Abstract
Over the past two decades, robotic and minimally invasive cardiac surgery has been continuously refined and is currently an alternative to traditional open-heart surgery for some patients. The parallel evolution of imaging modalities has made robotic surgery safer and more efficient. Here, we review the pre- and post-operative use of computed tomography (CT) in minimally invasive and robotic cardiac procedures.
Keywords: Cardiac surgical procedures, minimally invasive surgical procedures, X-ray computed tomography, mitral valve annuloplasty, vascular graft occlusion
Imaging in valve procedures
Robotic mitral valve surgeries have been done in the US since 2000; they can be performed safely by highly trained surgeons (1) and reduce hospitalization time (2). Pre-operative analysis of patients’ computed tomography (CT) scans has proven to be an invaluable tool to prepare for surgeries and screen for patients for whom minimally invasive surgery is contraindicated.
CT can aid in determining the risk of morbidity and mortality associated with cardiac surgery. The presence of coronary artery disease (CAD) in patients undergoing valve surgery worsens prognosis, and 64-slice CTA has been demonstrated to be a good tool to pre-operatively screen patients for CAD (3). In fact, the American College of Cardiology/American Heart Association recommends that candidates for major surgery should be preoperatively evaluated for ischemic heart disease (4). CT can be used for this purpose, and Russo et al proposed that CT could be the method of choice for assessing cardiac risk in patients who are candidates for major non-coronary cardiac surgery and do not present with symptoms of ischemic heart disease (4).
Another benefit of 64-slice CT is that it has been shown to provide geometric and anatomical data on the mitral valve and the subvalvular apparatus. In patients with mitral valve regurgitation, 64-slice CT identified asymmetric deformations of the valve, which is important for mitral valve restoration surgeries (5). Mitral valve leaflet geometry may even be used to screen patients for restrictive annuloplasty [a procedure in which an undersized annuloplasty ring is inserted, which improves the apposition of the mitral valve leaflets and reduces mitral regurgitation (6)]. A study of 51 patients undergoing restrictive annuloplasty demonstrated that a posterior leaflet angle ≥45 degrees was the best predictor of postoperative regurgitation [sensitivity 100%, specificity 97%, positive predictive value (PPV) 92%, negative predictive value (NPV) 100%] (7).
Considerable aortic atherosclerotic plaques, which are associated with elevated long-term mortality after cardiothoracic surgery (8), can also be detected with CT. Such findings often lead to a change in surgical strategy—Moodley et al. demonstrated that in the case of many patients (87%) who were found to have extensive aorto-iliac atherosclerosis or mitral annular calcification (which can also be detected with CT), the strategy was changed from minimally invasive robotic mitral valve surgery to a sternotomy, or the surgery was canceled (9). Calcification of the mitral annulus is a contraindication for robotic mitral valve repair (10).
CT can also be used to decide on an optimal cardiopulmonary bypass (CPB) strategy in minimally invasive surgery. During CPB in typical minimally invasive procedures, perfusion is achieved via the femoral artery, venous drainage via the femoral vein, and the aorta is occluded either by endoaortic balloon occlusion (EBO) or transthoracic cross-clamping (TTC). Aortic and pulmonary artery diameter is established via CT and the appropriate decisions are made—EBO use is not advised when the aortic diameter is >4.0 cm (10) and TTC should not be used when the pulmonary artery is enlarged or when the aorta is small and fragile (11). In order to successfully insert a venous cannula and achieve venous drainage during CPB, CT scans of the central veins must be analyzed to identify venous stenoses and occlusions, anomalies, or clots. Patients with venous occlusions may be poor candidates for robotic mitral valve surgery (10).
CT is also used to assess the ilio-femoral arteries. They are evaluated for their minimal luminal diameter, tortuosity, atherosclerotic burden, abnormal angulation, and evidence of prior accesses (10). Arteries with a minimal luminal diameter <7 mm are considered poor candidates for cannulation, and patch graft femoral cannulation or axillary artery cannulation may be considered instead (10). Figure 1 shows a preoperative three-dimesnional (3D) volume rendered image obtained from a CT scan, which reveals a right external iliac artery that is too tortuous and narrow (at its minimal luminal diameter) to be cannulated. In this case, the patient was cannulated on the left side instead.
Figure 1.
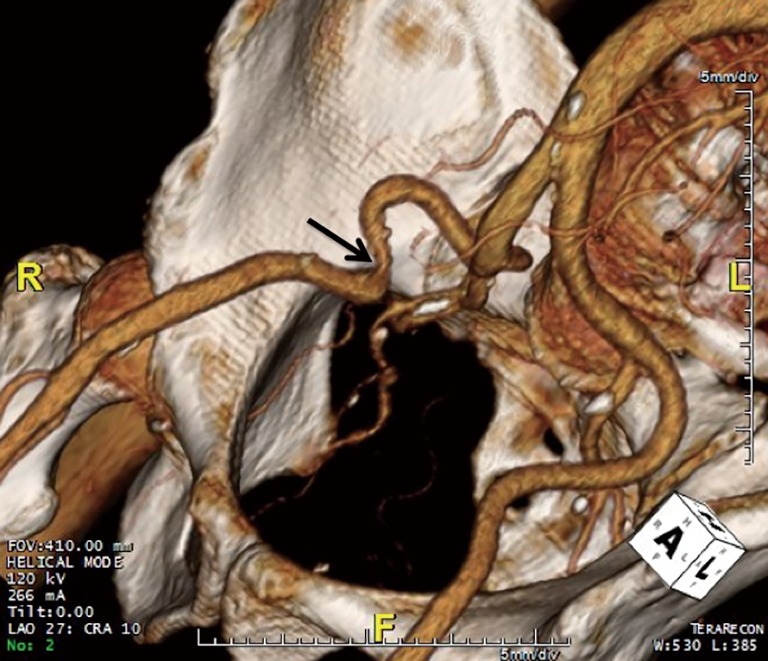
Three-dimensional (3D) volume rendered image obtained from a computed tomography (CT) scan showing a tortuous right external iliac artery with a narrowing (indicated by the arrow). In contrast, the left external iliac artery is normal.
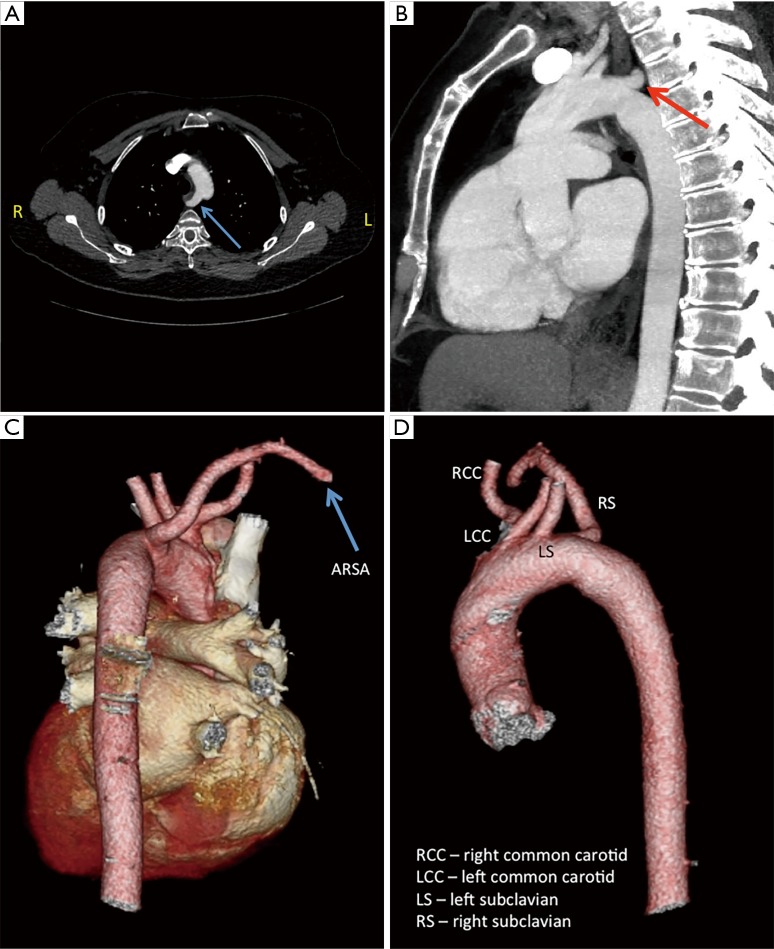
In another case, a patient’s CT showed an aberrant right subclavian artery (ARSA) (Figure 2). Normally, the right subclavian artery comes off the right brachiocephalic artery, the first branch off the aortic arch. An ARSA is the last branch off the aortic arch and typically takes a retroesophagel course to reach the right side. Due to the ARSA, it was decided to alter the cannulation and myocardial preservation strategy for the procedure due to the fact that the use of aortic endoballoon occlusion is facilitated by the ability to measure simultaneous blood pressure in both arms to ensure that the balloon has not migrated into the aortic arch. Furthermore, the patient had small femoral arteries unlikely to accommodate the large caliber (21 or 23 F) arterial femoral cannulae that would be required for use of the endoballoon. Therefore the case was done with fibrillatory arrest and no aortic cross-clamp.
Figure 2.
Computed tomography (CT) scans demonstrating an aberrant right subclavian artery (ARSA)—instead of taking origin from the brachiocephalic artery the ARSA is the last branch off the aortic arch and loops around to reach the right side. (A,B) ARSA is indicated by the arrow; (C,D) three-dimensional (3D) volume rendered images obtained from a CT scan.
Additional parts of the CT scans that should be assessed are the right pleura and right hemidiaphragm. Pleural thickening is an indication of chronic inflammation and potential pleural adhesions that would prevent collapse of the right lung during surgery. In such cases, the operation is often converted to a sternotomy (10).
The position of the right diaphragm should be analyzed on CT scans to determine appropriate port placement sites (12). If the diaphragm is too elevated, it can be pulled down via a suture through the central tendon (10). Also, since transesophageal echocardiography is used to monitor cannulation prior to surgery, CT images of the esophagus must be examined for stenosis and masses (10).
Another use of CT technology in minimally invasive cardiac procedures is to predict the outcome of transcatheter aortic valve replacement (TAVR). TAVR, first performed on a human patient in 2002, is a minimally invasive technique, which involves percutaneously delivering a heart valve with the aid of a catheter, and implanting it within a diseased aortic valve (13). Paravulvar regurgitation (PAR, leakage of blood around the valve) is a potential complication of the procedure and a multicenter trial showed that it was present in 11.8% of the patients at 30 days and in 10.5% at 1 year post surgery (14). Currently, the size of the artificial valve is determined by a 2D measurement of the aortic annulus using an echocardiogram. Wilson at al demonstrated that using 3D measurements of the aortic annulus obtained with multidetector CT might yield valuable information regarding prosthetic valve sizing. Two parameters turned out to be the best predictors of moderate or severe PAR—the difference between the circular prosthetic valve diameter and the CT-obtained mean annular diameter (since aortic valves are oval in shape, the mean diameter is the average of the long and short diameters); and the ratio of prosthetic valve area to the CT-obtained annular area [respectively: area under the receiver-operator curve (AUC): 0.81, 95% confidence interval (CI): 0.68–0.88; AUC: 0.80, 95% CI: 0.65–0.90] (15). Valves with diameters at least 1 mm larger than the mean annular diameter and areas at least 10% larger than the aortic annular area had significantly lower rates of PAR. However, further studies need to be conducted in order to demonstrate whether intentionally oversizing the prosthetic valves is clinically safe and feasible (15). The use of preoperative CT scanning can also assist in planning incisions for minimally invasive surgical aortic valve replacement (SAVR).
Preoperative imaging in other robotic cardiac surgery procedures
Vascular ring division procedures further highlight the preoperative benefit of CT. Robotically assisted totally endoscopic vascular ring division was shown to be a feasible and safe procedure (16) and CT and MRI are used preoperatively to detect vascular aberrations, airway compression, or esophageal impingement (17).
Patent ductus arteriosus repair is another procedure that can be done totally endoscopically with a robotic surgical system (16). It was established that ECG gated CT is able to clearly demonstrate a patent ductus arteriosus in adults, its size, and calcification level. Furthermore, virtual angioscopy (a CT scan postprocessing technique) allows visualization of calcification within the duct. As such, it can be utilized as a noninvasive preoperational screening method, since patients whose ducts are heavily calcified, distorted, or contain aneurysms are poor candidates for surgery (18).
CT virtual cardioscopy is an effective tool in visualizing intracardiac tumors. A case report demonstrated that a left atrial myxoma was visualized using CT virtual cardioscopy, and its anatomy was confirmed via angiocardiography and surgery (19). Since robotic atrial myxoma resections seems to be safe (20), virtual cardioscopy may become a standard for these procedures since it is superior to echocardiography and catheterization in examining the intracardiac anatomy and pathology in multiple planes (19).
Port location determination for robotic cardiac procedures
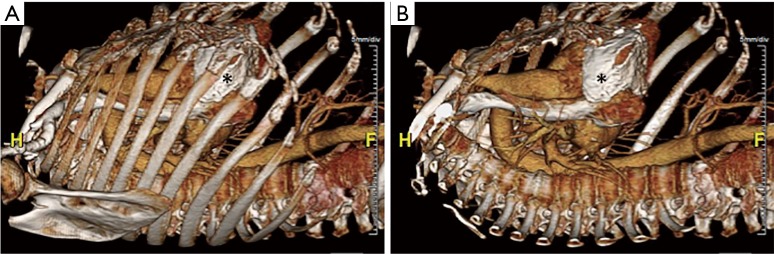
Robotic cardiac surgery involves placing instruments inside of patients via small ports, and as such, port placement is an important issue in surgery planning. 3D volume rendered images obtained from CT scans (Figure 3) allow the surgeon to analyze the patient’s anatomy and determine optimal locations for port placement. For instance, since pericardial calcification can be visualized on these images (Figure 3), this provides crucial information regarding port placement, or can rule out robotic surgery altogether if the pericardial calcifications are too widespread.
Figure 3.
Three-dimensional (3D) volume rendered images obtained from a computed tomography (CT) scan with ribs present (A) and digitally removed (B). Areas of pericardial calcifications can be easily seen and are indicated by asterisks.
Ongoing research aims to further improve port location selection methodologies. Wierzbicki et al. developed and validated a patient-specific method to model the motion of the heart (21), which might be incorporated into surgery planning in the future. Cannon et al. published an algorithm that can be used to automatically determine optimal port locations for coronary artery bypass grafting (CABG) surgeries. As inputs, this algorithm takes preoperative CT images of the patient and optimal instrument and endoscope angles at each surgical site, and determines the optimal port locations. The utilization of this algorithm avoids estimation of the patient’s intrathoracic anatomy, and was shown to significantly improve CABG surgery efficiency in validation experiment on a torso model, comparing the algorithmically determined port locations to ones determined by cardiac surgeons and the literature (22). Such algorithms may be used in the future to enhance robotic surgery.
CT for the assessment of graft patency after CABG surgery
Thousands of CABG surgeries are performed annually in the US and minimally invasive robotic CABG surgery has been performed in select institutions over the past 15 years (23). Unfortunately, coronary bypass graft occlusions are frequent and increase with time (24). A study of 1,074 recipients of saphenous vein grafts found that the 10-year patency of grafts was 61% (25). As such, patients need to be monitored post-surgery. Currently, the reference standard in the assessment of graft patency following CAGB is invasive coronary angiography (ICA) (26). ICA began with the work of F. Mason Sones, who pioneered cine coronary arteriography and noted a correlation between symptomatic coronary disease and major coronary artery stenosis (27). ICA involves the catherization of a patient and subsequent injection of radio opaque contrast while using X-rays to image the vasculature. Although minimally invasive, ICA comes with risks of myocardial infarction and death (28,29).
With the evolution of CT technology, the current debate is whether non-invasive computed tomography angiography (CTA) can replace catheter-based angiography in the detection of vascular occlusions. The importance of this debate is heightened for CABG patients because ICA in CABG patients is harder, more time consuming, and has a higher rate of complications (30). Furthermore, it may be easier to visualize grafts via CTA as compared to ICA (31). A large-scale meta-analysis comparing the diagnostic accuracy of ICA to CTA (with multisection CT scanners using a minimum of 16 sections) in obstructive bypass graft disease demonstrated that CTA was diagnostically reliable (sensitivity: 98%; specificity: 97%; PPV: 93%; NPV: 99%). The drawback was that some grafts (7.6%) were not assessable. The meta-analysis also demonstrated that diagnostic accuracy increased with the number of slices taken by the CT—the accuracy for 16- and 64-slice CTA was 90% and 96% respectively (P<0.001) (30).
Another study compared 64-slice CTA with ICA results in 131 patients with CABG (a total of 418 grafts were examined) and similar statistical parameters as the meta-analysis were obtained (sensitivity: 97%, specificity: 97%, PPV: 93%, NPV: 99%). Here, only 2% of the grafts were of non-assessable quality. The study also demonstrated that while the evaluability of bypass grafts in arrhythmic patients was significantly lower than in non-arrhythmic patients, it was still high (95 vs. 100%, P<0.01) (32).
An important milestone in CTA was the introduction of ECG gating—a protocol in which the patient is exposed to radiation only at specific points in the cardiac cycle. The first feasibility study utilizing ECG-gating on a 64-slice CT scanner demonstrated the diagnostic ability of the procedure [99% of coronary segments were of diagnostic quality when heart rate (HR) was below 63 bpm] and the fact that the effective radiation dose was very low (mean effective radiation dose: 2.1±0.6 mSv; range, 1.1–3.0 mSv) (33).
The 128-slice CT system with ECG gating was shown to perform well on graft analysis. In a study of 125 grafts (465 vessel sections), only 0.6% of the sections contained severe motion artifacts rendering the images non-diagnostic. The small percentage of poor images was even more remarkable in light of the fact that none of the patients were given medications for HR control before the CT procedures [many studies involving 64-slice CT administered HR controlling drugs prior to CT (32,34)]. Furthermore, as compared to typical 64-slice CTA with ECG gating, patients received a more than 8-fold lower radiation dose [2.3±0.3 mSv (entire thorax scanned) vs. 18.9±6.0 mSv (scan range from the aortic arch to the base of the heart)] (35).
The development of 256-slice CT enhanced image quality. In a study of well-matched patient cohorts, the image quality was better with 256-slice ECG gated CT as compared to 64-slice ECG gated CT (98.9% vs. 95.6% assessable coronary segments respectively, P< 0.05). The same study also demonstrated that while the average radiation dose was not significantly different between the two groups, the contrast medium volume was significantly reduced by 17% in patients undergoing 256-slice CT, attributable to shorter imaging times when 256-slice CT is used (36). This may be beneficial, since the risk of acute renal failure post angiography is elevated in diabetic patients and patients with serum creatinine >2.0, and is likely linked to iodinated contrast imaging agents (37).
Due to the evolution of CT technology, CTA has become a promising imaging method for the assessment of coronary graft patency, which avoids the risks of ICA. In 2006, an expert panel consisting of renowned medical societies published appropriateness criteria for cardiac CT and cardiac MRI, in which they rated the use of CT for evaluation of bypass grafts in the setting of chest pain syndrome as uncertain. However, in 2009, they changed their rating to appropriate, reflecting the improvement of CT (38).
It should be noted that a drawback of CTA is that while obstructions can be detected, their physiological consequences (i.e., whether or not they cause ischemia) are harder to interpret. For instance, Nakazato et al. demonstrated that 30–49% of lesions which appeared mild on an angiogram were ischemia causing; the converse was also true, only 27% of lesions which caused a 50–69% reduction in lumen size resulted in ischemia. The group then proposed the use of fractional flow reserve derived from computed tomography angiography (FFRCT) to gauge the physiologic consequence of lesions and demonstrated its favorable diagnostic potential (39).
Utilization of CT in pacemaker lead evaluation
Biventricular pacemakers can be used to treat heart failure and conduction delay, and epicardial lead placement is a procedure that can be safely performed via minimally invasive means (minithoracotomy or video-assisted/robotically-assisted endoscopic surgery) (40). Rarely, the leads migrate, penetrate the ventricle (and sometimes the pericardium) and have to be corrected. The method most commonly used to detect location of the leads is echocardiography; however, there are reported cases of echocardiographic findings being ambiguous. CT was used in its place, with excellent results (41).
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Cao C, Wolfenden H, Liou K, et al. A meta-analysis of robotic vs. conventional mitral valve surgery. Ann Cardiothorac Surg 2015;4:305-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woo YJ, Nacke EA. Robotic minimally invasive mitral valve reconstruction yields less blood product transfusion and shorter length of stay. Surgery 2006;140:263-7. 10.1016/j.surg.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 3.Meijboom WB, Mollet NR, Van Mieghem CA, et al. Pre-operative computed tomography coronary angiography to detect significant coronary artery disease in patients referred for cardiac valve surgery. J Am Coll Cardiol 2006;48:1658-65. 10.1016/j.jacc.2006.06.054 [DOI] [PubMed] [Google Scholar]
- 4.Russo V, Gostoli V, Lovato L, et al. Clinical value of multidetector CT coronary angiography as a preoperative screening test before non-coronary cardiac surgery. Heart 2007;93:1591-8. 10.1136/hrt.2006.105023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delgado V, Tops LF, Schuijf JD, et al. Assessment of mitral valve anatomy and geometry with multislice computed tomography. JACC Cardiovasc Imaging 2009;2:556-65. 10.1016/j.jcmg.2008.12.025 [DOI] [PubMed] [Google Scholar]
- 6.Marwick TH. Restrictive annuloplasty for ischemic mitral regurgitation: too little or too much? J Am Coll Cardiol 2008;51:1702-3. 10.1016/j.jacc.2008.01.032 [DOI] [PubMed] [Google Scholar]
- 7.Magne J, Pibarot P, Dagenais F, et al. Preoperative posterior leaflet angle accurately predicts outcome after restrictive mitral valve annuloplasty for ischemic mitral regurgitation. Circulation 2007;115:782-91. 10.1161/CIRCULATIONAHA.106.649236 [DOI] [PubMed] [Google Scholar]
- 8.Kurra V, Lieber ML, Sola S, et al. Extent of thoracic aortic atheroma burden and long-term mortality after cardiothoracic surgery: a computed tomography study. JACC Cardiovasc Imaging 2010;3:1020-9. 10.1016/j.jcmg.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 9.Moodley S, Schoenhagen P, Gillinov AM, et al. Preoperative multidetector computed tomograpy angiography for planning of minimally invasive robotic mitral valve surgery: impact on decision making. J Thorac Cardiovasc Surg 2013;146:262-8.e1. 10.1016/j.jtcvs.2012.06.052 [DOI] [PubMed] [Google Scholar]
- 10.Dass C, Simpson SA, Steiner RM, et al. Preprocedural Computed Tomography Evaluation for Minimally Invasive Mitral Valve Surgery: What the Surgeon Needs to Know. J Thorac Imaging 2015;30:386-96. 10.1097/RTI.0000000000000170 [DOI] [PubMed] [Google Scholar]
- 11.Chirichilli I, D'Ascoli R, Rose D, et al. Port Access (Thru-Port System) video-assisted mitral valve surgery. J Thorac Dis 2013;5 Suppl 6:S680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czesla M, Götte J, Weimar T, et al. Safeguards and pitfalls in minimally invasive mitral valve surgery. Ann Cardiothorac Surg 2013;2:849-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8. 10.1161/01.CIR.0000047200.36165.B8 [DOI] [PubMed] [Google Scholar]
- 14.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 15.Willson AB, Webb JG, Labounty TM, et al. 3-dimensional aortic annular assessment by multidetector computed tomography predicts moderate or severe paravalvular regurgitation after transcatheter aortic valve replacement: a multicenter retrospective analysis. J Am Coll Cardiol 2012;59:1287-94. 10.1016/j.jacc.2011.12.015 [DOI] [PubMed] [Google Scholar]
- 16.Suematsu Y, Mora BN, Mihaljevic T, et al. Totally endoscopic robotic-assisted repair of patent ductus arteriosus and vascular ring in children. Ann Thorac Surg 2005;80:2309-13. 10.1016/j.athoracsur.2005.05.078 [DOI] [PubMed] [Google Scholar]
- 17.Haramati LB, Glickstein JS, Issenberg HJ, et al. MR imaging and CT of vascular anomalies and connections in patients with congenital heart disease: significance in surgical planning. Radiographics 2002;22:337-47; discussion 48-9. 10.1148/radiographics.22.2.g02mr09337 [DOI] [PubMed] [Google Scholar]
- 18.Morgan-Hughes GJ, Marshall AJ, Roobottom C. Morphologic assessment of patent ductus arteriosus in adults using retrospectively ECG-gated multidetector CT. AJR Am J Roentgenol 2003;181:749-54. 10.2214/ajr.181.3.1810749 [DOI] [PubMed] [Google Scholar]
- 19.Chen HW, Chen SJ, Chiu IS. Computed tomographic virtual cardioscopy in a case of left atrial myxoma. Heart 2004;90:e8. 10.1136/hrt.2003.022046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao C, Yang M, Wang G, et al. Excision of atrial myxoma using robotic technology. J Thorac Cardiovasc Surg 2010;139:1282-5. 10.1016/j.jtcvs.2009.09.013 [DOI] [PubMed] [Google Scholar]
- 21.Wierzbicki M, Drangova M, Guiraudon G, et al. Validation of dynamic heart models obtained using non-linear registration for virtual reality training, planning, and guidance of minimally invasive cardiac surgeries. Med Image Anal 2004;8:387-401. 10.1016/j.media.2004.06.014 [DOI] [PubMed] [Google Scholar]
- 22.Cannon JW, Stoll JA, Selha SD, et al. Port Placement Planning in Robot-Assisted Coronary Artery Bypass. IEEE Trans Rob Autom 2003;19:912-7. 10.1109/TRA.2003.817502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao C, Harris C, Croce B. Robotic coronary artery bypass graft surgery. Ann Cardiothorac Surg 2016;5:594. 10.21037/acs.2016.11.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzgibbon GM, Kafka HP, Leach AJ, et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol 1996;28:616-26. 10.1016/0735-1097(96)00206-9 [DOI] [PubMed] [Google Scholar]
- 25.Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol 2004;44:2149-56. 10.1016/j.jacc.2004.08.064 [DOI] [PubMed] [Google Scholar]
- 26.Gabriel J, Klimach S, Lang P, et al. Should computed tomography angiography supersede invasive coronary angiography for the evaluation of graft patency following coronary artery bypass graft surgery? Interact Cardiovasc Thorac Surg 2015;21:231-9. 10.1093/icvts/ivv078 [DOI] [PubMed] [Google Scholar]
- 27.Proudfit WL, Shirey EK, Sones FM. Selective cine coronary arteriography. Correlation with clinical findings in 1,000 patients. Circulation 1966;33:901-10. 10.1161/01.CIR.33.6.901 [DOI] [PubMed] [Google Scholar]
- 28.Wyman RM, Safian RD, Portway V, et al. Current complications of diagnostic and therapeutic cardiac catheterization. J Am Coll Cardiol 1988;12:1400-6. 10.1016/S0735-1097(88)80002-0 [DOI] [PubMed] [Google Scholar]
- 29.Gobel FL, Stewart WJ, Campeau L, et al. Safety of coronary arteriography in clinically stable patients following coronary bypass surgery. Post CABG Clinical Trial Investigators. Cathet Cardiovasc Diagn 1998;45:376-81. [DOI] [PubMed] [Google Scholar]
- 30.Hamon M, Lepage O, Malagutti P, et al. Diagnostic performance of 16- and 64-section spiral CT for coronary artery bypass graft assessment: meta-analysis. Radiology 2008;247:679-86. 10.1148/radiol.2473071132 [DOI] [PubMed] [Google Scholar]
- 31.Trigo Bautista A, Estornell J, Ridocci F, et al. [Non-invasive assessment of coronary artery bypass grafts by computed tomography: comparison with conventional coronary angiography]. Rev Esp Cardiol 2005;58:807-14. 10.1157/13077232 [DOI] [PubMed] [Google Scholar]
- 32.Meyer TS, Martinoff S, Hadamitzky M, et al. Improved noninvasive assessment of coronary artery bypass grafts with 64-slice computed tomographic angiography in an unselected patient population. J Am Coll Cardiol 2007;49:946-50. 10.1016/j.jacc.2006.10.066 [DOI] [PubMed] [Google Scholar]
- 33.Husmann L, Valenta I, Gaemperli O, et al. Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J 2008;29:191-7. 10.1093/eurheartj/ehm613 [DOI] [PubMed] [Google Scholar]
- 34.Malagutti P, Nieman K, Meijboom WB, et al. Use of 64-slice CT in symptomatic patients after coronary bypass surgery: evaluation of grafts and coronary arteries. Eur Heart J 2007;28:1879-85. 10.1093/eurheartj/ehl155 [DOI] [PubMed] [Google Scholar]
- 35.Goetti R, Leschka S, Baumüller S, et al. Low dose high-pitch spiral acquisition 128-slice dual-source computed tomography for the evaluation of coronary artery bypass graft patency. Invest Radiol 2010;45:324-30. [DOI] [PubMed] [Google Scholar]
- 36.Klass O, Walker M, Siebach A, et al. Prospectively gated axial CT coronary angiography: comparison of image quality and effective radiation dose between 64- and 256-slice CT. Eur Radiol 2010;20:1124-31. 10.1007/s00330-009-1652-7 [DOI] [PubMed] [Google Scholar]
- 37.Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 2002;105:2259-64. 10.1161/01.CIR.0000016043.87291.33 [DOI] [PubMed] [Google Scholar]
- 38.Carbonaro S, Villines TC, Hausleiter J, et al. International, multidisciplinary update of the 2006 Appropriateness Criteria for cardiac computed tomography. J Cardiovasc Comput Tomogr 2009;3:224-32. 10.1016/j.jcct.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 39.Nakazato R, Park HB, Berman DS, et al. Noninvasive fractional flow reserve derived from computed tomography angiography for coronary lesions of intermediate stenosis severity: results from the DeFACTO study. Circ Cardiovasc Imaging 2013;6:881-9. 10.1161/CIRCIMAGING.113.000297 [DOI] [PubMed] [Google Scholar]
- 40.Navia JL, Atik FA, Grimm RA, et al. Minimally invasive left ventricular epicardial lead placement: surgical techniques for heart failure resynchronization therapy. Ann Thorac Surg 2005;79:1536-44; discussion -44. [DOI] [PubMed]
- 41.Henrikson CA, Leng CT, Yuh DD, et al. Computed tomography to assess possible cardiac lead perforation. Pacing Clin Electrophysiol 2006;29:509-11. 10.1111/j.1540-8159.2006.00385.x [DOI] [PubMed] [Google Scholar]