition. Within each identified scan, we delimited the beginning of oral response according to the audio-spectrogram (Figure 1). Response latencies were calculated based on the difference between the beginning of oral response and the beginning of the scan, given that pictures were presented at the beginning of each scan. Based on oral responses, the correct response rate (% of CR) and the mean reaction time (RT, in milliseconds) for correct responses were calculated. The run lasted 7.06 min.
Figure 1.
Shows the procedure to extract response times recorded during the object naming task under functional magnetic resonance imaging (fMRI).
Functional MRI Acquisition
The study was performed in a whole-body 3T MR scanner (Philips Achieva) with a 32-channel head coil. For functional scans, the manufacturer-provided gradient-echo/T2*-weighted EPI method was used. Forty-four interleaved axial slices parallel to the bi-commissural plane were acquired. Slice thickness was 3 mm. The in-plane voxel size was 2.3 × 2.3 mm (220 × 220 mm field of view acquired with 88 × 85 pixel data matrix, reconstructed with zero filling to 96 × 96 pixels). The main sequence parameters were TR = 2.5 s, TE = 30 ms, and flip angle = 80°. A T1-weighted high-resolution three dimensional anatomical volume was acquired, via a 3D T1 TFE sequence (field of view = 256 × 240 × 160 mm; resolution: 0.89 × 0.89 × 1 mm; acquisition matrix: 272 × 250 × 160 pixels; reconstruction matrix: 288 × 288 × 160 pixels).
Data Processing
Cognitive Scores and Naming Performance
Cognitive values were within normal neuropsychological ranges for all but one participant (MMSE < 24), who was then excluded from the study. Two other participants were also excluded owing to abnormal behavioral responses (RTs > 2 SD). Twenty-seven participants were thus retained. For recall, we aimed to determine: (1) which strategy was used by older adults to maintain equivalent naming accuracy than younger adults (semantic or executive); and (2) which HR mechanisms might explain longer naming latencies: (a) in older adults compared to younger adults; and (b) in slower older adults compared to faster older adults (in relation to cognitive reserve). For (1), we inferred the two strategies, semantic vs. executive, from HR indices reflecting patterns of HR. For their calculation, see below. The cut-off between YG and OG was based on the median age of 59 years. To test the effect of naming latencies, OG was divided into two groups based on the naming latencies, with a faster group (OG/RT+; n = 7, M = 830.52 ms, SD = 53.02) and a slower group (OG/RT−; n = 7, M = 1004.61 ms, SD = 68.57). The OG/RT+ and OG/RT− did not differ in terms of age (Z = −0.384, p = 0.710) and other cognitive scores (see Table 2), except for naming latencies (Z = −3.13, p = 0.001).
Table 2.
Non-parametrical analyses between faster older adults (OG/RT+) and slower older adults (OG/RT−) on the object naming task on inclusion criteria, cognitive scores and object naming performance.
| OG/RT+ N = 7 |
OG/RT− N = 7 |
Mann-Whitney test | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Z | p | ||
| Age | 70.71 | 9.14 | 71.71 | 4.46 | −0.384 | 0.710 | |
| Inclusion criteria | Socio-cultural | 3.85 | 0.37 | 3.85 | 0.37 | 0.00 | 1.00 |
| Laterality | 90.42 | 9.88 | 89.57 | 14.39 | −0.332 | 0.805 | |
| MMSE | 28.71 | 1.60 | 29.42 | 0.53 | −0.556 | 0.620 | |
| HAD_A | 5.71 | 1.70 | 6.71 | 2.21 | −1.110 | 0.318 | |
| HAD_D | 3.42 | 2.82 | 4.42 | 2.99 | −0.642 | 0.535 | |
| Dubois | 9.85 | 0.37 | 10.00 | 0.00 | −1.00 | 0.710 | |
| Naming | RTs | 830.52 | 53.02 | 1004.61 | 68.57 | −3.130 | 0.001 |
| %CR | 98.86 | 1.46 | 98.71 | 1.38 | −0.335 | 0.805 | |
| Perceptuo-motor | TMT-A | 42.00 | 14.25 | 52.28 | 7.56 | −1.599 | 0.128 |
| Executive | [TMT-B] minus [TMT-A] | 57.00 | 25.31 | 52.00 | 11.71 | −0.576 | 0.620 |
| Verbal fluency | 21.28 | 8.03 | 19.00 | 5.50 | −0.388 | 0.710 | |
| Language | Verbal automatisms | 35.14 | 2.03 | 34.42 | 1.98 | −0.581 | 0.620 |
| Mill-Hill | 40.00 | 2.51 | 10.14 | 1.67 | −0.258 | 0.805 | |
| Memory | Forward digit span | 8.71 | 2.21 | 8.71 | 2.28 | 0.000 | 1.000 |
| Backward digit span | 8.57 | 3.45 | 9.00 | 2.08 | −1.121 | 0.318 | |
| MacNair | 13.85 | 5.04 | 15.85 | 6.89 | −0.563 | 0.620 | |
Analyses show that the two groups differ only on RTs for naming (in bold). Abbreviation: OG, Older group; MMSE, Mini-Mental State Evaluation; HAD_A, Hospital Anxiety and Depression scale_Anxiety; HAD_D, Hospital Anxiety and Depression scale_Depression; TMT-A, Trail Making Test, version A; TMT-B, Trail Making Test, version B.
Objective 1: naming accuracy
YG and OG were compared on cognitive scores (i.e., language, memory, executive, and perceptuo-motor processes) and naming performance (RTs, %CR) using Mann-Whitney tests. We also performed Mann-Whitney tests on the HR indices between the YG and the OG, to determine which strategy was used to maintain naming accuracy.
Objective 2: naming latencies
Difference in HR indices between YG and OG (based on Objective 1) were controlled for by including RT as a covariate during naming, determining whether the age difference was explained by naming latencies. We also performed Mann-Whitney tests on HR indices between OG/RT+ and OG/RT− to evaluate the cognitive reserve mechanism at intra- and inter-hemispheric levels.
Neuroimaging
Cerebral activation during object naming
Pre-processing
Preprocessing was performed using SPM12 (Welcome Department of Imaging Neuroscience, London, UK1) implemented in MATLAB 2014 (Mathworks Inc., Sherborn, MA, USA). As the use of a study-specific template (SST) is recommended for aging studies to avoid methodological bias attributable to morphometric inter-individual differences (Huang et al., 2010; Fillmore et al., 2015), we first created an SST. The T1-weighted anatomical volumes were coregistered to the mean image created by the realignment of the functional images and segmented via DARTEL using the six tissue probability maps. We generated an SST by matching all of the tissue class images provided during the segmentation step, and then normalized this template to the MNI space. The T1-weighted anatomical volumes were normalized to the SST. Then temporal correction of the realigned functional images was performed. The deformation field generated by the segmentation was subsequently used for the normalization of functional volumes. Finally, each functional volume was smoothed by means of an 8-mm full width at half maximum (FWHM) Gaussian kernel.
Statistical first-level analyses
The fMRI signal was analyzed using the general linear model at an individual level (Friston et al., 1994, 1995). For each participant, two conditions of interest (task, control) were modeled as two regressors, constructed as box-car functions convolved with a canonical hemodynamic response. Movement parameters derived from realignment correction were entered in the design matrix as six additional regressors of no interest, in order to account for motion-related variability. The time series for each voxel were high-pass filtered (1/128 Hz cutoff).
Statistical second-level analyses
One-sample t-test group analysis was performed to obtain activation for the main contrast of interest (task > control) for all participants (N = 27; k = 5; p < 0.05 corrected; t = 5.9). Activated regions were identified and labeled via the macroscopic parcellation of the MNI single subject reference brain (Tzourio-Mazoyer et al., 2002).
ROI analysis
To determine the effect of age and naming latencies on intra and inter-hemispheric asymmetry, we first defined anatomical regions of interest (ROI) based on previously reported results for object naming (Indefrey and Levelt, 2004; Indefrey, 2011) and semantic memory (Binder et al., 2009). We obtained the following ROIs: (a) frontal level: inferior frontal gyrus (IFG), middle frontal gyrus (MFG), superior frontal gyrus (SFG), supplementary motor area (SMA); (b) temporal level: postero-inferior temporal/ fusiform gyrus (FG), middle-inferior temporal gyrus (ITG), middle temporal gyrus (MTG), anterior temporal lobe (ATL) and hippocampus; (c) parietal level: inferior parietal lobule (IPL). We also included the insula, given its role in lexical production (Indefrey and Levelt, 2004). Second, to get ROIs based on naming-task related activity, we defined the ROIs based on the peak activity in our population. To account for anatomo-functional variability of the activation related to age, we defined the coordinates of each ROI separately, for each participant, using the leave-one-out (LOSO) method (Esterman et al., 2010; Prevost et al., 2017). Accordingly, 27 one-sample T-tests analyses (Task > Control contrast) were run, each one leaving out one participant. Based on these analyses, we determined the peak of activation of each ROI for the participant left aside. Each ROI was symmetrically defined in the left and right hemispheres. Each ROI was defined as a sphere (6 mm radius; Heath et al., 2012), centered on the activation peak. For each ROI and each participant, we extracted the mean beta values (i.e., BOLD-contrast signal) for the task and the control condition. BOLD-contrast values, calculated from the difference task minus control, were retained for further analyses. These BOLD-contrast differential values were separated into two groups of category regions (CR), an anterior CR (grouping 4 frontal regions and insula) and a posterior CR (grouping 6 temporo-parietal regions). Table 3 shows the mean spatial coordinates and standard deviation for each ROI. Based on BOLD-contrast task-specific values for each right and left region located in either the anterior or the posterior CR, we defined four HR indices, two at an intra-hemispheric level (anterior vs. posterior) and two at an inter-hemispheric level (left vs. right). The intra-hemispheric HR indices were as follows: left anterior-posterior asymmetry index (LAP), calculated as the difference between left anterior and left posterior CR, and right anterior-posterior asymmetry index (RAP), calculated as the difference between right anterior and right posterior CR. For an illustrative purpose, we mention below the formula used for calculating the LAP index:
| (1) |
Table 3.
Mean and standard deviation for activation peaks for each region of interest (ROI) labeled according to the AAL atlas and to Brodmann Areas.
| x | y | z | AAL label | Brodmann Area | ||
|---|---|---|---|---|---|---|
| Anterior | Left insula | −33.7 (±1.25) | 22.0 (±4.34) | 9.0 (±2.03) | Insula_L | BA 13 |
| Right insula | 33.7 (±1.25) | 22.0 (±4.34) | 9.0 (±2.03) | Insula_R | BA 13 | |
| Left IFG | −54.9 (0.38) | 24 (±0.38) | 23.0 (±0) | Frontal_Inf_Tri_L | BA 45 | |
| Right IFG | 54.9 (±0.38) | 24 (±0.38) | 23.0 (±0) | Frontal_Inf_Tri_R | BA 45 | |
| Left MFG | −29.8 (±0.96) | 10.0 (±0) | 32.3 (±1.73) | Frontal_Mid_L | undefined | |
| Right MFG | 29.8 (±0.96) | 10.0 (±0) | 32.3 (±1.73) | Frontal_Inf_Oper_R | undefined | |
| Left SFG | −11.0 (±0) | 20.4 (±1.88) | 42.1 (±3.34) | Frontal_Sup_L | BA 32 | |
| Right SFG | 14.0 (±0) | 12.0 (±0.38) | 47.0 (±0) | Frontal_Sup_R | undefined | |
| Left SMA | −8.7 (±0.64) | 18.5 (±1.11) | 47.3 (±1.27) | Supp_Motor_Area_L | undefined | |
| Right SMA | 12.2 (±1.87) | 13.9 (±0.38) | 47.0 (±0) | Supp_Motor_Area_R | undefined | |
| Posterior | Left FG | −46.2 (±0.72) | −55.5 (±3.66) | −15.7 (±0.80) | Temporal_Inf_L | BA 37 |
| Right FG | 45.3 (±2.63) | −55.0 (±1.59) | −16.0 (±0) | Temporal_Inf_R | BA 37 | |
| Left ITG | −50.0 (±0) | −50.0 (±0) | −16.0 (±0) | Temporal_Inf_L | BA 20 | |
| Right ITG | 51.0 (±0) | −54.1 (±1.39) | −16.0 (±0) | Temporal_Inf_R | BA 20 | |
| Left MTG | −52.8 (±0, 96) | (±0.57) | −18.8 (±0.57) | Temporal_Mid_R | BA 21 | |
| Left ATL | −32.0 (±0) | 5.0 (±0) | −25.0 (±0) | Temporal_Pole_Sup_L | BA 38 | |
| Right ATL | 35.0 (±0) | 5.0 (±0) | −25.0 (±) | Temporal_Pole_Sup_R | undefined | |
| Left Hipocampus | −26.8 (±0.53) | −11.0 (0.38) | −22.0 (±) | Hippocampus_L | Hippocampus | |
| Right Hippocampus | 25.0 (±0) | −11.0 (±0) | −22.0 (±0) | Hippocampus_R | Hippocampus | |
| Left IPL | −49.8 (±0.53) | −29.2 (±0.8) | 31.5 (±1.6) | SupraMarginal_L | BA 40 | |
| Right IPL | 42.8 (±1.03) | −31.8 (±0.9) | 32.0 (±0) | Undefined | BA 40 |
Abbreviations: BA, Brodmann Area; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; SFG, superior frontal gyrus; SMA, supplementary motor area; FG, fusiform gyrus; ITG, inferior temporal gyrus; MTG, middle temporal gyrus; ATL, anterior temporal lobe; IPL, inferior parietal lobule.
including left anterior1 = left IFG, left anterior2 = left MFG, left anterior3 = left SFG, left anterior4 = left Insula, and left anterior5 = left SMA; left posterior1 = left FG, left posterior2 = left MTG, left posterior3 = left ITG, left posterior4 = left ATL, left posterior5 = left hippocampus, and left posterior6 = left IPL. The inter-hemispheric HR indices were as follows: anterior left-right asymmetry index (ALR), calculated as the difference between left anterior and right anterior CR, and posterior left-right asymmetry index (PLR), calculated as the difference between left posterior and right posterior CR. Each of these four HR indices was used for correlation analyses with age, naming performance, and cognitive scores.
Results
This section is structured in three parts: General results (aging effects on cognitive performance, naming performance and cerebral activity); Objective 1: Naming accuracy, and Objective 2: Naming latencies.
General Results
Cognitive Scores
Results of Man-Whitney analyses performed for cognitive scores in young and old age groups are presented in Table 4.
Table 4.
Non-parametrical analyses between younger participants (YG) and older participants (OG) on naming performance and cognitive scores.
| YG N = 13 |
OG N = 14 |
Mann-Whitney tests | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Z | p | ||
| Naming | RTs | 818.59 | 72.06 | 917.56 | 107.83 | −2.475 | 0.012 |
| %CR | 98.31 | 1.88 | 98.79 | 1.36 | −0.681 | 0.519 | |
| Perceptuo-motor | TMT-A | 30.61 | 9.66 | 47.14 | 12.19 | −3.108 | 0.001 |
| Executive | [TMT-B] minus [TMT-A] | 23.00 | 8.15 | 54.50 | 19.12 | −3.891 | <0.01 |
| Verbal fluency | 28.53 | 10.99 | 20.14 | 6.72 | −1.779 | 0.076 | |
| Language | Verbal automatisms | 30.66 | 3.25 | 34.78 | 1.96 | 0.321 | 0.001 |
| Mill-Hill | 38.23 | 4.30 | 40.07 | 2.05 | −0.933 | 0.375 | |
| Memory | Forward digit span | 10.08 | 2.67 | 8.71 | 2.16 | −1.319 | 0.212 |
| Backward digit span | 9.38 | 2.32 | 8.78 | 2.75 | −1.000 | 0.350 | |
| MacNair | 9.84 | 5.56 | 14.85 | 5.89 | −2.141 | 0.033 | |
Significant differences are in bold. Abbreviation: YG, Younger group; OG, Older group; TMT-A, Trail Making Test, version A; TMT-B, Trail Making Test, version B.
As illustrated in Table 4, we obtained significant differences between YG and OG on the verbal automatisms test (Z = −3.321, p = 0.001), MacNair questionnaire (Z = −2.141, p = 0.033), executive score (Z = −3.981, p < 0.05), and TMT A scores (Z = −3.108, p = 0.001). The difference between YG and OG was not significant for verbal fluency (Z = −1.779, p = 0.076), Mill-Hill scores (Z = −0.933, p = 0.375), and forward (Z = −1.319, p = 0.212) and backward (Z = −1.000, p = 0.350) digit span tests. Overall, OG showed preserved language and memory functioning despite increased subjective memory complaints, with decline of executive functioning and general cognitive slowing.
Naming Performance
As illustrated in Table 4, Man-Whitney analyses between YG and OG on RTs for object naming revealed that older adults were significantly slower than younger adults (Z = −2.473, p = 0.012). Importantly, no difference between YG and OG was observed for naming accuracy (Z = 0.681, p = 0.519).
fMRI Activation during Object Naming
The cerebral network for object naming was obtained by calculating the main contrast, Task vs. Control, that elicited bilateral activation of the FG, ITG and hippocampus, and left activation of the inferior frontal and left pre-central gyri (see Figure 2 and Table 5).
Figure 2.
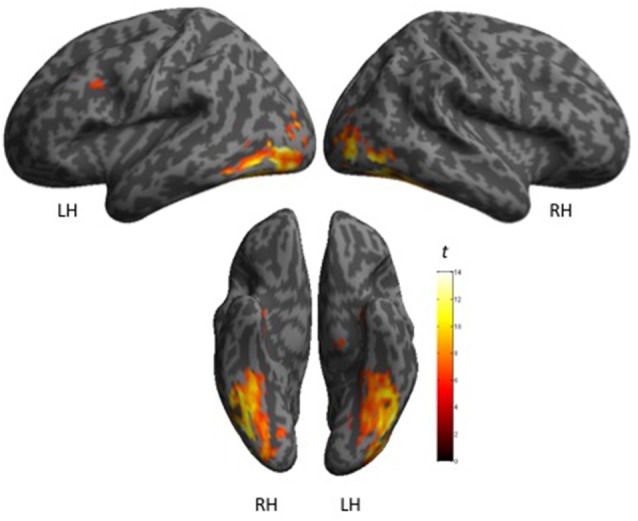
Shows maps of activation during object naming for all participants (N = 27), as projected onto 3D anatomical templates. The color scale indicates the t-value of the activation.
Table 5.
Shows main effect of the object naming in all participants (n = 27), in terms of peaks of activation for the contrast task > control (one sample t-test).
| Cluster | Label | Peak {mm} | Peak (statistics) | |||
|---|---|---|---|---|---|---|
| k | AAL | x | y | z | T | Z |
| 1172 | Occipital_Mid_L | −43 | −80 | −1 | 14.44 | 7.67 |
| Occipital_Inf_L | −46 | −61 | −13 | 12.67 | 7.25 | |
| Fusiform_L | −41 | −66 | −13 | 11.81 | 7.02 | |
| 1161 | Occipital_Inf_R | 39 | −80 | −10 | 14.04 | 7.58 |
| Fusiform_R | 37 | −71 | −13 | 12.77 | 7.28 | |
| Temporal_Inf_R | 44 | −57 | −16 | 12.53 | 7.22 | |
| 82 | Hippocampus_L | −23 | −6 | −22 | 8.20 | 5.81 |
| 79 | Hippocampus_R | 25 | −9 | −22 | 7.63 | 5.57 |
| ParaHippocampal_R | 16 | −4 | −22 | 7.24 | 5.39 | |
| 26 | Precentral_L | −39 | 8 | 29 | 7.50 | 5.51 |
| Frontal_Inf_Tri_L | −41 | 14 | 29 | 7.15 | 5.35 | |
| 60 | Temporal_Inf_R | −9 | −27 | −19 | 7.45 | 5.49 |
The most significant voxels of the cluster are in bold. For each peak, the number of voxels (k), the AAL label, the coordinates (x, y, z; in mm) and the T and Z values are indicated.
Objective 1: Naming Accuracy
Analyses between HR Indices and Age Groups
As illustrated in Table 6a, our results indicate a significant difference between YG and OG only for the LAP indices (Z = −2.329, p = 0.019). The difference was not significant for ALR (p = 0.128), PLR (p = 0.830) and RAP (p = 0.239) indices. Compared to the YG, the OG showed greater activity in the left temporo-parietal regions (YG, Mean = −0.21, SD = 0.22; OG, Mean = −0.48, SD = 0.29).
Table 6.
Non-parametrical analyses between (a) YG and OG, and (b) OG/RT+ and OG/RT− on hemispheric reorganization (HR) indices.
| (a) | LAP | RAP | ALR | PLR | |
|---|---|---|---|---|---|
| YG N = 13 | Mean | −0.21 | −0.32 | 0.13 | 0.02 |
| SD | 0.22 | 0.32 | 0.16 | 0.16 | |
| OG N = 14 | Mean | −0.48 | −0.49 | 0.03 | 0.02 |
| SD | 0.29 | 0.32 | 0.11 | 0.15 | |
| Z | −2.329 | −1.213 | −1.553 | −0.243 | |
| p | 0.019 | 0.239 | 0.128 | 0.830 | |
| (b) | LAP | RAP | ALR | PLR | |
| OG/RT+ N = 7 | Mean | −0.54 | −0.44 | −0.03 | 0.05 |
| SD | 0.28 | 0.28 | 0.08 | 0.11 | |
| OG/RT− N = 7 | Mean | −0.43 | −0.54 | 0.10 | −0.009 |
| SD | 0.32 | 0.36 | 0.10 | 0.20 | |
| Z | −0.575 | −0.319 | −2.236 | −0.192 | |
| p | 0.620 | 0.805 | 0.026 | 0.902 |
Significant differences are in bold. For LAP and RAP, negative values indicate posterior > anterior asymmetry and positive values indicate anterior > posterior asymmetry. For ALR and PLR, negative value indicate right > left asymmetry and positive value indicate left > right asymmetry. Abbreviation: YG, Younger group; OG, Older group; LAP, left anterior-posterior asymmetry; RAP, right anterior-posterior asymmetry; ALR, anterior left-right asymmetry; PLR, posterior left-right asymmetry.
Objective 2: Naming Latencies
When controlling for naming latencies (ANCOVA analysis), the difference between YG and OG on LAP indices (see above) remained significant (F(1, 26) = 6.450, p = 0.018). This result indicates that the increase left temporo-parietal asymmetry in OG was not related to increase naming latencies with age.
Mann-Whitney test comparing OG/RT+ and OG/RT− (see Table 6b) indicates that faster older participants (OG/RT+) showed less inter-hemispheric asymmetry on the ALR indices (Z = −2.236, p = 0.026) than slower older participants (OG/RT−). The difference was not significant for other HR indices (PLR, p = 0.902; LAP, p = 0.620; RAP, p = 0.805).
Discussion
We aimed to evaluate the effect of normal aging on lexical production, in terms of intra- and inter-HR (HR indices) of cerebral activation during an object naming task. Our main objective was to identify possible compensatory mechanisms that could explain the maintenance of accuracy (%CR, Objective 1) in older participants associated with increased lexical production response latencies (RTs, Objective 2). Our results showed increased recruitment of left posterior temporo-parietal cortex along the left anterior-posterior axis in older, compared to younger, participants. This result is in agreement with the semantic hypothesis, suggesting that older adults might enhance the recruitment of semantic knowledge to maintain naming accuracy. Moreover, in older participants, we found an anterior inter-hemispheric asymmetry that correlated with shorter RTs (i.e., better performance), suggesting that the bilateral anterior frontal regions might reflect compensatory executive-based mechanisms in relation to the cognitive reserve. The following section presents a more detailed and comprehensive discussion of our results, with two parts: (1) aging effects on cognitive performance; and (2) aging effects on HR patterns during object naming in relation to accuracy and response latencies.
Aging Effects on Cognitive Performance
Based on cognitive scores, our older participants were less efficient for executive functions and visual analysis, in line with other studies showing aging effects for executive functions (Tomer and Levin, 1993; Cepeda et al., 2001; Ashendorf et al., 2008; Turner and Spreng, 2012), perceptual processing and speed of sensory-motor processing (Cerella et al., 1990; Salthouse, 2004). However, in our study, working memory (backward digit span) and verbal short-term memory (forward-digit span) remained intact in older participants, contrary to other findings (Hultsch et al., 1992; Wang et al., 2011; Logie and Morris, 2014). The fact that, in this study, older adults were well educated and had high socio-cultural levels might explain the preservation of some cognitive abilities (Orsini et al., 1986). Despite adequate scores for both short and long term memory, older participants reported subjective memory complaints more frequently. However, it has been shown that subjective memory complaints may be unrelated to objective deficits (Bolla et al., 1991; Mol et al., 2006). Finally, verbal automatisms increased in older participants, suggesting an increase of overlearned knowledge and automatic retrieval processes with age. Age differences for verbal automatisms scores may point to a cohort effect that could potentially skew interpretations of the results (see Froger et al., 2009 who compared verbal automatisms between older groups only, for example). Overall, our findings are in agreement with previous studies suggesting that older adults show a greater decline in executive functions than in language functions (Glisky, 2007; Harada et al., 2013).
Aging Effects on Hemispheric Reorganization (HR) during Naming in Relation to Accuracy and Response Latency
The main contrast, Task minus Control, revealed that object naming elicited activation of the classical cerebral network reported for this task, including bilateral FG (perceptual and semantic processes; Whatmough et al., 2002; Mion et al., 2010; Ding et al., 2016), bilateral ITG (conceptual lexical retrieval; Indefrey, 2011), bilateral hippocampus (semantic memory retrieval; Sawrie et al., 2000; Binder and Desai, 2011), left IFG (lexico-semantic selection and phonological processes; Binder et al., 2009; Indefrey, 2011) and left pre-central gyrus (syllabification and articulatory processes, output phonology; Indefrey, 2011). Based on naming results, older participants were as accurate as younger ones, suggesting unimpaired sized vocabulary and lexical knowledge. This is consistent with other findings (Villardita et al., 1985; Wierenga et al., 2008; Boudiaf et al., 2016; see Goulet et al., 1994 for a review), allowing us to evaluate possible strategic differences between older and younger adults in manipulating lexical information during object naming. Indeed, a first indication of differential strategies according to age is that, despite normal accuracy, older adults were significantly slower than younger adults. The increased response times in older adults have been frequently reported by other studies and are attributable to various causes, including general slowing of processing speed (Salthouse, 1996; Feyereisen et al., 1998), decline of executive functioning (Craik and Byrd, 1982; West, 2000; Lustig et al., 2007), decline of working memory (Kemper and Sumner, 2001; Waters and Caplan, 2005), decline of perceptual processes (Baltes and Lindenberger, 1997; Schneider and Pichora-Fuller, 2000), and a deficit in lexical access (Bowles, 1989; Barresi et al., 2000; Mirman and Britt, 2013). Lima et al. (1991) proposed that a common general slowing of processing speed can explain aging effects for both lexical (domain-specific) and non-lexical (domain-general) processes. In the same vein, Rogalski et al. (2011) showed that longer RTs for naming in older participants were attributable to a slowing down of general processing, rather than to perceptual or contextual deficits. The idea that naming speed is related to executive and/or other general processes is also consistent with our previous findings (Baciu et al., 2016; Boudiaf et al., 2016) on lexical production with aging. Furthermore, we observed that the OG might be divided into two groups based on naming latencies, the faster OG (OG/RT+) and the slower OG (OG/RT−). The two subgroups differed only on naming latencies, raising the question of what mechanisms led to the situation in which the OG/RT+ group showed shorter RTs than the OG/RT− group in the context of cognitive reserve theory. The following section is divided into two parts, (a) the relation between preserved naming accuracy and HR mechanisms with aging (younger vs. older participants); and (b) the relation between naming latencies and HR mechanisms with aging and between older participants.
As described in the previous sections, our analyses were mainly based on the calculation of two intra- (LAP and RAP) and two inter-hemispheric (ALR and PLR) HR indices reflecting patterns of reorganization. Based on previous studies, we initially hypothesized that two strategies could be developed by older adults to perform lexical production: a semantic strategy suggesting supplementary involvement of semantic processes (Ansado et al., 2013; Lacombe et al., 2015) and/or an executive strategy assuming that in case of difficulty in performing the task, older adults would recruit executive functions to retrieve and generate words faster (Wierenga et al., 2008). We observed that LAP asymmetry was higher for older participants than younger ones, suggesting the use of a semantic strategy to perform naming with aging. Our findings are in line with Ansado et al. (2013) and Lacombe et al. (2015), as these authors suggest that older participants rely on preserved semantic mechanisms by over-recruiting left temporo-parietal regions to perform the task. We posit that this result, referred as the left anterior-posterior aging effect (LAPA), reflects a domain-specific (linguistic) strategy to maintain performance in older adults for tasks involving semantic processes. However, further studies are needed to determine the direct relationship between semantic processing and the LAPA effect. Our study only shows an indirect relation based on anatomical regions, but has the virtue of highlighting a new HR mechanism that was not discussed otherwise. The LAPA effect was not mediated by naming latencies, reinforcing is compensatory role to naming.
In the older group, participants with faster naming latencies (OG/RT+) showed reduced anterior inter-hemispheric asymmetry as compared to participants with slower latencies (OG/RT−). This suggests a compensatory role for the bilateral frontal regions, in line with the HAROLD model (Cabeza, 2002; Cabeza et al., 2002). Similar effects and interpretations were previously revealed for other processes, such as episodic memory, working memory, and inhibitory control (see Cabeza, 2002 for a review). Altogether, these findings indicate that bilateral frontal recruitment reflects domain-general, unspecific compensatory mechanisms occurring with age. Cappell et al. (2010) reported that the recruitment of right prefrontal cortex for a verbal working memory task was dependent on the task demands in older participants. Specifically, the right prefrontal cortex was additionally recruited for low task-demands and was associated with normal performance. With higher task demands, the recruitment of prefrontal cortex decreased in parallel with performance, reflecting its failure to adapt to increased task demands above a certain threshold. In our study, the over-activation of right frontal regions in faster older adults may reflect compensatory recruitment of executive and attentional processes (Weissman and Banich, 2000). However, when task demands become too high, older participants fail to recruit the right hemisphere, resulting in increased naming latencies. In relation to the cognitive reserve theory (Stern, 2002, 2009), we suggest that the ability to recruit bilateral frontal regions during naming reflects higher neural networks flexibility to cope with higher task demands. These preliminary results need to be validated by further experiments to understand better the differential recruitment of frontal executive functions in older adults, according to their performance and the level of task-demands. Indeed, task demands are classically induced by the task (e.g., low demand task conditions vs. high demand task conditions). For example, it would be interesting to evaluate the effect of task-demands induced by the task (e.g., using low and high frequency words) to determine the relationship between bilateral frontal recruitment (HAROLD model) and the ability to cope with task-demands (cognitive reserve).
Overall, our results suggest at least two co-existent HR patterns and two strategic mechanisms at play in object naming, according to age and to performance: the LAPA effect and the HAROLD model. The newly described model, the LAPA effect, entails an intra-hemispheric left asymmetry with greater involvement of temporo-parietal regions explained by supplementary access to semantic resources engaged to perform a lexical production task. In contrast, the HAROLD model, also tested in older adults, is related to more domain-general mechanisms, consisting of supplementary recruitment of frontal executive regions to maintain a good level of performance. Our findings—mainly the LAPA effect—add to other existing models already described in the literature, such as the CRUNCH (Reuter-Lorenz and Cappell, 2008) and STAC (Reuter-Lorenz and Park, 2014 for a revised version) models. However, these models suggest that multidimensional sources may contribute to the diverse mechanisms and strategies developed with aging, such as anatomo-functional properties (e.g., brain volume, white matter integrity, neural specificity), functional reorganization (e.g., bilateral frontal recruitment, neurogenesis), life experience (e.g., intellectual activities, stress), and learning or training. Thus, assessment of aging effects on cognitive tasks, should be considered in the context of many other factors including occupation, daily and social activities, nutrition, and exercise (Fratiglioni et al., 2004; Paillard-Borg et al., 2009; Allès et al., 2012; Small et al., 2012).
A main limitation of this study is related to the sample size, as only 27 participants took part in our experiment, limiting the statistical power of our analyses. As a consequence, we used non-parametric analyses that are adapted for small sample size, allowing us to compare subgroups (YG vs. OG; OG/RT+ vs. OG/RT−). Furthermore, the small sample size limited our analysis approach to selecting subject-specific ROIs for the computation of HR indices. Hence, having only one group of participants, it was not possible to select activity peaks from one sample, and to analyze BOLD-contrast signal changes from the another. The later approach is more conservative in removing serial testing effects (Kriegeskorte et al., 2010), especially when studying age effects. By using the leave-one-out methodology, we posit that the deep circularity issue, although not totally removed, is diminished (Esterman et al., 2010). Further studies are needed to improve detection sensitivity by increasing the number of participants.
Conclusion
Our findings suggest that aging has separate and differential effects on the mechanisms and cerebral substrates of lexical production: (a) a LAPA effect with asymmetric anterior-posterior activation and supplementary recruitment of temporo-parietal regions in older adults, suggesting the use of a semantic strategy; (b) bilateral frontal activation (i.e., the HAROLD pattern) in faster older adults, suggesting executive-based and domain-general strategies. The originality of our findings lies in both the description of a new pattern of HR occurring in aging, the LAPA effect, and in underscoring the fact that several mechanisms, involving different strategies and patterns of reorganization might coexist when performing a cognitive task, depending on difficulty, performance and age.
Author Contributions
EH was involved in design, data analysis, data interpretation, drafting and final approval of the submitted version and approval for all aspects of the project. NB was involved in design and data analysis, final approval of the submitted version and approval for all aspects of the work. EC was involved in data analysis, data interpretation, final approval of the submitted version and approval for all aspects of the project. CP was involved in data analysis, final approval of the submitted version and approval for all aspects of the work. NF was involved in design and neuropsychological evaluation, final approval of the submitted version and approval for all aspects of the work. AK was involved in drafting and final approval of the submitted version and approval for all aspects of the project. AJ was involved in data analysis, data interpretation, drafting work, final approval of the submitted version and approval for all aspects of the project. MB was involved in design, data interpretation, drafting work, final approval of the submitted version and approval for all aspects of the project.
Funding
This work has been funded by ARC2 “Qualité de vie and Vieillissement” from Région Rhônes-Alpes in France.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Grenoble MRI IRMaGE unit for enabling us to perform the fMRI acquisitions. This unit is partly funded by the French program “Investissement d’Avenir” run by “Agence Nationale pour la Recherche” (Grant “Infrastructure d’Avenir en Biologie Santé”, ANR-11-INBS-0006).
Footnotes
References
- Allès B., Samieri C., Féart C., Jutand M.-A., Laurin D., Barberger-Gateau P. (2012). Dietary patterns: a novel approach to examine the link between nutrition and cognitive function in older individuals. Nutr. Res. Rev. 25, 207–222. 10.1017/s0954422412000133 [DOI] [PubMed] [Google Scholar]
- Ansado J., Marsolais Y., Methqal I., Alary F., Joanette Y. (2013). The adaptive aging brain: evidence from the preservation of communication abilities with age. Eur. J. Neurosci. 37, 1887–1895. 10.1111/ejn.12252 [DOI] [PubMed] [Google Scholar]
- Ashendorf L., Jefferson A. L., O’Connor M. K., Chaisson C., Green R. C., Stern R. A. (2008). Trail Making Test errors in normal aging, mild cognitive impairment and dementia. Arch. Clin. Neuropsychol. 23, 129–137. 10.1016/j.acn.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baciu M., Boudiaf N., Cousin E., Perrone-Bertolotti M., Pichat C., Fournet N., et al. (2016). Functional MRI evidence for the decline of word retrieval and generation during normal aging. Age (Dordr) 38:3. 10.1007/s11357-015-9857-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes P. B., Lindenberger U. (1997). Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol. Aging 12, 12–21. 10.1037/0882-7974.12.1.12 [DOI] [PubMed] [Google Scholar]
- Barresi B. A., Nicholas M., Tabor Connor L., Obler L. K., Albert M. L. (2000). Semantic degradation and lexical access in age-related naming failures. Aging Neuropsychol. Cogn. 7, 169–178. 10.1076/1382-5585(200009)7:3;1-q;ft169 [DOI] [Google Scholar]
- Beauregard A. (1971). Le Test des Automatismes Verbaux. Issy-les-Moulineaux: Editions Scientifiques et Psychotechniques. [Google Scholar]
- Binder J. R., Desai R. H. (2011). The neurobiology of semantic memory. Trends Cogn. Sci. 15, 527–536. 10.1016/j.tics.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J. R., Desai R. H., Graves W. W., Conant L. L. (2009). Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex 19, 2767–2796. 10.1093/cercor/bhp055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma P., Weenink D. (2001). Praat speech processing software. Institute of Phonetics Sciences of the University of Amsterdam. Available Online at: http://www.praat.org
- Bolla K. I., Lindgren K. N., Bonaccorsy C., Bleecker M. L. (1991). Memory complaints in older adults. Fact or fiction? Arch. Neurol. 48, 61–64. 10.1001/archneur.1991.00530130069022 [DOI] [PubMed] [Google Scholar]
- Boudiaf N., Laboissière R., Cousin E., Fournet N., Krainik A., Baciu M. (2016). Behavioral evidence for a differential modulation of semantic processing and lexical production by aging: a full linear mixed-effects modeling approach. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 24, 1–22. 10.1080/13825585.2016.1257100 [DOI] [PubMed] [Google Scholar]
- Bowles N. L. (1989). Age and semantic inhibition in word retrieval. J. Gerontol. 44, P88–P90. 10.1093/geronj/44.3.p88 [DOI] [PubMed] [Google Scholar]
- Burke D. M., Shafto M. A. (2004). Aging and language production. Curr. Dir. Psychol. Sci. 13, 21–24. 10.1111/j.0963-7214.2004.01301006.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. (2002). Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol. Aging 17, 85–100. 10.1037/0882-7974.17.1.85 [DOI] [PubMed] [Google Scholar]
- Cabeza R., Anderson N. D., Locantore J. K., McIntosh A. R. (2002). Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage 17, 1394–1402. 10.1006/nimg.2002.1280 [DOI] [PubMed] [Google Scholar]
- Cappell K. A., Gmeindl L., Reuter-Lorenz P. A. (2010). Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex 46, 462–473. 10.1016/j.cortex.2009.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardebat D., Doyon B., Puel M., Goulet P., Joanette Y. (1990). Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level. Acta Neurol. Belg. 90, 207–217. [PubMed] [Google Scholar]
- Cavanaugh J. C., Grady J. G., Perlmutter M. (1983). Forgetting and use of memory aids in 20 to 70 year olds everyday life. Int. J. Aging Hum. Dev. 17, 113–122. 10.2190/h7l2-k3xk-h32k-vw89 [DOI] [PubMed] [Google Scholar]
- Cepeda N. J., Kramer A. F., Gonzalez de Sather J. (2001). Changes in executive control across the life span: examination of task-switching performance. Dev. Psychol. 37, 715–730. 10.1037/0012-1649.37.5.715 [DOI] [PubMed] [Google Scholar]
- Cerella J., Birren J. E., Schaie K. W. (1990). Aging and information-processing rate. Handbook of the psychology of aging 3, 201–221. 10.1016/b978-0-12-101280-9.50018-8 [DOI] [Google Scholar]
- Cohen G., Faulkner D. (1986). Memory for proper names: age differences in retrieval. Br. J. Dev. Psychol. 4, 187–197. 10.1111/j.2044-835x.1986.tb01010.x [DOI] [Google Scholar]
- Corrigan J. D., Hinkeldey N. S. (1987). Relationships between parts A and B of the trail making test. J. Clin. Psychol. 43, 402–409. [DOI] [PubMed] [Google Scholar]
- Craik F. I. M., Byrd M. (1982). “Aging and cognitive deficits,” in Aging and Cognitive Processes, eds Craik F. I. M., Trehub S. (New York, NY: Plenum Press; ), 191–211. [Google Scholar]
- Davis S. W., Dennis N. A., Daselaar S. M., Fleck M. S., Cabeza R. (2008). Que PASA? The posterior-anterior shift in aging. Cereb. Cortex 18, 1201–1209. 10.1093/cercor/bhm155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deltour J. J. (1993). “Echelle de vocabulaire de Mill Hill de JC Raven,” in Adaptation française et normes européennes du Mill Hill et du Standard Progressive Matrices de Raven (PM38), (Braine-le-Château: Editions l’application des techniques modernes; ). [Google Scholar]
- Diaz M. T., Rizio A. A., Zhuang J. (2016). The neural language systems that support healthy aging: integrating function, structure and behavior. Lang. Linguist. Compass 10, 314–334. 10.1111/lnc3.12199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J., Chen K., Chen Y., Fang Y., Yang Q., Lv Y., et al. (2016). The left fusiform gyrus is a critical region contributing to the core behavioral profile of semantic dementia. Front. Hum. Neurosci. 10:215. 10.3389/fnhum.2016.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B., Touchon J., Portet F., Ousset P. J., Vellas B., Michel B. (2002). “The 5 words”: a simple and sensitive test for the diagnosis of Alzheimer’s disease. Presse Med. 31, 1696–1699. [PubMed] [Google Scholar]
- Duffau H., Moritz-Gasser S., Mandonnet E. (2014). A re-examination of neural basis of language processing: proposal of a dynamic hodotopical model from data provided by brain stimulation mapping during object naming. Brain Lang. 131, 1–10. 10.1016/j.bandl.2013.05.011 [DOI] [PubMed] [Google Scholar]
- Esterman M., Tamber-Rosenau B. J., Chiu Y. C., Yantis S. (2010). Avoiding non-independence in fMRI data analysis: leave one subject out. Neuroimage 50, 572–576. 10.1016/j.neuroimage.2009.10.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard M. (2002). Ageing and lexical access to common and proper names in picture naming. Brain Lang. 81, 174–179. 10.1006/brln.2001.2515 [DOI] [PubMed] [Google Scholar]
- Feyereisen P., Demaeght N., Samson D. (1998). Why do object naming latencies increase with age: general slowing, greater sensitivity to interference, or task-specific deficits? Exp. Aging Res. 24, 21–51. 10.1080/036107398244346 [DOI] [PubMed] [Google Scholar]
- Fillmore P. T., Phillips-Meek M. C., Richards J. E. (2015). Age-specific MRI brain and head templates for healthy adults from 20 through 89 years of age. Front. Aging Neurosci. 7:44. 10.3389/fnagi.2015.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Fratiglioni L., Paillard-Borg S., Winblad B. (2004). An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 3, 343–353. 10.1016/s1474-4422(04)00767-7 [DOI] [PubMed] [Google Scholar]
- Friston K. J., Holmes A. P., Poline J. B., Grasby P. J., Williams S. C., Frackowiak R. S. J., et al. (1995). Analysis of fMRI time-series revisited. Neuroimage 2, 45–53. 10.1006/nimg.1995.1007 [DOI] [PubMed] [Google Scholar]
- Friston K. J., Holmes A. P., Worsley K. J., Poline J.-P., Frith C. D., Frackowiak R. S. J. (1994). Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 2, 189–210. 10.1002/hbm.460020402 [DOI] [Google Scholar]
- Froger C., Taconnat L., Landré L., Beigneux K., Isingrini M. (2009). Effects of level of processing at encoding and types of retrieval task in mild cognitive impairment and normal aging. J. Clin. Exp. Neuropsychol. 31, 312–321. 10.1080/13803390802112554 [DOI] [PubMed] [Google Scholar]
- Galdo-Alvarez S., Lindín M., Díaz F. (2009). Age-related prefrontal over-recruitment in semantic memory retrieval: evidence from successful face naming and the tip-of-the-tongue state. Biol. Psychol. 82, 89–96. 10.1016/j.biopsycho.2009.06.003 [DOI] [PubMed] [Google Scholar]
- Glisky E. L. (2007). “Changes in cognitive function in human aging,” in Brain Aging: Models, Methods, and Mechanisms, ed. Riddle D. R. (Boca Raton, FL: CRC Press/Taylor & Francis Group; ), 3–20. [Google Scholar]
- Goulet P., Ska B., Kahn H. J. (1994). Is there a decline in object naming with advancing age? J. Speech Lang. Hear. Res. 37, 629–644. 10.1044/jshr.3703.629 [DOI] [PubMed] [Google Scholar]
- Grady C. (2012). The cognitive neuroscience of ageing. Nat. Rev. Neurosci. 13, 491–505. 10.1038/nrn3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C. L., Maisog J. M., Horwitz B., Ungerleider L. G., Mentis M. J., Salerno J. A., et al. (1994). Age-related changes in cortical blood flow activation during visual processing of faces and location. J. Neurosci. 14, 1450–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood P. M. (2007). Functional plasticity in cognitive aging: review and hypothesis. Neuropsychology 21, 657–673. 10.1037/0894-4105.21.6.657 [DOI] [PubMed] [Google Scholar]
- Gutchess A. H., Welsh R. C., Hedden T., Bangert A., Minear M., Liu L. L., et al. (2005). Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J. Cogn. Neurosci. 17, 84–96. 10.1162/0898929052880048 [DOI] [PubMed] [Google Scholar]
- Harada C. N., Natelson Love M. C., Triebel K. L. (2013). Normal cognitive aging. Clin. Geriatr. Med. 29, 737–752. 10.1016/j.cger.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath S., McMahon K., Nickels L., Angwin A., MacDonald A., van Hees S., et al. (2012). The neural correlates of picture naming facilitated by auditory repetition. BMC Neurosci. 13:21. 10.1186/1471-2202-13-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helder E. J., Zuverza-Chavarria V., Whitman R. D. (2016). Executive functioning and lateralized semantic priming in older adults. Cogent Psychol. 3:1182687 10.1080/23311908.2016.1182687 [DOI] [Google Scholar]
- Huang C.-M., Lee S.-H., Hsiao T., Kuan W.-C., Wai Y.-Y., Ko H.-J., et al. (2010). Study-specific EPI template improves group analysis in functional MRI of young and older adults. J. Neurosci. Methods 189, 257–266. 10.1016/j.jneumeth.2010.03.021 [DOI] [PubMed] [Google Scholar]
- Hultsch D. F., Hertzog C., Small B. J., McDonald-Miszczak L., Dixon R. A. (1992). Short-term longitudinal change in cognitive performance in later life. Psychol. Aging 7, 571–584. 10.1037/0882-7974.7.4.571 [DOI] [PubMed] [Google Scholar]
- Hultsch D. F., MacDonald S. W., Dixon R. A. (2002). Variability in reaction time performance of younger and older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 57, P101–P115. 10.1093/geronb/57.2.p101 [DOI] [PubMed] [Google Scholar]
- Indefrey P. (2011). The spatial and temporal signatures of word production components: a critical update. Front. Psychol. 2:255. 10.3389/fpsyg.2011.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P., Levelt W. J. (2004). The spatial and temporal signatures of word production components. Cognition 92, 101–144. 10.1016/j.cognition.2002.06.001 [DOI] [PubMed] [Google Scholar]
- James L. E. (2004). Meeting Mr. Farmer versus meeting a farmer: specific effects of aging on learning proper names. Psychol. Aging 19, 515–522. 10.1037/0882-7974.19.3.515 [DOI] [PubMed] [Google Scholar]
- Kalafat M., Hugonot-Diener L., Poitrenaud J. (2003). Standardisation et étalonnage français du “Mini Mental State” (MMS) version GRECO. Revue de neuropsychologie 13, 209–236. [Google Scholar]
- Kemper S., Sumner A. (2001). The structure of verbal abilities in young and older adults. Psychol. Aging 16, 312–322. 10.1037/0882-7974.16.2.312 [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N., Lindquist M. A., Nichols T. E., Poldrack R. A., Vul E. (2010). Everything you never wanted to know about circular analysis, but were afraid to ask. J. Cereb. Blood Flow Metab. 30, 1551–1557. 10.1038/jcbfm.2010.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe J., Jolicoeur P., Grimault S., Pineault J., Joubert S. (2015). Neural changes associated with semantic processing in healthy aging despite intact behavioral performance. Brain Lang. 149, 118–127. 10.1016/j.bandl.2015.07.003 [DOI] [PubMed] [Google Scholar]
- Laver G. D., Burke D. M. (1993). Why do semantic priming effects increase in old age? A meta-analysis. Psychol. Aging 8, 34–43. 10.1037/0882-7974.8.1.34 [DOI] [PubMed] [Google Scholar]
- Lima S. D., Hale S., Myerson J. (1991). How general is general slowing? Evidence from the lexical domain. Psychol. Aging 6, 416–425. 10.1037/0882-7974.6.3.416 [DOI] [PubMed] [Google Scholar]
- Logie R. H., Morris R. G. (2014). Working Memory and Ageing. New York, NY: Psychology Press. [Google Scholar]
- Lövdén M., Bäckman L., Lindenberger U., Schaefer S., Schmiedek F. (2010). A theoretical framework for the study of adult cognitive plasticity. Psychol. Bull. 136, 659–676. 10.1037/a0020080 [DOI] [PubMed] [Google Scholar]
- Lustig C., Hasher L., Zacks R. T. (2007). Inhibitory deficit theory: recent developments in a “new view”. Inhib. Cogn. 17, 145–162. 10.1037/11587-008 [DOI] [Google Scholar]
- McNair D. M., Kahn R. J. (1983). “Self-assessment of cognitive deficits,” in Assessment in Geriatric Psychopharmacology, eds Crook T., Ferris S., Bartus R. (New Canaan, CT: Mark Powley Associates; ), 137–143. [Google Scholar]
- Metz-Lutz M. N., Kremin H., Deloche G., Hannequin D., Ferrand L., Perrier D., et al. (1991). Standardisation d’un test de dénomination orale: contrôle des effets de l’âge, du sexe et du niveau de scolarité chez les sujets adultes normaux. Rev. Neuropsychol. 1, 73–95. [Google Scholar]
- Mion M., Patterson K., Acosta-Cabronero J., Pengas G., Izquierdo-Garcia D., Hong Y. T., et al. (2010). What the left and right anterior fusiform gyri tell us about semantic memory. Brain 133, 3256–3268. 10.1093/brain/awq272 [DOI] [PubMed] [Google Scholar]
- Mirman D., Britt A. E. (2013). What we talk about when we talk about access deficits. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369:20120388. 10.1098/rstb.2012.0388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol M. E. M., van Boxtel M., Willems D., Jolles J. (2006). Do subjective memory complaints predict cognitive dysfunction over time? A six-year follow-up of the Maastricht Aging Study. Int. J. Geriatr. Psychiatry 21, 432–441. 10.1002/gps.1487 [DOI] [PubMed] [Google Scholar]
- Morcom A. M., Johnson W. (2015). Neural reorganization and compensation in aging. J. Cogn. Neurosci. 27, 1275–1285. 10.1162/jocn_a_00783 [DOI] [PubMed] [Google Scholar]
- Obler L. K., Albert M. L. (1980). Language and Communication in the Elderly: Clinical, Therapeutic, and Experimental Issues. Lexington, MA: Lexington Books. [Google Scholar]
- Obler L. K., Rykhlevskaia E., Schnyer D., Clark-Cotton M. R., Spiro A., III, Hyun J., et al. (2010). Bilateral brain regions associated with naming in older adults. Brain Lang. 113, 113–123. 10.1016/j.bandl.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Orsini A., Chiacchio L., Cinque M., Cocchiaro C., Schiappa O., Grossi D. (1986). Effects of age, education and sex on two tests of immediate memory: a study of normal subjects from 20 to 99 years of age. Percept. Mot. Skills 63, 727–732. 10.2466/pms.1986.63.2.727 [DOI] [PubMed] [Google Scholar]
- Paillard-Borg S., Wang H. X., Winblad B., Fratiglioni L. (2009). Pattern of participation in leisure activities among older people in relation to their health conditions and contextual factors: a survey in a Swedish urban area. Ageing Soc. 29, 803–821. 10.1017/s0144686x08008337 [DOI] [Google Scholar]
- Park D. C., Polk T. A., Park R., Minear M., Savage A., Smith M. R. (2004). Aging reduces neural specialization in ventral visual cortex. Proc. Natl. Acad. Sci. U S A 101, 13091–13095. 10.1073/pnas.0405148101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D. C., Reuter-Lorenz P. (2009). The adaptive brain: aging and neurocognitive scaffolding. Annu. Rev. Psychol. 60, 173–196. 10.1146/annurev.psych.59.103006.093656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K., Nestor P. J., Rogers T. T. (2007). Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 8, 976–987. 10.1038/nrn2277 [DOI] [PubMed] [Google Scholar]
- Perlmutter M. (1978). What is memory aging the aging of? Dev. Psychol. 14, 330–345. 10.1037/0012-1649.14.4.330 [DOI] [Google Scholar]
- Prevost M., Hot P., Muller L., Ruffieux B., Cousin E., Pichat C., et al. (2017). Neural correlates of the healthiness evaluation processes of food labels. Nutr. Neurosci. 11, 1–11. 10.1080/1028415X.2017.1309820 [DOI] [PubMed] [Google Scholar]
- Rajah M. N., D’Esposito M. (2005). Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain 128, 1964–1983. 10.1093/brain/awh608 [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P. A., Cappell K. A. (2008). Neurocognitive aging and the compensation hypothesis. Curr. Dir. Psychol. Sci. 17, 177–182. 10.1111/j.1467-8721.2008.00570.x [DOI] [Google Scholar]
- Reuter-Lorenz P. A., Park D. C. (2014). How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol. Rev. 24, 355–370. 10.1007/s11065-014-9270-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski Y., Peelle J. E., Reilly J. (2011). Effects of perceptual and contextual enrichment on visual confrontation naming in adult aging. J. Speech Lang. Hear. Res. 54, 1349–1360. 10.1044/1092-4388(2011/10-0178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T. A. (1996). The processing-speed theory of adult age differences in cognition. Psychol. Rev. 103, 403–428. 10.1037/0033-295x.103.3.403 [DOI] [PubMed] [Google Scholar]
- Salthouse T. A. (2004). What and when of cognitive aging. Curr. Dir. Psychol. Sci. 13, 140–144. 10.1111/j.0963-7214.2004.00293.x [DOI] [Google Scholar]
- Salthouse T. A., Mandell A. R. (2013). Do age-related increases in tip-of-the-tongue experiences signify episodic memory impairments? Psychol. Sci. 24, 2489–2497. 10.1177/0956797613495881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawrie S. M., Martin R. C., Gilliam F. G., Faught R. E., Maton B., Hugg J. W., et al. (2000). Visual confrontation naming and hippocampal function: a neural network study using quantitative 1H magnetic resonance spectroscopy. Brain 123, 770–780. 10.1093/brain/123.4.770 [DOI] [PubMed] [Google Scholar]
- Schneider B. A., Pichora-Fuller M. K. (2000). Implications of perceptual deterioration for cognitive aging research, in The Handbook of Aging and Cognition 2nd Edn. eds Craik F. I. M., Salthouse T. A. (Mahwah, NJ: Lawrence Erlbaum Associates; ), 155–219. [Google Scholar]
- Ska B., Goulet P. (1989). Trouble de dénomination lors du vieillissement normal. Langages 24, 112–127. 10.3406/lgge.1989.1562 [DOI] [Google Scholar]
- Small B. J., Dixon R. A., McArdle J. J., Grimm K. J. (2012). Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria Longitudinal Study. Neuropsychology 26, 144–155. 10.1037/a0026579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 8, 448–460. 10.1017/s1355617702813248 [DOI] [PubMed] [Google Scholar]
- Stern Y. (2009). Cognitive reserve. Neuropsychologia 47, 2015–2028. 10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh T. N. (2004). Trail making test A and B: normative data stratified by age and education. Arch. Clin. Neuropsychol. 19, 203–214. 10.1016/s0887-6177(03)00039-8 [DOI] [PubMed] [Google Scholar]
- Tomer R., Levin B. E. (1993). Differential effects of aging on two verbal fluency tasks. Percept. Mot. Skills 76, 465–466. 10.2466/pms.1993.76.2.465 [DOI] [PubMed] [Google Scholar]
- Turner G. R., Spreng R. N. (2012). Executive functions and neurocognitive aging: dissociable patterns of brain activity. Neurobiol. Aging 33, 826.e1–826.13. 10.1016/j.neurobiolaging.2011.06.005 [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Verhaeghen P. (2003). Aging and vocabulary scores: a meta-analysis. Psychol. Aging 18, 332–339. 10.1037/0882-7974.18.2.332 [DOI] [PubMed] [Google Scholar]
- Villardita C., Cultrera S., Cupone V., Mejìa R. (1985). Neuropsychological test performances and normal aging. Arch. Gerontol. Geriatr. 4, 311–319. 10.1016/0167-4943(85)90038-x [DOI] [PubMed] [Google Scholar]
- Votruba K. L., Persad C., Giordani B. (2016). Cognitive deficits in healthy elderly population with “normal” scores on the mini-mental state examination. J. Geriatr. Psychiatry Neurol. 29, 126–132. 10.1177/0891988716629858 [DOI] [PubMed] [Google Scholar]
- Wang M., Gamo N. J., Yang Y., Jin L. E., Wang X. J., Laubach M., et al. (2011). Neuronal basis of age-related working memory decline. Nature 476, 210–213. 10.1038/nature10243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters G., Caplan D. (2005). The relationship between age, processing speed, working memory capacity and language comprehension. Memory 13, 403–413. 10.1080/09658210344000459 [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1997). WAIS-III: Wechsler Adult Intelligence Scale. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Weissman D. H., Banich M. T. (2000). The cerebral hemispheres cooperate to perform complex but not simple tasks. Neuropsychology 14, 41–59. 10.1037/0894-4105.14.1.41 [DOI] [PubMed] [Google Scholar]
- West R. (2000). In defense of the frontal lobe hypothesis of cognitive aging. J. Int. Neuropsychol. Soc. 6, 727–729. 10.1017/s1355617700666109 [DOI] [PubMed] [Google Scholar]
- Whatmough C., Chertkow H., Murtha S., Hanratty K. (2002). Dissociable brain regions process object meaning and object structure during object naming. Neuropsychologia 40, 174–186. 10.1016/s0028-3932(01)00083-5 [DOI] [PubMed] [Google Scholar]
- Wierenga C. E., Benjamin M., Gopinath K., Perlstein W. M., Leonard C. M., Rothi L. J., et al. (2008). Age-related changes in word retrieval: role of bilateral frontal and subcortical networks. Neurobiol. Aging 29, 436–451. 10.1016/j.neurobiolaging.2006.10.024 [DOI] [PubMed] [Google Scholar]
- Wingfield A., Grossman M. (2006). Language and the aging brain: patterns of neural compensation revealed by functional brain imaging. J. Neurophysiol. 96, 2830–2839. 10.1152/jn.00628.2006 [DOI] [PubMed] [Google Scholar]
- Zelinski E. M., Gilewski M. J., Thompson L. W. (1980). “Do laboratory tests relate to self-assessment of memory ability in the young and old?,” in New Directions in Memory and Aging, eds Poon L. W., Fozard J. L., Cermak L. L. S., Arenberg D., Thompson L. W. (Hillsdale, NJ: Laurence Erlbaum; ), 519–544. [Google Scholar]
- Zigmond A. S., Snaith R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatr. Scand. 67, 361–370. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]