Abstract
Background
Despite biochemical euthyroidism, some levothyroxine (L-T4)-treated hypothyroid patients report persisting symptoms and some of these patients are tentatively treated with a combination of L-T4 and liothyronine (L-T3). Combination therapy and the appropriate choice of blood tests to monitor treatment are highly debated among specialists and patients.
Aim
To evaluate whether measuring serum triiodothyronine (S-T3) at baseline or during combination therapy can be used as an indicator of a positive effect from L-T4/L-T3 combination therapy.
Materials and Methods
Observational retrospective study of patients (n = 42) with persisting symptoms of hypothyroidism despite L-T4 therapy who had normal TSH levels and did not have any comorbidities that could explain their symptoms. All were then treated with L-T4/L-T3 combination therapy at a dose ratio of 17/1 according to European Thyroid Association guidelines. Based on patient-reported outcome, they were divided into responders and nonresponders.
Results
Five patients were lost to follow-up and thus excluded. At the 3-month follow-up, 11 were classified as nonresponders and 26 as responders. At 12 months these figures had changed to 13 (35%) and 24 (65%), respectively. When comparing responders versus nonresponders, no differences were seen at baseline or during follow-up in S-T3 and in free T3 estimates. Further, logistic regression showed no correlation between S-T3 and free T3 estimates and responder/nonresponder status.
Conclusion
Our data indicate that serum T3 measurements are not suitable to predict which patient will benefit from L-T4/L-T3 combination therapy, and treatment response cannot be followed by repeated T3 measurements either.
Key Words: Liothyronine, Levothyroxine, Serum triiodothyronine
Background
Hypothyroidism is a common disease that affects primarily women with a range of symptoms. Levothyroxine (L-T4) is the mainstay replacement therapy of choice according to guidelines, but some patients report persistent hypothyroid symptoms despite being biochemically euthyroid [1]. European Thyroid Association (ETA) guidelines [2] from 2012 suggest that L-T4/liothyronine (L-T3) combination therapy could be considered as an experimental approach for compliant patients with persisting complaints despite normalized TSH values for 6 months, no competing conditions explaining the symptoms, and who have received counseling on living with a chronical disease. However, L-T4/L-T3 combination therapy is still regarded as controversial among specialists [3].
A meta-analysis has found no difference in effectiveness between L-T4 mono- and L-T4/L-T3 combination therapy evaluated on quality of life (QOL) and neurocognitive function tests in unselected hypothyroid subjects [3]. Further, skeptics towards combination therapy argue that normal serum T3 (S-T3) levels can be achieved through L-T4 monotherapy even in athyreotic individuals, and therefore consider L-T4/L-T3 combination therapy as unnecessary [4].
However, we have reported a randomized controlled trial (RCT) with a cross-over design in which TSH levels in contrast to several other similar studies were kept constant in the groups receiving combination and monotherapy, respectively. In this study QOL was superior during combination therapy compared to monotherapy, and patient preference was in favor of combination therapy. This trial also showed changes in peripheral markers of thyroid function (sex hormone binding protein and pro-collagen-1-N-terminal peptide), suggesting that combination therapy is more metabolically active compared to monotherapy [5].
This divergent effect has been explained by reduced thyroidal T3 secretion due to the underlying hypothyroid state, as well by a reduced peripheral 5′-deiodination of T4 into T3. The latter might be due to polymorphisms in thyroid hormone transporters or deiodinase-2 genes in a subgroup of patients [6,7,8], or a as a result of a downregulation of deiodinase activity resulting in an increase in the S-T4/S-T3 ratio which is often seen in T4-treated hypothyroid patients [9,10].
Consequently, it has been suggested to measure S-T3, either the total concentration or the free concentration, to monitor patients treated for hypothyroidism [9]. This debate has been extended to social media, where patients share thoughts about both preferences in monitoring of thyroid function as well as choice of treatment [11], and unfortunately has led to a high degree of autonomous self-medication among patients, with an increasing pressure on general practitioners and endocrinologists to increase prescription rates of combination therapy and S-T3 measuring [12].
There is a need for better ways to predict which patients will benefit from combination therapy. Therefore we aimed to evaluate whether the use of T3 measurements predicts the effect from L-T4/L-T3 combination therapy in a group of hypothyroid patients with persisting hypothyroid symptoms on L-T4 monotherapy.
Materials and Methods
This is an observational, retrospective study of patients referred to a specialized outpatient thyroid clinic due to a dissatisfactory effect of L-T4 monotherapy. Medical charts from all patients referred with the diagnosis hypothyroidism from September 2012 to December 2013 were screened. Only patients referred from general practitioners due to persistent hypothyroid symptoms and thus a potentially unsatisfactory effect of L-T4 therapy, were included. All patients had a routine physical examination (including BMI) and a blood sample drawn.
TSH (normal range: 0.35–4.00 mU/L, interassay coefficient of variation [CV] = 19.6 mU/L and intra-assay CV = 8.17%), serum total T4 (normal range: 70–140 nmol/L, intra-assay CV = 1.19% and interassay CV = 1.47%), and S-T3 (normal range: 1.0–2.6 nmol/L, intra-assay CV = 11.1% and interassay CV = 29.6%) were all measured by competitive immune analysis through chemiluminescence technology (ADVIA Centaur, USA). A T3 uptake test (normal range: 0.85–1.15, intra-assay CV = 2.58% and interassay CV = 1.42%) was measured by enzyme-enhanced chemiluminescence immunoassay (IMMULITE Automated Immunoassay System, Unilabs, Denmark) and serum free T3 estimate by multiplying S-T3 with the T3 uptake test.
Medical history was recorded and a routine extended battery of testing for further diagnostics was initiated in order to rule out a competing disease or depression, including celiac disease, adrenal insufficiency, or other autoimmune conditions.
The local treatment guidelines corresponds to the ETA guidelines [2], i.e., combination treatment of L-T4 and L-T3 is given corresponding to a 17/1 ratio (weight/weight), and L-T3 is given twice daily, in the morning and at bedtime, using 5-µg L-T3 tablets.
A responder was defined semiquantitatively as a patient experiencing a clinically relevant reduction in hypothyroid symptoms and improvement of QOL, which persist for 12 months of therapy, resulting in a wish of continued combination therapy. A nonresponder was a patient who did not achieve improvement of symptoms and therefore chose to revert to L-T4 monotherapy. No structured questionnaire was used for this particular evaluation, which was thus based entirely on the subjective feelings of the patient.
An initial evaluation of the effect was made after 3 months of therapy, and if the patient was a responder, treatment was re-evaluated at 6 and 12 months. If the effect of the treatment diminished, the patient was reclassified as a nonresponder. Nonresponders reverted to their initial L-T4 regime without further follow-up.
This study was approved by the Danish Data Protection Agency.
Statistics
Data were analyzed with parametric testing if data were Gaussian distributed, otherwise nonparametric testing was used. Results were expressed as means or medians and range, accordingly. Groups were compared using a t test for parametric and Mann-Whitney test for nonparametric data. A two-sided p value <0.05 was considered significant. IBM SPSS 22 software was used for data analysis.
Results
One hundred and sixteen patients (110 females) were referred due to persistent hypothyroid symptoms and a potentially unsatisfactory effect of L-T4 monotherapy. Baseline data are shown in Table 1.
Table 1.
Baseline characteristics of all referred patients
| Patients, n | Mean/median | Range | |
|---|---|---|---|
| Median age, years | 116 | 51 | 22 – 86 |
| Mean BMI | 71 | 28.4 | 18.25 – 44.6 |
| Median TSH at referral, mU/L | 116 | 1.1 | 0.01 – 6.10 |
| Mean total T4, nmol/L | 116 | 110 | 54 – 178 |
| Median total T3, nmol/L | 116 | 1.2 | 0.6 – 2.30 |
| Mean free T3 estimate | 116 | 1.1 | 0.55 – 2.10 |
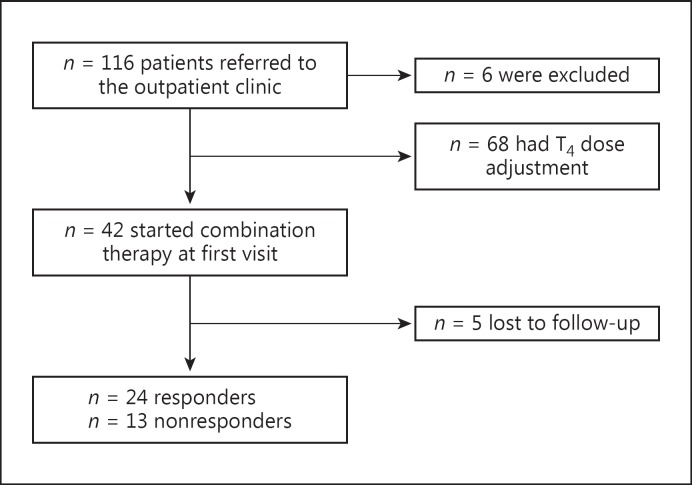
Six patients were excluded due to stress (n = 1), depression (n = 2), iron deficiency anemia (n = 1), and vitamin D deficiency (n = 1). One woman planned pregnancy and was also excluded (Fig. 1). Thirty-two patients (28%) of the 116 included patients were overtreated at the time of referral, defined as having serum TSH (S-TSH) below 0.35 mU/L, and 17 (15%) were classified as undertreated with a S-TSH above 4.00 mU/L (range: 4–56 mU/L).
Fig. 1.
Patient flow.
The individual relationship between patients and independent doctors did lead to situations where combination therapy was initiated although the patients were not completely euthyroid (2 with TSH ≤0.1 mU/L, 7 with TSH 0.1–0.34 mU/L, and 1 with TSH 4.2 mU/L).
Forty-two patients started L-T4/L-T3 therapy. Of these, 5 patients were lost to follow-up and were excluded from the final calculation. This left 37 patients starting on combination therapy with 12 months of follow-up.
After 3 months of therapy 26 patients (70%) were classified as responders and 11 patients (30%) were classified as nonresponders.
After 12 months, 24 patients (65%) were classified as responders, while 13 patients (35%) experienced no beneficial effect from combination therapy, 2 of whom ended their contact with the outpatient clinic before evaluation of thyroid status.
During combination therapy, when comparing baseline to evaluation, T4 levels decreased (p = 0.046), and S-T3 and free T3 estimate levels increased (p = 0.003 and p = 0.002, respectively), which was expected (Table 2).
Table 2.
Patients eligible for L-T4/L-T3 combination therapy at referral (mean/median and range)
| Total | Responders | Nonresponders | p value | |
|---|---|---|---|---|
| At baseline | (n = 37) | (n = 24) | (n = 13) | |
| Age, years | 49 (22 – 72) | 57 (36 – 79) | 0.06 | |
| BMIa | 28.3 (18.3 – 44.6) | 28.4 (21.8 – 44.6) | 28.1 (18.3 – 35.5) | 0.91 |
| TSHb, mIU/L | 0.85 (0.08 – 4.2) | 0.78 (0.08 – 4.20) | 0.92 (0.1 – 3.06) | 0.10 |
| T4, nmol/L | 109 (69 – 147) | 108.1 (69 – 143) | 109.8 (85 – 147) | 0.78 |
| T3b, nmol/L | 1.20 (0.7 – 2.00) | 1.2 (0.7 – 1.9) | 1.2 (0.8 – 2.00) | 0.94 |
| Free T3 estimateb | 1.12 (0.55 – 2.1) | 1.16 (0.55 – 1.82) | 1.08 (0.76 – 2.10) | 0.63 |
| After L-T4/L-T3 combination therapy | (n = 35) | (n = 24) | (n = 11) | |
| TSHb, mIU/L | 0.43 (0.01 – 4.02) | 0.57 (0.01 – 4.02) | 0.33 (0.02 – 2.42) | 0.37 |
| T4, nmol/L | 95 (43 – 155) | 90.0 (43 – 155) | 106 (81 – 130) | 0.04c |
| T3b, nmol/L | 1.50 (0.8 – 2.4) | 1.5 (0.8 – 2.4 | 1.3 (0.9 – 2.30) | 0.50 |
| Free T3 estimateb | 1.38 (0.73 – 2.54) | 1.41 (0.73 – 2.54) | 1.33 (0.86 – 2.02) | 0.56 |
| Delta | (n = 35) | (n = 24) | (n = 11) | |
| TSHb, mIU/L | 0.30 (–3.94 to 3.79) | 0.23 (–3.91 to 3.79) | 0.47 (–1.07 to 2.18) | 0.48 |
| T4, nmol/L | –13.8 (–76 to 36) | –18 (–76 to 24) | –4.5 (–36 to 36) | 0.54 |
| T3, nmol/L | 0.27 (–0.5 to 1.70) | 0.28 (–0.5 to 1.70) | 0.25 (–0.30 to 0.9) | 0.85 |
| Free T3 estimate | 0.24 (–0.7 to 1.62) | 0.24 (–0.77 to 1.62) | 0.22 (–0.23 to 0.68) | 0.89 |
n = 16 responders, 7 nonresponders.
Median when data is not Gaussian distributed.
Significant result.
Comparing responders to nonresponders, we did not find any significant difference in S-T3, free T3 estimate at baseline, or changes during follow-up, nor did we find any differences in S-TSH or BMI. Further, linear logistic regression showed no correlation with responder/nonresponder status and the variables S-T3 and free T3 estimate measured at admission, at the 3-month evaluation, and as a change over time.
Discussion
In this retrospective study, we did not find that measurement of S-T3 and free T3 estimate, neither at baseline nor during follow-up, predicted a positive effect of combination therapy.
The background for a possible effect of combination therapy is not fully understood [1] and is continuously debated with opponents to combination therapy arguing that euthyroidism may be achieved through L-T4 monotherapy [4,13]. On the other hand, a theoretical argument for an effect is a possible reduced peripheral 5′-deiodination of T4 into T3, due to polymorphisms in thyroid hormone transporters or deiodinase-2 genes in a subgroup of patients [6,7,8], or a as a result of a downregulation of deiodinase activity in L-T4-treated patients and thus an increase in the S-T4/S-T3 ratio [9,10], This might explain why only some patients have an unsatisfying effect from L-T4 monotherapy and benefit from combination therapy on QOL factors [14]. We did not analyze thyroid hormone transporters or deiodinase genes, and therefore we have no data on a possible effect from these on response to combination therapy.
A double-blinded RCT study on 697 L-T4-treated hypothyroid patients randomized for usual L-T4 or a combination of L-T4/L-T3 confirmed the missing correlation between free T3 estimates and QOL, but did find a correlation between QOL and TSH as well as QOL and free T4 estimate [15]. In accordance, in a similar study of 59 patients, we found no correlation between QOL and T3 levels, but could not confirm the described correlation between QOL and TSH or T4 levels [14].
The present study has several limitations. A significant placebo/credo effect on QOL and psychological well-being from combination therapy is known [14], and this study is not an RCT but instead only observational. However, the patients were followed for at least 12 months, thus avoiding a short-lasting placebo effect. We did not have an exact measurement of QOL, only a semiquantitative subjective evaluation from patients. However, our data (65% responders in this selected group) are similar to results in an RCT on a similar patient group in which 49% preferred the L-T4/L-T3 combination therapy versus 15% L-T4 therapy, and 36% were indifferent [14]. Further, tissue T3 concentrations were not measured, which could be a way forward for the understanding of the clinical phenomenon dealt with in this study. This, however, would demand invasive techniques.
Finally, we did not measure serum-free T3 directly, but measured free T3 estimates based on total T3 and a T3 uptake. Neither of these 2 principles of measuring free T3 estimates are perfect, a problem that has been discussed since the 1980s. Most laboratory tests measuring free T3 do not make an actual measurement of free T3, which is rather difficult to measure and vitiated by errors [16,17], but instead use a laboratory kit which includes measurements of protein binding and a computerized calculation of free T3.
The study only included 37 patients and a possible type 2 error could be present, but the difference in delta total T3 of 0.03 nmol/L and delta in the free T3 estimate of 0.02 when comparing responders and nonresponders indicate that no clinical significant difference is present.
Having mentioned these limitations, we feel that our data can be generalized to other outpatient clinics treating hypothyroid patients since our study population was in essence regular hypothyroid patients, representing a well-known and rather prevalent unsatisfied subgroup.
Among the patients (n = 116) submitted to the outpatient clinic due to persistent symptoms, 43% did not start combination therapy due to being overtreated with a S-TSH less than 0.1 mU/L or undertreated with a S-TSH of 4–56 mU/L – this is in accordance with Eligar et al. [18], underlining the importance of careful follow-up and adjustment of medication in this patient group.
Conclusion
Our data indicate that S-T3 measurements are not suitable to predict which patient will benefit from L-T4/L-T3 combination therapy, and that treatment response cannot be followed by repeated measurements of T3 either.
Ethics Statement
This study has no ethical implications. The study has been registered at the Danish Data Protection Agency.
Disclosure Statement
The authors declare no conflicts of interests.
Acknowledgement
This paper was economically supported by the Mørck foundation (Denmark) and Department of Internal Medicine, Herlev Hospital, Denmark.
References
- 1.Biondi B, Wartofsky L. Combination treatment with T4 and T3: toward personalized replacement therapy in hypothyroidism? J Clin Endocrinol Metab. 2012;97:2256–2271. doi: 10.1210/jc.2011-3399. [DOI] [PubMed] [Google Scholar]
- 2.Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012 ETA Guidelines: the Use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur Thyroid J. 2012;1:55–71. doi: 10.1159/000339444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grozinsky-Glasberg S, Fraser A, Nahshoni E, Weizman A, Leibovici L. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2006;91:2592–2599. doi: 10.1210/jc.2006-0448. [DOI] [PubMed] [Google Scholar]
- 4.Jonklaas J, Davidson B, Bhagat S, Soldin SJ. Triiodothyronine levels in athyreotic individuals during levothyroxine therapy. JAMA. 2008;299 doi: 10.1001/jama.299.7.769. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt U, Nygaard B, Jensen EW, Kvetny J, Jarlov A, Faber J. Peripheral markers of thyroid function: the effect of T4 monotherapy vs T4/T3 combination therapy in hypothyroid subjects in a randomized crossover study. Endocr Connect. 2013;2:55–60. doi: 10.1530/EC-12-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, Dayan CM. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab. 2009;94:1623–1629. doi: 10.1210/jc.2008-1301. [DOI] [PubMed] [Google Scholar]
- 7.Peeters RP, van Toor H, Klootwijk W, de Rijke YB, Kuiper GG, Uitterlinden AG, Visser TJ. Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects. J Clin Endocrinol Metab. 2003;88:2880–2888. doi: 10.1210/jc.2002-021592. [DOI] [PubMed] [Google Scholar]
- 8.van der Deure WM, Appelhof BC, Peeters RP, Wiersinga WM, Wekking EM, Huyser J, Schene AH, Tijssen JG, Hoogendijk WJ, Visser TJ, Fliers E. Polymorphisms in the brain-specific thyroid hormone transporter OATP1C1 are associated with fatigue and depression in hypothyroid patients. Clin Endocrinol (Oxf) 2008;69:804–811. doi: 10.1111/j.1365-2265.2008.03267.x. [DOI] [PubMed] [Google Scholar]
- 9.Hoermann R, Midgley JE, Giacobino A, Eckl WA, Wahl HG, Dietrich JW, Larisch R. Homeostatic equilibria between free thyroid hormones and pituitary thyrotropin are modulated by various influences including age, body mass index and treatment. Clin Endocrinol (Oxf) 2014;81:907–915. doi: 10.1111/cen.12527. [DOI] [PubMed] [Google Scholar]
- 10.McAninch EA, Bianco AC. New insights into the variable effectiveness of levothyroxine monotherapy for hypothyroidism. Lancet Diabetes Endocrinol. 2015;3:756–758. doi: 10.1016/S2213-8587(15)00325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stop the Thyroid Madness. 2016. www.stopthethyroidmadness.com
- 12.Michaelsson LF, Medici BB, la Cour JL, Selmer C, Roder M, Perrild H, Knudsen N, Faber J, Nygaard B. Treating hypothyroidism with thyroxine/triiodothyronine combination therapy in Denmark: following guidelines or following trends? Eur Thyroid J. 2015;4:174–180. doi: 10.1159/000437262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts CG, Ladenson PW. Hypothyroidism. Lancet. 2004;363:793–803. doi: 10.1016/S0140-6736(04)15696-1. [DOI] [PubMed] [Google Scholar]
- 14.Nygaard B, Jensen EW, Kvetny J, Jarlov A, Faber J. Effect of combination therapy with thyroxine (T4) and 3,5,3′-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. Eur J Endocrinol. 2009;161:895–902. doi: 10.1530/EJE-09-0542. [DOI] [PubMed] [Google Scholar]
- 15.Saravanan P, Visser TJ, Dayan CM. Psychological well-being correlates with free thyroxine but not free 3,5,3′-triiodothyronine levels in patients on thyroid hormone replacement. J Clin Endocrinol Metab. 2006;91:3389–3393. doi: 10.1210/jc.2006-0414. [DOI] [PubMed] [Google Scholar]
- 16.van Doorn J, Roelfsema F, van der Heide D. Concentrations of thyroxine and 3,5,3′-triiodothyronine at 34 different sites in euthyroid rats. Endocrinology. 1985;117:1201–1208. doi: 10.1210/endo-117-3-1201. [DOI] [PubMed] [Google Scholar]
- 17.Soukhova N, Soldin OP, Soldin SJ. Isotope dilution tandem mass spectrometric method for T4/T3. Clin Chim Acta. 2004;343:185–190. doi: 10.1016/j.cccn.2004.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eligar V, Taylor PN, Okosieme OE, Leese GP, Dayan CM. Thyroxine replacement: a clinical endocrinologist's viewpoint. Ann Clin Biochem. 2016;53:421–433. doi: 10.1177/0004563216642255. [DOI] [PubMed] [Google Scholar]