Summary
Obesity is a very common condition; however, the effect of excess body weight on the appropriate dose of immunoglobulin has not been defined empirically. The proposed pharmacokinetic differences between lean and obese patients and the opportunity to reduce costs has led to the proposition that obese patients should receive proportionally lower doses of immunoglobulin once a certain threshold is reached. Here the theoretical factors which could affect dosing in obese patients are considered alongside the available empirical evidence. The available evidence indicates that obesity may affect the pharmacokinetics of immunoglobulin; however, the effect is likely to be too small to have a clinically important effect on dosing. Wide interpatient individuality and highly variable clinical need mean that obesity should not play a major factor in dosing considerations. However, patients who are obese are more likely to have multiple cardiovascular risk factors and their weight indicates a large dose. This puts these patients at a higher risk of adverse reactions, and therefore caution is advised.
Keywords: dosing, immunoglobulin, IVIg, obesity, primary immunodeficiency
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Clinical challenges in the management of patients with B cell immunodeficiencies. Clinical and Experimental Immunology 2017, 188: 323–5.
The role of genomics in common variable immunodeficiency disorders. Clinical and Experimental Immunology 2017, 188: 326–32.
When to initiate immunoglobulin replacement therapy (IGRT) in antibody deficiency: a practical approach. Clinical and Experimental Immunology 2017, 188: 333–41.
Progressive multi‐focal leucoencephalopathy – driven from rarity to clinical mainstream by iatrogenic immunodeficiency. Clinical and Experimental Immunology 2017, 188: 342–52.
Chronic norovirus infection and common variable immunodeficiency. Clinical and Experimental Immunology 2017, 188: 363–70.
Introduction
It is recommended to dose immunoglobulin (Ig) for both replacement and autoimmune indications with reference to actual body weight 1, 2; however, clinicians have long queried whether this is the optimal dosing approach for all patients 3, 4. In general, how dosing can be optimized to maximize efficacy while minimizing cost and the risk of adverse events is an important question. In this short review the focus will be on the challenges the obese patient population presents and how dosing can be optimized in this group.
Optimum dosing of immunoglobulin remains a hotly debated topic in general for both replacement and autoimmune indications 5, 6, so tackling this topic in a subset of patients who are not well represented in research 7, 8 is challenging. Zuckerman et al. 9 advocate that obese patients should be considered as a special population and included as standard in all clinical trials, as well as introducing the requirement to record body mass index (BMI) in all post‐authorization safety studies (PASS). This idea, although currently peripheral, is likely to become more accepted as obese people represent a significant and growing proportion of the global population, and this is particularly pronounced in areas of high immunoglobulin (Ig) use. The World Health Organization (WHO) estimated that more than 20% of people were obese in Europe in 2010 10, and the Centre for Disease Control published figures showing that >36% of people in the United States were obese in 2014 11.
The WHO defines obesity as a body mass index of above 30 kg/m2 and overweight as >25 kg/m2 12. In non‐athletes BMI remains a strong indicator of body fat composition and health risk while also being a readily available parameter, and therefore that is how the term obesity is used here. The usefulness of BMI has been criticized severely within the sporting world due to the insensitivity to muscle and bone mass 13; however, for the largely sedentary patient population receiving immunoglobulin it is appropriate.
Dosing adaptations are made routinely to take into consideration factors which affect drug metabolism and excretion (e.g. hepatic and renal function); however, very little consideration is given to obesity, which has the potential to affect all aspects of drug pharmacokinetics and has a much higher prevalence.
Current dosing practice by clinical immunologists in primary immunodeficiency (PID) is largely unaffected by obesity and generally reflects guidelines with a standard starting dose of 0·4–0·8 g/kg/month alongside evidence‐based alterations made based on clinical factors (e.g. presence of bronchiectasis 14). Further optimization is then undertaken in the maintenance phase based largely on clinical response, but also on laboratory parameters, tolerability and patient preference. Whether the presence of obesity specifically affects these general factors remains a topic of debate.
The situation in higher‐dose indications is slightly different, where significant emphasis is put on reducing the starting dose from the (largely arbitrary) 2 g/kg starting point and also the use of maximum total doses due to safety considerations. This is important, as although the original use of Ig was in PID indications, such as common variable immunodeficiency disorder (CVID) and X‐linked agammaglobulinaemia (XLA), these now represent a fraction of the overall need, with neurological and haematological conditions playing an increasing role. Most patients receiving Ig for neurological conditions (e.g. the peripheral neuropathies; multi‐focal motor neuropathy (MMN) and chronic inflammatory demyelinating polyneuropathy (CIDP)) are immunocompetent and often receive much larger doses than replacement (PID) patients. This means that following Ig administration the IgG levels in a peripheral neuropathy patient may be very high, which will affect the pharmacokinetics and safety considerations in these patients. Use in secondary immunodeficiency (particularly iatrogenic) has become more important in recent years 15, but the dosing and risks in these patients are similar to PID patients, with the exception of myeloma, where a very high baseline IgG plasma concentration may be present and therefore hyperviscosity must be considered.
Theoretical factors which may influence the pharmacokinetics of IgG in obese patients
There is a reasonable body of literature investigating the empirical effects of obesity on the pharmacokinetics of small molecule drugs allowing some general principles to emerge, but consensus does not yet exist 16, 17, 18. The situation in immunoglobulin administration is more difficult due to the paucity of data and the unique characteristics of Ig, therefore this section is a summary of established physiological differences between lean and obese patients which may influence the pharmacokinetics and therefore dosing of immunoglobulin (see also Fig. 1).
Figure 1.
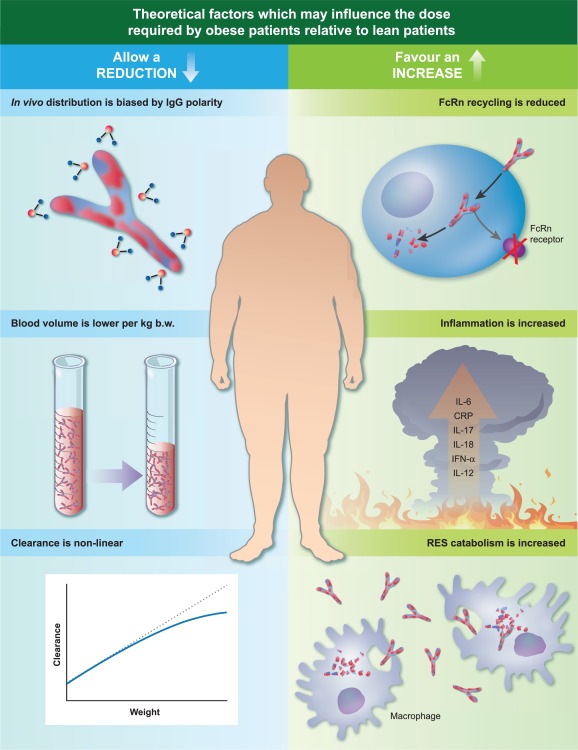
Theoretical factors which may affect immunoglobulin (Ig) pharmacokinetics in obese patients. IgG is a polar molecule, and therefore a preference for the aqueous spaces (e.g. blood) would be accentuated in obese patients. This preference may be additionally pronounced because obese patients have a proportionally (per kg body weight (b.w.)) lower blood volume compared to lean patients. Furthermore, protein drug clearance is proportionally lower as weight increases, which may contribute to an extended half‐life for Ig. The chronic inflammatory state seen in obese patients may necessitate a higher dose to control symptoms, as would an increase in Ig catabolism and reduced recycling due to a shorter t 1/2.
Hydrophilicity
Immunoglobulin G (IgG) is a relatively polar molecule with a small volume of distribution (VD), which generally means that the drug molecules accumulate preferentially in aqueous compartments, e.g. plasma 19. Therefore, penetration of the active ingredient into adipose tissue/lipophilic environments has been postulated to be poor, which could result in a higher plasma concentration in obese patients despite the same dose/kg. Of course, IgG distribution is not simply the function of a partition co‐efficient in vivo, and IgG can be particularly influenced by active transport and inflammation 20, 21, 22. For monoclonal antibodies the distribution is altered specifically by binding to the target antigen; however, this is not the case for polyvalent normal human immunoglobulin 23.
Blood volume
Perfusion of adipose tissue relative to lean tissue is known to be poor, which may exacerbate the partition effect discussed above through a reduced opportunity for a plasma concentrated drug to access adipose tissue 24. Likely to be of more consequence is the fact that total blood volume relative to actual body weight is reduced in obese patients compared with lean patients (50 versus 75 ml/kg) 25. The effect of lower ml/kg blood volume on protein drug pharmacokinetics was demonstrated well by Wang et al. 26 in laboratory animals who received the same g/kg dose, but the obese animals exhibited higher plasma drug concentrations. Of relevance to the route of adminstration was that this applied only to intravenous (i.v.) dosing and was not replicated when the animals were dosed by the subcutaneous (sc.) route.
Neonatal Fc receptor (FcRn) recycling
FcRn; which protects IgG from lysosomal degradation, facilitates creation of an intracellular protein reservoir and subsequently recycles IgG back into the circulation, is responsible for the long half‐life of IgG in vivo 27. It is distributed widely throughout the body, although there is some evidence that expression in adipose tissue is lower than in other tissues (e.g. skin and muscle) 28, 29, which could result in a lower capability of obese patients to recycle IgG.
Clearance
Clearance of protein drugs may not be linear with body weight. It is hypothesized that although clearance increases with body weight, at higher weights the increase may be non‐linear 30, which would mean that heavier patients would have a slightly reduced rate of clearance per kg body weight which could result in an extended IgG half‐life. This effect comes with some significant caveats, which are that it would apply similarly to those of high body weights due to height, muscle mass or obesity and also that it is extrapolated from general protein drug behaviour rather than from the characteristics of IgG.
Obese patients have chronically enhanced inflammation
Adipose tissue is not simply an energy storage compartment; it is a metabolically and immunologically active tissue 31. In normal adipose tissue (lean patients), pro‐ and anti‐inflammatory adipokines balance to create metabolic and immunological homeostasis. However, in obesity the balance tips to production of excess chemokine (C‐C motif) ligand 2 (CCL2), interleukin (IL)‐1β, IL‐6, IL‐8, IL‐12, IL‐17, IL‐18, tumour necrosis factor (TNF)‐α, interferon (IFN)‐γ and C‐reactive protein (CRP), which are transported to the systemic circulation and precipitate a chronic inflammatory state which causes, predisposes to and exacerbates conditions such as type 2 diabetes, cancer and cardiovascular diseases 31, 32, 33, 34, 35, 36, 37. This is part of the metabolic syndrome found in obesity which is characterized by high waist circumference, raised blood pressure, raised triglycerides, reduced high‐density lipoprotein cholesterol and raised blood glucose 38. Although the metabolic syndrome is simply a collection of risk factors, the associated inflammatory state exacerbates immune dysregulation, including pathogenic antibody production by functionally altered B cells 39, and may increase the functional requirement for IgG replacement in obese patients suffering from PIDs. In the obese state increased levels of activated and effector proinflammatory CD4+ and CD8+ T cells and reduced levels of immunoregulatory T cells (Tregs) are found in visceral adipose and contribute to immune dysregulation 40, 41. It is clear that in PID the autoimmune and inflammatory complications are driven by the underlying genetic/molecular defects particular to the pathophysiology of each patient (e.g. cytotoxic T lymphocyte antigen (CTLA)‐4 deficiency); however, it is plausible that additional obesity mediated immune dysregulation, which is well documented, may worsen the prognosis or exacerbate symptoms. Indicators that this is likely can be seen in the detrimental effect obesity has on response to vaccines 42 and increased mortality following infection with influenza 31, 43. Interestingly, there is a mortality advantage of obesity in sepsis which is hypothesized to be due primarily to the haemodynamic benefit of the hypertensive state and protection from a catabolism‐mediated nutritional deficit; however, there was also a role for the distinct immunological status of the obese patient 44. It would therefore be interesting to know if these effects have an impact on any of the indications for which Ig is prescribed, but particularly in primary immunodeficiencies such as CVID, where proliferative, inflammatory and autoimmune complications cause significant morbidity 45 and can prove a more difficult challenge for treating physicians than classical infectious complications 46. The consequence of these combined effects may be the need for a greater than proportional increase in dose in the obese PID patient to ensure that both the underlying condition and the obesity‐mediated immune dysregulatory exacerbation and are attended to sufficiently.
Catabolism of IgG
In concert with the increased inflammatory propensity of the obese patient discussed above, the population of activated macrophages is also expanded significantly in excess adipose tissue 47. As the primary elimination mechanism of IgG molecules is via the reticuloendothelial system 48, this is likely to contribute to increased catabolism of IgG and a reduced half‐life in comparison with lean patients.
Established physiological differences between lean and obese patients are described above, although some amelioration of opposing factors is likely and the cumulative impact of these individual factors on Ig pharmacokinetics, and therefore dosing, remains theoretical.
Cost and safety
The particular physiology of obese patients contributes to theoretical factors which could mean that either an increase or a decrease in dose is warranted. However, pharmacokinetic factors are not the only considerations when formulating the most appropriate treatment for any group of patients or in particular any one individual.
Ig is a high‐cost medicine, and in many indications where it is utilized successfully dosing studies have not been performed; e.g. the 2 g/kg starting dose in some autoimmune indications may not be fully evidence‐based. There is now some evidence which supports the reduction of Ig doses from the starting dose to a lower maintenance dose in immunomodulatory conditions 49. Observational studies across both lean and obese patients in CIDP indicate that although the dose range and frequency used in maintenance is wide, 1 g/kg/month is sufficient for stability 50. It should be noted, however, that very high doses may be necessary in some acute conditions e.g. 3 g/kg over 2–5 days in divided doses for toxic epidermal necrolysis 51. Under these circumstances, where a very high dose is necessary, safety is the primary concern.
The management of chronic autoimmune conditions requires that responsiveness to Ig therapy is first demonstrated followed by a dose appropriate to maintain that response. This therefore requires that a sufficiently high initial dose is used to ensure that all potential responders are detected. Following this, it is appropriate that clinicians titrate the starting dose so that it strikes the right balance of cost and effectiveness in maintenance therapy. This question is driven by a twofold desire to reduce health system expenditure and optimize patient care, and has been successful in achieving this in individual patients 5, 14, 52, 53, 54. The desire by some budget holders to solely reduce costs exerts a blind downward pressure on the dose used. However, the balance between safety and effectiveness is much more subtle, and requires the lowest possible dose which achieves the best possible outcome for the patient during both the short and long term. It is important to note that costs to a health system are much more related to the maintenance dose and the long‐term wellbeing of the patient. The best way to ensure that both of these are optimized is through an individualized dosing regimen.
The question of drug safety is particularly relevant for obese patients due to the larger doses used and the generally higher risk of adverse reactions, which is associated with obesity and other co‐morbidities common in this population (e.g. diabetes mellitus, hypertension, history of vascular disease). It must also be remembered that, irrespective of weight, the question of safety is likely to be much more pressing in the patient groups receiving larger doses, i.e. in general those patients being treated with Ig for immunomodulatory reasons with doses of 1–2 g/kg. Some practical mitigation steps should be followed where risk factors are present. These include adequate hydration of the patient prior to the infusion, maintaining the appropriate infusion rate, deferring the infusion if the patient has an active infection, dividing very large doses and, in cases where a particular risk is apparent, blood viscosity can be monitored or weight loss advised. While cutting costs and reducing risk are important arguments for short‐term impact, many of the conditions where Ig is used are chronic diseases with high levels of long‐term morbidity and mortality. The balance which must be struck is illustrated in Fig. 2, and should be considered in the context of the primary purpose of giving any medicine which is to improve the quality and duration of patients' lives. An important goal of Ig administration is to reduce the risk of lesions to physical structures which are irreversible and refractory to treatment (e.g. bronchiectasis in CVID 55 and permanent axonal damage in MMN 56), therefore highlighting the necessity of optimal dosing in the long term.
Figure 2.
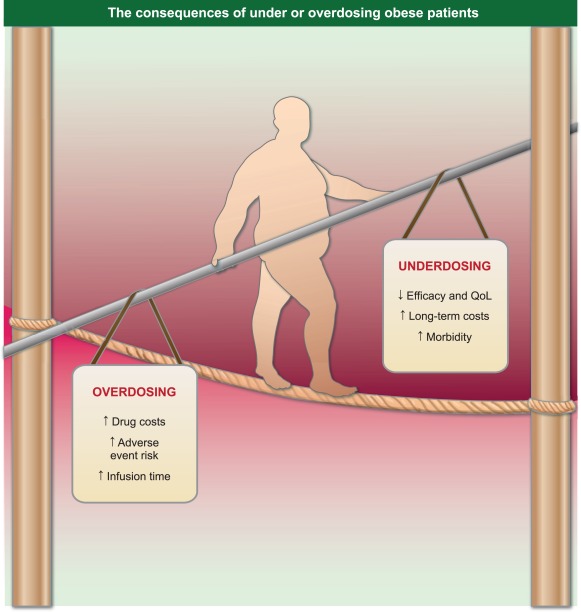
The consequences of under‐ or overdosing can be felt by both the treating clinicians and the health system, but will primarily affect the patient. General dose recommendations are useful to the treating physician; however, the balance necessary for each patient must be made on a individual basis where all factors can be considered. QoL: Quality of life.
Although safety is always paramount, patient preference may also be important here as larger doses result in longer infusion times or the use of higher infusion rates. The latter, particularly, may reduce tolerability (e.g. increased levels of transient, reversible side effects such as chills or headache) and may necessitate divided doses, which are inconvenient and can limit options in the use of alternative routes of administration (e.g. s.c. Ig infusion or rapid push). Due to innovation by Ig manufacturers, modern‐high percentage Ig preparations can be used to reduce infusion time 57, novel routes (e.g. facilitated s.c. Ig) can be used to give large doses while avoiding a high Cmax and wear‐off effects 58 while standard or rapid‐push s.c. Ig offers almost constant plasma concentrations coupled with autonomy and home therapy in countries where this is practised 59, 60. These options give the patient and clinician greater flexibility to optimize patient safety but also satisfaction.
Patient outcome – the need for empirical data
How does the multitude of factors discussed above coalesce in published clinical practice? The effects of obesity on the pharmacokinetics of drugs is contradictory, and even when only protein drugs are considered no consensus can be found within the published literature 61. Therefore, in order to attempt to understand the role of obesity in dosing immunoglobulin in replacement and immunomodulatory indications we must consider studies which investigate this area specifically. A search of the literature since 2000 revealed six studies of immunoglobulin which considered obese patients specifically or BMI (or approaches which discounted adipose tissue when dosing, e.g. ideal body weight (IBW)).
A UK‐based retrospective audit involving 107 CVID patients across four centres did not find any relationship between annual dose and trough level despite normalizing for weight and BMI 62. The lack of any association may indicate that the sample size and power of this study may have been too small to detect the positive correlation between change in plasma concentration and Ig dose, which has been demonstrated in a number of contemporary studies 52, 60, 63, 64, 65. Ig studies are at particular risk of this due to the very high interindividual patient variability, which has been documented repeatedly 14, 52, 56, 57, 63.
A US study involving 173 PID patients receiving s.c. Ig showed that in both lean and obese patients the increase in serum Ig concentration was proportional to the dose administered and the increase in plasma Ig concentration/g of administered SCIg was the same in both cohorts. These data allowed the author to conclude that there was no difference in the pharmacokinetics of replacement SCIg between obese versus lean patients and therefore there was no justification for adjusting the dose in obese patients relative to lean patients 60.
Both these studies considered patients receiving Ig replacement therapy for primary immunodeficiencies, but a significant portion of Ig is now used in high‐dose immunomodulation therapy. This distinction is important, not only because of the high volume of Ig use in neurological conditions (43% of the total use in the UK in 2014 66), but also because this patient group may be pharmacokinetically distinct, as these patients are largely immunocompetent and generally receive higher doses which lead to higher than physiological IgG plasma concentrations during an extended period 5, 52, 63, 67. Therefore, data are required for both replacement and immunomodulation dosing regimens.
A smaller study published in 2015 63 investigated the effect of obesity on dosing for both replacement and autoimmune indications. Although covering a wide dose and plasma concentration range is a positive aspect of this study, it must also come with the caveat that this necessitated the inclusion of patients with different diseases and therefore different pathophysiologies and outcome measures. This was a study in which 31 obese patients were matched with a clinically equivalent lean control at centres where all patients are initiated on an actual body weight dose and then titrated as appropriate based on clinical outcome. This ensured that the minimum dose necessary to optimize the clinical outcome of the patients was administered, and allowed the doses necessary to achieve this to be determined retrospectively. The study found that there was no difference in the obese and lean cohorts at lower replacement doses, but at higher autoimmune doses the obese patients achieved a higher plasma concentration for each gram of Ig administered. This indicated that there is a real pharmacokinetic difference in lean and obese patients at a population level, but only at higher doses. However, the impact on clinical outcome in individuals was not so straightforward. While some obese patients in the cohort benefited from low but clinically effective doses, others required high doses to achieve good outcomes (outcome was measured by number of infections in PID and validated functional scores in the peripheral neuropathy patients). This illustrates that while a pharmacokinetic effect of obesity appears to exist it does not necessarily translate into clinical outcome. This suggests that other patient‐specific factors are more important. A study of 15 chronic inflammatory demyelinating polyneuropathy patients whose dose was adjusted to the minimum required to achieve ‘best clinical response' found that patients exhibited large interpatient variability in Ig pharmacokinetics but small intrapatient variability 52. A statistically significant correlation was shown for the relationship between Ig dose and change in IgG plasma concentration; however, no influence was found when weight and BMI were considered.
One centre reported treating all patients prescribed Ig with an IBW dose over the period a year. Unfortunately, the only outcome reported from this study was the total amount of immunoglobulin diverted from patients and no clinical outcome data were recorded. Although the reduction in dose reduced drug costs, no conclusion can be made on whether the patients experienced positive or negative outcomes as a result 68.
A report of 11 adults, including four who were obese, indicated that the increase in plasma Ig concentration was directly proportional to the actual body weight‐adjusted dose 64. Although no differentiation was made between lean and obese patients in the published work, a personal communication indicated that the obese patients had a larger increase in IgG plasma concentration for the same dose when compared with lean patients. Although this was a very small cohort and unlikely to be statistically significant, it mirrors the data published by Hodkinson et al. 63. No patient outcome data were reported in this study, and the indications were not published, so the potential for insights is limited.
An important gap in all these studies was that they did not report any tolerability or safety‐related outcomes, a dose comparison in the same patients (e.g. IBW versus standard of care) or a large cohort of obese patients, and therefore the impact of dose in obesity remains unclear. It should be noted that even if such studies did report tolerability data it is unlikely that they would be sufficiently powerful to detect subtle differences in the safety of different dosing strategies.
Implications for clinical practice
There is a balance to be made between the risks of overdosing and underdosing patients (Fig. 2). It is unavoidable that patients will be under‐ or overdosed if a fixed weight‐based dose is applied to Ig, which has such a large interpatient variability 14, 52, 56, 57, 63, 69, 70. It is essential, therefore, that consideration is given to both clinical and laboratory outcomes to determine whether the delicate balance has been met.
Where doses can be reduced (or the dose interval extended) without compromising efficacy this should be practised, although it should also be taken into consideration that dose titration of immunoglobulin is an inexact science and it is difficult to determine the long‐term outcome of any given dosing strategy. There is no validated surrogate measure of long‐term benefit for Ig replacement or immunomodulation as can be relied upon when controlling blood sugar in diabetes or when lowering blood pressure to manage cardiovascular risk.
Although questions remain, it has been demonstrated in PID that optimized trough levels of Ig offer a greater level of protection from infection 71, 72, 73, and this is key 74 to protect from long‐term irreversible damage and therefore also from increased morbidity. Suboptimally dosed patients also cost the health system more in the long term 75. For these reasons, individual doses should be optimized rationally, not due to short‐term cost‐saving considerations alone, and kept under regular review, as the patient's circumstances may change over time.
The theoretical complexity of the interactions between dose, efficiacy, interpatient pharmacokinetic variation, safety and cost suggest that a prospective study providing empirical data would be useful to determine the optimal dosing strategy 76. However, most patient populations which would benefit from immunoglobulin therapy are small, and as differential efficacy would be difficult and lengthy to ascertain such an undertaking is unlikely. A more feasible study would be a prospective pharmacokinetic analysis to determine the relationship between dose, body weight/BMI and clearance. Although such a study would be mechanistically interesting and would solve the core pharmacokinetic question, it would not elucidate the link with patient outcomes in terms of safety and efficacy and therefore would leave open questions.
Although there is no definitive consensus in the published literature regarding the effect obesity has on the dose required, the research taken as a whole suggests that the impact on efficacy is small and unlikely to justify a general dose modification. When this is coupled with the well‐documented wide interpatient variability in Ig pharmacokinetics, it is clear that no blanket recommendation can be applied to this population. Although the evidence does not support a blanket dose reduction or cap based solely on obesity, it should be an important consideration for clinicians that obese patients who are being treated for an autoimmune indication may be receiving a large dose while having multiple risk factors.
In summary, clinicians should continue to have the freedom to use their clinical judgement and experience to optimize dosing of Ig on an individual basis for all patients (irrespective of weight). This will ensure that effectiveness is maximized and will reduce the risk of adverse drug reactions which will, in turn, ensure that costs for the health system as a whole in the short and long term are minimized.
Disclosure
J. H. is an employee of Biotest AG, which manufactures immunoglobulin medicinal products.
Acknowledgements
I would like to thank Dr. Jörg Schuttrümpf, Professor Artur Bauhofer, Dr. Rainer Schmeidl and Dr. Ellen Rentz for their valuable comments and sharing their expertise in this area.
References
- 1. European Medicines Agency (EMA) . Guideline on core SmPC for human normal immunoglobulin for subcutaneous and intramuscular administration. London, UK: EMA; 2015.
- 2. European Medicines Agency (EMA) . Guideline on core SmPC for human normal immunoglobulin for intravenous administration. London, UK: EMA; 2010.
- 3. Kasperek M, Wetmore R. Considerations in the use of intravenous immune globulin products. Clin Pharm 1990; 9:909. [PubMed] [Google Scholar]
- 4. Woolfrey S, Dewar M. Treatment of idiopathic thrombocytopenic purpura using a dose of immunoglobulin based on lean body mass. Ann Pharmacother 1993; 27:510. [DOI] [PubMed] [Google Scholar]
- 5. Berger M, Allen JA. Optimizing IgG therapy in chronic autoimmune neuropathies: a hypothesis driven approach. Muscle Nerve 2015; 51:315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kerr J, Quinti I, Eibl M et al. Is dosing of therapeutic immunoglobulins optimal? A review of a three‐decade long debate in Europe. Front Immunol 2014; 5:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hall R, Jean G, Sigler M, Shah S. Dosing considerations for obese patients receiving cancer chemotherapeutic agents. Ann Pharmacother 2013; 47:1666–74. [DOI] [PubMed] [Google Scholar]
- 8. Pai MP. Drug dosing based on weight and body surface area: mathematical assumptions and limitations in obese adults. Pharmacotherapy 2012; 32:856–68. [DOI] [PubMed] [Google Scholar]
- 9. Zuckerman M, Greller HA, Babu KM. A review of the toxicologic implications of obesity. J Med Toxicol 2015; 11:342–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization (WHO) . Global status report on non‐communicable diseases 2010. Geneva: WHO; 2011.
- 11. Ogden C, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. US Department of Health and Human Services; 2015. [PubMed] [Google Scholar]
- 12.WHO Expert Consultation. Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363:157–63. [DOI] [PubMed] [Google Scholar]
- 13. Ackland TR, Lohman TG, Sundgot‐Borgen J, Maughan RJ, Meyer NL, Stewart AD, Muller W. Current status of body composition assessment in sport: review and position statement on behalf of the ad hoc research working group on body composition health and performance, under the auspices of the I.O.C. Medical Commission. Sports Med 2012; 42:227–49. [DOI] [PubMed] [Google Scholar]
- 14. Lucas M, Lee M, Lortan J, Lopez‐Granados E, Misbah S, Chapel H. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allerg Clin Immunol 2010; 125:1354–60. [DOI] [PubMed] [Google Scholar]
- 15. Dhalla F, Misbah SA. Secondary antibody deficiencies. Curr Opin Allergy Clin Immunol 2015; 15:505–13. [DOI] [PubMed] [Google Scholar]
- 16. Knibbe CA, Brill MJ, van Rongen A, Diepstraten J, van der Graaf PH, Danhof M. Drug disposition in obesity: toward evidence‐based dosing. Annu Rev Pharmacol Toxicol 2015; 55:149–67. [DOI] [PubMed] [Google Scholar]
- 17. Alobaid AS, Hites M, Lipman J, Taccone FS, Roberts JA. Effect of obesity on the pharmacokinetics of antimicrobials in critically ill patients: a structured review. Int J Antimicrob Agents 2016; 47:259–68. [DOI] [PubMed] [Google Scholar]
- 18. Harskamp‐van Ginkel MW, Hill KD, Becker KC et al. Drug dosing and pharmacokinetics in children with obesity: a systematic review. JAMA Pediatr 2015; 169:678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang W, Wang E, Balthasar J. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 2008; 84:548–58. [DOI] [PubMed] [Google Scholar]
- 20. Poduslo JF, Curran GL, Berg CT. Macromolecular permeability across the blood–nerve and blood–brain barriers. Proc Natl Acad Sci USA 1994; 91:5705–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ubogu EE. The molecular and biophysical characterization of the human blood–nerve barrier: current concepts. J Vasc Res 2013; 50:289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Freiberger T, Grodecká L, Ravcuková B et al. Association of FcRn expression with lung abnormalities and IVIG catabolism in patients with common variable immunodeficiency. Clin Immunol 2010; 136:419–25. [DOI] [PubMed] [Google Scholar]
- 23. Dostalek M, Gardner I, Gurbaxani BM, Rose RH, Chetty M. Pharmacokinetics, pharmacodynamics and physiologically‐based pharmacokinetic modelling of monoclonal antibodies. Clin Pharmacokinet 2013; 52:83–124. [DOI] [PubMed] [Google Scholar]
- 24. Han P, Duffull S, Kirkpatrick C, Green B. Dosing in obesity: a simple solution to a big problem. Clin Pharmacol Ther 2007; 82:505–8. [DOI] [PubMed] [Google Scholar]
- 25. Leykin Y, Miotto L, Pellis T. Pharmacokinetic considerations in the obese. Best Pract Res Clin Anaesthesiol 2011; 25:27–36. [DOI] [PubMed] [Google Scholar]
- 26. Wang W, Chen N, Shen X et al. Lymphatic transport and catabolism of therapeutic proteins after subcutaneous administration to rats and dogs. Drug Metab Dispos 2012; 40:952–62. [DOI] [PubMed] [Google Scholar]
- 27. Martins JP, Kennedy PJ, Santos HA, Barrias C, Sarmento B. A comprehensive review of the neonatal Fc receptor and its application in drug delivery. Pharmacol Ther 2016; 161:22–39. [DOI] [PubMed] [Google Scholar]
- 28. Shi S. Biologics: an update and challenge of their pharmacokinetics. Curr Drug Metab 2014; 15:271–90. [DOI] [PubMed] [Google Scholar]
- 29. Borvak J, Richardson J, Medesan C et al. Functional expression of the MHC class I‐related receptor, FcRn, in endothelial cells of mice. Int Immunol 1998; 10:1289–98. [DOI] [PubMed] [Google Scholar]
- 30. Mould DR. The pharmacokinetics of biologics: a primer. Dig Dis 2015; 33 (Suppl. 1):61–9. [DOI] [PubMed] [Google Scholar]
- 31. Grant RW, Dixit VD. Adipose tissue as an immunological organ. Obesity (Silver Spring) 2015; 23:512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mancuso P. The role of adipokines in chronic inflammation. Immunotargets Ther 2016; 5:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferrante AW. Jr. The immune cells in adipose tissue. Diabetes Obes Metab 2013; 15 (Suppl. 3):34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Djuric Z. Obesity‐associated cancer risk: the role of intestinal microbiota in the etiology of the host proinflammatory state. Transl Res 2017; 179:155–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boulange CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med 2016; 8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yudkin JS. Adipose tissue, insulin action and vascular disease: inflammatory signals. Int J Obes Relat Metab Disord 2003; 27 (Suppl. 3):S25–8. [DOI] [PubMed] [Google Scholar]
- 37. Ramos EJ, Xu Y, Romanova I et al. Is obesity an inflammatory disease? Surgery 2003; 134:329–35. [DOI] [PubMed] [Google Scholar]
- 38. Alberti KG, Eckel RH, Grundy SM et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120:1640–5. [DOI] [PubMed] [Google Scholar]
- 39. Exley MA, Hand L, O'Shea D, Lynch L. Interplay between the immune system and adipose tissue in obesity. J Endocrinol 2014; 223: R41–8. [DOI] [PubMed] [Google Scholar]
- 40. Donohoe CL, Lysaght J, O'Sullivan J, Reynolds JV. Emerging concepts linking obesity with the hallmarks of cancer. Trends Endocrinol Metab 2017; 28:46–62. [DOI] [PubMed] [Google Scholar]
- 41. Feuerer M, Herrero L, Cipolletta D et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009; 15:930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Milner JJ, Sheridan PA, Karlsson EA, Schultz‐Cherry S, Shi Q, Beck MA. Diet‐induced obese mice exhibit altered heterologous immunity during a secondary 2009 pandemic H1N1 infection. J Immunol 2013; 191:2474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith AG, Sheridan PA, Harp JB, Beck MA. Diet‐induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr 2007; 137:1236–43. [DOI] [PubMed] [Google Scholar]
- 44. Pepper DJ, Sun J, Welsh J, Cui X, Suffredini AF, Eichacker PQ. Increased body mass index and adjusted mortality in ICU patients with sepsis or septic shock: a systematic review and meta‐analysis. Crit Care 2016; 20:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chapel H, Lucas M, Lee M et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood 2008; 112:277–86. [DOI] [PubMed] [Google Scholar]
- 46. Maglione PJ. Autoimmune and lymphoproliferative complications of common variable immunodeficiency. Curr Allergy Asthma Rep 2016; 16:19. [DOI] [PubMed] [Google Scholar]
- 47. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Investig 2003; 112:1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wohlrab J. Pharmacokinetic characteristics of therapeutic antibodies. J Dtsch Dermatol Ges 2015; 13:530–4. [DOI] [PubMed] [Google Scholar]
- 49. Rajabally YA. Long‐term immunoglobulin therapy for chronic inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve 2015; 51:657–61. [DOI] [PubMed] [Google Scholar]
- 50. Misbah SA. Effective dosing strategies for therapeutic immunoglobulin: managing wear‐off effects in antibody replacement to immunomodulation. Clin Exp Immunol 2014; 178 (Suppl. 1):70–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Enk A, Hadaschik E, Eming R et al. European Guidelines (S1) on the use of high‐dose intravenous immunoglobulin in dermatology. J Dtsch Dermatol Ges 2016; 30:1657–69. [DOI] [PubMed] [Google Scholar]
- 52. Rajabally YA, Wong SL, Kearney DA. Immunoglobulin G level variations in treated chronic inflammatory demyelinating polyneuropathy: clues for future treatment regimens? J Neurol 2013; 260:2052–6. [DOI] [PubMed] [Google Scholar]
- 53. Bonagura VR, Marchlewski R, Cox A, Rosenthal DW. Biologic IgG level in primary immunodeficiency disease: the IgG level that protects against recurrent infection. J Allergy Clin Immunol 2008; 122:210–2. [DOI] [PubMed] [Google Scholar]
- 54. Bonilla FA. IgG replacement therapy, no size fits all. Clin Immunol 2011; 139:107–9. [DOI] [PubMed] [Google Scholar]
- 55. Verma N, Grimbacher B, Hurst JR. Lung disease in primary antibody deficiency. Lancet Respir Med 2015; 3:651–60. [DOI] [PubMed] [Google Scholar]
- 56. Vlam L, Cats EA, Willemse E et al. Pharmacokinetics of intravenous immunoglobulin in multifocal motor neuropathy. J Neurol Neurosurg Psychiatry 2014; 85:1145–8. [DOI] [PubMed] [Google Scholar]
- 57. Krivan G, Konigs C, Bernatowska E, Salama A, Wartenberg‐Demand A, Sonnenburg C, Linde R. An open, prospective trial investigating the pharmacokinetics and safety, and the tolerability of escalating infusion rates of a 10% human normal immunoglobulin for intravenous infusion (IVIg), BT090, in patients with primary immunodeficiency disease. Vox Sang 2015; 109:248–56. [DOI] [PubMed] [Google Scholar]
- 58. Ponsford M, Carne E, Kingdon C et al. Facilitated subcutaneous immunoglobulin (fSCIg) therapy – practical considerations. Clin Exp Immunol 2015; 182:302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Perraudin C, Bourdin A, Spertini F, Berger J, Bugnon O. Switching patients to home‐based subcutaneous immunoglobulin: an economic evaluation of an interprofessional drug therapy management program. J Clin Immunol 2016; 36:502–10. [DOI] [PubMed] [Google Scholar]
- 60. Shapiro R. Subcutaneous immunoglobulin (16 or 20%) therapy in obese patients with primary immunodeficiency: a retrospective analysis of administration by infusion pump or subcutaneous rapid push. Clin Exp Immunol 2013; 173:365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gill KL, Machavaram KK, Rose RH, Chetty M. Potential sources of inter‐subject variability in monoclonal antibody pharmacokinetics. Clin Pharmacokinet 2016; 55:789–805. [DOI] [PubMed] [Google Scholar]
- 62. Khan S, Grimbacher B, Boecking C et al. Serum trough IgG level and annual intravenous immunoglobulin dose are not related to body size in patients on regular replacement therapy. Drug Metab Lett 2011; 5:132–6. [DOI] [PubMed] [Google Scholar]
- 63. Hodkinson JP, Lucas M, Lee M et al. Therapeutic immunoglobulin should be dosed by clinical outcome rather than by body weight in obese patients. Clin Exp Immunol 2015; 181:179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Anderson CR, Olson JA. Correlation of weight‐based i.v. immune globulin doses with changes in serum immunoglobulin G levels. Am J Health Syst Pharm 2015; 72:285–9. [DOI] [PubMed] [Google Scholar]
- 65. Ensom MH, Stephenson MD. A two‐center study on the pharmacokinetics of intravenous immunoglobulin before and during pregnancy in healthy women with poor obstetrical histories. Hum Reprod 2011; 26:2283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Medical Data Solutions and Services (MDSAS) . Immunoglobulin database report. London: Department of Health; 2015:1–31.
- 67. Milford Ward A, Sheldon J, Rowbottom A, Wild GD. Protein reference unit handbook In: Milford Ward A, Sheldon J, Rowbottom A, Wild GD, eds. Clinical immunochemistry, 9th edn. PRU publications: Sheffield, 2007:104. [Google Scholar]
- 68. Rocchio MA, Hussey AP, Southard RA, Szumita PM.. Impact of ideal body weight dosing for all inpatient i.v. immune globulin indications. Am J Health Syst Pharm 2013; 70:751–2. [DOI] [PubMed] [Google Scholar]
- 69. Kreuz W, Erdos M, Rossi P, Bernatowska E, Espanol T, Marodi L. A multi‐centre study of efficacy and safety of Intratect(R), a novel intravenous immunoglobulin preparation. Clin Exp Immunol 2010; 161:512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. van Doorn PA, Kuitwaard K, Jacobs BC. Serum IgG levels as biomarkers for optimizing IVIg therapy in CIDP. J Peripher Nerv Syst 2011; 16 (Suppl. 1):38–40. [DOI] [PubMed] [Google Scholar]
- 71. Orange JS, Grossman WJ, Navickis RJ, Wilkes MM. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: a meta‐analysis of clinical studies. Clin Immunol 2010; 137:21–30. [DOI] [PubMed] [Google Scholar]
- 72. Ballow M. Optimizing immunoglobulin treatment for patients with primary immunodeficiency disease to prevent pneumonia and infection incidence: review of the current data. Ann Allergy Asthma Immunol 2013; 111: S2–5. [DOI] [PubMed] [Google Scholar]
- 73. Berger M. Incidence of infection is inversely related to steady‐state (trough) serum IgG level in studies of subcutaneous IgG in PIDD. J Clin Immunol 2011; 31:924–6. [DOI] [PubMed] [Google Scholar]
- 74. Brent J, Guzman D, Bangs C et al. Clinical and laboratory correlates of lung disease and cancer in adults with idiopathic hypogammaglobulinaemia. Clin Exp Immunol 2016; 184:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Haddad E, Berger M, Wang EC, Jones CA, Bexon M, Baggish JS. Higher doses of subcutaneous IgG reduce resource utilization in patients with primary immunodeficiency. J Clin Immunol 2012; 32:281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pan SD, Zhu LL, Chen M, Xia P, Zhou Q. Weight‐based dosing in medication use: what should we know? Patient Prefer Adherence 2016; 10:549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


