Abstract
A 45-year-old woman was found to have a pancreatic tumor by abdominal ultrasound performed for a medical check-up. Abdominal contrast-enhanced computed tomography showed a hypovascular tumor measuring 30 mm in diameter in the pancreatic tail. Endoscopic ultrasound-guided fine needle aspiration was performed. An extragastric growing gastrointestinal stromal tumor was thereby diagnosed preoperatively, and surgical resection was planned. Laparoscopic surgery was attempted but conversion to open surgery was necessitated by extensive adhesions, and distal pancreatectomy, splenectomy, and partial gastrectomy were performed. The histological diagnosis was an intra-abdominal desmoid tumor. A desmoid tumor is a fibrous soft tissue tumor arising in the fascia and musculoaponeurotic tissues. It usually occurs in the extremities and abdominal wall, and only rarely in the abdominal cavity. We experienced a case with an intra-abdominal desmoid tumor that was histologically diagnosed after laparotomy, which had been preoperatively diagnosed as an extragastric growing gastrointestinal stromal tumor. Although rare, desmoid tumors should be considered in the differential diagnosis of intra-abdominal tumors. Herein, we report this case with a literature review.
Key Words: Intra-abdominal desmoid tumor, Pancreas, Gastrointestinal stromal tumor
Case Report
A 45-year-old woman had previously been in good health. In January 2005, she was found to have a pancreatic tumor by abdominal ultrasound performed for a routine medical check-up and was referred to our hospital. She had no chief complaint. Her past history and family history were unremarkable. Physical findings were as follows: height 161 cm; weight, 64.7 kg; blood pressure, 110/65 mm Hg; heart rate, 61 beats/min. The abdomen was soft and flat, with no tenderness and no palpable masses. The results of blood tests were unremarkable. Her pancreatic enzyme levels and tumor markers were within normal limits.
Abdominal ultrasound showed a well-defined hypoechoic mass measuring 30 mm in diameter with internal heterogeneity in the pancreatic tail (Fig. 1a). There was no obvious blood flow signal in the mass. There were no findings of main pancreatic duct dilatation. Abdominal contrast-enhanced computed tomography (CT) showed a mass lesion measuring approximately 30 mm in diameter, extending from the gastric wall to the pancreatic tail (Fig. 1b). The mass was well defined and showed delayed enhancement. The beak sign was present, suggesting the lesion to be extrapancreatic. Abdominal magnetic resonance imaging (MRI) showed a mass of hypointensity on T1-weighted imaging and slight hyperintensity on T2-weighted imaging (Fig. 1c, d). Fluorodeoxyglucose-positron emission tomography (FDG-PET) showed slight accumulation of FDG at the site of the lesion (Fig. 2a). No other significant accumulation was detected. Conventional endoscopy showed a smooth bulge in the posterior wall of the middle gastric body, suggesting an extragastric compression (Fig. 2b). Endoscopic ultrasound (EUS) showed a hypoechoic mass measuring 30 mm in diameter located between the pancreatic tail and the stomach. The internal echo was heterogeneous and suspected to be partially continuous with the fourth layer of the stomach. Therefore, the mass was suspected to be an extragastric growing submucosal tumor. EUS-guided fine needle aspiration showed a slight aggregation of spindle-shaped cells intermingled with blood components in a portion of the sample. The cells were positive for vimentin and negative for c-kit (Fig. 3). Although the biopsy did not provide a definitive diagnosis because the amount of tissue obtained was insufficient, a nontumor tissue mass, such as one comprised of myofibroblasts, or a soft tissue tumor was suspected.
Fig. 1.
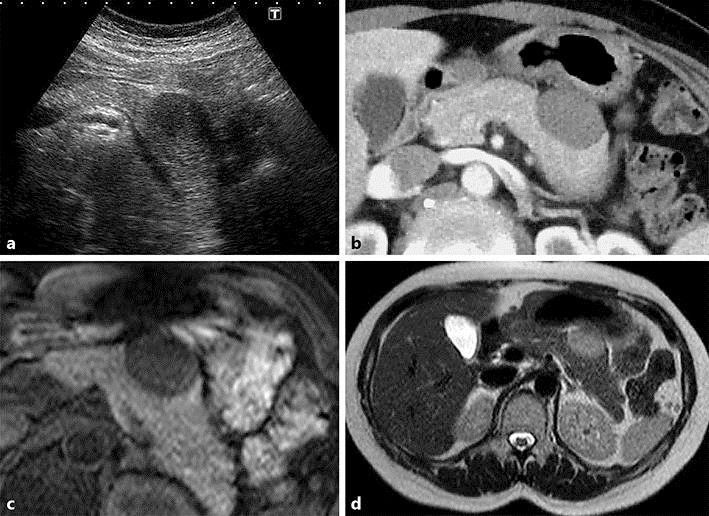
a Abdominal ultrasound shows a well-defined hypoechoic mass measuring 30 mm in diameter with internal heterogeneity in the pancreatic tail. b Abdominal contrast-enhanced CT (early phase) shows a well-defined mass lesion measuring approximately 30 mm in diameter, extending from the gastric wall to the pancreatic tail. c, d Abdominal MRI shows a mass lesion with low signal intensity on T1-weighted imaging (c) and slightly high signal intensity on T2-weighted imaging (d).
Fig. 2.
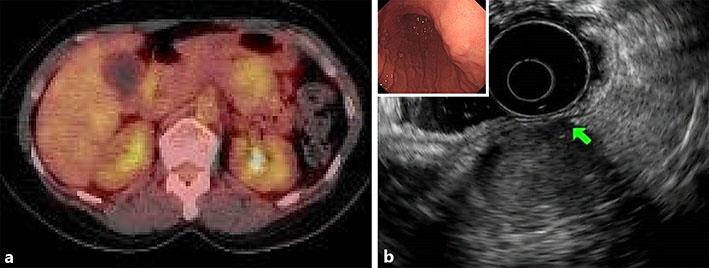
a FDG-PET shows slight accumulation of FDG at the site of the lesion. No other significant accumulation is seen. b Conventional endoscopy shows a smooth bulge in the posterior wall of the middle gastric body. Endoscopic ultrasound shows a hypoechoic mass measuring 30 mm in diameter located between the pancreatic tail and the stomach. The internal echo is heterogeneous and suspected to be partially continuous with the fourth layer of the stomach (arrow).
Fig. 3.
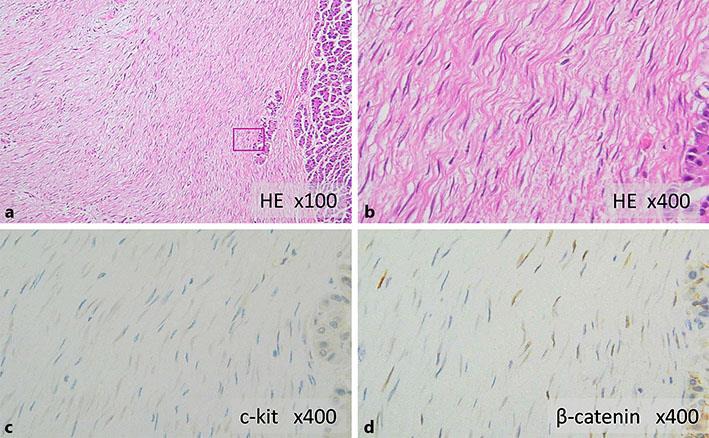
a, b Histopathological findings show cord-like proliferation of spindle-shaped cells with minimal atypia, and the intercellular spaces are filled with thick bundles of collagen fibers. a HE stain, ×100. b HE stain, ×400. Immunostaining shows that the cells are negative for c-kit (c) and positive for nuclear β-catenin (d). c c-kit, ×400. d β-catenin, ×400.
Surgery was indicated on the basis of imaging and histopathological findings. In June 2015, the patient underwent tumor resection. Laparoscopic observation revealed extensive adhesion of the tumor to the stomach and pancreas. Therefore, laparoscopic surgery was converted to open surgery because complete removal of the tumor by endoscopy was considered to be impossible. The tumor was located in the pancreatic body. The edge of the tumor was relatively well defined, but the tumor was strongly adhesive to the transverse mesocolon and posterior wall of the gastric body. Resection of the pancreatic body and tail with splenectomy, plus partial gastrectomy, was performed.
In macroscopic findings of the resected specimen, the tumor was a well-defined white solid mass measuring 45 × 40 × 55 mm. The tumor extended from the gastric wall and most of its mass was embedded in the pancreas. In histopathological findings (Fig. 3), the lesion showed cord-like proliferation of spindle-shaped cells with minimal atypia, and thick bundles of collagen fibers filled the intercellular spaces. The tumor was in contact with the proper muscle layer and the subserosal layer of the stomach and showed pancreatic interlobular invasion-like proliferation in the area adjacent to the pancreas. The following immunostaining results were obtained: c-kit negative, s-100 negative, CD34 negative, desmin negative, actin-SM positive, and β-catenin positive. Based on these findings, a desmoid tumor was diagnosed.
Discussion
A desmoid tumor is a fibrous soft tissue tumor arising in the fascia and musculoaponeurotic tissues. Stout et al. [1] proposed that a desmoid tumor is characterized by proliferation of well-differentiated fibroblasts, the presence of collagen fibers in the intercellular space, an infiltrative growth pattern, lack of malignant findings, absence of metastasis, and local recurrence. According to the new WHO classification, a desmoid tumor is histologically classified as benign but clinically classified as intermediate between a benign and a malignant tumor because it shows an infiltrative growth pattern without a capsule and local recurrence often develops, though not distant metastases [2].
The reported incidence rates for desmoid tumor range from 2.4 to 4.3 per 1,000,000 persons/year. It is rare, accounting for 0.03% of all neoplasms and less than 3% of all soft tissue tumors. Desmoid tumors are divided into 3 groups by location, i.e., abdominal wall desmoid tumors (49%), extra-abdominal wall desmoid tumors (43%), and intra-abdominal desmoid tumors (8%), the last of which is reportedly the least common [3]. Our present patient had an intra-abdominal desmoid tumor. Among patients with intra-abdominal desmoid tumors, the male to female ratio is 6 to 4 and the mean age is 44 ± 16 years. The most frequent site is reportedly the small bowel mesentery (72%); of note, 45% of patients have a history of abdominal surgery and 22% have Gardner syndrome [4].
Abnormalities affecting the genes governing tissue repair have been suggested to be the basic underlying cause of desmoid tumor development. It is assumed that desmoid tumors develop when stimulating factors are added to these genetic abnormalities. These stimulating factors include (1) adenomatous polyposis coli gene abnormalities such as familial adenomatous polyposis (FAP) and Gardner syndrome, (2) mechanical stimulation such as laparotomy and abdominal injury, and (3) changes in estrogen receptors during pregnancy and after delivery [5]. The present patient had no history of laparotomy, abdominal injury, underlying diseases, or pregnancy, and the cause was thus unknown.
In general, preoperative diagnosis of desmoid tumor is difficult. Desmoid tumors are often asymptomatic until they have grown quite large. Therefore, the most frequent chief complaint is an abdominal mass, followed by abdominal distension, abdominal pain, and vomiting that are caused by mechanical compression due to the tumor [6]. There are no characteristic imaging findings for desmoid tumors. The ultrasound appearance of desmoid tumor is often a hypoechoic mass with internal homogeneity. On CT, a desmid tumor usually appears as a solid mass with an essentially homogeneous density, although the degree of enhancement varies among tumors. In the present case, CT scan showed delayed enhancement. On MRI, a desmoid tumor typically shows high signal intensity or heterogeneous signal intensity on T2-weighted images. High signal intensity reportedly indicates a high density of cellular components, while low signal intensity indicates a low density of cellular components and corresponds to the area with abundant collagen [6]. In the present case, the tumor was visualized as a slightly high intensity area on T2-weighted images. Localization of a desmoid tumor is often difficult because most are detected after the tumor has become large. In the present case, diagnosis of the extrapancreatic lesion was facilitated by the presence of the beak sign in the pancreas. However, extragastric growing gastrointestinal stromal tumor (GIST) was suspected because EUS showed the lesion to be continuous with the fourth layer of the gastric wall. In addition, there is a reported case in which FDG-PET showed marked accumulation of FDG and a malignant tumor was suspected, but it was histologically diagnosed as a desmoid tumor [7]. The present patient was suspected to have a malignant tumor because FDG-PET showed slight accumulation, and surgery was thus performed. In addition, EUS-FNA was performed in an attempt to make a definitive diagnosis because GIST had been suspected preoperatively. However, the diagnosis of GIST could not be confirmed. Thus, the preoperative diagnosis is difficult because there are no characteristic imaging findings for diagnosing desmoid tumors. We advocate including this disease in the differential diagnosis of intra-abdominal tumors.
Surgical resection is the first-line treatment. Desmoid tumor is often discovered after it has already become quite large, and resection of adjacent organs due to tumor invasion is often required. In the present case, as the tumor was embedded in the pancreas and showed extensive adhesions, resection of the pancreatic body and tail with splenectomy was performed. Given that the desmoid tumor recurrence rate is high even after complete resection, careful postoperative follow-up is essential. While there are treatment options other than resection, such as radiation and pharmacological therapies (anticancer drugs, anti-estrogen drugs, molecularly targeted agents, and nonsteroidal anti-inflammatories) [8, 9], there is currently no established treatment.
Though patients with desmoid tumors not associated with FAP reportedly have a relatively good prognosis [10], the mortality rate for all cases with intra-abdominal desmoid tumors is 30%, and the recurrence and mortality rates of desmoid tumor patients with FAP are 22–85% and 10–60%, respectively [11]. In addition, desmoid tumor is the second most frequent cause of death following malignancy development in patients with FAP. Thus, an elevated risk of recurrence should be considered in patients with desmoid tumors even after surgery, and regular follow-up is required for early detection of recurrence. The present patient did not have FAP and remains recurrence free to date. However, given the high recurrence rate of desmoid tumors, we are continuing meticulous follow-up of this patient.
We experienced a case with an intra-abdominal desmoid tumor embedded in the pancreas, which was preoperatively diagnosed as an extragastric growing GIST. Although a detailed examination was performed, the preoperative diagnosis was difficult, suggesting that making the clinical diagnosis of this tumor would be challenging. Herein, we have presented this case with a review of the relevant literature.
Statement of Ethics
All experiments involving animals and human subjects were designed and performed in compliance with the relevant laws regarding the humane care and use of subjects.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Stout AP, Lattes R. Tumors of the Soft Tissues, Atlas of Tumor Pathology, Second Series Fascicle. Washington: Armed forces Institute of Pathology; 1967. [Google Scholar]
- 2.Hayry P, Reitamo JJ, Totterman S, et al. The desmoids tumor. II. Analysis of factors possibly contributing to the etiology and growth behavior. Am J Clin Pathol. 1982;77:674–680. doi: 10.1093/ajcp/77.6.674. [DOI] [PubMed] [Google Scholar]
- 3.Reitamo JJ, Häyry P, Nykyri E, et al. The desmoid tumor. I. Incidence, sex-, age- and anatomical distribution in the Finnish population. Am J Clin Pathol. 1982;77:665–673. doi: 10.1093/ajcp/77.6.665. [DOI] [PubMed] [Google Scholar]
- 4.Belliveau P, Graham AM. Mesenteric desmoid tumor in Gardner's syndrome treated by sulindac. Dis Colon Rectum. 1984;27:53–54. doi: 10.1007/BF02554079. [DOI] [PubMed] [Google Scholar]
- 5.Reitamo JJ, Scheinin TM, Hayry P. The desmoid syndrome. New aspects in the cause, pathogenesis and treatment of the desmoid tumor. Am J Surg. 1986;151:230–237. doi: 10.1016/0002-9610(86)90076-0. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi K, Hirakata R, Maeda S, et al. Spontaneous isolated intra-abdominal mesenteric fibromatosis. Case report. Eur J Surg. 1991;157:293–296. [PubMed] [Google Scholar]
- 7.Basu S, Nair N, Banavali S. Uptake characteristics of fluorodeoxyglucose (FDG) in deep fibromatosis and abdominal desmoids: potential clinical role of FDG-PET in the management. Br J Radiol. 2007;80:750–756. doi: 10.1259/bjr/53719785. [DOI] [PubMed] [Google Scholar]
- 8.Lim CL, Walker MJ, Mehta RR, et al. Estrogen and antiestrogen binding sites in desmoids tumors. Eur J Cancer Clin Oncol. 1986;22:583–587. doi: 10.1016/0277-5379(86)90047-7. [DOI] [PubMed] [Google Scholar]
- 9.Hansmann A, Adolph C, Vogel T, et al. High dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer. 2004;100:612–620. doi: 10.1002/cncr.11937. [DOI] [PubMed] [Google Scholar]
- 10.Clark SK, Phillips RK. Desmoids in familial adenomatous polyposis. Br J Surg. 1996;83:1494–1504. doi: 10.1002/bjs.1800831105. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Bigas MA, Mahoney MC, Karakousis CP, et al. Desmoid tumors in patients with familial adenomatous polyposis. Cancer. 1994;74:1270–1274. doi: 10.1002/1097-0142(19940815)74:4<1270::aid-cncr2820740415>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]


