Abstract
Phosphoenolpyruvate carboxykinase, EC 4.1.1.32 (PEPCK), was purified 43-fold from the grass Panicum maximum. Michaelis constants (Km) were determined for the exchange reaction, the carboxylation reaction, and the decarboxylation reaction. The Km values for oxaloacetate and ATP in the decarboxylation reaction were found to be lower than the Km values for the substrates used in the exchange reaction and in the carboxylation reaction. Phosphoenolpyruvate carboxylase was not detectable in the purified PEPCK preparation.
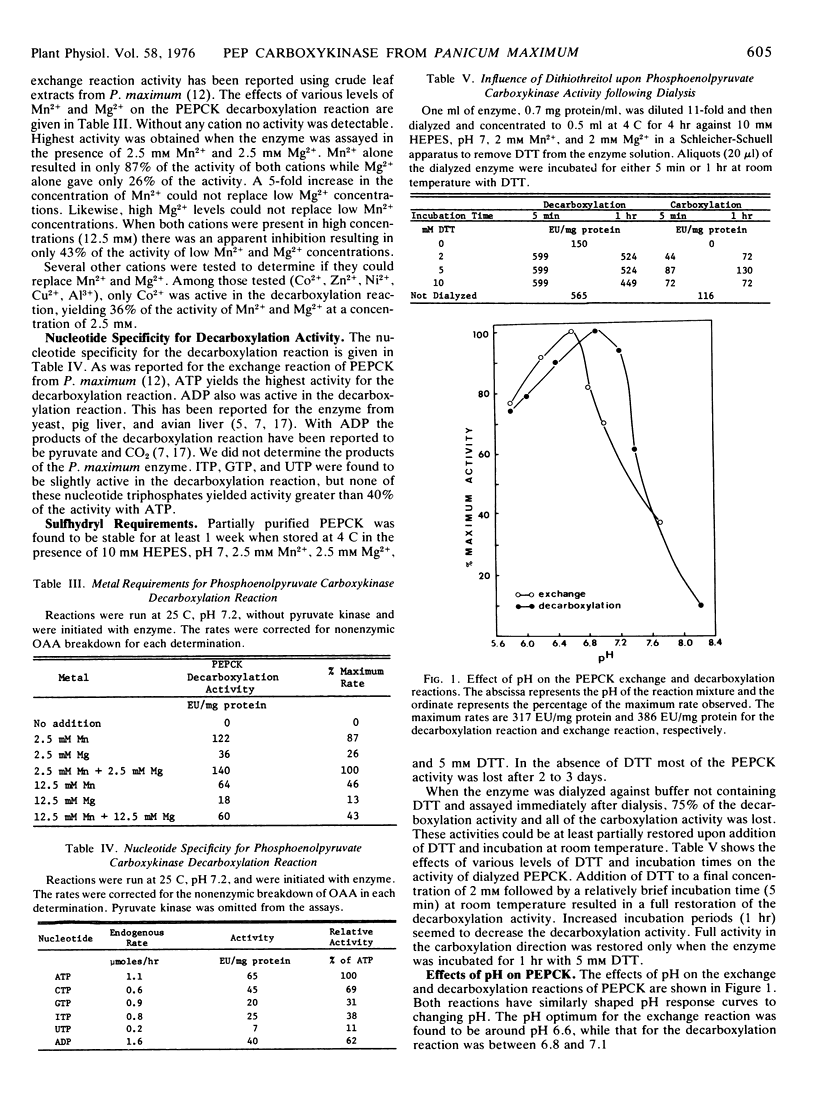
Studies on the nucleotide specificity of the oxaloacetate decarboxylation reaction indicate that ATP serves as the best nucleotide for this reaction and that ADP is about 60% as effective as ATP. The pH optimum for decarboxylase activity is near 6.8. The decarboxylation reaction has a divalent cation requirement with both Mn2+ and Mg2+ needed for full activity.
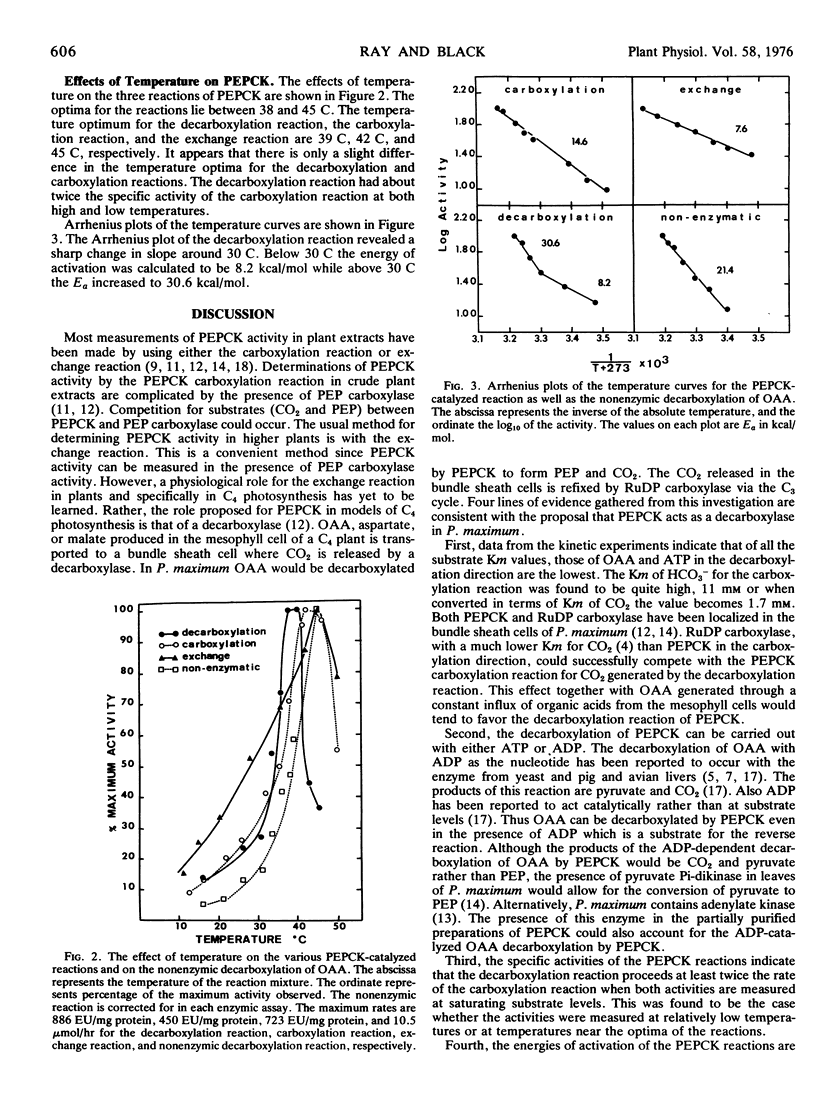
Temperature curves of the three PEPCK reactions indicate optimum activities between 38 and 45 C. There is a pronounced drop in the decarboxylation and carboxylation activities as the temperature is decreased from these optima. Below 30 C the energy of activation was 8.2 kcal/mol for the decarboxylation reaction.
These studies are consistent with the proposal that under physiological conditions PEPCK catalyzes the decarboxylation of oxaloacetate in the bundle sheath cells of Panicum maximum leaves during C4 dicarboxylic acid photosynthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard F. J., Hanson R. W. Purification of phosphoenolpyruvate carboxykinase from the cytosol fraction of rat liver and the immunochemical demonstration of differences between this enzyme and the mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1969 Oct 25;244(20):5625–5630. [PubMed] [Google Scholar]
- Benedict C. R., Beevers H. Formation of sucrose from malate in germinating castor beans. I. Conversion of malate to phosphoenol-pyruvate. Plant Physiol. 1961 Sep;36(5):540–544. doi: 10.1104/pp.36.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes G. pH Dependence of the Km(CO(2)) of Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1975 Nov;56(5):630–633. doi: 10.1104/pp.56.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannata J. J., De Flombaum M. A. Phosphenolpyruvate carboxykinases from bakers' yeast. Kinetics of phosphoenolpyruvate formation. J Biol Chem. 1974 Jun 10;249(11):3356–3365. [PubMed] [Google Scholar]
- Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. II. Purification and properties of liver mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1966 May 25;241(10):2413–2420. [PubMed] [Google Scholar]
- Chang H. C., Maruyama H., Miller R. S., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. 3. Investigation of the kinetics and mechanism of the mitochondrial phosphoenolpyruvate carboxykinase-catalyzed reaction. J Biol Chem. 1966 May 25;241(10):2421–2430. [PubMed] [Google Scholar]
- Cooper T. G., Benedict C. R. PEP carboxykinase exchange reaction in photosynthetic bacteria. Plant Physiol. 1968 May;43(5):788–792. doi: 10.1104/pp.43.5.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Tchen T. T., Wood H. G., Benedict C. R. The carboxylation of phosphoenolpyruvate and pyruvate. I. The active species of "CO2" utilized by phosphoenolpyruvate carboxykinase, carboxytransphosphorylase, and pyruvate carboxylase. J Biol Chem. 1968 Jul 25;243(14):3857–3863. [PubMed] [Google Scholar]
- Dittrich P., Campbell W. H., Black C. C. Phosphoenolpyruvate carboxykinase in plants exhibiting crassulacean Acid metabolism. Plant Physiol. 1973 Oct;52(4):357–361. doi: 10.1104/pp.52.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Kanai R., Black C. C. Phosphoenolpyruvate carboxykinase in leaves of certain plants whick fix CO 2 by the C 4 -dicarboxylic acid cycle of photosynthesis. Biochem Biophys Res Commun. 1971 Oct 15;45(2):278–285. doi: 10.1016/0006-291x(71)90814-x. [DOI] [PubMed] [Google Scholar]
- Hatch M. D. An assat for PEP carboxykinase in crude tissue extracts. Anal Biochem. 1973 Mar;52(1):280–285. doi: 10.1016/0003-2697(73)90350-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mazelis M., Vennesland B. Carbon Dioxide Fixation into Oxalacetate in Higher Plants. Plant Physiol. 1957 Nov;32(6):591–600. doi: 10.1104/pp.32.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noce P. S., Utter M. F. Decarboxylation of oxalacetate to pyruvate by purified avian liver phosphoenolpyruvate carboxykinase. J Biol Chem. 1975 Dec 10;250(23):9099–9105. [PubMed] [Google Scholar]
- Ruffner H. P., Kliewer W. M. Phosphoenolpyruvate carboxykinase activity in grape berries. Plant Physiol. 1975 Jul;56(1):67–71. doi: 10.1104/pp.56.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. O., Slack C. R., McPherson H. G. Plants under Climatic Stress: VI. Chilling and Light Effects on Photosynthetic Enzymes of Sorghum and Maize. Plant Physiol. 1974 Nov;54(5):696–701. doi: 10.1104/pp.54.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UTTER M. F., KURAHASHI K., ROSE I. A. Some properties of oxalacetic carboxylase. J Biol Chem. 1954 Apr;207(2):803–819. [PubMed] [Google Scholar]


