Clinical outcomes and management of cystic fibrosis (CF) have undergone startling and dramatic advances in the last two decades. These improvements can be linked to the advances in CF basic science starting with the identification of the disease-causing gene defect (the gene producing cystic fibrosis transmembrane conductance regulator protein [CFTR]) to more recent animal models such as the CF pig, that have led to an improved understanding of the early pathophysiologic events leading to lung disease (1). By developing a robust clinical trial network (the CF Foundation Therapeutics Development Network in the United States and Clinical Trials Network in Europe), advances in the laboratory have been translated into the repurposing of existing therapeutics and the development of novel therapeutics, with 11 medications approved specifically for CF in either Europe or the United States. CF basic and clinical research has led the way in demonstrating how advances in the laboratory can lead to advances in clinical care and outcomes. Therapeutics in CF have evolved from treating downstream events such as chronic lower airway infection to addressing the primary defect in CF by modifying mutant CFTR protein with the approval of both ivacaftor and ivacaftor–lumacaftor by the U.S. Food and Drug Administration (2, 3). The CF community is now challenged with how to continue to advance the science in CF to ensure that every patient with CF can live a full and normal life free of disease. In this Perspective, we highlight the changing demographics of the CF population, in addition to advances in therapeutics and challenges we face in continuing to improve the outcomes of our patients.
Epidemiology and Changing Demographics
The CF population is evolving with new diagnostic and therapeutic practices. Worldwide, patients with CF are living longer, with an increasing proportion of patients who are adults (4, 5). In the United States, the proportion of patients who are adults (age ≥18 yr) exceeded 50% in 2014 (6). In concert with improved survival, CF diagnosis occurs earlier in life with universal newborn screening (NBS) in many countries and, as of 2010, all states in the United States (7). In 2010, more than half (57.5%) of persons with CF in the United States were diagnosed by NBS (6). NBS has been linked to overall improved health and prevention of structural lung disease, and studies comparing patient outcomes before and after NBS implementation show benefits to lung function and nutrition status (8). In Australia, members of the AREST-CF (Australian Respiratory Early Surveillance Team for Cystic Fibrosis) study group evaluated infants diagnosed through NBS and found evidence for inflammation and infection of the airways as early as 3 months of age (9, 10), highlighting the importance of early identification and treatment for patients with CF. Earlier age at diagnosis and diagnosis of asymptomatic infants through NBS has contributed to the inclusion of a milder phenotype in recent cohorts of patients with CF (6). NBS appears to increase the diagnosis rate of CFTR-related metabolic syndrome (CRMS); this disorder describes asymptomatic infants who have a positive CF NBS result and indeterminate diagnostic testing results. Using this CF Foundation consensus definition, 1,540 and 309 infants in the U.S. CF Foundation Patient Registry in 2010–2012 met the criteria for CF and CRMS, respectively (CF:CRMS ratio, 5:1), thus altering the dynamic makeup of the CF population (11). Even before the advent of universal NBS, the clinical phenotype of persons with CF had changed. Respiratory Pseudomonas aeruginosa infection has decreased over the past two decades with increased emphasis on eradication (12). Pulmonary function is known to be correlated with nutritional status and with P. aeruginosa infection (13), which may explain the mechanism of improved pulmonary function in this cohort.
The dynamic nature of the CF population has been highlighted by significant improvements in survival (5, 14). U.S. data have demonstrated that CF survival improved from 2000 to 2010 at a rate of 1.8%/year, and a median survival of 56 years is projected if the mortality rate continues to decrease at the rate observed between 2000 and 2010 (5). The median predicted survival in the United States has increased from 28.9 years in 1999 to 39.3 years in 2014 (6). Superior patient outcomes are likely related to advances in multidisciplinary care focused on early and aggressive treatment of pulmonary exacerbations (15), airway clearance, optimized nutritional status (16), identification and treatment of complications (17), and promotion of medication adherence (18). These advances in clinical care are made possible in part by the improved understanding of CF pathophysiology and the development of treatments targeting the consequences of the underlying defect (19).
Not only has the population of patients with CF aged, but increasing numbers of persons with milder phenotypes of CF have been diagnosed through NBS (including CRMS, described previously) and through advances in genotyping of CFTR mutations. In subjects with CF who are 40 years of age and older, the median age at diagnosis has been reported to be 48.8 years (20); these persons are much more likely to have a nonclassical, milder CF phenotype (21). In addition to these changes, survival even among patients with CF with severe lung disease (defined as an FEV1 less than 30% of predicted) is improving, with median survival without lung transplantation improving from 1.2 to 5.3 years from 1991 to 2002 (22, 23). The rapidly changing demography of the CF population due to NBS, nutritional interventions, genotyping, and improved therapies has major implications for research. Novel therapies must bridge the widening CF phenotype, and clinical trials must be more sensitive to evaluating efficacy in those with less severe disease.
Novel Approved Therapeutics and Drugs on the Horizon
Historically, CF therapies were centered on controlling symptomatic complications from the loss of CFTR protein function (18). More recently, emphasis has turned to precision medicine for tailoring individual patient-driven therapies to improve outcomes (24), and several new therapeutic agents have been approved or are currently being studied for individuals with CF. The approval of CFTR modulators and potentiators, for example, underscores the potential for novel therapeutics targeting the underlying CFTR defect. These therapies can largely be broken down into mutation-specific agents or mutation-agnostic treatments that aim to restore normal function regardless of the specific CFTR defect. Clinical trials are also currently underway, examining additional treatments focused on lung inflammation, chronic lung infection, and pancreatic enzyme replacement therapy.
CFTR-based Therapeutic Approaches
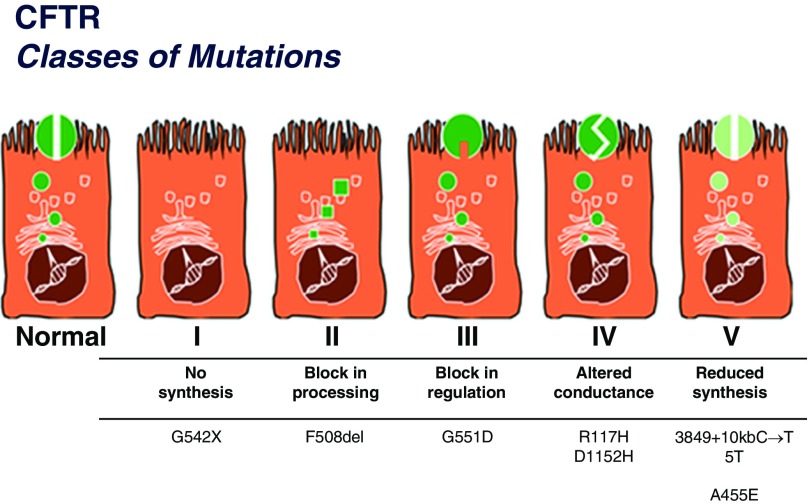
To date, more than 2,000 disease-causing and benign CFTR mutations have been described (25). A classification system was created on the basis of the molecular mechanisms of each mutation contributing to CFTR dysfunction (26); although simplistic (with several CFTR mutations spanning multiple classes), this system is relevant to therapeutic strategies aimed at restoring normal CFTR function (Figure 1). The six classes are as follows: class I (no CFTR synthesis/nonsense mutations), class II (premature protein degradation), class III (protein dysregulation/gating mutations), class IV (decreased channel conductance), class V (decreased production), and class VI (destabilization of CFTR at the cell surface). Thus far, therapies targeting class I–IV mutations have been produced with varied results. As therapies evolve, a more complex system may be required.
Figure 1.
Classes of cystic fibrosis transmembrane conductance regulator protein (CFTR) mutations. Note that class VI is not included in this figure. Adapted by permission from http://www.umd.be/CFTR/W_CFTR/gene.html with initial adaptation from Welsh and Smith (26) and Scriver and colleagues (63). Reprinted from Reference 6, by permission from the Cystic Fibrosis Foundation.
The first major breakthrough to address the basic defect in CF came for those patients with class III mutations. Although class III mutations account for only approximately 5% of the CF population worldwide, the most promising mutation-specific therapy (ivacaftor, a CFTR potentiator) has demonstrated significant clinical benefit (27). Ivacaftor improves chloride transport through CFTR channels by increasing the channel open probability (or gating) at the cell surface (28). In patients with the G551D mutation (the most common class III mutation) on at least one CFTR allele, ivacaftor was associated with improvements in lung function in as early as 2 weeks that were sustained at the end of the trial (48 wk) (2). Reductions were also seen in pulmonary exacerbations, patient-reported respiratory symptoms, and concentration of sweat chloride (2), and children aged 6–11 years with a G551D mutation also experienced improvements with ivacaftor in pulmonary function, weight, and CFTR activity (29). Additional work demonstrated significant improvements in lung function, body mass index, sweat chloride, and CF quality-of-life scores in patients with a non-G551D gating mutation (30), although marginal effects were seen in those with the class IV altered conductance mutation R117H (31). Assessments of physiological effects of ivacaftor postapproval note that ivacaftor restores mucociliary clearance, normalizes duodenal pH, and has been associated with a reduction in culturing P. aeruginosa in patients with G551D mutations (32, 33).
Class II defects are found in 90% of patients with CF, and result in synthesis of immature CFTR protein and inappropriate release from the endoplasmic reticulum (34). F508del, the most common CFTR mutation, leads to rapid protein degeneration and minimal if any protein presence at the plasma membrane (24). Ivacaftor alone was not efficacious in patients homozygous for the F508del mutation (35), likely reflecting its mutation-specific effects. The CFTR corrector lumacaftor was developed specifically to repair this defect by increasing functional CFTR protein at the cell surface. Lumacaftor restores protein function in human F508del homozygous epithelial cells in vitro (36), and the combination therapy lumacaftor–ivacaftor was shown to moderately benefit patients, aged 12 years and older, with homozygous F508del CF despite in vitro evidence that the combination has a limited physiological effect in airway epithelial cells (37, 38). Citing a 2.6–4.0% improvement in FEV1 percent predicted and a reduction in pulmonary exacerbations by 30–39% (3), on July 2, 2015, lumacaftor–ivacaftor was approved by the U.S. Food and Drug Administration in this population. VX-661, another investigational CFTR corrector, has shown activity in phase 2 trials, and preliminary data indicate improvements in lung function and sweat chloride. Investigators are currently enrolling patients at least 12 years of age and either heterozygous or homozygous for the F508del mutation in several phase 3 trials exploring VX-661 in combination with ivacaftor (NCT02516410; NCT02392234; NCT02412111; NCT02508207). The arrival and approval of these latest CF treatments is not without controversy. The high cost of care coupled with marginal clinical benefit in those with the R117H or F508del mutation has led to restricted access for some, based on third-party insurers’ reluctance to pay for the drug.
Class I mutations are responsible for approximately 10% of CF cases worldwide, and up to 60% of cases in Israel (39). These nonsense mutations arise from premature stop codons that disrupt ribosomal translation of the CFTR protein. Preclinical evaluation of a new drug identified by high-throughput screening (ataluren, or PTC124), using a CF mouse model, found that functional CFTR could be generated in a nonsense mutation (40). Ataluren was shown to improve mechanistic markers of CFTR function. In addition, although a phase 3 trial found no overall change in lung function or pulmonary exacerbations, a post hoc analysis of patients receiving ataluren and not using inhaled tobramycin found improvements in both clinical measures (41). At present, a phase 3 trial of ataluren is underway in patients with CF not receiving aminoglycoside antibiotics (NCT02139306).
Non–CFTR-based Therapeutic Approaches
Whereas the goal of CFTR modulation is to restore CFTR function in a mutation-specific fashion, mutation-agnostic techniques—including gene therapy and gene editing—aim to provide patients with CF with wild-type CFTR. These treatments should be effective irrespective of CFTR mutation. After the discovery of the CFTR gene in 1989, numerous gene therapy clinical trials have been performed, with minimal direct clinical benefit established (42). However, rather than emphasizing clinical end points, these early studies were performed primarily to illustrate the clinical safety and proof of concept for gene therapy/editing techniques. Interestingly, a phase 2b study used repeated nebulization of nonviral vectors to deliver the CFTR gene to respiratory epithelial cells and found a small but significant improvement in FEV1 (3.7%) in the treatment group compared with placebo that was sustained for 12 months (43). This study not only illustrated potential clinical benefit, but also provided in vivo, proof of principle for further nonviral vector studies.
Genome-editing techniques have also showed promise in permanently correcting the CFTR mutation. Zinc finger nuclease homology-directed repair works by correcting mutations in the coding region of the CFTR gene by creating a double-stranded DNA break upstream of the defect and combining it with wild-type CFTR DNA (44). This approach has led to wild-type CFTR expression in vitro as well as restoration of CFTR chloride channel function in human bronchial epithelial cells (45). Another genome-editing system, CRISPR (clustered regularly interspaced short palindromic repeats)–Cas9 (CRISPR-associated protein 9), allows for DNA cleavage in a sequence-specific manner and homologous recombination with a corrected endogenous CFTR locus. Lung epithelial cells with abnormal CFTR protein modified by the CRISPR–Cas9 technique demonstrated recovered function and expression of CFTR (46). Although exciting, advances in gene therapy and gene editing require further study (e.g., to ensure efficient and adequate drug delivery to diseased and obstructed airways) and also expose important ethical issues that must be addressed before clinical use.
Another innovative, non–CFTR mutation–specific therapeutic approach to treating the basic defect in CF is the use of epithelial sodium channel (ENaC) inhibitors. ENaC is a sodium epithelial channel that is hyperactive in CF airways and contributes to increased sodium resorption, a decreased periciliary liquid layer, and stickier airway surface liquid that contributes to mucus stasis and airway obstruction (47). The goal of ENaC inhibitors is to reverse this process. Amiloride was the first sodium channel blocker tested, and although it had been shown to improve the rheological properties of mucus, a randomized, double-blind, placebo-controlled trial using nebulized amiloride in patients with CF failed to show improvement in lung function or secondary study end points (48). Additional ENaC inhibitors, including P-1037 (NCT02343445) and GS-9411 (NCT00999531), are being developed, and investigations into molecular regulators of ENaC function uncovered an ENaC modulator that showed inhibition of the protein diglyceride kinase 1 that decreased ENaC activity and normalized sodium and fluid absorption in CF airways. Excessive blocking of ENaC function can lead to unwanted consequences, including fluid accumulation in the lungs and subsequent pulmonary edema (49), and thus any potential future therapeutic agent targeting the ENaC sodium channel to normalize airway surface liquid needs to address this issue.
Last, clinical trials are underway investigating additional treatments targeting lung inflammation, chronic lung infection, and nutrition, and it is hoped that several of these studies will be completed by the end of 2016. These novel therapeutics—although encouraging with respect to their clinical benefits—should be added on rather than substituted for the chronic CF therapies (including airway clearance, mucolytics, and inhaled antibiotics) shown to be beneficial in CF. For example, no studies have evaluated the effectiveness of CFTR modulators compared with recommended therapies for the maintenance of health, and until future studies address these concerns, adherence to previously established CF treatment regimens remains paramount.
Challenges to Clinical Trial Design for Future Therapeutics
The changing landscape of CF demographics, prognosis, and therapies presents unique challenges for conducting clinical research. The classic randomized, double-blind, placebo-controlled trial has been, and is, the gold standard for demonstrating the safety and efficacy of new therapies (50). However, the small patient population and chronic nature of CF have made it difficult to conduct large-size or short-duration studies afforded other diseases (e.g., cardiology and cancer, respectively). Despite that, the path to regulatory approval has been successful for a number of therapies, in part due to the orphan disease status designation, and through careful study design and patient and end-point selection. FEV1 and pulmonary exacerbation are standard clinical outcomes that have been used to demonstrate efficacy in relatively efficient (small-sized) trials—especially when selectively recruiting patients with more severe disease (lower FEV1 and frequent exacerbations). Although these methods have been successful for antiinfectives and early CFTR modulators in CF, the increased use of chronic CF therapies and improved care necessitates that CF trial design adapt to accommodate a milder CF phenotype and settings where placebo-controlled studies are not feasible.
Advances in CF have led to longer median survival (5), improved lung function, and reduced morbidities; these advances have in turn created a challenging environment for further development of novel therapeutics because it is inherently more difficult to show efficacy on clinical measures that are nearly normal, particularly in children with CF. For this setting, and specifically for future CFTR modulators that may show only modest improvements in the short term over currently approved correctors and potentiators (3), sensitive outcome measures and investment into the development of robust biomarkers will be instrumental. Relying on subtle long-term changes in clinical measures (FEV1 rate of decline over 1 yr or longer) can be financially prohibitive and tax patient resources and their ability to participate in other trials. To speed drug development, it is critical to validate markers of mechanism of action through large-scale bridging studies to promote these biomarkers to surrogate end-point status (51). Either as a biomarker or surrogate, robust measures of mechanism of action in CF such as sweat chloride, inflammation, or lung injury (measured by multiple breath washout or lung imaging [52]) can enrich trials and move novel therapeutics to approval more safely and quickly. International collaborations between academic investigators, philanthropic foundations, private industry, and regulatory agencies will facilitate progress by synthesizing registry and trial data sources, sharing biospecimens, and finding creative solutions to speed registration trials safely and effectively.
The increasingly complex regimen of chronic CF therapies (53, 54) and comorbidities that require supplemental care (e.g., liver disease, CF-related diabetes, and osteopenia) make tightly controlled trials for approval and registration more difficult to conduct because of the underlying medical complications that could exclude patients from enrollment. The escalation of underlying standard of care in CF limits the broad applicability of placebo-controlled efficacy trials (55). A postapproval study of continuous alternating therapy of inhaled aztreonam and tobramycin versus inhaled tobramycin and placebo was stopped early because of enrollment difficulties in a patient population increasingly managed with multiple inhaled antibiotics; patients and their providers were reluctant to participate in a placebo-controlled trial, potentially due to concerns about worsening symptoms and lung function in the absence of inhaled antibiotics (56). Comparative effectiveness research is an appealing alternative to traditional controlled trials aimed to answer clinical questions through pragmatic trials with limited inclusion/exclusion criteria, or via observational data such as patient registries (57, 58). However, such trials cannot replace initial efficacy studies. In addition, comparative effectiveness research studies involving registry data or observational studies can be fraught with indication bias, and patient and physician perceptions can be difficult to overcome even when there is little evidence supporting the current standard of care (15).
Creative solutions are needed to counter the current and future challenges of conducting clinical research in CF and moving novel therapeutics from bench to bedside. Biomarker and end-point development and the increased implementation of pragmatic research are only two pieces of the puzzle. Inventive study designs that might include randomized withdrawal (59) or adaptive treatment algorithms will need to be more seriously considered to meet the efficiency needs of CF therapeutic development (60); thus, investigators, data safety monitoring committees, and sponsors need to become more versed in the nuance of these complex study designs. To date, CF studies are increasingly implementing formal group sequential stopping rules to improve the safety and ethics of ongoing trials and decrease the average sample size of trials. N-of-1 study designs provide a unique opportunity to apply patient-centric “precision medicine” to rare CFTR mutations that might otherwise be excluded from larger scale clinical trials (61); such studies can be pooled and leveraged for population-level inference in addition to their application in individual patient care decisions. The participation rate in clinical trials is high among those with CF (62), and patient, family, and physician engagement in therapeutic trials is highly encouraged and valued in the CF community. With equipoise, preparation, and innovation the potential obstacles to conducting clinical trials can and will be overcome to continue delivering safe, life-improving therapies to those with CF.
Conclusions
The clinical presentation and natural history of CF are rapidly evolving. These changes are clearly due to advances in care over the last 20 years. None of the advances we have seen so far in CF clinical outcomes have included the host of novel therapeutics entering the CF armamentarium. One can already envision CF transitioning from a childhood illness to a chronic illness of adulthood. Novel therapeutics in CF have been transformative, demonstrating how a commitment to basic science can translate into therapeutic advances. Without the initial investment into understanding the underpinnings of a disease, advances in therapeutics are unlikely to follow. The future is bright for both patients with CF and their families. To continue to make advances that address the primary defect in CF, we are going to need to be both smarter and more agile in our design of clinical trials.
Footnotes
S.L.H. receives funding from the Cystic Fibrosis Foundation (HELTSH15A0 and OBSERV13A0) and the National Institutes of Health (NIH) (R01DK095738, P30DK089507, UM1HL119073, R01DK095869, and R01HL124053). J.C. receives funding from the Cystic Fibrosis Foundation (COGEN16A0). K.J.R. receives funding from the Cystic Fibrosis Foundation (RAMOS16A0) and the NIH (F32 HL131246-01). C.H.G. receives funding from the Cystic Fibrosis Foundation, the NIH (R01HL103965, R01HL113382, R01AI101307, U M1HL119073, and P30DK089507), and the U.S. Food and Drug Administration (R01FD003704).
Author Contributions: Drafting the manuscript for important intellectual content: all authors.
Originally Published in Press as DOI: 10.1164/rccm.201606-1250PP on October 6, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, Moninger TO, Michalski AS, Hoffman EA, Zabner J, et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345:818–822. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wainwright CE, Elborn JS, Ramsey BW. Lumacaftor–ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373:1783–1784. doi: 10.1056/NEJMc1510466. [DOI] [PubMed] [Google Scholar]
- 4.Elborn JS, Bell SC, Madge SL, Burgel PR, Castellani C, Conway S, De Rijcke K, Dembski B, Drevinek P, Heijerman HG, et al. Report of the European Respiratory Society/European Cystic Fibrosis Society task force on the care of adults with cystic fibrosis. Eur Respir J. 2016;47:420–428. doi: 10.1183/13993003.00592-2015. [DOI] [PubMed] [Google Scholar]
- 5.MacKenzie T, Gifford AH, Sabadosa KA, Quinton HB, Knapp EA, Goss CH, Marshall BC. Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the Cystic Fibrosis Foundation Patient Registry. Ann Intern Med. 2014;161:233–241. doi: 10.7326/M13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cystic Fibrosis Foundation Patient Registry. Bethesda, MD: Cystic Fibrosis Foundation; 2014. Annual data report 2014. [Google Scholar]
- 7.Wagener JS, Zemanick ET, Sontag MK. Newborn screening for cystic fibrosis. Curr Opin Pediatr. 2012;24:329–335. doi: 10.1097/MOP.0b013e328353489a. [DOI] [PubMed] [Google Scholar]
- 8.Farrell PM, Kosorok MR, Rock MJ, Laxova A, Zeng L, Lai HC, Hoffman G, Laessig RH, Splaingard ML Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Pediatrics. 2001;107:1–13. doi: 10.1542/peds.107.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Linnane BM, Hall GL, Nolan G, Brennan S, Stick SM, Sly PD, Robertson CF, Robinson PJ, Franklin PJ, Turner SW, et al. Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) Lung function in infants with cystic fibrosis diagnosed by newborn screening. Am J Respir Crit Care Med. 2008;178:1238–1244. doi: 10.1164/rccm.200804-551OC. [DOI] [PubMed] [Google Scholar]
- 10.Sly PD, Brennan S, Gangell C, de Klerk N, Murray C, Mott L, Stick SM, Robinson PJ, Robertson CF, Ranganathan SC Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180:146–152. doi: 10.1164/rccm.200901-0069OC. [DOI] [PubMed] [Google Scholar]
- 11.Ren CL, Fink AK, Petren K, Borowitz DS, McColley SA, Sanders DB, Rosenfeld M, Marshall BC. Outcomes of infants with indeterminate diagnosis detected by cystic fibrosis newborn screening. Pediatrics. 2015;135:e1386–e1392. doi: 10.1542/peds.2014-3698. [DOI] [PubMed] [Google Scholar]
- 12.Salsgiver EL, Fink AK, Knapp EA, LiPuma JJ, Olivier KN, Marshall BC, Saiman L. Changing epidemiology of the respiratory bacteriology of patients with cystic fibrosis. Chest. 2016;149:390–400. doi: 10.1378/chest.15-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konstan MW, Butler SM, Wohl ME, Stoddard M, Matousek R, Wagener JS, Johnson CA, Morgan WJ Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr. 2003;142:624–630. doi: 10.1067/mpd.2003.152. [DOI] [PubMed] [Google Scholar]
- 14.Stephenson AL, Tom M, Berthiaume Y, Singer LG, Aaron SD, Whitmore GA, Stanojevic S. A contemporary survival analysis of individuals with cystic fibrosis: a cohort study. Eur Respir J. 2015;45:670–679. doi: 10.1183/09031936.00119714. [DOI] [PubMed] [Google Scholar]
- 15.Flume PA, Mogayzel PJ, Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, Marshall BC Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802–808. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 16.Borowitz D, Robinson KA, Rosenfeld M, Davis SD, Sabadosa KA, Spear SL, Michel SH, Parad RB, White TB, Farrell PM, et al. Cystic Fibrosis Foundation. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155(6) Suppl:S73–S93. doi: 10.1016/j.jpeds.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med. 2000;162:891–895. doi: 10.1164/ajrccm.162.3.9904075. [DOI] [PubMed] [Google Scholar]
- 18.Cohen-Cymberknoh M, Shoseyov D, Kerem E. Managing cystic fibrosis: strategies that increase life expectancy and improve quality of life. Am J Respir Crit Care Med. 2011;183:1463–1471. doi: 10.1164/rccm.201009-1478CI. [DOI] [PubMed] [Google Scholar]
- 19.Ong T, Ramsey BW. Update in cystic fibrosis 2014. Am J Respir Crit Care Med. 2015;192:669–675. doi: 10.1164/rccm.201504-0656UP. [DOI] [PubMed] [Google Scholar]
- 20.Rodman DM, Polis JM, Heltshe SL, Sontag MK, Chacon C, Rodman RV, Brayshaw SJ, Huitt GA, Iseman MD, Saavedra MT, et al. Late diagnosis defines a unique population of long-term survivors of cystic fibrosis. Am J Respir Crit Care Med. 2005;171:621–626. doi: 10.1164/rccm.200403-404OC. [DOI] [PubMed] [Google Scholar]
- 21.Nick JA, Chacon CS, Brayshaw SJ, Jones MC, Barboa CM, St Clair CG, Young RL, Nichols DP, Janssen JS, Huitt GA, et al. Effects of gender and age at diagnosis on disease progression in long-term survivors of cystic fibrosis. Am J Respir Crit Care Med. 2010;182:614–626. doi: 10.1164/rccm.201001-0092OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George PM, Banya W, Pareek N, Bilton D, Cullinan P, Hodson ME, Simmonds NJ. Improved survival at low lung function in cystic fibrosis: cohort study from 1990 to 2007. BMJ. 2011;342:d1008. doi: 10.1136/bmj.d1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. N Engl J Med. 1992;326:1187–1191. doi: 10.1056/NEJM199204303261804. [DOI] [PubMed] [Google Scholar]
- 24.Clancy JP, Jain M. Personalized medicine in cystic fibrosis: dawning of a new era. Am J Respir Crit Care Med. 2012;186:593–597. doi: 10.1164/rccm.201204-0785PP. [DOI] [PubMed] [Google Scholar]
- 25.Cystic Fibrosis Centre at the Hospital for Sick Children in Toronto. Cystic Fibrosis Mutation Database. [updated 2011 Apr 25; accessed 2016 May 31]. Available from: http://www.genet.sickkids.on.ca/app.
- 26.Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993;73:1251–1254. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- 27.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, Sagel SD, Hornick DB, Konstan MW, Donaldson SH, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss RB, Flume PA, Elborn JS, Cooke J, Rowe SM, McColley SA, Rubenstein RC, Higgins M VX11-770-110 (KONDUCT) Study Group. Efficacy and safety of ivacaftor in patients with cystic fibrosis who have an Arg117His-CFTR mutation: a double-blind, randomised controlled trial. Lancet Respir Med. 2015;3:524–533. doi: 10.1016/S2213-2600(15)00201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies JC, Wainwright CE, Canny GJ, Chilvers MA, Howenstine MS, Munck A, Mainz JG, Rodriguez S, Li H, Yen K, et al. VX08-770-103 (ENVISION) Study Group. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med. 2013;187:1219–1225. doi: 10.1164/rccm.201301-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Boeck K, Munck A, Walker S, Faro A, Hiatt P, Gilmartin G, Higgins M. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros. 2014;13:674–680. doi: 10.1016/j.jcf.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Vertex Pharmaceuticals (Canada) Inc. Laval, PQ, Canada: Vertex Pharmaceuticals (Canada) Inc.; 2012. Product monograph: Kalydeco. [Google Scholar]
- 32.Heltshe SL, Mayer-Hamblett N, Burns JL, Khan U, Baines A, Ramsey BW, Rowe SM GOAL (G551D Observation-AL) Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor. Clin Infect Dis. 2015;60:703–712. doi: 10.1093/cid/ciu944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, Sagel SD, Khan U, Mayer-Hamblett N, Van Dalfsen JM, et al. GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190:175–184. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Virant-Young D, Thomas J, Woiderski S, Powers M, Carlier J, McCarty J, Kupchick T, Larder A. Cystic fibrosis: a novel pharmacologic approach to cystic fibrosis transmembrane regulator modulation therapy. J Am Osteopath Assoc. 2015;115:546–555. doi: 10.7556/jaoa.2015.112. [DOI] [PubMed] [Google Scholar]
- 35.Flume PA, Liou TG, Borowitz DS, Li H, Yen K, Ordoñez CL, Geller DE VX 08-770-104 Study Group. Ivacaftor in subjects with cystic fibrosis who are homozygous for the F508del-CFTR mutation. Chest. 2012;142:718–724. doi: 10.1378/chest.11-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack J, Cao D, Neuberger T, Singh A, Olson ER, Wine J, et al. VX-809, a CFTR corrector, increases the cell surface density of functional F508del-CFTR in pre-clinical models of cystic fibrosis [abstract] Pediatr Pulmonol. 2009;44:154. [Google Scholar]
- 37.Cholon DM, Quinney NL, Fulcher ML, Esther CR, Jr, Das J, Dokholyan NV, Randell SH, Boucher RC, Gentzsch M. Potentiator ivacaftor abrogates pharmacological correction of ΔF508 CFTR in cystic fibrosis. Sci Transl Med. 2014;6:246ra96. doi: 10.1126/scitranslmed.3008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veit G, Avramescu RG, Perdomo D, Phuan PW, Bagdany M, Apaja PM, Borot F, Szollosi D, Wu YS, Finkbeiner WE, et al. Some gating potentiators, including VX-770, diminish ΔF508-CFTR functional expression. Sci Transl Med. 2014;6:246ra97. doi: 10.1126/scitranslmed.3008889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerem B, Chiba-Falek O, Kerem E. Cystic fibrosis in Jews: frequency and mutation distribution. Genet Test. 1997;1:35–39. doi: 10.1089/gte.1997.1.35. [DOI] [PubMed] [Google Scholar]
- 40.Du M, Liu X, Welch EM, Hirawat S, Peltz SW, Bedwell DM. PTC124 is an orally bioavailable compound that promotes suppression of the human CFTR-G542X nonsense allele in a CF mouse model. Proc Natl Acad Sci USA. 2008;105:2064–2069. doi: 10.1073/pnas.0711795105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerem E, Konstan MW, De Boeck K, Accurso FJ, Sermet-Gaudelus I, Wilschanski M, Elborn JS, Melotti P, Bronsveld I, Fajac I, et al. Cystic Fibrosis Ataluren Study Group. Ataluren for the treatment of nonsense-mutation cystic fibrosis: a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir Med. 2014;2:539–547. doi: 10.1016/S2213-2600(14)70100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griesenbach U, Pytel KM, Alton EW. Cystic fibrosis gene therapy in the UK and elsewhere. Hum Gene Ther. 2015;26:266–275. doi: 10.1089/hum.2015.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alton EW, Armstrong DK, Ashby D, Bayfield KJ, Bilton D, Bloomfield EV, Boyd AC, Brand J, Buchan R, Calcedo R, et al. UK Cystic Fibrosis Gene Therapy Consortium. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. 2015;3:684–691. doi: 10.1016/S2213-2600(15)00245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee CM, Flynn R, Hollywood JA, Scallan MF, Harrison PT. Correction of the ΔF508 mutation in the cystic fibrosis transmembrane conductance regulator gene by zinc-finger nuclease homology-directed repair. Biores Open Access. 2012;1:99–108. doi: 10.1089/biores.2012.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crane AM, Kramer P, Bui JH, Chung WJ, Li XS, Gonzalez-Garay ML, Hawkins F, Liao W, Mora D, Choi S, et al. Targeted correction and restored function of the CFTR gene in cystic fibrosis induced pluripotent stem cells. Stem Cell Rep. 2015;4:569–577. doi: 10.1016/j.stemcr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–658. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- 48.Pons G, Marchand MC, d’Athis P, Sauvage E, Foucard C, Chaumet-Riffaud P, Sautegeau A, Navarro J, Lenoir G Amiloride-AFLM Collaborative Study Group. French multicenter randomized double-blind placebo-controlled trial on nebulized amiloride in cystic fibrosis patients. Pediatr Pulmonol. 2000;30:25–31. doi: 10.1002/1099-0496(200007)30:1<25::aid-ppul5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 49.Althaus M, Clauss WG, Fronius M. Amiloride-sensitive sodium channels and pulmonary edema. Pulm Med. 2011;2011:830320. doi: 10.1155/2011/830320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Center for Biologics Evaluation and Research (CDER), Center for Drug Evaluation and Research (CBER), U.S. Food and Drug Administration. Guidance for industry: providing clinical evidence of effectiveness for human drug and biological products. 1998. [1998 May; accessed 2016 Nov 3]. Available from: http://www.fda.gov/downloads/Drugs/GuidanceCompliance%20RegulatoryInformation/Guidances/UCM078749.pdf+Providing+clinical+evidence+of+effectiveness+for+human+and+bio&client=FDAgov&site=FDAgov&lr=&proxystylesheet=FDAgov&output=xml_no_dtd&ie=UTF-8&access=p&oe=UTF-8.
- 51.Fleming TR, Powers JH. Biomarkers and surrogate endpoints in clinical trials. Stat Med. 2012;31:2973–2984. doi: 10.1002/sim.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenow T, Oudraad MC, Murray CP, Turkovic L, Kuo W, de Bruijne M, Ranganathan SC, Tiddens HA, Stick SM Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) PRAGMA-CF: a quantitative structural lung disease computed tomography outcome in young children with cystic fibrosis. Am J Respir Crit Care Med. 2015;191:1158–1165. doi: 10.1164/rccm.201501-0061OC. [DOI] [PubMed] [Google Scholar]
- 53.Flume PA, O’Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ, Jr, Willey-Courand DB, Bujan J, Finder J, Lester M, Quittell L, et al. Cystic Fibrosis Foundation, Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957–969. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 54.Mogayzel PJ, Jr, Naureckas ET, Robinson KA, Mueller G, Hadjiliadis D, Hoag JB, Lubsch L, Hazle L, Sabadosa K, Marshall B Pulmonary Clinical Practice Guidelines Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187:680–689. doi: 10.1164/rccm.201207-1160oe. [DOI] [PubMed] [Google Scholar]
- 55.Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros. 2009;8:91–96. doi: 10.1016/j.jcf.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flume PA, Clancy JP, Retsch-Bogart GZ, Tullis DE, Bresnik M, Derchak PA, Lewis SA, Ramsey BW. Continuous alternating inhaled antibiotics for chronic pseudomonal infection in cystic fibrosis. J Cyst Fibros. 2016;15:809–815. doi: 10.1016/j.jcf.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, Elbert A, Petren KM, Marshall BC. The Cystic Fibrosis Foundation Patient Registry: design and methods of a national observational disease registry. Ann Am Thorac Soc. 2016;13:1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 58.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290:1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 59.Balfour-Lynn IM, Lees B, Hall P, Phillips G, Khan M, Flather M, Elborn JS CF WISE (Withdrawal of Inhaled Steroids Evaluation) Investigators. Multicenter randomized controlled trial of withdrawal of inhaled corticosteroids in cystic fibrosis. Am J Respir Crit Care Med. 2006;173:1356–1362. doi: 10.1164/rccm.200511-1808OC. [DOI] [PubMed] [Google Scholar]
- 60.Meurer WJ, Lewis RJ, Berry DA. Adaptive clinical trials: a partial remedy for the therapeutic misconception? JAMA. 2012;307:2377–2378. doi: 10.1001/jama.2012.4174. [DOI] [PubMed] [Google Scholar]
- 61.Zucker DR, Ruthazer R, Schmid CH. Individual (n-of-1) trials can be combined to give population comparative treatment effect estimates: methodologic considerations. J Clin Epidemiol. 2010;63:1312–1323. doi: 10.1016/j.jclinepi.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goss CH, Rubenfeld GD, Ramsey BW, Aitken ML. Clinical trial participants compared with nonparticipants in cystic fibrosis. Am J Respir Crit Care Med. 2006;173:98–104. doi: 10.1164/rccm.200502-273OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B. The metabolic and molecular bases of inherited disease, 8th ed. New-York: McGraw-Hill; 2001. pp. 5121–5188.