Abstract
Nivolumab is a recently approved medication for the treatment of unresectable malignant melanoma. Many immune-related adverse events (irAEs) associated with nivolumab have been reported, such as pneumonitis, hepatitis, dermatitis, and thyroiditis. Prednisolone can effectively treat irAEs. However, it is unclear how or if nivolumab should be administered to patients after they have experienced an irAE. Herein, we show 2 patients who underwent pneumonitis as irAE. Case 1 demonstrated a cryptogenic organizing pneumonia pattern in the CT scan and case 2 had a diffuse alveolar damage (DAD) pattern. Oral corticosteroids improved chest shadow of CT scan in both cases. However, when nivolumab was re-administrated, case 1 demonstrated no symptoms, but case 2 demonstrated pneumonia again. From our cases, it is difficult to re-administrate nivolumab for the patients with pneumonitis which shows a DAD pattern in CT, even if oral corticosteroids improve their symptoms.
Key Words: Nivolumab, Immune-related adverse event, Re-administration, Unresectable malignant melanoma, Cryptogenic organizing pneumonia, Diffuse alveolar damage, Pneumonitis
Introduction
Antibodies against programmed cell death-1 (PD-1) are newly approved medications for the treatment of unresectable malignant melanoma. Nivolumab, a PD-1 inhibitor, improves overall survival among melanoma patients compared to conventional therapy, such as dacarbazine [1]. However, treatment with nivolumab is associated with a variety of immune-related adverse events (irAEs) [2]. Anti PD-1 agents, such as nivolumab, cause fewer irAEs compared to CTLA-4 inhibitors (e.g., ipilimumab), and the clinical benefits appear to be superior to ipilimumab [2, 3]. IrAEs due to immune system inhibitors are very different from toxicities observed with conventional cytotoxic chemotherapy. The possible reactions include the development of rashes, vitiligo, colitis, interstitial pneumonia (IP), hepatitis, thyroiditis, nephritis, and hypophysitis [4]. Initial therapies for irAEs in both ipilimumab and nivolumab have been proposed in some literatures. However, it is actually difficult to decide to re-administrate nivolumab after a patient has recovered from an irAE. In our institution, 27 patients have received nivolumab treatment, and some patients experienced severe irAEs, such as hepatitis, colitis, and pneumonitis. Nivolumab was discontinued, and the patients improved following treatment with systemic corticosteroids. However, it was unclear whether to resume nivolumab therapy. Nevertheless, we decided to resume nivolumab therapy after obtaining informed consent from the patients. Today, the treatment of irAE seems to be a current topic for oncologists. Since no studies have evaluated the effects of nivolumab re-administration following an irAE episode, we present the results from 2 cases of nivolumab re-administration to facilitate the development of guidelines for managing this clinical scenario.
Patients
Patients were enrolled from 2014 to 2015 at the Tokyo Metropolitan Cancer and Infectious Diseases Center of the Komagome Hospital. The following patient data were collected: age, sex, primary lesion information (see Table 1 for a complete list of patient characteristics). A molecular investigation revealed the absence of the BRAF V600E mutation in all patients. In all cases, nivolumab was administered every 3 weeks at a dose of 2 mg/kg, which differs from the dosage approved by the FDA.
Table 1.
Details of the patients
| Case | Age, years | Sex | Primary lesion | Metastatic site | CT pattern | Treatment for irAE | Interval to re-administration | Recurrence of irAE |
|---|---|---|---|---|---|---|---|---|
| 1 | 62 | M | Finger | Bone Brain |
COP | PSL 1.0 mg/kg/day | 5 weeks | – |
| 2 | 75 | M | Rectum | Lungs Liver Bone |
DAD | PSL 1.0 mg/kg/day | 9 weeks | – |
COP, cryptogenic organizing pneumonia; DAD, diffuse alveolar damage; irAE, immune-related adverse event.
Case Presentations
Case 1
A 62-year-old male with digital melanoma was treated with nivolumab for bone metastasis. After 6 courses of nivolumab, the patient became febrile to 38°C and complained of mild respiratory discomfort. A repeat CT scan revealed bilateral lower lobe lung consolidations that were consistent with cryptogenic organizing pneumonia (COP) pattern of nivolumab-induced IP (grade 1) (Fig. 1a). Therefore, systemic corticosteroid therapy was administered (1 mg/kg/day), and the dose was gradually reduced as the lung lesions improved (Fig. 1b). The patient was then re-treated with nivolumab and oral corticosteroids (0.15 mg/kg/day) without any further evidence of IP.
Fig. 1.
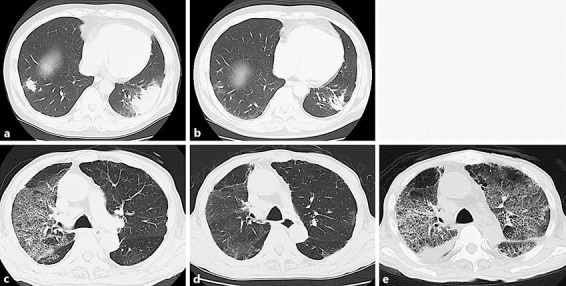
CT scans of both cases. a In case 1, a focal patch of subpleural consolidation was observed after nivolumab re-administration. b Pneumonitis gradually improved with corticosteroids. c In case 2, diffuse ground glass opacity was observed in the lungs. d Pneumonitis had clearly improved. e Diffuse bilateral pneumonitis recurred in the lungs after nivolumab re-administration.
Case 2
A 75-year-old male with rectal melanoma was referred to our hospital. The patient presented with bone, lung, and liver metastases. We began treatment with nivolumab for unresectable melanoma. Four weeks following the first administration of nivolumab, the patient suddenly developed difficulty in breathing. A CT scan revealed widespread bilateral ground-glass opacity of his lungs consistent with a diffuse alveolar damage (DAD) pattern (grade 2) (Fig. 1c). He was treated with systemic corticosteroids (1 mg/kg/day) for nivolumab-induced IP. Following improvement of his respiratory status (Fig. 1d), nivolumab therapy was resumed with oral corticosteroids (0.25 mg/kg/day). However, 3 days later, he experienced a sudden exacerbation of his respiratory status. A repeat CT scan revealed recurrent nivolumab-induced IP (Fig. 1e). Steroid pulse therapy with 1,000 mg of methylprednisolone was initiated.
Discussion
IrAEs due to PD-1 inhibitors are mainly grade 1 or 2 in severity and can be managed with algorithms developed for irAEs associated with ipilimumab [4, 5]. General principles for the optimal management of irAEs include early recognition and correct use of immunosuppressive agents based on the severity of the episode. IrAEs are reversible with prompt recognition. However, failing to manage these cases appropriately can lead to severe toxicity and death [6]. Treatment for irAEs generally consists of the administration of immunosuppressive agents, such as corticosteroids or other agents such as TNF-α inhibitors [4]. Although re-administration of nivolumab is allowed in grade1–2 irAE, it is often abandoned in grade 3–4. With the consent of the patients, we re-administered nivolumab to 2 patients after the irAEs improved because of a lack of alternative therapy such as vemurafenib at that time. In case 2, pneumonitis was severe and diffusely spread throughout both lungs. Nonetheless, corticosteroid therapy was effective, and the pneumonitis improved. Therefore, the patient was re-administered nivolumab with corticosteroids.
In our cases, IP was more severe than colitis and required more time to respond to treatment. Because it is not desirable to discontinue therapy in cases of advanced melanoma, we attempted to resume nivolumab therapy early along with corticosteroids to prevent irAE recurrence. Based on the clinical responses, it seems feasible to re-administer nivolumab without corticosteroids in some cases. However, in cases of pneumonitis, great care must be taken because of the possibility of recurrent IP even with concurrent corticosteroid use. There are several IP patterns. Based on these classifications [7], case 1 demonstrated a pattern consistent with COP. The pattern in case 2 was consistent with DAD. In general, COP is more responsive to treatment with corticosteroids than DAD. Eventually, we do not recommend re-initiating treatment with nivolumab in patients with pneumonitis that is consistent with DAD according to a CT scan. In particular, the second CT scan in case 2 revealed severe recurrent pneumonitis. Therefore, we decided to begin steroid pulse therapy.
When both the occurrence and improvement of irAEs were rapid compared to what is usually observed, it is feasible to re-administer nivolumab with a rapid dose reduction of corticosteroids. However, this is not a desirable approach in our cases of IP, even with the continued use of corticosteroids. Particularly, for cases of IP that are consistent with a DAD pattern, we suggest that the corticosteroid dose is gradually reduced over time, and nivolumab should be discontinued (replaced with another agent). We report 2 cases of nivolumab re-administration after irAEs. The occurrence of IP is associated with a slow or incomplete response and is likely to recur following nivolumab re-administration in combination with corticosteroids, particularly in patients with IP (DAD pattern). In this report, we recommend that in the event of an irAE, corticosteroids are administered first. The occurrence of IP is associated with a slow or incomplete response and is likely to recur following nivolumab re-administration in combination with corticosteroids, particularly in patients with IP (DAD pattern).
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding Sources
None.
References
- 1.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 5.Fecher LA, Agarwala SS, Hodi FS, et al. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18:733–743. doi: 10.1634/theoncologist.2012-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560–575. doi: 10.3978/j.issn.2218-6751.2015.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS) and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]


