Abstract
Background
New surgical techniques for nephrectomy mainly related to early diagnosis made possible by advances in imaging studies have been developed in recent decades. However, postoperative renal dysfunction is a constant concern because of the major problems faced by healthcare services and by the patients themselves. To assess risk factors for developing acute renal failure (ARF) in patients submitted to nephrectomy in a university hospital.
Methods
Seventy-seven patients submitted to nephrectomy for benign and malignant diseases in a university hospital were evaluated in respect to preoperative and postoperative creatinine clearance. Demographic (gender, age), clinical (cancer, diabetes, high blood pressure, chronic kidney disease) and surgical (anesthesia time, open or laparoscopic surgery) variables were also analyzed.
Results
Of the 77 patients, 72 met the inclusion criteria. Of these, ten (13.8%) had a diagnosis of chronic renal failure (CRF), 30 (48%) had stage I ARF and one (16.1%) had stage II ARF. The anesthesia time, type of surgery (open or laparoscopy), total or partial nephrectomy, the side of the procedure, hypertension, diabetes, CRF, renal cancer, preoperative and postoperative creatinine concentrations were analyzed. Only the difference between preoperative and postoperative creatinine clearance was clinically significant (P<0.001).
Conclusions
An altered preoperative renal function is a risk factor for the development of ARF in nephrectomized patients.
Keywords: Renal insufficiency, nephrectomy, risk factors
Introduction
Since 1963, radical nephrectomy has been the gold standard to treat localized renal tumors (1). However, the approaches to renal tumors has changed mainly due to new techniques involving advances in imaging that allow early diagnosis (2). This increase has prompted discussions about the best method to preserve renal function in patients submitted to surgery.
Previous studies have shown significantly increased risk of renal failure, defined as creatinine serum concentrations greater than 2 mg/dL in patients submitted to radical nephrectomy compared to those submitted to partial nephrectomy (3). On assessing renal function after kidney donation, recent studies show a higher risk of developing chronic kidney disease (CKD) compared to the general population (4). With 60% of patients developing renal dysfunction, several studies have attempted to identify risk factors for developing acute renal failure (ARF) after surgical procedures (5,6).
On considering the difficulties faced by hospitals including prolonged hospital stays with consequent high costs and by kidney disease patients such as worsening quality of life and work absenteeism, this study evaluates risk factors for the development of ARF in patients submitted to nephrectomy in a university hospital.
Methods
Patients and methods
Seventy-seven patients submitted to nephrectomy for both benign as malignant conditions were evaluated in the Urology Service of Hospital de Base in São José do Rio Preto, SP, from January 2015 to February 2016. Of the patients evaluated, five were excluded from the study due to lack of post-operative tests or loss of follow-up in the service. This study was approved by the Research Ethics Committee of the Medicine School in São José do Rio Preto (FAMERP-CAAE: 60833616.6.0000.5415).
Demographic (gender, age), clinical (type of neoplasm, diabetes, high blood pressure, CKD) and surgical data (time of anesthesia, open surgery or laparoscopy) were collected from the medical records of patients. The results of preoperative creatinine concentrations of 72 patients were analyzed and creatinine clearance was estimated using the Crockroft and Gault formula; the result was multiplied by 0.85 for female patients (7).
Postoperative analysis was based on creatinine levels measured between the 2nd and 30th days after surgery with the lowest value of creatinine found during this period being used. The criteria utilized to maintain patients in hospital for further creatinine measurements were based on the clinical condition of the patient, especially in cases of prior CKD. The mean follow-up until creatinine stabilization and the characterization of ARF was 30 days after the procedure.
Non-parametric tests (Spearman correlation) were used to investigate possible correlations in creatinine clearance after the procedure (qualitative data) and parametric tests (Pearson correlation) examined differences between preoperative and postoperative creatinine (quantitative data). All analyses were performed using the SPSS program version 23 for Windows (IBM, New York, United States). An alpha error of 5% was considered acceptable.
Results
The age of the patients submitted to nephrectomy ranged from 22 to 82 years (mean: 56±12 years) with 56% of the cases being male and 44% female. Of the 72 patients included in this study, 65% had renal neoplasms, 69% underwent open surgery and the mean surgical time, independent of the type of procedure, was 189 minutes (Table 1).
Table 1. Clinical and surgical data of patients submitted to open surgery and laparoscopic nephrectomy.
| Variable | n [%] |
|---|---|
| Neoplasia | 47 [65] |
| Hypertension | 35 [48] |
| Diabetes | 15 [20] |
| Chronic kidney disease | 10 [14] |
| Open surgery | 50 [69] |
| Laparoscopic surgery | 22 [31] |
The mean anesthesia time of patients submitted to laparoscopy (238 min) was significantly greater than the time for open surgery (167 min: P=0.0005). The open surgery group of nephrectomized patients had higher rates of hypertension (58%) and diabetes (26%) (Table 2).
Table 2. Comparison of demographic, clinical and operative variables of patients submitted to nephrectomy by open surgery and laparoscopy.
| Variable | Open surgery | Laparoscopic surgery | P value |
|---|---|---|---|
| Age (year) (mean ± SD) | 59±22 | 47±20 | <0.0001* |
| Anesthetic time (min) | 167 | 238 | 0.0005* |
| Side [n (%)] | |||
| Right | 26 [52] | 10 [46] | 0.7985** |
| Left | 24 [48] | 12 [54] | |
| Neoplasia [n (%)] | 38 [76] | 9 [40] | – |
| Hypertension [n (%)] | 29 [58] | 6 [27] | – |
| Diabetes [n (%)] | 13 [26] | 2 [9] | – |
| Preoperative CKD [n (%)] | 6 [12] | 4 [18] | – |
| Mean preoperative clearance (mL/min/1.73 m2) | 68 | 78 | 0.2094* |
| Mean postoperative clearance (mL/min/1.73 m2) | 54 | 65 | 0.0932* |
*, student’s t test; **, chi-square test. SD, standard deviation; CKD, chronic kidney disease.
Of the 72 patients submitted to nephrectomy, 10 (13.8%) had a previous diagnosis of chronic renal failure (CRF). Furthermore, according to the Acute Kidney Injury Network (AKIN) criteria 8 used to classify renal function, renal failure was acute in 5 (50%) with 1 (10%) classified as stage I, none as stage II, and 4 (40%) as stage III.
Using the same criteria, of the 62 patients who had no diagnosis of CKD, 31 (50%) did not evolve with acute renal injury in the postoperative period, 30 (48%) progressed with Stage I ARF, 1 (16.1%) Stage II ARF and no patients developed Stage III ARF.
Among the patients who did not have preoperative CKD, 42 (67%) already had renal function below 90 mL/min/1.73 m2 before the procedure. In patients with normal renal function prior to surgery, 65% (n=20) had reduced renal function after surgery.
Using the Spearman correlation, there were significant associations of postoperative creatinine clearance with CRF (P<0.0001), neoplasia (P=0.0298) and serum creatinine (P<0.0001) before the surgical procedure (Table 3). Moreover, a statistically significant correlation was found on comparing preoperative and postoperative creatinine clearance (P<0.0001) (Figure 1).
Table 3. Correlation analysis to evaluate the postoperative clearance.
| Variable | P value | 95% CI | r |
|---|---|---|---|
| Anesthesia time | 0.3207 | −0.1231 to 0.3472 | 0.1187 |
| Open surgery | 0.0992 | −0.4148 to 0.04454 | −0.1959 |
| Laparoscopic surgery | 0.0992 | −0.04454 to 0.4148 | 0.1959 |
| Right side | 0.468 | −0.3186 to 0.1546 | −0.08688 |
| Left side | 0.468 | −0.1546 to 0.3186 | 0.08688 |
| Partial | 0.9153 | −0.2503 to 0.2262 | −0.01276 |
| Total | 0.9153 | −0.2262 to 0.2503 | 0.01276 |
| CKD | <0.0001 | −0.7117 to −0.3839 | −0.5701 |
| Diabetes | 0.4455 | −0.3226 to 0.1503 | −0.09133 |
| Hypertension | 0.3681 | −0.3373 to 0.1341 | −0.1076 |
| Neoplasia | 0.0298 | −0.4660 to −0.01903 | −0.2562 |
| Preoperative creatinine | <0.0001 | −0.6318 to −0.2529 | −0.4633 |
| Postoperative creatinine | <0.0001 | −0.7559 to −0.4625 | −0.6313 |
CI, confidence interval; CKD, chronic kidney disease.
Figure 1.
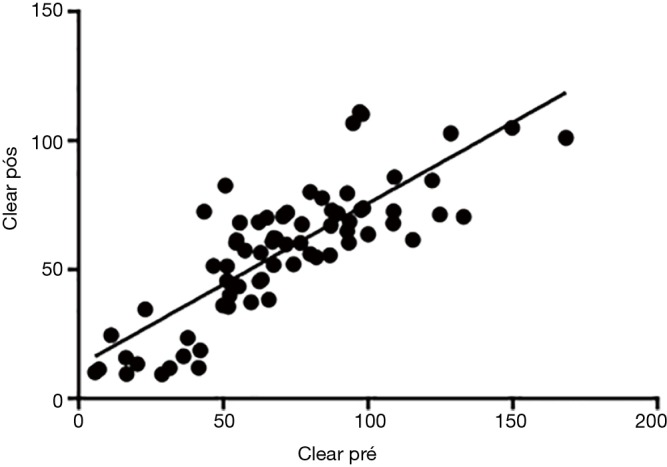
Analysis Pearson correlation between pre and postoperative clearance (r=0.8116; 95% CI, 0.7142–0.8782; P<0.0001).
Discussion
This study showed that the main risk factor for ARF in nephrectomized patients was preexisting CKD. Although preoperative creatinine clearance was statistically significant, the correlation coefficient was not high. However, analysis of preoperative and postoperative creatinine clearance identified a notable positive correlation, confirming that preexistent CKD is a risk factor for postoperative deterioration of renal function.
The only risk factor associated with the development of acute kidney injury was preoperative hypertension (8). According to Naik et al. (9), chronic hypertension increases the risk of lability of the blood pressure in the preoperative period, thereby contributing to perioperative renal failure. These authors also report that more studies are needed to evaluate how control of the blood pressure before surgery influences CRF. However, in the current study, control of the preoperative pressure was not investigated.
Forbes et al. (10) reported surgical times that do not differ significantly from those found in this study. It is noteworthy that the current study was conducted in a university hospital with a medical residency program in urology that may have increased surgical time due to the learning curve. Surgical time did not correlate significantly with worsening renal function, i.e., the duration of anesthesia did not influence postoperative changes in renal function.
Examining renal dysfunction in general surgery, Gaber et al. (11) showed that 64% of patients had reductions in the glomerular filtration rate before surgery, thereby supporting the results obtained in this series, in which 67% of patients did not have a diagnosis of CKD before surgery, but had some type of renal damage as shown by the creatinine clearance. As preoperative renal function is a predictor of postoperative ARF, our results reinforce the importance of the evaluation of renal function prior to nephrectomy. For Lane et al. (12) this clinical condition occurs in older patients with previous diseases, who often have preexisting CRF at the time of surgery.
Of the patients without preoperative renal injury, 65% had reduced postoperative clearance that did not drop to below 70 mL/min/1.73 m2. Rajan et al. (13) found that 39% of his patients had ARF after partial nephrectomy. However, Thompson et al. (5) and Weight et al. (6) reported that 60% of patients had some type of kidney dysfunction after surgery. In this study, even with slightly higher rates in patients without impaired renal function before surgery, serious injuries in need of an intervention that might compromise the length of stay and patient survival were not observed. However, acute kidney injury should be avoided in patients undergoing radical nephrectomy with normal preoperative renal function because of the risk of progression to CKD (14). Renal injury after surgery can be minimized with appropriate procedures, including pre-operative care, the proper surgical technique and postoperative resuscitation (15,16).
One limitation of this study is the retrospective approach that has a selection bias due to non-randomization. Variables such as blood pressure were not monitored to verify the severity and control of hypertension. Anesthesia time is biased as the procedures were performed by surgeons at different stages of training, including residents, and so this time is probably longer than would be expected with just experienced surgeons. The number of cases of each type of procedure and respective diseases was not studied and so whether the type of procedure performed for a given disease influenced the postoperative renal function was not investigated.
In conclusion, impaired preoperative renal function is a risk factor for the development of ARF in patients submitted to nephrectomy in a university hospital.
Acknowledgements
None.
Ethical Statement: This study was approved by the Research Ethics Committee of the Medicine School in São José do Rio Preto (FAMERP-CAAE: 60833616.6.0000.5415).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Robson CJ, Churchill BM, Anderson W. The Results of Radical Nephrectomy for Renal Cell Carcinoma. J Urol 2017;197:S111-3. 10.1016/j.juro.2016.10.095 [DOI] [PubMed] [Google Scholar]
- 2.Lee CT, Katz J, Shi W, et al. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol 2000;163:730-6. 10.1016/S0022-5347(05)67793-2 [DOI] [PubMed] [Google Scholar]
- 3.McKiernan J, Simmons R, Katz J, et al. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology 2002;59:816-20. 10.1016/S0090-4295(02)01501-7 [DOI] [PubMed] [Google Scholar]
- 4.Mjøen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int 2014;86:162-7. 10.1038/ki.2013.460 [DOI] [PubMed] [Google Scholar]
- 5.Thompson RH, Lane BR, Lohse CM, et al. Renal function after partial nephrectomy: effect of warm ischemia relative to quantity and quality of preserved kidney. Urology 2012;79:356-60. 10.1016/j.urology.2011.10.031 [DOI] [PubMed] [Google Scholar]
- 6.Weight CJ, Larson BT, Fergany AF, et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol 2010;183:1317-23. 10.1016/j.juro.2009.12.030 [DOI] [PubMed] [Google Scholar]
- 7.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31-41. 10.1159/000180580 [DOI] [PubMed] [Google Scholar]
- 8.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naik BI, Colquhoun DA, McKinney WE, et al. Incidence and risk factors for acute kidney injury after spine surgery using the RIFLE classification. J Neurosurg Spine 2014;20:505-11. 10.3171/2014.2.SPINE13596 [DOI] [PubMed] [Google Scholar]
- 10.Forbes CM, Rendon RA, Finelli A, et al. Disease progression and kidney function after partial vs. radical nephrectomy for T1 renal cancer. Urol Oncol 2016;34:486.e17-e23. 10.1016/j.urolonc.2016.05.034 [DOI] [PubMed] [Google Scholar]
- 11.Gaber AO, Moore LW, Aloia TA, et al. Cross-sectional and case-control analyses of the association of kidney function staging with adverse postoperative outcomes in general and vascular surgery. Ann Surg 2013;258:169-77. 10.1097/SLA.0b013e318288e18e [DOI] [PubMed] [Google Scholar]
- 12.Lane BR, Poggio ED, Herts BR, et al. Renal function assessment in the era of chronic kidney disease: renewed emphasis on renal function centered patient care. J Urol 2009;182:435-43; discussion 443-4. 10.1016/j.juro.2009.04.004 [DOI] [PubMed] [Google Scholar]
- 13.Rajan S, Babazade R, Govindarajan SR, et al. Perioperative factors associated with acute kidney injury after partial nephrectomy. Br J Anaesth 2016;116:70-6. 10.1093/bja/aev416 [DOI] [PubMed] [Google Scholar]
- 14.Cho A, Lee JE, Kwon GY, et al. Post-operative acute kidney injury in patients with renal cell carcinoma is a potent risk factor for new-onset chronic kidney disease after radical nephrectomy. Nephrol Dial Transplant 2011;26:3496-501. 10.1093/ndt/gfr094 [DOI] [PubMed] [Google Scholar]
- 15.Choi YS, Park YH, Kim YJ, et al. Predictive factors for the development of chronic renal insufficiency after renal surgery: a multicenter study. Int Urol Nephrol 2014;46:681-6. 10.1007/s11255-013-0534-8 [DOI] [PubMed] [Google Scholar]
- 16.Shin S, Han Y, Park H, et al. Risk factors for acute kidney injury after radical nephrectomy and inferior vena cava thrombectomy for renal cell carcinoma. J Vasc Surg 2013;58:1021-7. 10.1016/j.jvs.2013.02.247 [DOI] [PubMed] [Google Scholar]


