Abstract
Rationale: Matrix metalloproteinase-1 (MMP-1) and mast cells are present in the airways of people with asthma.
Objectives: To investigate whether MMP-1 could be activated by mast cells and increase asthma severity.
Methods: Patients with stable asthma and healthy control subjects underwent spirometry, methacholine challenge, and bronchoscopy, and their airway smooth muscle cells were grown in culture. A second asthma group and control subjects had symptom scores, spirometry, and bronchoalveolar lavage before and after rhinovirus-induced asthma exacerbations. Extracellular matrix was prepared from decellularized airway smooth muscle cultures. MMP-1 protein and activity were assessed.
Measurements and Main Results: Airway smooth muscle cells generated pro–MMP-1, which was proteolytically activated by mast cell tryptase. Airway smooth muscle treated with activated mast cell supernatants produced extracellular matrix, which enhanced subsequent airway smooth muscle growth by 1.5-fold (P < 0.05), which was dependent on MMP-1 activation. In asthma, airway pro–MMP-1 was 5.4-fold higher than control subjects (P = 0.002). Mast cell numbers were associated with airway smooth muscle proliferation and MMP-1 protein associated with bronchial hyperresponsiveness. During exacerbations, MMP-1 activity increased and was associated with fall in FEV1 and worsening asthma symptoms.
Conclusions: MMP-1 is activated by mast cell tryptase resulting in a proproliferative extracellular matrix. In asthma, mast cells are associated with airway smooth muscle growth, MMP-1 levels are associated with bronchial hyperresponsiveness, and MMP-1 activation are associated with exacerbation severity. Our findings suggest that airway smooth muscle/mast cell interactions contribute to asthma severity by transiently increasing MMP activation, airway smooth muscle growth, and airway responsiveness.
Keywords: asthma, extracellular matrix, airway remodeling, airway smooth muscle, mast cells
At a Glance Commentary
Scientific Knowledge on the Subject
Matrix metalloproteinase-1 (MMP-1) and mast cells are minimally present in normal airways but both are present in the airway smooth muscle bundles of patients with asthma. Despite evidence that both are related to asthma severity, the underlying mechanisms are uncertain. MMP-1 is a matrix processing collagenase that requires activation by proteases. We hypothesized mast cell proteases could activate MMP-1, resulting in structural changes that promote airway remodeling.
What This Study Adds to the Field
MMP-1 can be activated by mast cell tryptase. Active MMP-1 is capable of processing airway smooth muscle–derived extracellular matrix, which enhances airway smooth muscle proliferation. In patients with asthma, mast cells are associated with airway smooth muscle growth. MMP-1 protein is related to bronchial reactivity, and MMP-1 activity increases during asthma exacerbations, for which its level is related to exacerbation severity. Interrupting mast cell/airway smooth muscle interactions has the potential to reduce airway remodeling in asthma.
Asthma is characterized by airway inflammation, bronchial hyperresponsiveness (BHR), and variable airway obstruction. Structural changes, collectively termed airway remodeling, are associated with BHR, airflow obstruction, worsening asthma symptoms, increased β2-agonist use, and exacerbations, and can persist despite optimal asthma treatment (1–4). Airway remodeling often starts in childhood with airflow limitation associated with persistence of asthma and abnormal lung function in adulthood (5, 6). Changes observed in airway remodeling include epithelial desquamation, goblet cell hyperplasia, reticular basement membrane thickening, increased airway smooth muscle (ASM) mass, and abnormal extracellular matrix (ECM) deposition (1, 7). Airway remodeling has been seen as a consequence of inflammation, although airway contraction, in the absence of inflammation, can also drive aspects of remodeling (8). Because airway structural changes may support inflammation (9) it is likely that airway inflammation, bronchoconstriction, remodeling, and fixed airflow obstruction interact in a linear, parallel, or combined manner to drive the asthma phenotype.
Mast cells, particularly activated, degranulated forms, are more common within ASM bundles of those with asthma (10–12) and generate proinflammatory cytokines including IL-4, IL-5, and IL-13, which regulate IgE production, eosinophilic inflammation, and profibrogenic cytokines, including transforming growth factor-β and basic fibroblast growth factor-2. Preformed serine proteases including tryptase, chymase, and carboxy-peptidase are secreted from granules and interact with various cell types via proteolytically activated receptors (PARs) (11). Collectively, mast cell mediators contribute to airway inflammation, hyperresponsiveness and remodeling causing bronchoconstriction, ASM cell proliferation, and inflammatory cell recruitment (13).
Our overarching hypothesis is that the airway environment in asthma sustains airway remodeling (14–16). We have shown that components of the ECM in asthmatic airways can promote remodeling by affecting proliferation (17), migration (16), apoptosis (18), matrix metalloproteinase (MMP) activation (16), and β-agonist signaling of ASM cells (19). Here we hypothesized that mast cell proteases are responsible for MMP-1 activation in asthma and used a combination of in vitro and human studies to examine the interactions between mast cells, MMP-1, airway remodeling, and asthma symptoms. Some of this work has previously been presented as abstracts (20, 21).
Methods
Patients and Control Subjects
Two asthma cohorts with matched control groups were studied. All subjects gave written informed consent. The relationship between ASM growth and MMP-1 expression was examined in 16 subjects with mild or moderate asthma, defined by the Global Initiative for Asthma criteria (22), without a history of exacerbation, change in therapy, or use of oral steroids for at least 6 weeks and 11 nonsmoking, age-matched control subjects without lung disease. Further study details are included in the online supplement. Those with asthma underwent spirometry, bronchial provocation testing, and modified Juniper Asthma Control Questionnaire (23). All subjects underwent flexible bronchoscopy with bronchial washings and biopsies taken from the right bronchus intermedius. The study was approved by the Nottingham Research Ethics Committee (12/EM/0199). The relationship between asthma exacerbations and MMP-1 activation was examined in a second group of 11 subjects with mild and 17 with moderate asthma with exacerbations induced by rhinovirus inoculation compared with 11 healthy control subjects.
The study has been described in detail previously (24). Briefly, nonsmokers with mild or moderate asthma, defined by Global Initiative for Asthma criteria, and nonsmoking, nonatopic healthy volunteers without a recent viral illness or asthma exacerbation, were recruited. Symptom scores, lung function, bronchoscopy, and bronchoalveolar lavage (BAL) were performed 2 to 4 weeks before inoculation with rhinovirus 16. Following virus inoculation, symptom scores were recorded daily and lung function, bronchoscopy, and BAL were repeated after 4 days. The study was approved by the St Mary’s Hospital ethics committee (09/H0712/59).
Cells and Tissues
Bronchial biopsy tissue was either processed for immunohistochemistry or used for culture of ASM cells as previously described with cells used at passage five or less (see Figure E1 in the online supplement) (15).
Mast cell supernatants were prepared from HMC-1 mast cells activated using phorbol 12-myristate 13-acetate (50 ng/ml) and calcium ionophore (A23187; 25 ng/ml) for 16 hours. Control mast cell supernatants had phorbol 12-myristate 13-acetate and calcium ionophore added after removal of the cell pellet. Inhibition of proteases was performed by preincubation of supernatants with protease inhibitors used at the manufacturer’s recommended concentrations. Full details are included in the online supplement.
MMP-1 Assays
Total MMP-1 protein (including pro and active species) and TIMP1 were measured using Duoset ELISAs (R&D Systems, Abingdon, UK). MMP-1 activity was measured using a Human Active MMP-1 Fluorokine E Kit (R&D Systems). Generation of the active 43-kD MMP-1 species was assessed by Western blotting (14). Proteolytic cleavage of MMP-1 by tryptase was examined by incubation of recombinant pro–MMP-1 (10 ng; R&D Systems) and recombinant human mast cell tryptase (0.1–2 IU; Promega, Southampton, UK) in physiologic buffered saline for 30 minutes at 37°C. Products were resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis and visualized by silver staining.
ECM Preparations
ASM cell-derived ECM was prepared as described (14). Further details are given in the online supplement.
In Vitro Cell Proliferation, Adhesion, and Apoptosis Assays
Cell proliferation was determined by both 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay and cell counting as described (16) with ASM cultured on ECM preparations for 48 hours in the presence of 1% fetal bovine serum. For adhesion assays, fluorescently labeled ASM cells were seeded onto ECM preparations and adherent cells measured by fluorescence over 2–18 hours. Apoptosis was assessed using an In Situ Cell Death Detection kit, AP (Roche, West Sussex, UK). Full details are provided in the online supplement.
MMP-1 siRNA Knockdown
siRNA was used as previously described (14) and reduced MMP-1 protein by greater than 70% (see Figure E2).
Quantification of Airway Mast Cells and Proliferating ASM Cells
Endobronchial biopsies were immunostained as previously described (15) with images captured using a Digital Nanozoomer (Hamamatsu Photonics UK Ltd., Welwyn Garden City, UK). All slides were assessed by a respiratory pathologist (I.S.). ASM bundles were identified by immunostaining with anti α-smooth muscle actin (Roche) and ASM cell nuclei counted using DAPI staining. In adjacent sections, proliferating ASM and tryptase-positive mast cells were identified by anti-Ki67 and anti-mast-cell tryptase antibodies, respectively (Roche). Proliferating ASM and mast cells within ASM bundles were quantified per 100 ASM cells by an observer blinded to the clinical details.
Statistical Analyses
Data were tested for normality and comparisons made by Student’s t test or Mann-Whitney U test as appropriate. Multiple comparisons were made by two-way analysis of variance. Correlations between parameters were analyzed by linear regression. Statistical analysis was performed using GraphPad Prism 6 for windows version 6.07 (1992–2015; GraphPad Software, Inc., La Jolla, CA). A P value less than 0.05 was considered to be significant.
Results
MMP-1 Is Expressed in the Airways of Patients with Asthma
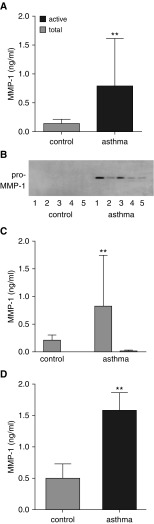
Details of the 16 patients and 11 control subjects in the airway remodeling study are shown in Table 1. The expression of total MMP-1 protein (ELISA) was 5.4-fold greater in bronchial washings from patients with asthma than those from control subjects (control: mean, 0.14 ng/ml; 95% confidence interval [CI], 0.095–0.197; asthma: mean, 0.79 ng/ml; 95% CI, 0.35–1.21; P = 0.002) (Figure 1A). Western blotting of these samples identified a single 54-kD band consistent with pro–MMP-1 in those with asthma but not in healthy control subjects. The 43-kD active MMP-1 species was not detected in either group (Figure 1B). In keeping with the Western blot analysis, MMP-1 activity was not detected in control subjects and barely present in those with stable asthma (Figure 1C).
Table 1.
Characteristics of Patients and Healthy Subjects Studied in the Bronchial Hyperresponsiveness/Airway Remodeling Study
| Asthma | Control | |
|---|---|---|
| N | 16 | 11 |
| Age, yr | 31 (9.4) | 36 (10.2) |
| Male/female | 9/7 | 6/5 |
| Disease duration, yr | 16 (9.9) | N/A |
| Atopy, present/absent | 8/8 | N/A |
| ACQ5 score | 2.28 (0.63) | N/A |
| BMI, kg/m2 | 25.1 (3.2) | 24.9 (2.8) |
| ICS dose, μg/d | 412 (296) | 0 |
| PC20 methacholine, mg/ml | ||
| 0–5 | 2 | |
| 5–8 | 5 | |
| 8–16 | 9 | |
| FEV1 % predicted | ||
| Prebronchodilator | 90.5 (9.5) | |
| Post-bronchodilator | 92.5 (11.5) | |
| Exhaled nitric oxide, ppb | 49.6 (30.2) |
Definition of abbreviations: ACQ5 = Juniper 5 asthma control score; BMI = body mass index; ICS = inhaled corticosteroid; N/A = not applicable; PC20 = provocative concentration of methacholine.
Values represent number in group or mean (SD) as appropriate.
Figure 1.
Matrix metalloproteinase (MMP)-1 expression in asthma. (A) Total MMP-1 protein measured by ELISA in bronchial washings from 16 patients with mild or moderate asthma, defined by the Global Initiative for Asthma criteria and 11 healthy control subjects. Those with asthma have significantly higher MMP-1 levels. **P = 0.002, Mann-Whitney U test. (B) Western blot of bronchial washings from five representative patients with asthma and five healthy control subjects. A single band of 55 kD consistent with pro–MMP-1 is present in those with asthma but not detectable in healthy control subjects. (C) Total and active MMP-1 activity measured by fluorokine activity assay in patients described in A. Total MMP-1 protein is low in healthy control subjects and significantly elevated in those with asthma, **P < 0.01. MMP-1 activity is undetectable in control subjects and low in those with asthma. (D) Total MMP-1 protein measured by ELISA in cell culture supernatants from airway smooth muscle cells grown from bronchial biopsies from patients with mild or moderate asthma and healthy control subjects. **P = 0.002, Mann-Whitney U test.
ASM cells were cultured from endobronchial biopsies of six of these patients with asthma and six healthy control subjects. Total MMP-1 protein was 3.3-fold higher in ASM culture supernatants from those with asthma than from healthy control subjects (control: mean, 0.48 ng/ml; 95% CI, 0.23–0.74; asthma: mean, 1.57 ng/ml; 95% CI, 0.30–1.23; P = 0.002) (Figure 1D).
ASM Cell MMP-1 Is Activated by Mast Cell–derived Tryptase
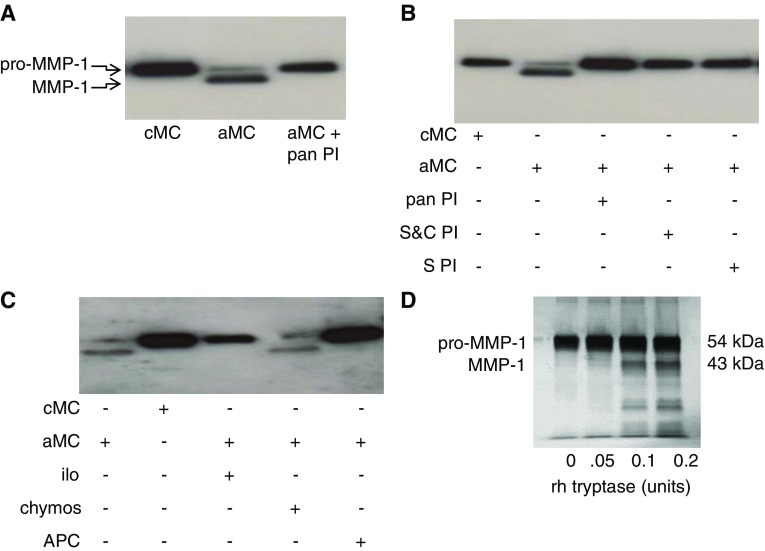
When the supernatants of unstimulated primary ASM cells from patients with asthma were examined by Western blotting a single band consistent with pro–MMP-1 was detected (Figure 2A). ASM cells were then incubated with the supernatants of activated or nonactivated HMC-1 mast cells. Activated, but not control, mast cell supernatants resulted in the appearance of the active 43-kD MMP-1 species, which could be inhibited using a pan protease inhibitor (Figure 2A). To determine which protease was responsible for MMP-1 activation, we used more specific protease inhibitors. Generation of active MMP-1, detected by Western blotting, was blocked by panserine protease and specific tryptase inhibitors, but not a chymase inhibitor (Figures 2B and 2C). The generation of active MMP-1 was associated with an increase in MMP-1 activity (see Figure E3). To determine if mast cell tryptase activated MMP-1 by direct cleavage, we coincubated recombinant mast cell tryptase and pro–MMP-1. Increasing concentrations of mast cell tryptase were associated with production of a cleaved MMP-1 protein at 43 kD consistent with active MMP-1 (Figure 2D).
Figure 2.
Mast cell tryptase activates matrix metalloproteinase (MMP)-1. (A) Western blot for MMP-1 in airway smooth muscle supernatants treated with control or activated mast cell supernatants. Activated mast cell supernatants cause MMP-1 activation as shown by the appearance of the smaller MMP-1 band, which is blocked by a broad-spectrum protease inhibitor. (B and C) MMP-1 activation by activated mast cell supernatants is inhibited by inhibitors of serine and cysteine proteases, serine proteases, metalloproteinases, and tryptase, but not chymase. (D) Silver stain of sodium dodecyl sulfate–polyacrylamide gel electrophoresis showing recombinant human tryptase cleaves pro–MMP-1 in vitro shown by the dose-dependent appearance of a 43-kD band. aMC = activated mast cell supernatants; APC = inhibitor of tryptase; chymos = inhibitor of chymase; cMC = control mast cell supernatants; ilo = inhibitor of metalloproteinases; pan PI = broad-spectrum protease inhibitor; rh = recombinant human; S&C PI = inhibitor of serine and cysteine proteases; S PI = inhibitor of serine protease.
Activated Mast Cell Treatment of ASM Cells Generates a Proproliferative ECM
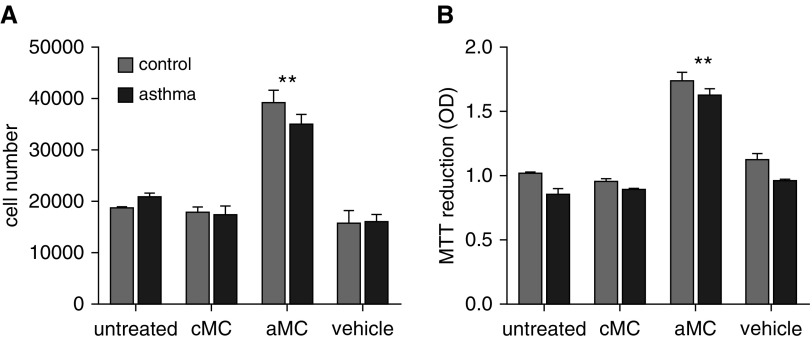
To determine if MMP-1 activation would lead to proteolytic modification of ASM cell-derived ECM and promote ASM growth, we generated ASM-derived ECM preparations from patients with asthma and control subjects following treatment with activated or control mast cell supernatants. ASM cells were then seeded onto these preparations and cell proliferation measured in the presence of 1% serum. Activated mast cell–treated ASM cells produced a matrix that enhanced proliferation of control and asthma-derived ASM over 48 hours (mean difference for control cells, 0.73; 95% CI of difference, 0.52–0.93; P < 0.001) (mean difference for asthma cells, 0.78; 95% CI of difference, 0.58–0.99; P < 0.001) as assessed by cell counting when compared with control mast cell supernatants. Similar results were obtained using MTT assay (Figure 3; see Figures E4 and E5). Neither control mast cell supernatants nor the activation vehicle alone affected ASM proliferation. Activated mast cell supernatants had no overall effect on ASM adhesion or apoptosis on control or asthma-derived ECM (see results in the online supplement; see Figures E6 and E7).
Figure 3.
Mast cell treatment of airway smooth muscle (ASM) generates proproliferative extracellular matrix. ASM from patients with asthma or control subjects was treated with control or activated mast cell supernatants or mast cell activation vehicle during extracellular matrix deposition. ASM cells were removed from extracellular matrix preparations, and normal ASM was seeded for 48 hours in the presence of 1% serum. Cell proliferation was quantitated by (A) 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (B) and cell counting. ASM cell proliferation was enhanced by activated, but not control, mast cell treatment. **P = 0.0001, two-way analysis of variance. aMC = activated mast cell supernatants; cMC = control mast cell supernatants; MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; OD = optical density.
Proproliferative Matrix Is Dependent on Tryptase-activated MMP-1
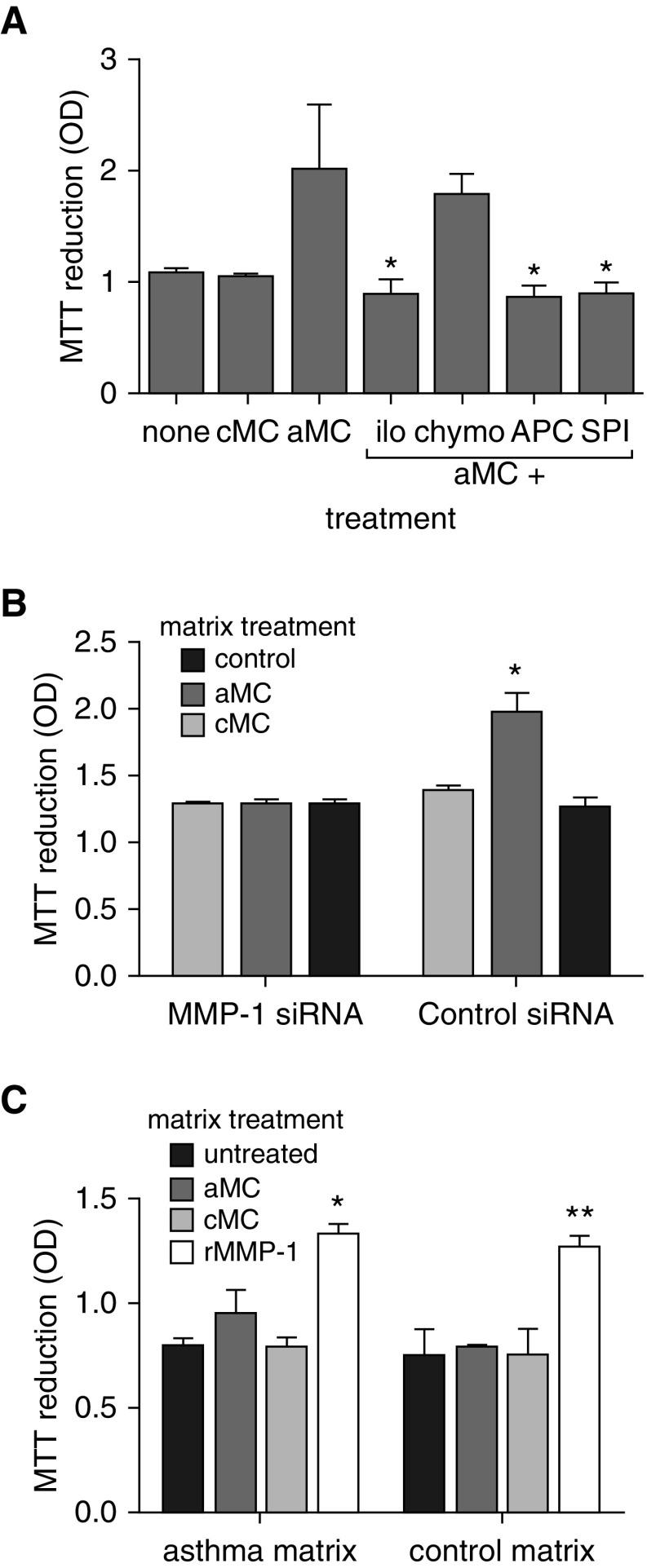
To determine if the proproliferative matrix generated by activated mast cells was dependent on mast cell tryptase activation of MMP-1, mast cell supernatants were incubated with a range of protease inhibitors before treatment of ASM cells. The excess ASM proliferation, estimated by MTT reduction, induced by activated mast cells, was blocked by a pan protease inhibitor and inhibitors of serine proteases, metalloproteinases, and tryptase but not chymase (Figure 4A). We then treated ASM with siRNA targeting MMP-1 or control siRNA during ECM deposition to confirm that MMP-1 was the responsible protease. Knockdown of MMP-1 but not control siRNA blocked the excess proliferation generated by activated mast cell supernatants estimated by MTT reduction (Figure 4B). Furthermore, direct application of activated MMP-1 (10 ng/ml) to untreated ASM-derived matrix significantly enhanced the proliferative capacity of both asthma and control-derived matrix, whereas activated and control mast cell supernatants, in the absence of ASM cells, had no effect (Figure 4C).
Figure 4.
Proproliferative matrix is dependent on airway smooth muscle (ASM)-derived matrix metalloproteinase (MMP)-1. (A) ASM treated with control (cMC) or activated (aMC) mast cell supernatants during extracellular matrix (ECM) deposition was coincubated with an MMP inhibitor, chymase inhibitor, tryptase inhibitor, or a serine protease inhibitor. Enhanced ASM proliferation generated by aMC treatment was abrogated by inhibitors of MMPs, serine proteases, and tryptase but not chymase. *Difference from aMC, P = 0.029, Mann-Whitney U test. (B) ECM preparations were generated by ASM cells treated by an MMP-1–specific or control siRNA. During ECM deposition cells were treated with cMC or aMC. The MMP-1–specific siRNA abrogated the enhanced proliferation generated by aMC treatment. *P = 0.04 Mann-Whitney U test. (C) ASM-derived ECM from asthma-derived or control cells were decellularized and then left untreated or incubated with cMC or aMC or active recombinant MMP-1 (rMMP-1). ASM cells were then seeded and allowed to grow for 48 hours. Mast cell supernatants had no direct effect on the ECM in the absence of ASM, but matrix-driven proliferation was enhanced by direct application of MMP-1. *P = 0.013, **P < 0.0001 Mann-Whitney U test. APC = inhibitor of tryptase; chymos = inhibitor of chymase; ilo = inhibitor of metalloproteinases; MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; OD = optical density; rMMP-1 = recombinant MMP-1; siRNA = small interfering RNA; SPI = inhibitor of serine protease.
Relationship between MMP-1 Activation and Asthma Exacerbations
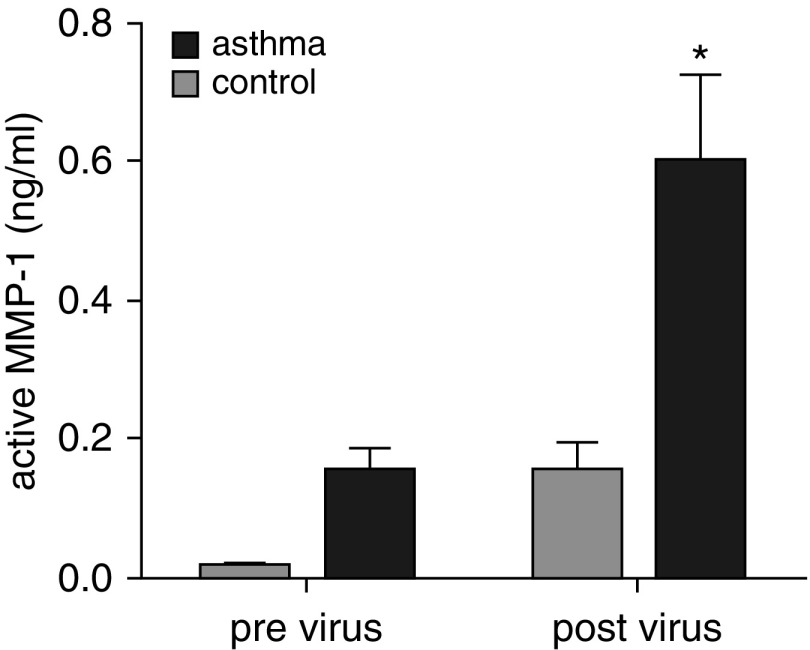
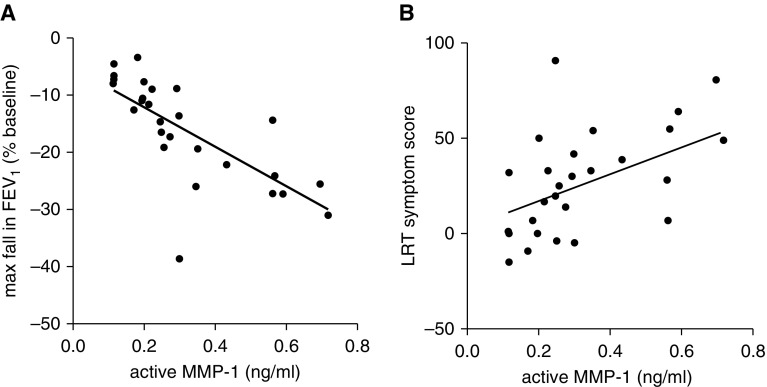
To understand the relevance of these in vitro findings to human asthma, we examined MMP-1 activation in BAL fluid of patients with asthma who developed exacerbations in response to rhinovirus inoculation. After virus inoculation, lower respiratory symptom scores increased and more severe reductions in lung function were observed in the subjects with asthma as previously reported (24). After viral inoculation, mean MMP-1 activity increased 3.9-fold (n = 28; difference, 0.445; 95% CI of difference, 0.052–0.84; P = 0.03) in patients with asthma and 11-fold in healthy control subjects, although the latter was not significant (n = 11; difference, 0.14; 95% CI of difference, −0.025 to 0.53) (Figure 5). Importantly, the degree of MMP-1 activation after exacerbation in those with asthma was strongly correlated with more severe falls in FEV1 (r2 = 0.54; P < 0.0001) and peak lower respiratory symptom score severity (r2 = 0.24; P = 0.007) (Figure 6). BAL TIMP1 protein was similar in control subjects and those with asthma and did not change significantly in response to viral challenge (see Figures E8 and E9).
Figure 5.
Matrix metalloproteinase (MMP)-1 is activated during exacerbations of asthma. MMP-1 activity in bronchoalveolar lavage fluid of healthy control subjects and patients with asthma was measured by MMP-1 activity assay at baseline and 4 days after inoculation with rhinovirus. MMP-1 activity was elevated by viral infection and was higher in those with asthma at baseline and after viral inoculation. *Before versus after viral inoculation for asthma subjects, P < 0.05. Two-way analysis of variance with Tukey correction for multiple comparisons.
Figure 6.
Matrix metalloproteinase (MMP)-1 activation is associated with postexacerbation FEV1 and asthma symptoms. (A) Rhinovirus-induced maximal fall in FEV1 in patients with asthma is correlated with the concentration of active MMP-1 in bronchoalveolar lavage fluid. r2 = 0.55, P = 0.0001. (B) Increased lower respiratory symptom scores during rhinovirus-induced exacerbation are associated with MMP-1 activation. r2 = 0.24, P = 0.007. LRT = lower respiratory tract.
Association between ASM Proliferation and Mast Cells in Asthma
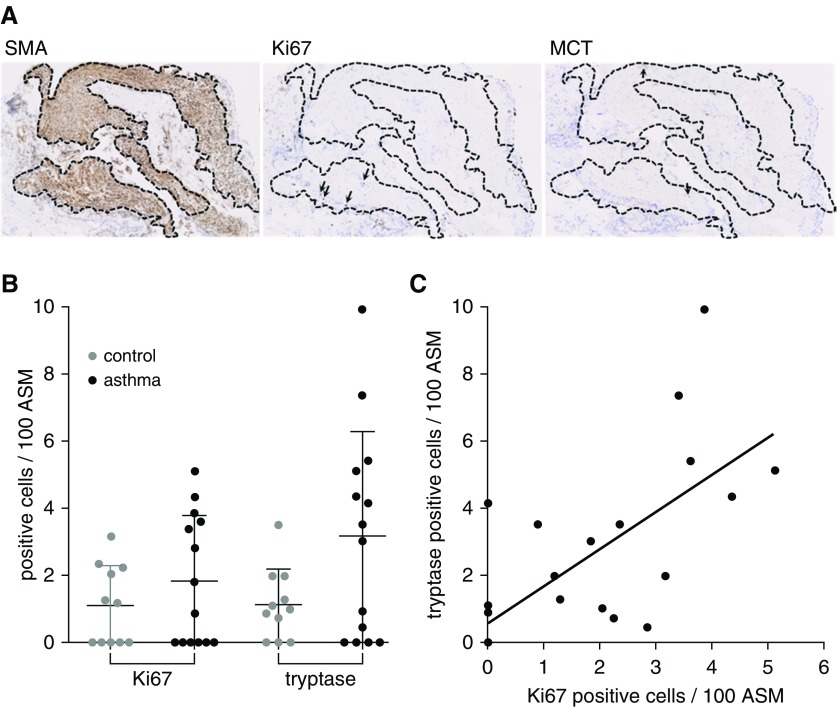
Having shown that mast cell tryptase dependent MMP-1 activation leads to proproliferative ECM remodeling in vitro, we examined the association between tryptase-positive mast cells and ASM proliferation in human airways. We quantitated proliferating ASM cells and tryptase-positive mast cells in the ASM bundles of the first group of 16 patients with asthma and 11 healthy control subjects (Figure 7A). Bronchial biopsies contained ASM in 12 and 10 cases, respectively. In these samples, both proliferating ASM cells and tryptase-positive mast cells tended to be more common in those with than without asthma, although the differences between groups were not significant (Figure 7B). Across individuals, the number of proliferating ASM cells was positively correlated with mast cell infiltration in both those with and without asthma (r2 = 0.49; P < 0.0001) (Figure 7C).
Figure 7.
Mast cells are associated with airway smooth muscle proliferation in asthma. (A) Bronchial biopsies from patients with mild or moderate asthma, defined by the Global Initiative for Asthma criteria, were cut in serial sections, airway smooth muscle (ASM) bundles were identified by α-smooth muscle actin staining, smooth muscle was localized in consecutive slides, and proliferating ASM cells and mast cells identified by ki67 and mast cell tryptase staining, respectively (arrows). (B) ki67- and tryptase-positive cells in ASM bundles were expressed per 100 ASM cells in healthy control subjects and those with asthma. Those with asthma tended to have more proliferating ASM and mast cells. (C) Tryptase-positive mast cells are correlated with proliferating ASM cells. r2 = 0.5, P = 0.0001. MCT = mast cell tryptase; SMA = α-smooth muscle actin.
MMP-1 Levels Are Associated with Bronchial Reactivity in Stable Asthma
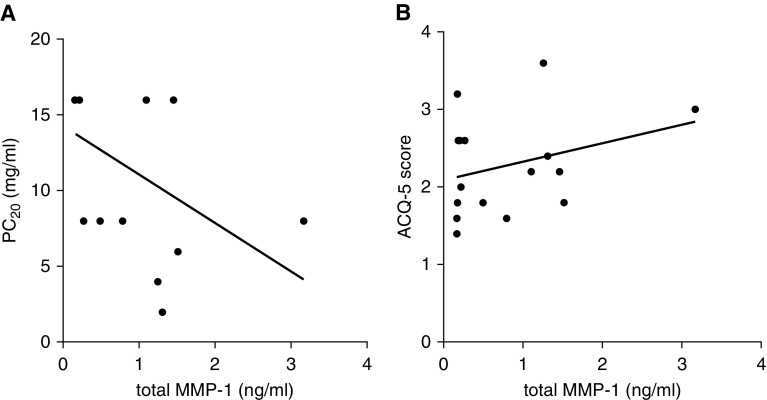
Because collagenase expression and MMP-1 activity have been related to airway contraction in vitro (25, 26) we examined the relationship between airway MMP-1 expression and airway contraction induced by methacholine in the first group of 16 patients with asthma. Increasing airway responsiveness to methacholine was associated with increasing total airway MMP-1 levels (r2 = 0.256; P = 0.04) (Figure 8A). In these stable patients, Juniper Asthma Control Questionnaire scores were not associated with total MMP-1 level (r2 = 0.09; P = 0.23) (Figure 8B).
Figure 8.
Matrix metalloproteinase (MMP)-1 is associated with bronchial hyperresponsiveness in asthma. (A) In patients with stable asthma, total MMP-1 in bronchial washings was associated with enhanced sensitivity to methacholine in bronchial challenge testing. r2 = 0.26, P = 0.045. (B) Modified Juniper Asthma Questionnaire score was not significantly related to MMP-1 levels. r2 = 0.09, P = 0.23. ACQ-5 = Juniper Asthma Questionnaire; PC20 = provocative concentration of methacholine.
Discussion
MMP-1 and mast cells are minimally present in normal airways but both are present in the ASM bundles of patients with asthma (27, 28). Here we have identified a potential mechanism linking MMP-1 activation by airway mast cells with ECM remodeling, ASM growth, airway contraction, and asthma severity. We have shown that MMP-1 is directly activated by mast cell tryptase and that this activation remodels ECM to generate a proproliferative substrate for ASM cells. In patients with stable asthma, the presence of tryptase-positive mast cells is associated with enhanced ASM proliferation and MMP-1 expression is directly correlated with airway narrowing in response to bronchoconstrictor stimuli. During exacerbations, MMP-1 activation is associated with exacerbation severity. These findings suggest that the interaction between MMP-1, mast cells, and ECM remodeling may contribute to airway contraction, remodeling, and worsening symptoms in patients with asthma.
Enhanced expression of MMP-1 has been described in response to several mediators associated with asthma and airway remodeling including collagen, tenascin, cyclical strain, leukotriene D4, tumor necrosis factor-α, and platelet-derived growth factor (27, 29–32), although the significance of this in asthma is unknown. In common with other MMPs, proteolytic removal of the prodomain is required for MMP-1 activation. Although MMP-1 activation by serine proteases has been described previously (33), in our study we demonstrated by direct interaction of the two proteins, that mast cell tryptase cleaves the prodomain of MMP-1 in vitro and supernatants from activated (degranulated) mast cells activate MMP-1. MMP-1 cleaves triple helical fibrillar collagens including collagens 1, 2, 3, 7, 8, and 10 and gelatin as part of ECM turnover, but can also generate bioactive mediators by collagen processing including the neutrophil chemoattractant matrikine Pro-Gly-Pro (34). In addition to collagens, MMP-1 has other matrix substrates including aggrecan, proteoglycan, versican, and perlecan (35) and can activate or inactivate bioactive proteins including insulin-dependent growth factor binding proteins 2, 3, and 5; PAR 1; CXCL8; and CXCL12 (36). The advantage of using biosynthesized ASM matrix in our study is the presence of multiple protein substrates in the preparation, making this system more physiologic than individual ECM substrates. This does mean we are unable to define which ECM-related proteins have been proteolytically processed and are responsible for the effect. It is likely that bioactive neoepitope generation, or ECM-associated growth factor processing, alters signaling to ASM (37). Our findings do show there is an absolute requirement for ASM cell–derived MMP-1 in generation of the proproliferative ECM by tryptase and that the direct action of mast cell–derived proteases is not sufficient to generate proliferative ECM in the absence of ASM-derived MMP-1.
Although we cannot directly link the ECM remodeling by MMP-1 and ASM growth seen in vitro to humans with asthma and airway MMP-1 may not completely reflect ASM-derived MMP-1: our findings, using primary asthma-derived ASM cells, are consistent with this mechanism acting in asthma. Tryptase-mediated activation of MMP-1 is one pathway by which mast cells could contribute to airway remodeling. Mast cells and mast cell degranulation are associated with severe asthma. In vivo it is likely that other mast cell mediators including prostaglandins and tryptase, by activation of PAR2, as previously observed in vitro, may stimulate ASM proliferation and contraction to worsen asthma symptoms (38, 39). We have previously shown that MMP-1 also supports ASM contraction in vitro and others have shown enhanced contraction of collagenase-treated bronchial rings (14, 40). In patients with stable asthma we found that airway MMP-1 is associated with airway narrowing in response to methacholine and that during exacerbations, activation of MMP-1 is associated with exacerbation severity judged by increasing symptoms and fall in FEV1. The mechanisms of airway narrowing and the relationship between airway remodeling, airway contraction, and BHR are complex. Our findings, and those of others linking alterations in the ECM to ASM growth (18), contraction (19), and ASM mass with BHR (41), could be consistent with MMP-1 activity contributing to changes in cell/matrix coupling and ECM stiffness altering resistive ASM loading to result in increased airway narrowing (42) (Figure 9).
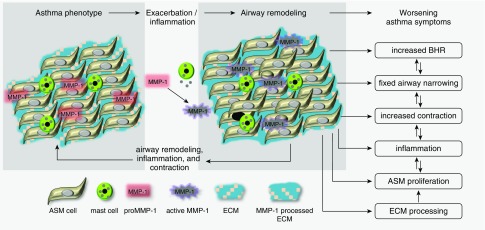
Figure 9.
Summary of findings and hypothesis. The airway phenotype of those with asthma differs from normal in many respects, including increased mast cell numbers and pro–matrix metalloproteinase (MMP)-1 expression. Exacerbations, other inflammatory stimuli, and possibly contraction cause mast cell degranulation, tryptase release, and MMP-1 activation. Active MMP-1 causes extracellular matrix processing to support airway smooth muscle proliferation. These changes result in fixed airflow obstruction, increased bronchial hyperresponsiveness, airway contraction, and worsening asthma symptoms. Interactions between airway remodeling, bronchial hyperresponsiveness, and airway contraction act at multiple levels, leading to worsening asthma symptoms. ASM = airway smooth muscle; BHR = bronchial hyperresponsiveness; ECM = extracellular matrix.
Longitudinal studies are generally consistent with the presence, but not progression, of airway obstruction with the minority of studies suggesting airflow obstruction increases with disease duration (2). No longitudinal studies have examined ASM or number or ECM mass over time in those with asthma and there are conflicting data on rates of ASM proliferation in asthma (5, 6, 43). Some suggest ASM proliferation is not elevated in patients with asthma (44), whereas others have demonstrated that ASM proliferation in bronchial biopsies is both enhanced in asthma and related to asthma severity (45). These contradictory findings both in vitro and in vivo may relate to a combination of methodologic and study population differences. Our findings, showing no difference in ASM proliferation in situ between those with asthma and control subjects overall, may reflect the exclusion of those with severe asthma. Importantly, using a mast cell number we observe an association with ASM proliferation. This suggests specific aspects of the asthma phenotype drive ASM proliferation, rather than enhanced ASM proliferation being a feature of all with asthma at all times in their disease. Structural changes in the airway are the net result of many processes including both ASM proliferation and death, ECM deposition, and resorption. The association of ASM growth and mast cells in vitro and in vivo is consistent with the generation of an environment that would alter this balance to sustain airway remodeling in the asthmatic airway (46).
Importantly, our findings suggest a mechanism driving asthma severity that is largely independent of Th2-mediated inflammation and are of relevance to developing therapies for the less well understood Th2-low asthma endotype, which is poorly responsive to current treatments (47). For most of those with asthma, airway remodeling is not completely preventable with current asthma treatments and alternatives are required to target this complication of chronic asthma. Inhibition of MMPs in humans has failed as a therapeutic strategy because of the poor specificity of MMP inhibitors and effects caused by MMPs having multiple effects on beneficial and injurious processes. Targeting the mast cell/smooth muscle interaction may be a potential option to prevent MMP-1-mediated airway remodeling in patients with asthma. Recently, Hinks and coworkers (48) have shown airway MMP-1 protein and MMP/TIMP ratio to be particularly associated with elevated body mass index in asthma. Further study is required to determine the importance of this pathway in specific asthma endotypes.
In summary we have shown that tryptase-dependent MMP-1 activation can remodel the ECM to support ASM proliferation. In the airways of patients with asthma, tryptase-positive mast cells are colocalized with proliferating ASM cells and MMP-1 expression is associated with BHR and MMP-1 activation with asthma exacerbations and exacerbation severity. Disrupting mast cell/ASM interactions may be a potential therapeutic option against airway remodeling.
Acknowledgments
Acknowledgment
The authors thank Drs. W. Chang and S. Anwar for help with bronchoscopies, Mrs. W. Tarpey for patient recruitment, and Prof. Anne Tattersfield for critical review of the manuscript.
Footnotes
Supported by the Medical Research Council (G1100163 and MR/M004643/1, S.R.J.). The experimental infection study was supported by ERC FP7 Advanced Grant 233015, MRC Centre Grant G1000758, and National Institute of Health Research Biomedical Research Centre Grant P26095 (S.L.J.). S.L.J. is supported by a Chair from Asthma UK (CH11SJ) and is a National Institute of Health Research Senior Investigator.
Author Contributions: S.R.J. conceived the study. S.R.J., D.E.S., and T.W.H. planned the work. S.L.J. ran the exacerbation study. S.N., C.R., and D.J.J. saw the patients. S.N., D.C., D.J.J., C.P., and C.K.B. performed the laboratory work. I.S. and S.N. evaluated the pathology slides. S.R.J., S.N., C.K.B., C.P., S.L.J., D.E.S., and T.W.H. interpreted the results and contributed to the manuscript. All authors approved the final manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201604-0822OC on December 14, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Benayoun L, Druilhe A, Dombret M-C, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–1368. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 2.Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339:1194–1200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- 3.Lindqvist A, Karjalainen E-M, Laitinen LA, Kava T, Altraja A, Pulkkinen M, Halme M, Laitinen A. Salmeterol resolves airway obstruction but does not possess anti-eosinophil efficacy in newly diagnosed asthma: a randomized, double-blind, parallel group biopsy study comparing the effects of salmeterol, fluticasone propionate, and disodium cromoglycate. J Allergy Clin Immunol. 2003;112:23–28. doi: 10.1067/mai.2003.1500. [DOI] [PubMed] [Google Scholar]
- 4.Shaw D, Green R, Berry M, Mellor S, Hargadon B, Shelley M, McKenna S, Thomas M, Pavord I. A cross-sectional study of patterns of airway dysfunction, symptoms and morbidity in primary care asthma. Prim Care Respir J. 2012;21:283–287. doi: 10.4104/pcrj.2012.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, Wise RA, Szefler SJ, Sharma S, Kho AT, et al. CAMP Research Group. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med. 2016;374:1842–1852. doi: 10.1056/NEJMoa1513737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 7.Jeffery PK, Laitinen A, Venge P. Biopsy markers of airway inflammation and remodelling. Respir Med. 2000;94:S9–S15. doi: 10.1016/s0954-6111(00)90127-6. [DOI] [PubMed] [Google Scholar]
- 8.Grainge CL, Lau LCK, Ward JA, Dulay V, Lahiff G, Wilson S, Holgate S, Davies DE, Howarth PH. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med. 2011;364:2006–2015. doi: 10.1056/NEJMoa1014350. [DOI] [PubMed] [Google Scholar]
- 9.Alkhouri H, Poppinga WJ, Tania NP, Ammit A, Schuliga M. Regulation of pulmonary inflammation by mesenchymal cells. Pulm Pharmacol Ther. 2014;29:156–165. doi: 10.1016/j.pupt.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 11.Bradding P, Arthur G. Mast cells in asthma: state of the art. Clin Exp Allergy. 2016;46:194–263. doi: 10.1111/cea.12675. [DOI] [PubMed] [Google Scholar]
- 12.Beasley R, Roche WR, Roberts JA, Holgate ST. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am Rev Respir Dis. 1989;139:806–817. doi: 10.1164/ajrccm/139.3.806. [DOI] [PubMed] [Google Scholar]
- 13.Fajt ML, Wenzel SE. Mast cells, their subtypes, and relation to asthma phenotypes. Ann Am Thorac Soc. 2013;10:S158–S164. doi: 10.1513/AnnalsATS.201303-064AW. [DOI] [PubMed] [Google Scholar]
- 14.Rogers NK, Clements D, Dongre A, Harrison TW, Shaw D, Johnson SR. Extra-cellular matrix proteins induce matrix metalloproteinase-1 (MMP-1) activity and increase airway smooth muscle contraction in asthma. PLoS One. 2014;9:e90565. doi: 10.1371/journal.pone.0090565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markwick LJ, Clements D, Roberts ME, Ceresa CC, Knox AJ, Johnson SR. CCR3 induced-p42/44 MAPK activation protects against staurosporine induced-DNA fragmentation but not apoptosis in airway smooth muscle cells. Clin Exp Allergy. 2012;42:1040–1050. doi: 10.1111/j.1365-2222.2012.04019.x. [DOI] [PubMed] [Google Scholar]
- 16.Henderson N, Markwick LJ, Elshaw SR, Freyer AM, Knox AJ, Johnson SR. Collagen I and thrombin activate MMP-2 by MMP-14-dependent and -independent pathways: implications for airway smooth muscle migration. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1030–L1038. doi: 10.1152/ajplung.00317.2006. [DOI] [PubMed] [Google Scholar]
- 17.Johnson S, Knox A. Autocrine production of matrix metalloproteinase-2 is required for human airway smooth muscle proliferation. Am J Physiol. 1999;277:L1109–L1117. doi: 10.1152/ajplung.1999.277.6.L1109. [DOI] [PubMed] [Google Scholar]
- 18.Freyer AM, Johnson SR, Hall IP. Effects of growth factors and extracellular matrix on survival of human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2001;25:569–576. doi: 10.1165/ajrcmb.25.5.4605. [DOI] [PubMed] [Google Scholar]
- 19.Freyer AM, Billington CK, Penn RB, Hall IP. Extracellular matrix modulates beta2-adrenergic receptor signaling in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2004;31:440–445. doi: 10.1165/rcmb.2003-0241OC. [DOI] [PubMed] [Google Scholar]
- 20.Naveed S, Clements D, Jackson D, Shaw D, Johnston S, Johnson SR. S92 matrix metalloproteinase-1 activation by mast cell tryptase causes airway remodelling and is associated with bronchial hyper-responsiveness in patients with asthma. Thorax. 2015;70:A52. [Google Scholar]
- 21.Naveed S, Jackson DJ, Clements D, Reynolds C, Shaw D, Johnston SL, Johnson SR. Mast cell tryptase activates matrix metalloproteinase-1 causing matrix remodelling, airway smooth muscle growth and airway obstruction during asthma exacerbations [abstract] Am J Respir Crit Care Med. 2016:193–A6174. [Google Scholar]
- 22.Global Initiative for Asthma. Global strategy for asthma management and prevention. Workshop report 2004. Available from: www.Ginasthma.Org/local/uploads/files/ginawr04clean2_1.
- 23.Juniper EF, Svensson K, Mörk A-C, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99:553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Jackson DJ, Makrinioti H, Rana BMJ, Shamji BWH, Trujillo-Torralbo M-B, Footitt J. Jerico D-R, Telcian AG, Nikonova A, Zhu J, et al. Il-33–dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med. 2014;190:1373–1382. doi: 10.1164/rccm.201406-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bramley AM, Roberts CR, Schellenberg RR. Collagenase increases shortening of human bronchial smooth muscle in vitro. Am J Respir Crit Care Med. 1995;152:1513–1517. doi: 10.1164/ajrccm.152.5.7582286. [DOI] [PubMed] [Google Scholar]
- 26.Karlinsky JB, Snider GL, Franzblau C, Stone PJ, Hoppin FG., Jr In vitro effects of elastase and collagenase on mechanical properties of hamster lungs. Am Rev Respir Dis. 1976;113:769–777. doi: 10.1164/arrd.1976.113.6.769. [DOI] [PubMed] [Google Scholar]
- 27.Rajah R, Nunn SE, Herrick DJ, Grunstein MM, Cohen P. Leukotriene D4 induces MMP-1, which functions as an IGFBP protease in human airway smooth muscle cells. Am J Physiol. 1996;271:L1014–L1022. doi: 10.1152/ajplung.1996.271.6.L1014. [DOI] [PubMed] [Google Scholar]
- 28.Cataldo DD, Gueders M, Munaut C, Rocks N, Bartsch P, Foidart JM, Noël A, Louis R. Matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases mRNA transcripts in the bronchial secretions of asthmatics. Lab Invest. 2004;84:418–424. doi: 10.1038/labinvest.3700063. [DOI] [PubMed] [Google Scholar]
- 29.Ito I, Fixman ED, Asai K, Yoshida M, Gounni AS, Martin JG, Hamid Q. Platelet-derived growth factor and transforming growth factor-beta modulate the expression of matrix metalloproteinases and migratory function of human airway smooth muscle cells. Clin Exp Allergy. 2009;39:1370–1380. doi: 10.1111/j.1365-2222.2009.03293.x. [DOI] [PubMed] [Google Scholar]
- 30.Schuliga M, Ong SC, Soon L, Zal F, Harris T, Stewart AG. Airway smooth muscle remodels pericellular collagen fibrils: implications for proliferation. Am J Physiol Lung Cell Mol Physiol. 2010;298:L584–L592. doi: 10.1152/ajplung.00312.2009. [DOI] [PubMed] [Google Scholar]
- 31.Margulis A, Nocka KH, Brennan AM, Deng B, Fleming M, Goldman SJ, Kasaian MT. Mast cell-dependent contraction of human airway smooth muscle cell-containing collagen gels: influence of cytokines, matrix metalloproteases, and serine proteases. J Immunol. 2009;183:1739–1750. doi: 10.4049/jimmunol.0803951. [DOI] [PubMed] [Google Scholar]
- 32.Hasaneen NA, Zucker S, Cao J, Chiarelli C, Panettieri RA, Foda HD. Cyclic mechanical strain-induced proliferation and migration of human airway smooth muscle cells: role of EMMPRIN and MMPs. FASEB J. 2005;19:1507–1509. doi: 10.1096/fj.04-3350fje. [DOI] [PubMed] [Google Scholar]
- 33.Saunders WB, Bayless KJ, Davis GE. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci. 2005;118:2325–2340. doi: 10.1242/jcs.02360. [DOI] [PubMed] [Google Scholar]
- 34.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 35.Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem. 1996;271:10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- 36.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 37.Ricard-Blum S, Salza R. Matricryptins and matrikines: biologically active fragments of the extracellular matrix. Exp Dermatol. 2014;23:457–463. doi: 10.1111/exd.12435. [DOI] [PubMed] [Google Scholar]
- 38.Balzar S, Fajt ML, Comhair SAA, Erzurum SC, Bleecker E, Busse WW, Castro M, Gaston B, Israel E, Schwartz LB, et al. Mast cell phenotype, location, and activation in severe asthma. Data from the Severe Asthma Research Program. Am J Respir Crit Care Med. 2011;183:299–309. doi: 10.1164/rccm.201002-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger P, Tunon-De-Lara JM, Savineau JP, Marthan R. Selected contribution: tryptase-induced PAR-2-mediated Ca(2+) signaling in human airway smooth muscle cells. J Appl Physiol (1985) 2001;91:995–1003. doi: 10.1152/jappl.2001.91.2.995. [DOI] [PubMed] [Google Scholar]
- 40.Khan MA, Ellis R, Inman MD, Bates JH, Sanderson MJ, Janssen LJ. Influence of airway wall stiffness and parenchymal tethering on the dynamics of bronchoconstriction. Am J Physiol Lung Cell Mol Physiol. 2010;299:L98–L108. doi: 10.1152/ajplung.00011.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodruff PG, Dolganov GM, Ferrando RE, Donnelly S, Hays SR, Solberg OD, Carter R, Wong HH, Cadbury PS, Fahy JV. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med. 2004;169:1001–1006. doi: 10.1164/rccm.200311-1529OC. [DOI] [PubMed] [Google Scholar]
- 42.An SS, Bai TR, Bates JH, Black JL, Brown RH, Brusasco V, Chitano P, Deng L, Dowell M, Eidelman DH, et al. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J. 2007;29:834–860. doi: 10.1183/09031936.00112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.James AL, Elliot JG, Jones RL, Carroll ML, Mauad T, Bai TR, Abramson MJ, McKay KO, Green FH. Airway smooth muscle hypertrophy and hyperplasia in asthma. Am J Respir Crit Care Med. 2012;185:1058–1064. doi: 10.1164/rccm.201110-1849OC. [DOI] [PubMed] [Google Scholar]
- 44.Ward JE, Harris T, Bamford T, Mast A, Pain MCF, Robertson C, Smallwood D, Tran T, Wilson J, Stewart AG. Proliferation is not increased in airway myofibroblasts isolated from asthmatics. Eur Respir J. 2008;32:362–371. doi: 10.1183/09031936.00119307. [DOI] [PubMed] [Google Scholar]
- 45.Hassan M, Jo T, Risse P-A, Tolloczko B, Lemière C, Olivenstein R, Hamid Q, Martin JG. Airway smooth muscle remodeling is a dynamic process in severe long-standing asthma. J Allergy Clin Immunol. 2010;125:1037–1045. doi: 10.1016/j.jaci.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 46.Chernyavsky IL, Croisier H, Chapman LA, Kimpton LS, Hiorns JE, Brook BS, Jensen OE, Billington CK, Hall IP, Johnson SR. The role of inflammation resolution speed in airway smooth muscle mass accumulation in asthma: insight from a theoretical model. PLoS One. 2014;9:e90162. doi: 10.1371/journal.pone.0090162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fahy JV. Type 2 inflammation in asthma: present in most, absent in many. Nat Rev Immunol. 2015;15:57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hinks TSC, Brown T, Lau LCK, Rupani H, Barber C, Elliott S, Ward JA, Ono J, Ohta S, Izuhara K, et al. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. J Allergy Clin Immunol. 2016;138:61–75. doi: 10.1016/j.jaci.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]