Abstract
In the subapical region of dark-grown pea epicotyls about 40% of the total polysomes are associated with membranes. The presence of poly(A) in polysomal mRNA was detected by hybridization of unlabeled RNA with 3H-poly(U). Both free mRNA and messenger ribonucleoprotein particles in polysomes hybridize with 3H-poly(U) quantitatively. The binding of 3H-poly(U) to polysomes is increased by treatment with the detergent sodium dodecyl sulfate. Since detergent influenced the 3H-poly(U) binding more in membrane-bound polysomes than in free, there may be more protein(s) associated with the poly(A) portion of the mRNA in membrane-bound polysomes. Analysis of the poly(A) segments isolated from the mRNA of these two classes of polysomes indicates that there are discrete classes of poly(A) and they appear to be differentially associated with free and membrane-bound polysomes. Mean size distribution of poly(A) in free polysomes is larger than in membrane-bound polysomes.
Following treatment (2 days) with the plant growth hormone indoleacetic acid, there is a gradual decrease in the mean length of total poly(A), which appears to correspond to a decrease in the size of the polysomes and their associated mRNA.
Full text
PDF
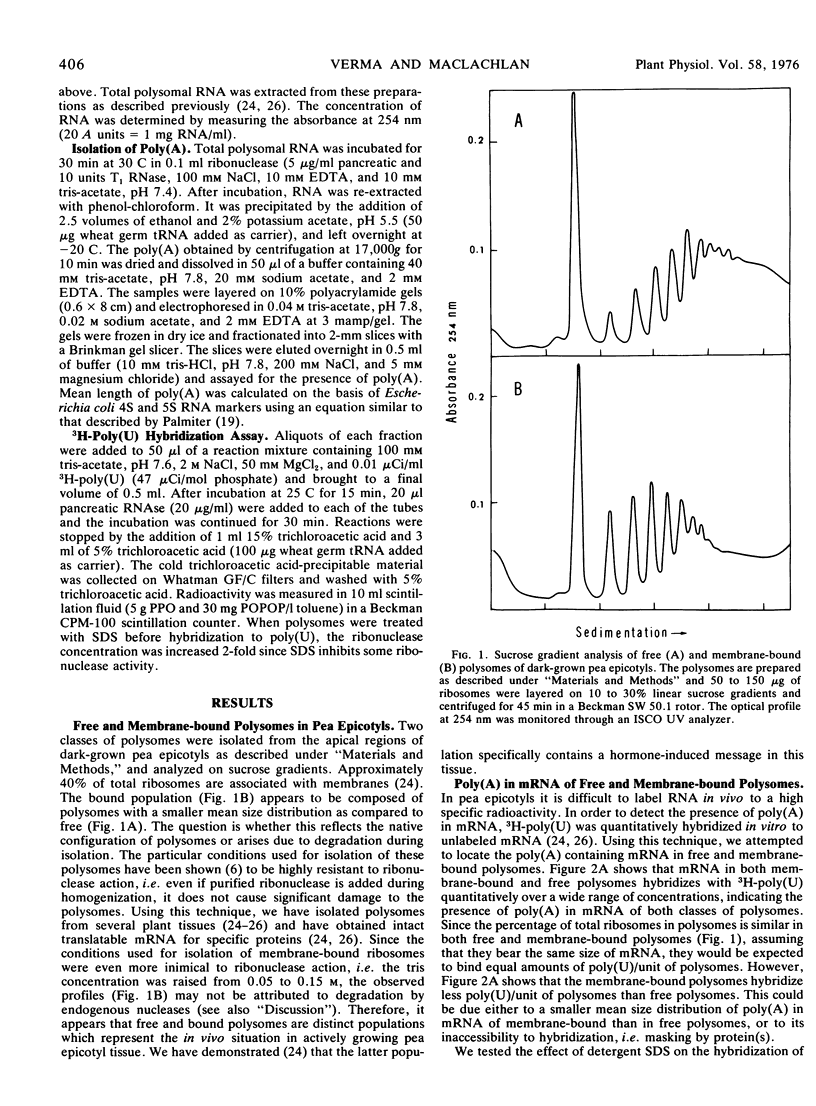
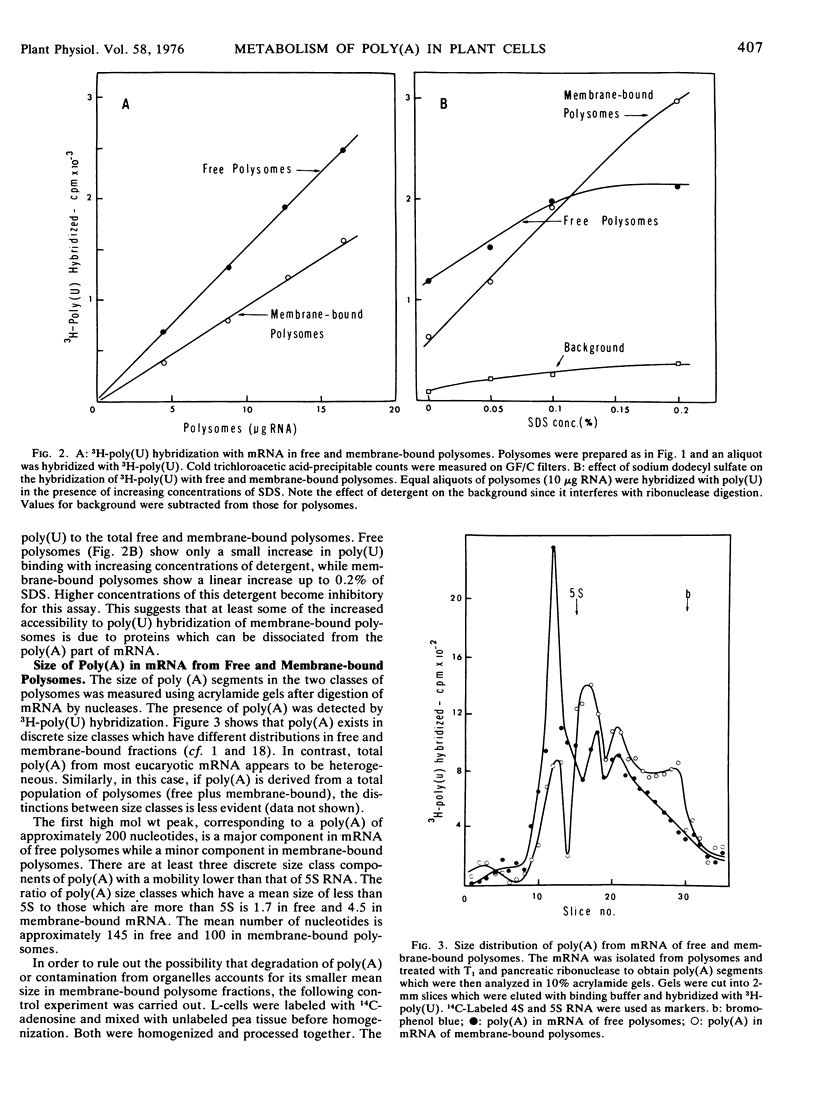
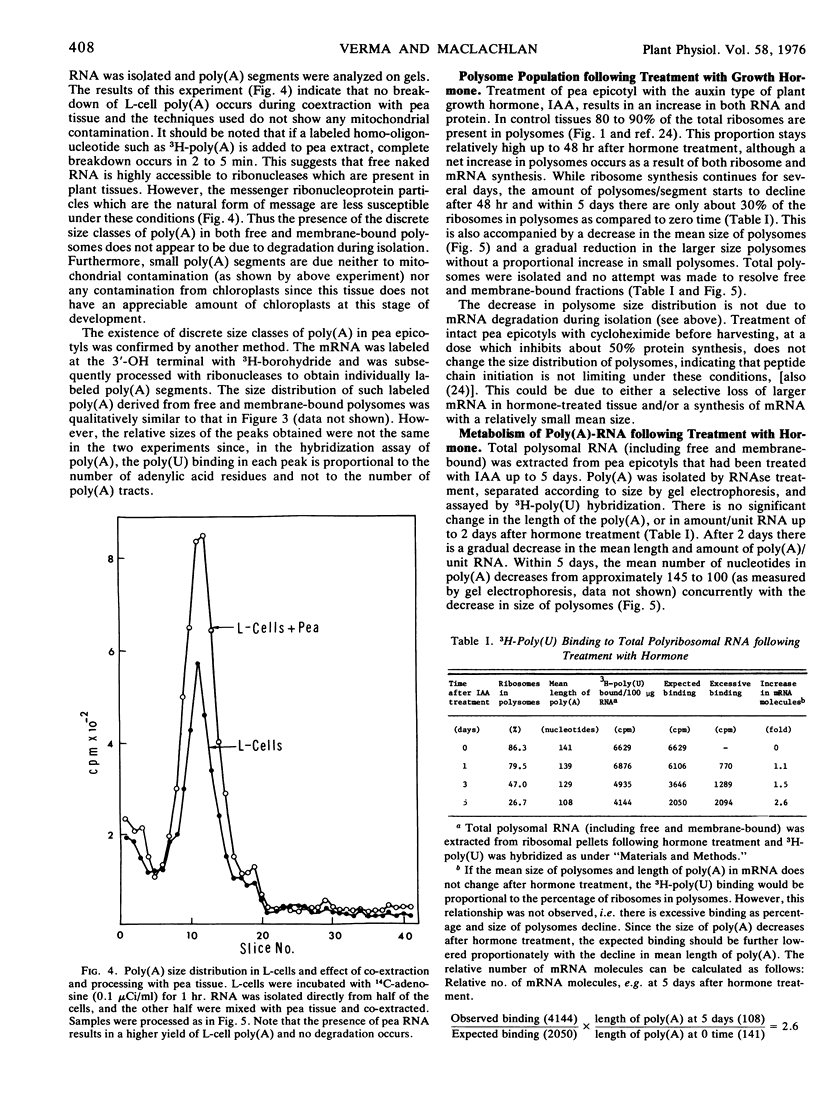
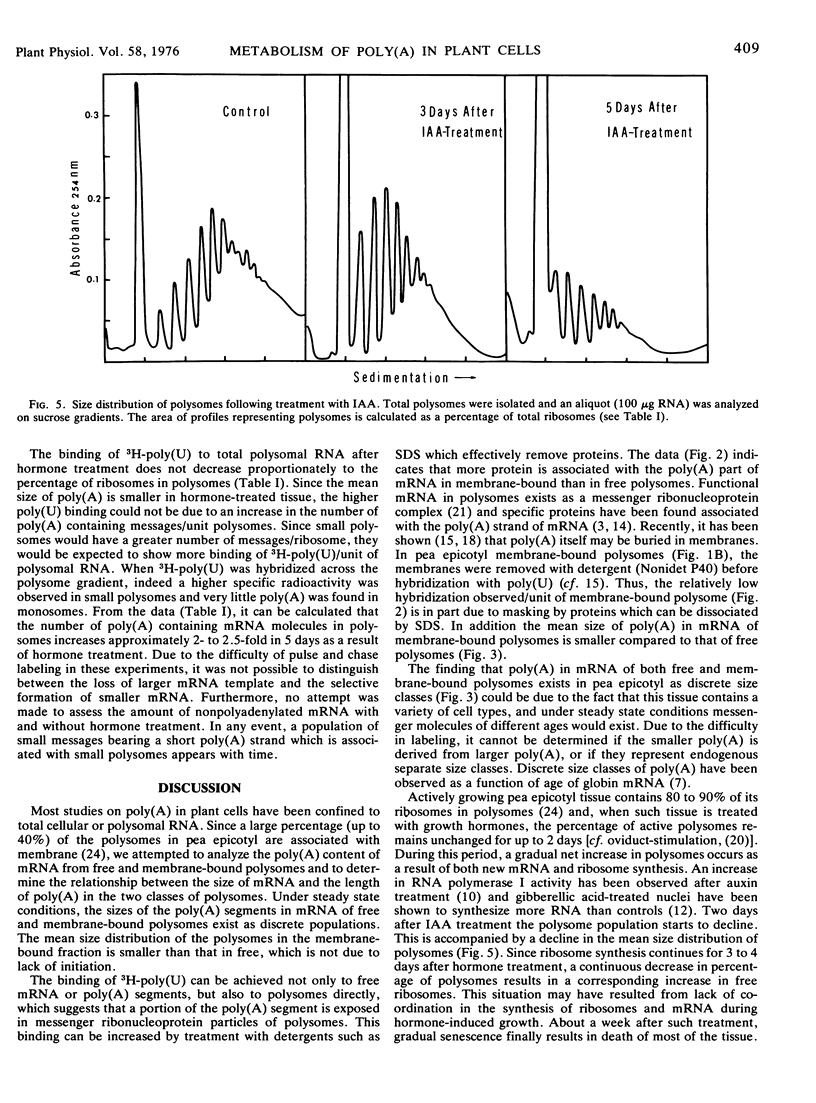

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C., Pemberton R., Delovitch T. Presence of polyadenylic acid sequences in RNA of membrane-bound polyribosomes. FEBS Lett. 1972 Oct 1;26(1):320–322. doi: 10.1016/0014-5793(72)80602-1. [DOI] [PubMed] [Google Scholar]
- Blobel G. A protein of molecular weight 78,000 bound to the polyadenylate region of eukaryotic messenger RNAs. Proc Natl Acad Sci U S A. 1973 Mar;70(3):924–928. doi: 10.1073/pnas.70.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne H., Christou N. V., Verma D. P., Maclachlan G. A. Purification and characterization of two cellulases from auxin-treated pea epicotyls. J Biol Chem. 1975 Feb 10;250(3):1012–1018. [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., Larkins B. A., Knight R. H. Polyribosomes from peas: an improved method for their isolation in the absence of ribonuclease inhibitors. Plant Physiol. 1972 Nov;50(5):581–584. doi: 10.1104/pp.50.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Morrison M. R., Merkel C. G., Lingrel J. B. Poly(A) size class distribution in globin mRNAs as a function of time. Nature. 1975 Feb 27;253(5494):749–751. doi: 10.1038/253749a0. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R., Perry R. P. Relative occurrence of polyadenylic acid sequences in messenger and heterogeneous nuclear RNA of L cells as determined by poly (U)-hydroxylapatite chromatography. J Mol Biol. 1972 Dec 14;72(1):91–98. doi: 10.1016/0022-2836(72)90070-8. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R., Perry R. P. The isolation and characterization of steady-state labeled messenger RNA from L-cells. Biochim Biophys Acta. 1972 Dec 6;287(2):361–366. doi: 10.1016/0005-2787(72)90386-3. [DOI] [PubMed] [Google Scholar]
- Guilfoyle T. J., Hanson J. B. Greater Length of Ribonucleic Acid Synthesized by Chromatin-bound Polymerase from Auxin-treated Soybean Hypocotyls. Plant Physiol. 1974 Jan;53(1):110–113. doi: 10.1104/pp.53.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins T. J., Mercer J. F., Goodwin P. B. Poly(A) sequences in plant polysomal RNA. Nat New Biol. 1973 Nov 21;246(151):68–70. doi: 10.1038/newbio246068a0. [DOI] [PubMed] [Google Scholar]
- Johri M. M., Varner J. E. Enhancement of RNA synthesis in isolated pea nuclei by gibberellic acid. Proc Natl Acad Sci U S A. 1968 Jan;59(1):269–276. doi: 10.1073/pnas.59.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan S. W., Brawerman G. A particle associated with the polyadenylate segment in mammalian messenger RNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3247–3250. doi: 10.1073/pnas.69.11.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande M. A., Adesnik M., Sumida M., Tashiro Y., Sabatini D. D. Direct association of messenger RNA with microsomal membranes in human diploid fibroblasts. J Cell Biol. 1975 Jun;65(3):513–528. doi: 10.1083/jcb.65.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan C. O., App A. A., Still C. C. The presence of polyadenylate sequences in the ribonucleic acid of a higher plant. Biochem Biophys Res Commun. 1973 Jul 17;53(2):588–595. doi: 10.1016/0006-291x(73)90702-x. [DOI] [PubMed] [Google Scholar]
- Milcarek C., Penman S. Membrane-bound polyribosomes in HeLa cells: association of polyadenylic acid with membranes. J Mol Biol. 1974 Oct 25;89(2):327–338. doi: 10.1016/0022-2836(74)90522-1. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Christensen A. K., Schimke R. T. Organization of polysomes from pre-existing ribosomes in chick oviduct by a secondary administration of either estradiol or progesterone. J Biol Chem. 1970 Feb 25;245(4):833–845. [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA-protein complexes and newly synthesized ribosomal subunits: analysis of free particles and components of polyribosomes. J Mol Biol. 1968 Jul 14;35(1):37–59. doi: 10.1016/s0022-2836(68)80035-x. [DOI] [PubMed] [Google Scholar]
- Schmid B. D., Siegel N. R., Vanderhoef L. N. The Isolation and Characterization of Adenosine Monophosphate-rich Polynucleotides Synthesized by Soybean Hypocotyl Cells: Their Relation to Messenger Ribonucleic Acid. Plant Physiol. 1975 Feb;55(2):277–281. doi: 10.1104/pp.55.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiness D., Darnell J. E. Polyadenylic acid segment in mRNA becomes shorter with age. Nat New Biol. 1973 Feb 28;241(113):265–268. doi: 10.1038/newbio241265a0. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Maclachlan G. A., Byrne H., Ewings D. Regulation and in vitro translation of messenger ribonucleic acid for cellulase from auxin-treated pea epicotyls. J Biol Chem. 1975 Feb 10;250(3):1019–1026. [PubMed] [Google Scholar]
- Verma D. P., Marcus A. Activation of Protein Synthesis upon Dilution of an Arachis Cell Culture from the Stationary Phase. Plant Physiol. 1974 Jan;53(1):83–87. doi: 10.1104/pp.53.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Nash D. T., Schulman H. M. Isolation and in vitro translation of soybean leghaemoglobin mRNA. Nature. 1974 Sep 6;251(5470):74–77. doi: 10.1038/251074a0. [DOI] [PubMed] [Google Scholar]


