Abstract
Background
Standard cine-cardiac magnetic resonance (CMR) imaging is commonly used to evaluate cardiac structure, geometry and function. Prior studies have shown that automated segmentation via partial voxel interpolation (PVI) accurately quantifies phantom-based cardiac chamber volumes and necropsy left ventricular myocardial mass. Despite this, the applicability and usefulness of PVI in the determination of physiologic parameters of the aorta such as aortic stiffness has yet to be investigated.
Methods
Routine CMR was conducted with a 1.5T (GE) scanner with pulse sequences similar to that of standard CMR (parameters: TR 3.4 msec, TE 1.14 msec, flip angle 60°, temporal resolution ~30–40 msec). Views were obtained in standard cardiac-oriented longitudinal or axial views (2, 3 and 4 chambers). Within non-dilated regions of the descending thoracic aorta, aortic area was quantified via a novel PVI automated process (LV-METRIC), which discerns relative amounts of blood pool in each voxel. Aortic stiffness, as calculated from brachial artery pulse pressure and aortic area at maximal and minimal dimensions, was evaluated in 60 total segments (one segment per patient). All segments were in the descending aorta and were not aneurysmal.
Results
Sixty patients in total were studied, including 50 that had genetically-related aortic disorder [35 bicuspid aortic valve (BAV), 15 Marfan syndrome (MFS)]. Ten normal controls without aortic disease were included for comparison purposes. All patients (n=60) had evaluable CMR images for assessment of the descending aorta with use of automated segmentation. Patients with BAV and MFS were similar to controls in age, systolic blood pressure, brachial artery pulse pressure, smoking status or hypercholesterolemia (all P=NS). There were more women (P<0.001), lower body mass index (P=0.008), and greater height (P<0.001) in the MFS cohort compared to BAV and controls. Descending aortic area in either systole (maximal) or diastole (minimal) was similar among all three cohorts. However, change in aortic area (ΔArea) throughout the cardiac cycle was substantially lower in MFS than control subjects (P<0.001). In contrast, change in aortic area throughout the cardiac cycle was not significantly different between BAV vs. controls (P=0.62). Aortic stiffness was increased among MFS patients versus control subjects (P=0.014). When comparing MFS to BAV subjects, a comparable trend was observed (P=0.09). No statistical difference was evident in aortic stiffness in patients with BAV versus control subjects (P=0.29).
Conclusions
The application of PVI to standard CMR imaging can assess abnormal descending aorta functional indices in normal caliber segments in MFS subjects. Future prospective studies with larger subject populations are warranted to further determine the overall utility of automated aortic segmentation as a possible early biomarker of aortic dysfunction before overt dilatation.
Keywords: Marfan syndrome (MFS), magnetic resonance imaging (MRI), aorta, arterial stiffness
Introduction
Thoracic aortic aneurysms (TAAs) predispose patients to catastrophic aortic complications such as aortic dissection or rupture (1-3). The past decade has witnessed advances in surgical management and pharmacotherapy; however, out of hospital mortality remains high whereby approximately 40% of patients still die in the field, highlighting the importance of more effective risk stratification to prevent aortic complications (4). Current risk stratification is based on initial and serial assessment of aortic diameter with noninvasive imaging, with use of aortic size to guide timing of prophylactic surgery (5). However, size alone imperfectly captures the underlying pathogenesis of TAA. Recent studies have demonstrated that among patients with genetically-mediated TAAs (the leading cause of TAAs), up to 60% of dissections occurred in aortic segments with diameters below cutoffs for surgery in current guidelines (6).
Marfan syndrome (MFS) is the most common genetic cause of TAAs and stems from mutations of fibrillin-1 (FBN1) (7). Given known prognostic limitations of aortic size, attention has shifted beyond aortic sizing to functional abnormalities that may mediate clinical manifestations such as altered biomechanics (8). TAA complications occur when biomechanical wall stress exceeds the strength of the aortic wall. Higher aortic stiffness is defined as a lesser degree of vascular expansion for a given pulse pressure during systole (9,10). Although prior studies have demonstrated abnormal aortic stiffness in patients with MFS, many of these studies have been limited by the use of 2-dimensional (2D) imaging with ultrasound, which may result in off-axis views of the aorta (11,12).
Standard cine-cardiac magnetic resonance (CMR) is commonly employed to evaluate cardiac geometry and function. In this study, we applied an automated segmentation CMR algorithm, developed and validated by our group, that affords partial voxel interpolation (PVI) of aortic lumen size (cross-sectional areas) throughout the cardiac cycle (systole and diastole) to assess aortic stiffness (13,14). In prior studies, PVI yielded improved agreement of CMR measures with phantom-based cardiac volumetric indices and necropsy left ventricular myocardial mass. The value of PVI for evaluation of physiologic parameters of the aorta has not been investigated. In addition to the use of this algorithm, we employed standard CMR pulse sequences used in mostly all cardiac magnetic resonance imaging (MRI) exams today (10,15). Our technique fundamentally differs from many other studies, which have evaluated aortic stiffness using flow based (e.g., velocity-encoded) imaging that is prone to various imaging-related artifacts (11,16).
In this study, we sought to evaluate an established biomechanical index, aortic stiffness, by standard CMR in subjects with MFS without advanced aortic disease in comparison to patients with bicuspid aortic valve (BAV) and apparently normal adults. We hypothesized that aortic stiffness would be increased (in aortic regions with normal size) in patients with MFS compared to patients with BAV as well as normative controls.
Methods
Patient population
This study included patients who underwent CMR for guideline-based clinical indications between 2005 and 2015. Extensive clinical data were recorded for all patients, including major cardiovascular risk factors, blood pressure parameters, and body size indices. MRIs of patients with MFS and BAV were assessed for evaluable images of the aorta. MFS patients undergo routine MR imaging as part of an established aortopathy program at Cornell that also ensured accurate diagnostic classification. Fifteen patients with MFS and 35 with BAV were included. Exclusion criteria included age less than 18 years, previous aortic surgery or dissection, or image artifacts that impaired accurate assessment of aortic contours. A pre-existing cohort of ten healthy age-matched subjects served as controls. Exclusion criteria in this cohort were known aortic disease, coronary artery disease, valvular disease, heart failure, antihypertensive treatment, systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg. The study was approved by the Weill Cornell Medical College institutional review board (No. 1505016188). Written informed consent was obtained from each participant.
Body mass index was calculated and pulse pressure was determined from brachial artery blood pressure. This retrospective study involving preexisting MRI and clinical data was performed in strict adherence to our Institutional Review Board.
Magnetic resonance image acquisition.
Routine CMR was conducted with a 1.5T (GE) scanner with pulse sequences similar to that of standard CMR (sequence parameters: TR 3.4 msec, TE 1.14 msec, angle 60ο, temporal and spatial resolution ~30–40 msec and ~2.0×1.5 mm, thickness ~6.0 mm, inter-slice width ~4.0 mm) (17). Views were obtained in standard cardiac-oriented longitudinal or axial views (2, 3 and 4 chambers).
Automated segmentation with LV-METRIC
LV-METRIC segmentation algorithm was used to quantify MR images of the thoracic aorta. The multi-step algorithmic approach has been previously published (17). Prior reported uses of this algorithm pertain mostly to cardiac chamber assessment and diastolic function ascertainment.
Aortic dimensions and stiffness measurements
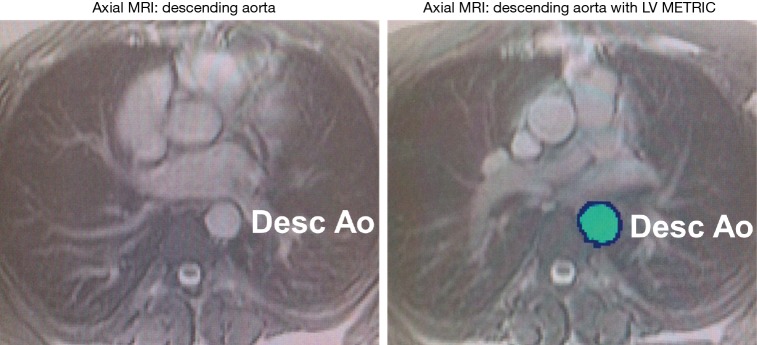
Aortic diameter and area were systematically recorded in normal caliber regions within the mid-descending aorta (Figure 1). Maximal and minimal luminal areas of the aorta and pulse pressure via brachial artery recordings were used to derive aortic stiffness, an index of vascular elasticity, of the mid-descending aorta. The following formula was used: Stiffness index (β) = ln (Ps – Pd)/([As − Ad]/Ad). Ps and Pd are systolic and diastolic pressures, respectively (determined by brachial artery pressure). As and Ad refer to aortic area during end-systole and end-diastole. Cine-CMR images were used to quantify within each aortic segment: measurements at end of systole and end of diastole were obtained via previously validated automated MRI segmentation algorithm (LV-METRIC) (Figure 1). In prior validation studies, LV-METRIC closely agrees with phantom-based cardiac volumetric indices and is high reproducible for cardiac chamber geometry measurements (13,14). Per-patient analyses were performed by a skilled investigator (>5 years experience) who was blinded to diagnosis and clinical characteristics.
Figure 1.
Assessment of aortic physiology via partial voxel automated segmentation. Axial cine-MRI without automation (A) and with automation (B) in the mid-descending aorta. Partial voxel interpolation (PVI) of the aortic segment is assessed at both the largest (systole) and smallest (diastole) area size.
Statistical analysis
All demographic data are presented as percentage or mean with standard deviations unless otherwise specified. Univariate analysis was performed using chi-square and analysis of variance to evaluate difference in baseline characteristics. Univariate analysis was performed using analysis of variance with the Sidak post-hoc test to compare descending aortic physiologic indices (maximal area in systole and diastole, delta area, and aortic stiffness) among control subjects and patients with BAV and MFS. Skewed data were log-transformed for statistical analysis. Multivariate regression models were then used to evaluate the relationship of aortic stiffness with BAV and MFS adjusted for covariates. Covariates were selected in different regression models based on potential confounders that included age, gender, height, systolic and diastolic blood pressure, diastolic aortic area, hypertension, and body mass index. A 2-tailed P value <0.05 was considered significant. All statistics and analyses were performed using SPSS (version 21) 2014, SPSS Inc. Chicago, IL, USA.
Results
Study subjects and baseline characteristics
Sixty patients in total were studied, including 50 that had genetic aortic disorders (n=35 BAV, n=15 MFS) (Table 1). Ten clinically normal controls without aortic disease were included for comparison purposes. All patients had evaluable CMR images for assessment of the descending aorta (n=60) with use of automated segmentation, LV-METRIC.
Table 1. Patient demographics.
| Baseline characteristics | Controls (n=10) | BAV (n=35) | MFS (n=15) | P value |
|---|---|---|---|---|
| Age, years | 42.9±12.8 | 43.0±11.1 | 36.9±13.2 | 0.257 |
| Women, % | 20 | 11.4** | 66.7#,* | <0.001 |
| Systolic blood pressure (mmHg) | 111.7±9.5 | 120.4±14.2 | 115.9±12.3 | 0.256 |
| Diastolic blood pressure (mmHg) | 70±7 | 75.1±7.8 | 67.5±10.9# | 0.023 |
| Pulse pressure (mmHg) | 42±3 | 46.3±11.4 | 49.4±10.5 | 0.317 |
| Hypertension, % | 0 | 33.3** | 16.7* | 0.124 |
| Hypercholesterolemia, % | 60 | 47.6 | 0# | 0.099 |
| Current smoker, % | 0 | 14.3 | 16.7 | 0.466 |
| Body mass index (kg/m2) | 31.6±11.6 | 26.3±4.1** | 23.2±4.3*,# | 0.008 |
| Height (inches) | 65.33±3.14 | 68.91±2.79 | 71.67±3.09*,# | <0.001 |
Data are presented as mean ± SD or %. P value (right column) indicates among groups differences. Symbols represent between groups differences (P≤0.05): *, MFS vs. controls; **, BAV vs. controls; #, MFS vs. BAV; MFS, Marfan syndrome; BAV, bicuspid aortic valve.
Patients with BAV and MFS were similar to the control cohort in age, systolic blood pressure, and pulse pressure (all P=NS). There were no significant differences in major cardiovascular risk factors including smoking status and hypercholesterolemia between study aortopathy subjects (BAV and MFS) and controls (P=NS). By design and according to our pre-specified exclusion criteria, control subjects did not have hypertension (as defined by clinical diagnosis or use of anti-hypertensives).
Notably, there were more women (P<0.001) and overall lower body mass index (P=0.008) in the MFS cohort compared to either BAV subjects or controls. As expected, height was significantly higher in patients with MFS versus BAV and controls.
Decreased aortic lumen excursion in MFS
Descending aortic parameters for MFS, BAV and controls are included in Table 2. Maximal area in systole and diastole within the descending aorta (assessed in a normal caliber segments) was comparable among the three groups (all P=NS). However, cyclical change in area of the aorta (ΔArea) was significantly decreased in MFS versus control subjects (P<0.001). In contrast, change in aortic area throughout the cardiac cycle was not significantly different when comparing BAV vs. control patients (P=0.62).
Table 2. Aortic physiology parameters as determined by standard cine-CMR imaging.
| Parameters | Controls (n=10) | BAV (n=35) | MFS (n=15) | P value (BAV vs. control) | P value (MFS vs. control) |
|---|---|---|---|---|---|
| Maximal area (systole) | 3.51±0.48 | 3.45±0.76 | 3.15±0.99 | 0.803 | 0.303 |
| Minimal area (diastole) | 2.77±0.35 | 2.76±0.75 | 2.77±0.99 | 0.979 | 0.992 |
| ΔArea | 0.74±0.18 | 0.68±0.36 | 0.39±0.21 | 0.623 | <0.001 |
| Aortic stiffness | 0.25±0.10 | 0.35±0.22 | 0.64±0.34 | 0.294 | 0.014 |
Data are presented as mean ± SD. CMR, cardiac magnetic resonance; BAV, bicuspid aortic valve; MFS, Marfan syndrome.
Increased aortic stiffness in normal-caliber aortic segments in MFS
Aortic stiffness, as calculated by brachial artery pulse pressure and aortic area at end of systole and en of diastole, was obtained in 60 total segments (one segment per patient). All segments were in the mid-descending aorta and were not aneurysmal. Aortic stiffness was increased among MFS patients versus control subjects (P=0.014) (Table 2), with a comparable trend between MFS versus BAV patients (P=0.09). No significant difference in aortic stiffness was noted when the BAV group was compared to control subjects (P=0.29). Analysis of covariance with height or an indicator variable for gender confirmed the independent association of MFS with greater aortic stiffness (P=0.019 vs. controls, P=0.002 vs. BAV patients).
Discussion
This study provides several new findings concerning aortic disease in MFS and automated CMR assessment. First, we show that patients with MFS exhibit abnormal aortic biomechanics (increased arterial stiffness) in normal-caliber aortic segments. Moreover, we show that routine cine-CMR with automated segmentation and PVI can discern abnormal aortic physiology.
To our knowledge, this is the first study to assess the utility of automated segmentation with PVI (LV-METRIC) for evaluation of physiologic indices of the aorta. Our group has previously shown that automated segmentation is important for assessment of LV chamber size and volume. In a prior validation study, LV-METRIC reduced processing time for analysis by over 90% compared to a manual approach, and closely agreed with both phantom derived chamber volumes and necropsy LV mass (8,15). LV-METRIC is an automated algorithm that relies on two assumptions: (I) MRI-based signal intensity of blood pool is different compared to surrounding and adjacent tissue; and (II) blood pool is surrounded by solid tissue (e.g., myocardium or vascular endothelium). This approach vastly differs from other existing automated segmentation algorithms, which may typically have sophisticated but limiting assumptions with regard to geometry or contour deformation (6,7). LV-METRIC does not use geometric assumptions and hence, accommodates differences in shape and remodeling patterns. Herein, we demonstrate potential clinical application of PVI via LV-METRIC to detect early aortic disease.
In addition, increased stiffness is a prognostically-validated measure of arterial dysfunction and carries the potential to identify susceptible regions before actual dilatation, and to distinguish structurally stable aneurysms from those at risk for expansion/rupture. A few prior studies have demonstrated increased stiffness in dilated thoracic aorta regions and improvement with targeted treatment—underscoring the relevance of stiffness as a marker of aortic disease (12,18). Despite this, it remains unknown how or even whether aortic stiffness relates to aortic growth. Presently, to our knowledge, no longitudinal studies with quantitative evaluation of stiffness in the thoracic aorta have been conducted.
In our study, we measured aortic stiffness using a sophisticated automated segmentation algorithm (LV-METRIC) capable of measuring dynamic changes in aortic size throughout the cardiac cycle. Our imaging approach employed standard MRI pulse sequences (SSFP) used in nearly all cardiovascular MRI exams today (10,15) and fundamentally differs from most prior studies, which have demonstrated a role for aortic stiffness using flow based (velocity-encoded) imaging—an approach that can potentially be inaccurate due to off axis imaging, obliquely contoured flow vortices, or mis-registration between phase contrast (flow) datasets and complementary pulse sequences used to assess aortic wall thickness or stiffness.
Although there are known changes in aortic physiology in MFS, temporal changes in stiffness encompassing all thoracic aortic regions have yet to be investigated. Prior studies of aortic stiffness have been limited by: small sample sizes, cross-sectional and retrospective design, use of 2D ultrasound, and use of brachial artery blood pressure (11,19). Moreover, there are no studies that have systematically assessed aortic stiffness with precise measures afforded by comprehensive 3D imaging (e.g., cine-MRI) and central aortic pressures. Studies that are larger and longitudinal in design are warranted to evaluate the prognostic ability of CMR-based automated segmentation, especially with more accurate measures afforded by central aortic blood pressure, as a possible surrogate marker of preclinical aortic dysfunction.
Despite decades of substantial progress in clinical aortic imaging, medical care and surgical techniques, patients with TAA complications continue to have high rates of morbidity and mortality. Based on current TAA consensus guidelines, current risk stratification is reliant on routine noninvasive imaging assessment of aortic anatomy—aortic size. Size is the guiding metric for use in diagnosis and surveillance, and criterion for elective surgical intervention (5). The standard anatomic-based imaging approaches, which mainly focus on detection of aortic size, reflect relatively late stage pathology, when structural dilation has already occurred. Beyond anatomic imaging, focusing on key and exacerbating pathological functional processes such as biomechanics requires application of functional imaging techniques, such as MRI. This approach has the potential to accelerate diagnosis, refine prognosis, and guide treatments. The ability to identify aortic segments at high risk of accelerated growth would be tremendously valuable, both in guiding selection of patients for prophylactic surgical repair and for optimizing timing of intervention to prevent aortic complications.
Limitations
This study is not without limitations. First, patients were identified from an existing MRI database making our study subject to limitations inherent to retrospective investigations (20). Despite this, we maintained a systematic and strict approach to determine inclusion eligibility. Second, not all aortic segments were evaluable via MRI. We deliberately chose patients with normal-caliber descending aortas to study pre-aneurysmal segments; exclusion of dilated segments and apparent reliance on axial images rather than double-oblique reconstructions would have taken out proximal aortic segments in many MFS and BAV patients. As such, our results on the descending aorta may not be extrapolated to more proximal aortic segments. Third, the use central aortic pulse pressure, a method extensively studied and prognostically-validated, is superior to brachial artery pulse pressure—which differs to a variable extent among individuals from the pressure within the central aorta—and may afford more accurate measures of stiffness. Fourth, it is possible that otherwise eligible patients with MFS may have been misclassified as having hypertension—and excluded from the present analysis—due to the standard use of anti-hypertensives (beta blockade and/or angiotensin receptor blockers) in this population (21). Fifth, aortic stiffness may have been higher in the MFS group due to the higher prevalence of women with smaller BMIs. In light of the known inverse relations of height and diastolic aortic area with aortic stiffness, due to effects of greater arterial reflected waves, the independent association of aortic stiffness with MFS in analyses that adjusted for these parameters in our study supports our hypothesis that increased stiffness is intrinsic and not related to body size (22). In addition, there was no significant association between gender and stiffness; however, this analysis is underpowered due to our small MFS sample size (n=15). Lastly, our study was limited to patients with MFS and BAV, so our findings may not necessarily be applicable to other cohorts. However, MFS is a leading etiology of genetically-associated TAA, affecting up to 65,000 individuals in the United States and 1,400,000 worldwide (23). Moreover, our findings provide important hypothesis-generating data concerning aortic stiffening as a novel predictor of TAA growth that can be broadly tested in future studies encompassing cohorts with both genetic and sporadically-mediated TAA.
Conclusions
This study shows that the use of an automated segmentation algorithm (e.g., LV-METRIC) permits ascertainment of aortic volumetric indices across a sample of subjects who underwent clinically-indicated CMR. In addition, our results show that abnormal biomechanics occurs early in the course of disease progression and prior to overt anatomic aortic dilatation in MFS. CMR can detect abnormal physiologic parameters of the aorta in non-dilated segments in patients with MFS. Further testing is warranted in longitudinal studies to determine the prognostic usefulness of CMR-based segmentation, especially with precise measures of central aortic pressure, as surrogate and early marker of aortic dysfunction and to determine whether abnormal biomechanics increases risk for accelerated aortic growth.
Acknowledgements
None.
Ethical Statement: The study was approved by the Weill Cornell Medical College institutional review board (No. 1505016188). Written informed consent was obtained from each participant.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Parish LM, Gorman JH, 3rd, Kahn S, et al. Aortic size in acute type A dissection: implications for preventive ascending aortic replacement. Eur J Cardiothorac Surg 2009;35:941-5; discussion 5-6. 10.1016/j.ejcts.2008.12.047 [DOI] [PubMed] [Google Scholar]
- 2.Trimarchi S, Jonker FH, Hutchison S, et al. Descending aortic diameter of 5.5 cm or greater is not an accurate predictor of acute type B aortic dissection. J Thorac Cardiovasc Surg 2011;142:e101-7. [DOI] [PubMed] [Google Scholar]
- 3.Kuzmik GA, Sang AX, Elefteriades JA. Natural history of thoracic aortic aneurysms. J Vasc Surg 2012;56:565-71. 10.1016/j.jvs.2012.04.053 [DOI] [PubMed] [Google Scholar]
- 4.Criado FJ. Aortic dissection: a 250-year perspective. Tex Heart Inst J 2011;38:694-700. [PMC free article] [PubMed] [Google Scholar]
- 5.Jondeau G, Detaint D, Tubach F, et al. Aortic event rate in the Marfan population: a cohort study. Circulation 2012;125:226-32. 10.1161/CIRCULATIONAHA.111.054676 [DOI] [PubMed] [Google Scholar]
- 6.Weinsaft JW, Devereux RB, Preiss LR, et al. Aortic Dissection in Patients With Genetically Mediated Aneurysms: Incidence and Predictors in the GenTAC Registry. J Am Coll Cardiol 2016;67:2744-54. 10.1016/j.jacc.2016.03.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson GD, Devereux R, Loeys B, et al. Report of the National Heart, Lung, and Blood Institute and National Marfan Foundation Working Group on research in Marfan syndrome and related disorders. Circulation 2008;118:785-91. 10.1161/CIRCULATIONAHA.108.783753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Judge DP, Dietz HC. Marfan's syndrome. Lancet 2005;366:1965-76. 10.1016/S0140-6736(05)67789-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roman MJ, Devereux RB, Schwartz JE, et al. Arterial stiffness in chronic inflammatory diseases. Hypertension 2005;46:194-9. 10.1161/01.HYP.0000168055.89955.db [DOI] [PubMed] [Google Scholar]
- 10.Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007;50:197-203. 10.1161/HYPERTENSIONAHA.107.089078 [DOI] [PubMed] [Google Scholar]
- 11.Teixido-Tura G, Redheuil A, Rodriguez-Palomares J, et al. Aortic biomechanics by magnetic resonance: early markers of aortic disease in Marfan syndrome regardless of aortic dilatation? Int J Cardiol 2014;171:56-61. 10.1016/j.ijcard.2013.11.044 [DOI] [PubMed] [Google Scholar]
- 12.Nollen GJ, Groenink M, Tijssen JG, et al. Aortic stiffness and diameter predict progressive aortic dilatation in patients with Marfan syndrome. Eur Heart J 2004;25:1146-52. 10.1016/j.ehj.2004.04.033 [DOI] [PubMed] [Google Scholar]
- 13.Codella NC, Lee HY, Fieno DS, et al. Improved left ventricular mass quantification with partial voxel interpolation: in vivo and necropsy validation of a novel cardiac MRI segmentation algorithm. Circ Cardiovasc Imaging 2012;5:137-46. 10.1161/CIRCIMAGING.111.966754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaji K, Codella NC, Prince MR, et al. Automated segmentation of routine clinical cardiac magnetic resonance imaging for assessment of left ventricular diastolic dysfunction. Circ Cardiovasc Imaging 2009;2:476-84. 10.1161/CIRCIMAGING.109.879304 [DOI] [PubMed] [Google Scholar]
- 15.Roman MJ, Devereux RB, Kizer JR, et al. High central pulse pressure is independently associated with adverse cardiovascular outcome the strong heart study. J Am Coll Cardiol 2009;54:1730-4. 10.1016/j.jacc.2009.05.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fattori R, Bacchi Reggiani L, Pepe G, et al. Magnetic resonance imaging evaluation of aortic elastic properties as early expression of Marfan syndrome. J Cardiovasc Magn Reson 2000;2:251-6. 10.3109/10976640009148688 [DOI] [PubMed] [Google Scholar]
- 17.Codella NC, Cham MD, Wong R, et al. Rapid and accurate left ventricular chamber quantification using a novel CMR segmentation algorithm: a clinical validation study. J Magn Reson Imaging 2010;31:845-53. 10.1002/jmri.22080 [DOI] [PubMed] [Google Scholar]
- 18.Bhatt AB, Buck JS, Zuflacht JP, et al. Distinct effects of losartan and atenolol on vascular stiffness in Marfan syndrome. Vasc Med 2015;20:317-25. 10.1177/1358863X15569868 [DOI] [PubMed] [Google Scholar]
- 19.Dormand H, Mohiaddin RH. Cardiovascular magnetic resonance in Marfan syndrome. J Cardiovasc Magn Reson 2013;15:33. 10.1186/1532-429X-15-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof 2013;10:12. 10.3352/jeehp.2013.10.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacro RV, Dietz HC, Mahony L. Atenolol versus Losartan in Marfan's Syndrome. N Engl J Med 2015;372:980-1. [DOI] [PubMed] [Google Scholar]
- 22.O'Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension 2005;45:652-8. 10.1161/01.HYP.0000153793.84859.b8 [DOI] [PubMed] [Google Scholar]
- 23.Groth KA, Hove H, Kyhl K, et al. Prevalence, incidence, and age at diagnosis in Marfan Syndrome. Orphanet J Rare Dis 2015;10:153. 10.1186/s13023-015-0369-8 [DOI] [PMC free article] [PubMed] [Google Scholar]