Significance
To what extent is brain organization driven by innate genetic constraints, and how dependent is it on individual experience during early development? We show that an area of the visual system that processes both hands and tools can develop without sensorimotor experience in manipulating tools with one’s hands. People born without hands show typical hand–tool conjoined activity, in a region connected to the action network. Taken with findings from studies with people born blind, who also show intact hand and tool specialization in the visual system, these findings suggest that no specific sensory or motor experience is crucial for domain-specific organization of visual cortex. Instead, the results suggest that functional brain organization is largely innately determined.
Keywords: body image, brain development, motor deprivation, tool use, visual cortex
Abstract
The visual occipito-temporal cortex is composed of several distinct regions specialized in the identification of different object kinds such as tools and bodies. Its organization appears to reflect not only the visual characteristics of the inputs but also the behavior that can be achieved with them. For example, there are spatially overlapping responses for viewing hands and tools, which is likely due to their common role in object-directed actions. How dependent is occipito-temporal cortex organization on object manipulation and motor experience? To investigate this question, we studied five individuals born without hands (individuals with upper limb dysplasia), who use tools with their feet. Using fMRI, we found the typical selective hand–tool overlap (HTO) not only in typically developed control participants but also in four of the five dysplasics. Functional connectivity of the HTO in the dysplasics also showed a largely similar pattern as in the controls. The preservation of functional organization in the dysplasics suggests that occipito-temporal cortex specialization is driven largely by inherited connectivity constraints that do not require sensorimotor experience. These findings complement discoveries of intact functional organization of the occipito-temporal cortex in people born blind, supporting an organization largely independent of any one specific sensory or motor experience.
The visual occipito-temporal cortex contains multiple domain-sensitive regions (1) that are highly reproducible across individuals. Much is known about these regions’ large-scale organization. Consistent with the patterns of neuropsychological dissociations showing a fundamental distinction between animate and inanimate objects (2, 3), neuroimaging results have shown that the animate/inanimate distinction is the primary organizational dimension in the occipito-temporal cortex (4–8), with a secondary distinction within the inanimate domain between navigation-relevant (e.g., large nonmanipulable objects and scenes) and small, manipulable inanimate objects (9–12). What are the principles that guide this organization? One possibility is that it is the direct result of experience, critically dependent on the individual’s life experiences and expertise (13–17). An alternative is that experience merely modulates an already existing innately determined structure, driven by connectivity constraints (4, 18–21) between regions within the occipito-temporal cortex and downstream areas specialized in processing specific object types.
A notable example supporting the close link between visual form processing and its downstream use is a specific region in the occipito-temporal cortex that shows spatially overlapping preferences for hands and tools (22, 23). This overlapping specialization is obviously not based on visual similarity alone because hands and tools are visually quite distinct. Nor is it due to general domain specialization because animate and inanimate objects are otherwise distinct (24–26). Instead, the overlap may reflect the importance of processing their shapes concomitantly during tool manipulation, and the extent to which the objects extend the body effectors (23). Furthermore, this region of the occipito-temporal cortex belongs to a functional network encompassing sensorimotor and fronto-parietal regions implicated in tool manipulation and action (27, 28), again stressing the link between the occipito-temporal cortex and object manipulation.
What drives this specialization? And what is the role of experience in shaping visual cortex organization? There is evidence that visual experience is not necessary for developing object preferences in the occipito-temporal cortex. The visual cortex of congenitally blind individuals shows the typical organization of object domain selectivity (18, 20, 29–31), including for body shapes and tools (32, 33). Although the preserved specialization for body shapes and tools does not depend on visual experience this does not mean that it does not require other sensorimotor experience for its emergence. Thus, for example, it is not unreasonable to assume that specialization for body shapes and tools in visual cortex may depend on such sensorimotor experience as palpating and using tools with the hands. The alternative hypothesis is that domain-specific specialization is innately determined and does not depend on any specific (ontogenetic) sensorimotor experience for its emergence.
Here, we ask whether this organization is critically dependent on sensorimotor experience. We do this by studying whether a similar domain overlap, between hands and tools, is found in individuals born without hands (individuals with upper limb dysplasia), who have no hand motor experience, and instead use tools with their feet.
Results
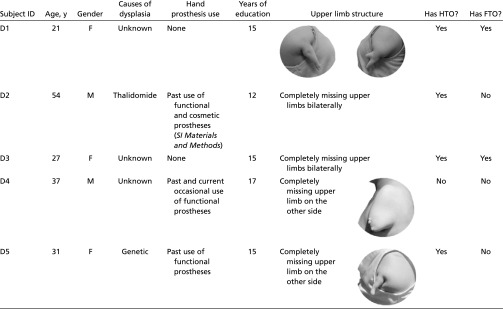
We studied a group of five people born with severely shortened or completely absent arms and no functional hands (dysplasics) (Table S1) and a group of typically developed controls to assess the role of sensorimotor experience in driving the visual cortex overlap between the hand and tool regions.
Table S1.
Characteristics of the dysplasic subjects
 |
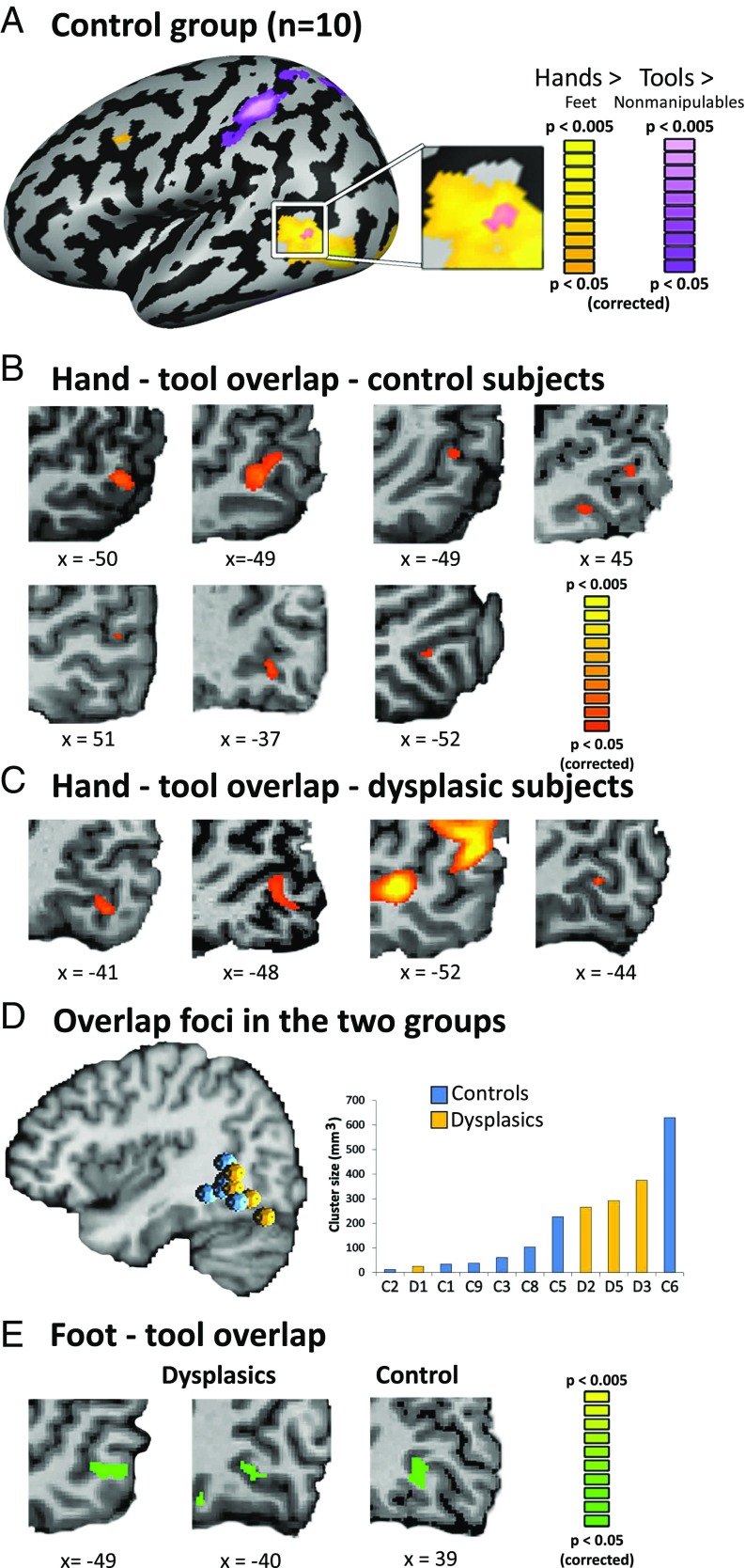
We first applied a strict contrast of hand selectivity (vs. viewing images of feet) and tool selectivity (vs. nonmanipulable artifacts), and replicated, in the control group, the established overlap between the regions in the occipito-temporal cortex (Fig. 1A). In individual participant analyses, 7 out of the 10 control participants showed this overlap (Fig. 1B), in agreement with previous findings (22), although two of them showed this overlap in the right rather than the left hemisphere (Table S2 for Talairach coordinates of the individual peaks). Importantly, among the dysplasics, four of the five participants also showed this overlap (Fig. 1C; dysplasic subjects 1,2,3, and 5). The hand–tool overlap (HTO) area shows a clear preference for viewing hands—it is spatially distinct from an area showing preference for viewing feet, and it is anterior to motion-selective regions (Figs. S1 and S2). Furthermore, its activity patterns for hands and feet are reliably distinguishable, for both dysplasic and control subjects: the activity patterns were statistically different in all but one control subject when classifying hand and foot responses using a multivariate pattern analysis (MVPA) approach within each subject’s HTO (average classification accuracy of 72.5% in the dysplasic subjects and 70.7% in the control subjects; P < 0.05).
Fig. 1.
Hand–tool visual cortex overlap does not require hands. (A) The hand and tool selectivity overlap (HTO) in the lateral occipito-temporal cortex is replicated in typically developed control subjects (n = 10, RFX GLM, P < 0.05, corrected for multiple comparisons). Yellow marks hand selectivity over feet, and purple marks tool selectivity over nonmanipulable artifacts. (B) The HTO, in the occipito-temporal cortex, can be found at the individual level in the majority (7 of 10) of the controls (for cortical surface views, see Fig. S2). (C) A majority of the dysplasics (4 of 5) born without hands show an overlap between hand and tool selectivity in the visual cortex. This suggests that motor experience is not critical for the formation and specialization of the visual-cortex hand action-related representations. (D) The location (Left; spheres denote the location of individual subjects HTO peaks) and size (Right) of the HTO in the dysplasics (marked yellow) did not differ from that of the control subjects (marked blue). (E) In addition to the overlap between hand and tool selectivity, 2 of the dysplasics (2 of 5) and 1 of the controls (1 of 10) show an overlap of feet and tools selectivity [a foot–tool overlap (FTO)]. This potentially suggests visual cortex organization is also somewhat plastic to changes due to different individual sensorimotor experience.
Table S2.
Hand–tool selectivity overlap (HTO) peaks
| Subject no. | Tal x | Tal y | Tal z | Overlap volume, mm3 |
| C1 | −52 | −52 | −4 | 34 |
| C2 | 51 | −58 | 2 | 12 |
| C3 | −50 | −59 | 11 | 61 |
| C5 | −50 | −64 | −7 | 227 |
| C6 | −49 | −56 | −1 | 630 |
| C8 | −48 | −58 | 3 | 103 |
| C9 | 45 | −62 | −2 | 38 |
| D1 | −44 | −65 | −6 | 25 |
| D2 | −48 | −61 | −2 | 266 |
| D3 | −52 | −64 | 6 | 375 |
| D5 | −41 | −70 | −15 | 292 |
Fig. S1.
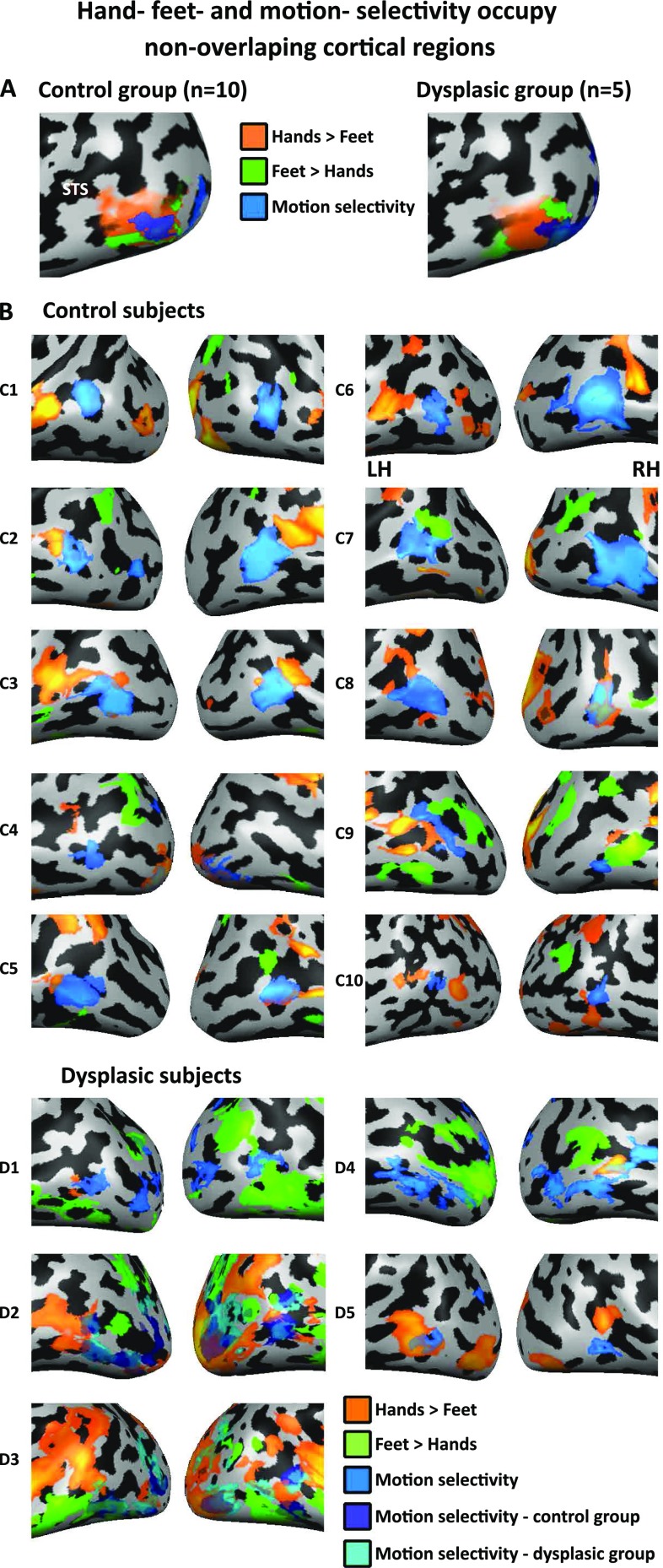
Hand, feet, and motion selectivity occupy distinct cortical regions. (A) Hand (orange), foot (green), and motion selectivity (blue) are viewed on the lateral occipito-temporal cortex in both groups. Hand and foot selectivity are compared on a winner-takes-all approach (each condition is compared with baseline at P < 0.05, FDR corrected, and the preferred body part is then selected for each vertex), revealing an inferior and posterior (dorsal) area, which show a preference for viewing feet over viewing hands in both groups. Motion selectivity was measured with an additional localizer (Materials and Methods; P < 0.05, FDR corrected). Importantly, hands- and feet-preferential responses do not all result from an overlap with motion-selective regions. (B) Hand (orange; hands > feet), foot (green; feet > hands), and motion (blue; moving > stationary rings) selectivity (measured by direct contrast) are depicted in individual subjects, largely reproducing the pattern seen in the group results of having distinct hand and foot preferences, which do not overlap with motion selectivity. For two dysplasic subjects who do not have individual motion selectivity localizers (D2, D3), group motion selectivity data from both groups is presented (in blue and cyan for the control and dysplasic groups, respectively; greatly overlapping with one another).
Fig. S2.
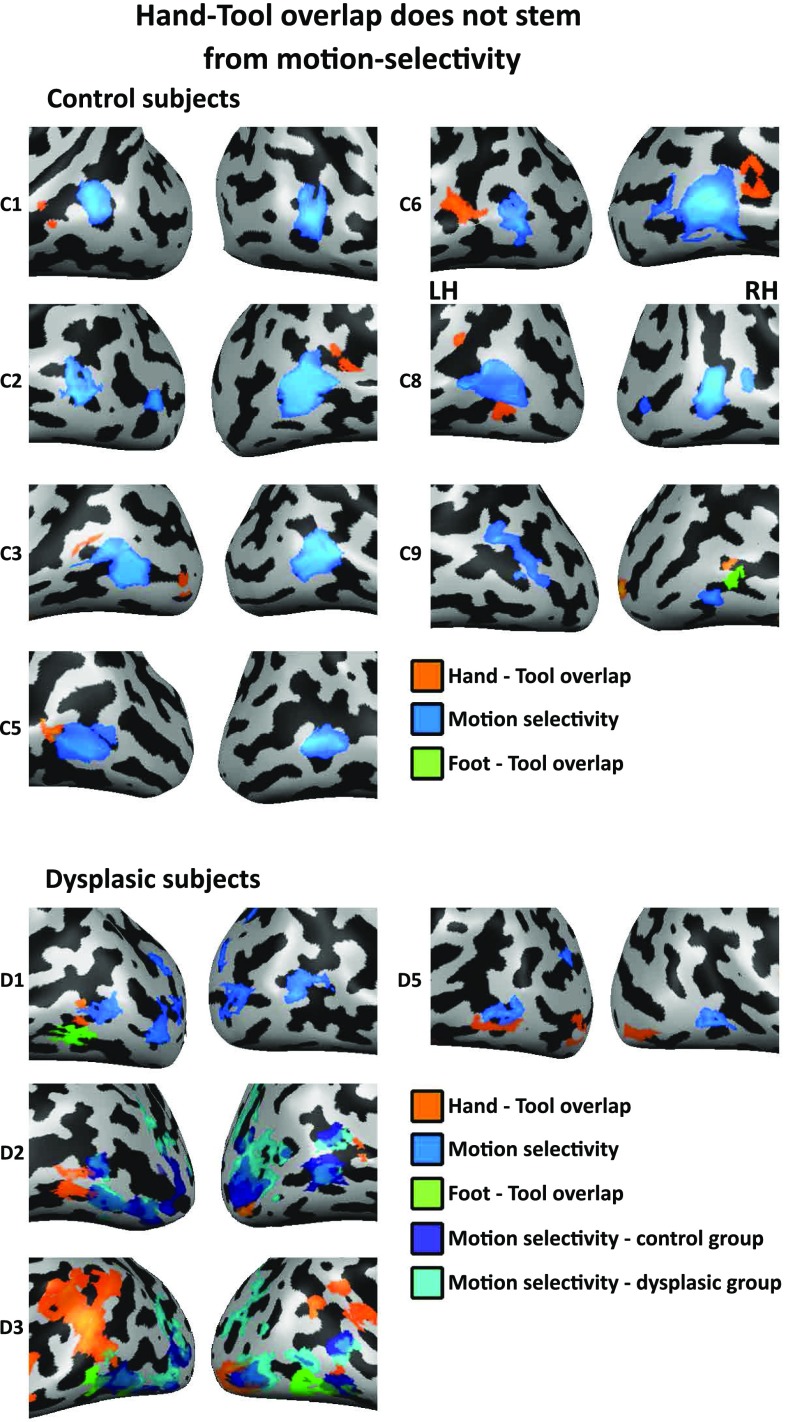
The hand–tool overlap (HTO) does not stem from motion selectivity. HTO (orange; hands > feet AND tools > nonmanipulable artifacts), foot–tool overlap (FTO) (green; feet > hands AND tools > nonmanipulable artifacts), and motion selectivity (blue; moving > stationary rings) are viewed on the lateral occipito-temporal cortex in each of the individual subjects (P < 0.05, corrected). For two dysplasic subjects who do not have individual motion selectivity localizers (D2, D3), group motion selectivity data from both groups is presented (in blue and cyan for the control and dysplasic groups, respectively; greatly overlapping with one another). The HTOs and FTOs do not overlap with motion selectivity. Therefore, general motion selectivity cannot account for the hand and tool conjoint selectivity in the dysplasics.
Neither the location nor the size of the HTO was statistically different between the groups (Fig. 1D for the overlap loci and size distribution), as assessed by Bayesian analysis. Bayes factor (BF) was below 1 (BF = 0.76 for HTO location and BF = 0.55 for HTO size), favoring the absence of difference between the groups. Therefore, the typical HTO development does not require manual motor experience.
What drives the emergence of the HTO in the dysplasics? One explanation of domain-specific specialization in visual cortex is based on the observation that objects differ in the kinds of computations necessary for their recognition and their use, and that the different computations recruit distinct brain regions. For example, the grasping response for using a hammer involves different neural regions than those engaged in the navigation of a visual scene among large nonmanipulable objects, implying the recruitment of distinct neural circuits for their effective processing (34). A possible implication of this fact is that visual regions become specialized for processing specific visual properties in part because of their connectivity with relevant downstream regions. Additionally, to the extent that object domains typically share visual characteristics that distinguish them from other object domains (35–37), this would result in domain-specific visual regions that are associated with distinct neural circuits (4, 18).
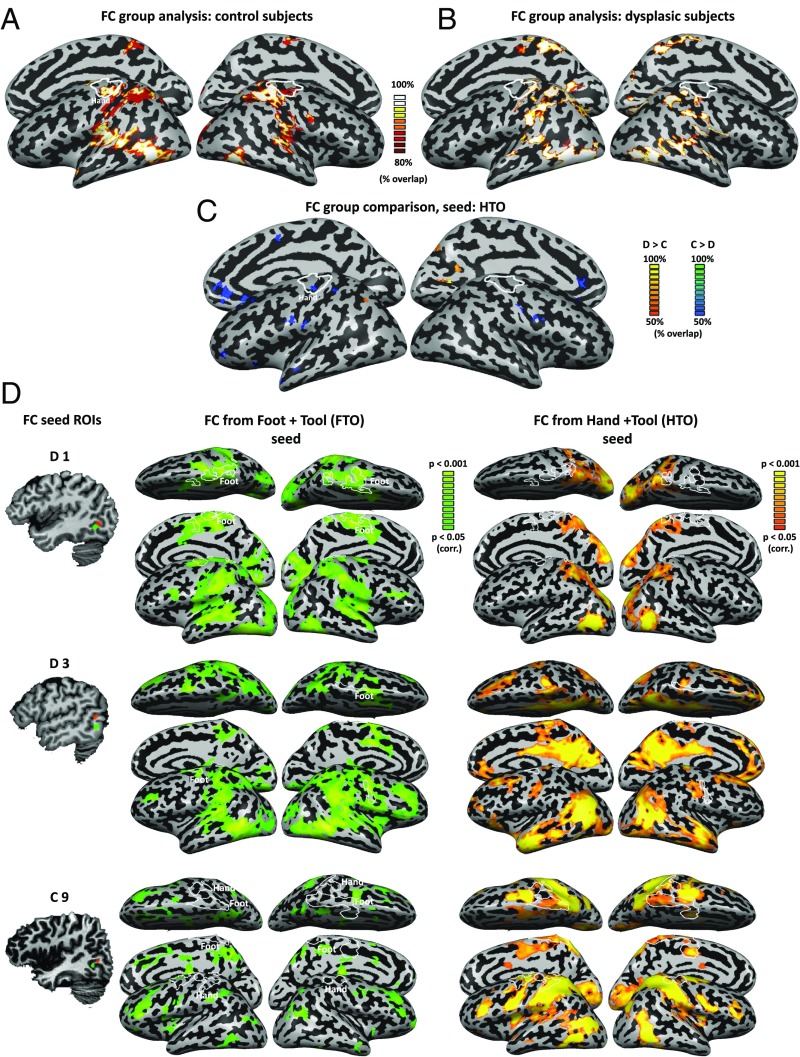
Can the same connectivity pattern blueprint exist in the dysplasics, despite the absence of hands? Or do different mechanisms and different connectivity patterns (unrelated to dorsal stream and action networks) explain the same finding in this case? To address these questions, we computed the functional connectivity from each control subject’s individual HTO peak in an independent resting-state measurement. Group analysis of the connectivity patterns replicate the results from previous studies (22, 27): We found that the HTO is significantly functionally connected to vast regions in the visual system (Fig. 2A), both in the ventral and dorsal stream, extending as far as the primary sensorimotor cortex, specifically its hand region (Fig. 2A, independent functional motor localizer marked in white). Computing the functional connectivity from HTO in the dysplasics shows a very similar pattern (Fig. 2B), connecting the HTO to widespread areas in the parietal lobe. The connectivity pattern is largely retained, such that a permissive comparison of the groups [Bayesian standardized difference test comparison of each dysplasic subject to the control group (38) and probabilistic mapping of differences in as little as two subjects; see Materials and Methods for detail] shows only sparse small foci of differential functional connectivity (Fig. 2C). Notably, a small yet significant difference between the connectivity pattern of the two groups exists in functional connectivity to the sensorimotor hand area, being weaker in the dysplasics (Fig. 2 B and C). Stronger functional connectivity in the dysplasics can be found in the dorsal stream, in a small focus in the left superior parietal lobule, and also in the right early visual cortex (Fig. 2C).
Fig. 2.
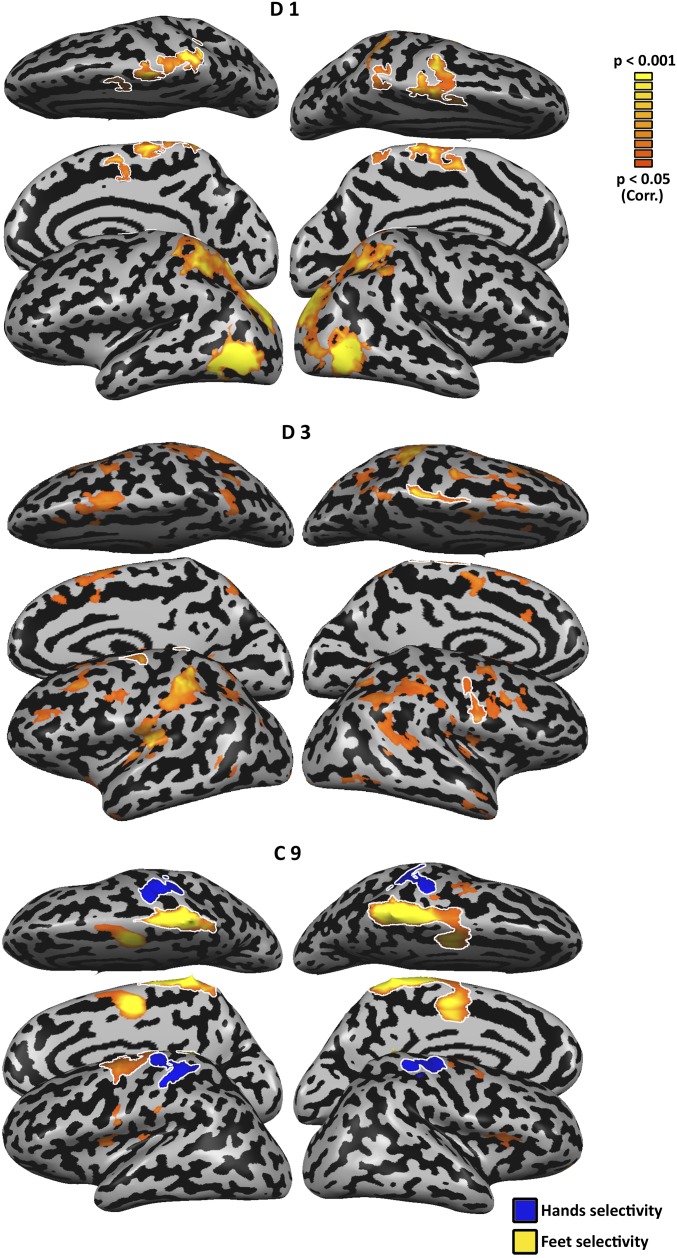
Connectivity from the occipito-temporal cortex reflects intact visual mechanisms alongside their plasticity. (A) Functional connectivity from the HTO in the control subjects (probabilistic mapping of the functional connectivity of individual subjects, reflecting the percentage of subjects showing this pattern) replicates the network of visual cortical regions engaged in visuomotor tool use processing, extending to the sensorimotor cortex of the hand region (independent localizer marked in white). (B) A highly similar network is connected to the HTO of the dysplasics, across the ventral and dorsal visual streams. One difference between the groups can be seen in the absence of functional connectivity to the primary sensorimotor cortex in the dysplasics. (C) A direct comparison of functional connectivity from the HTO between the dysplasics and control subjects was computed by Bayesian standardized difference test comparison of each dysplasic subject to the control group. The figure denotes a probabilistic mapping of differences, such that only two dysplasic subjects would have to show a difference from the control subjects for a voxel to be marked. Even at this permissive overlap threshold, relatively sparse small foci of differential functional connectivity can be found. These show heightened functional connectivity in the dysplasics in the visual cortex (including the superior parietal lobe), and decreased functional connectivity in the sensorimotor cortices, including the hand sensorimotor cortex (independent localizer: marked in white). (D) Functional connectivity was computed in three subjects, two dysplasics (D1 and D3), and one control (C9), which showed both an HTO and a foot–tool overlap (FTO). The dysplasics show functional connectivity from the FTO but little from HTO to their sensorimotor cortex [including their foot-selective regions, marked in white; these include atypical lateral foot responses (63); for full motor response maps, see Figs. S3 and S4]. In contrast, the control subject shows the reverse trend, of functional connectivity to the sensorimotor cortex only from her HTO. Therefore, the unique experience of the dysplasics in foot–tool use can manifest in functional connectivity linking the FTO to the motor cortex.
What are the implications of using tools with an atypical body part, the feet, on the representation of tools and the body? Interestingly, two of the five dysplasics (Fig. 1E), in addition to having a HTO, also showed an overlap of foot- and tool-selective responses in the occipito-temporal cortex. However, such a foot–tool overlap (FTO) was not limited to dysplasics, as one of the control subjects also showed both FTO and HTO (Fig. 1E). For subjects who showed this overlap, the FTO was more inferior in location to the HTO (see peaks in Fig. 2D; Table S2), in accord with the general body part topographical organization in the visual cortex (39) (Figs. S1A and S2). Multivariate pattern analysis accurately classified response patterns for hands and feet in the FTO of all three subjects (P < 0.05 in all cases).
Despite the similarity in activation of the FTOs, plotting the functional connectivity from these FTOs on a subject-by-subject level reveals a clear dissociation between the two dysplasics and the control subject. The dysplasics show functional connectivity from the FTO but little functional connectivity from the HTO to their sensorimotor cortex (including their individually localized foot regions, marked in white; Fig. 2D; for motor response maps, see Figs. S3 and S4). In contrast, the control subject shows the reverse trend: functional connectivity to the sensorimotor cortex only from her HTO. Therefore, even though a tool–foot overlap can be found in people who do not use tools with their feet, the added extensive experience with viewing or interacting with tools using the feet may change the functional connectivity such that the visual cortex FTO would also functionally connect to the relevant sensorimotor cortex. Interestingly, the FTO was found in the two dysplasics who have never used prostheses, potentially stressing the need for very extreme experience to modify the innate connectivity pattern.
Fig. S3.
Motor responses in single subjects presented in Fig. 2. Motor foot selectivity (flexing the feet > flexing shoulders, stomach and mouth muscles; P < 0.05, corrected; depicted in orange) is presented for dysplasics D1 and D3, and control C9. The responses near the central sulcus are outlined and were used in Fig. 2 to identify the individual foot-selective regions. For subject C9, hand selectivity (flexing the hands > flexing shoulders, stomach and mouth muscles; P < 0.05, corrected; depicted in blue) is also presented.
Fig. S4.
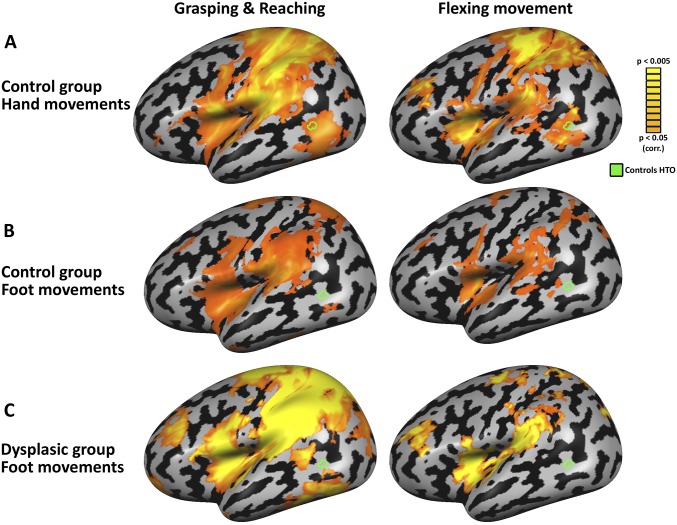
The HTO responds to unseen hand movements in typically developed subjects, but not to foot movements. (A) Motor responses for hand movements in the control group (RFX, P < 0.05, corrected) are presented for two motor control experiments (vs. baseline; see Materials and Methods for detail). The left panel depicts activation during grasping and reaching actions, and the right panel depicts activation during performance of simple hand-flexing movements. Both movement patterns of the hands activate the HTO of the control subjects. (B) Foot actions in the control group (RFX, P < 0.05, corrected) do not activate the HTO, showing this region’s motor responsivity is specific and aligned to its visual selectivity. (C) Foot actions in the dysplasic group (P < 0.05, corrected) do not activate the HTO, suggesting the HTO’s development in the dysplasics does not result from foot-related motor imagery or simulation. Interestingly, the motor foot responses of the dysplasics spread to the lateral sensorimotor cortex, to areas usually occupied by hand motor selectivity, which are not activated by the control subjects (compare B). This is in accord with a past report of the overtake of the hand motor area in congenitally dysplasic individuals (63).
Discussion
In recent years, it has been found that viewing hands and tools recruits overlapping cortical areas in the occipito-temporal cortex. A fundamental question concerns the nature of the principles driving this topographical bias. One possible explanation is that the HTO emerges through repeated sensorimotor experience of using tools with the hands. Consistent with this view is the observation that the HTO is located in a region of the occipito-temporal cortex showing preferential functional connectivity (22, 27) with the network of left-lateralized (40) areas of the dorsal visual stream and motor cortex involved in tool manipulation. These include the anterior intraparietal area [involved, among other tasks, in producing accurate grip (41–43)] and the ventral premotor cortex (44–46). Furthermore, the fact that similar tool-selective and body-selective responses have been found in the same part of the occipito-temporal cortex in individuals deprived of visual experience from birth (32, 33, 47, 48), invites the inference that, because vision is not necessary for developing theses preferences, the burden for shaping this region’s functional specialization is carried by motor experience. Our finding, however, calls for a reexamination of this experience-based account. We found that individuals born without hands who, therefore, have never grasped or manipulated tools with the hands, show the typical overlap between hands and tools selectivity in the occipito-temporal cortex, indistinguishable in volume or location from that of the controls. They also showed a largely similar functional connectivity of this region to the left intraparietal sulcus involved in tool use. This was found even in subjects who have never used prostheses (two dysplasic subjects) and in a subject who has only used functional (hook-like) prostheses, which cannot be used to manipulate tools (see detail in Materials and Methods). Therefore, these subjects had no experience in manipulating tools with instruments visually similar to hands. A clear example of the independence of HTO from manual motor experience is ID3, who has completely absent upper limbs, has no prostheses experience, and shows intact HTO (the largest overlap volume among the dysplasics) and functional connectivity (Fig. 2D). We can thus conclude that this specialization of the high-level visual cortex for hands and tools does not require manual tool use experience for its emergence.
If ontogenetic experience, be it visual or motor, is not the crucial force driving the topographical specialization for tools and hands in the occipito-temporal cortex, how can we explain that tools and hands processing overlaps in this brain region? Our results encourage the hypothesis that the organization of evolutionarily relevant object domains in high-level visual cortex is mainly driven by the differential connectivity of its different regions with different downstream networks in the service of behavior (4, 18). The hypothesis is that the brain could have evolved specialized representational systems in high-level visual cortex, which are particularly sensitive to object features that are strongly associated with different object domains. On this view, the HTO would have emerged because of the potential advantage that accrues from the efficient processing of hands and tools as parts of a common (or closely intertwined), specialized system. This system, in turn, is connected to the dorsal, action-processing areas to allow quick and efficient shaping of hands to grasp and use tools. Once evolved, this innately determined system would manifest itself ontogenetically even in the absence of any of the specific inputs, as in the case of the dysplasics, that originally contributed to the full usefulness of the pattern.
The results reported here and the results obtained with congenitally blind individuals (32, 33, 47) show that motor and visual experience are singly unnecessary for the full development of the hand and tool specialization in the occipito-temporal cortex. However, it is possible that visual experience (e.g., in the dysplasics) and motor experience (e.g., in the blind subjects) are each sufficient for the development of hand and tool specialization in the occipito-temporal cortex. Still, to account for the topographic arrangement of the hand and tool specialization, we would need to assume that the region in question is preferentially disposed to encode information about those object categories independently of a specific type of sensory input. This would, in turn, suggest that the nature of representations in this region would need to be of a form that is accessible through different modalities, including written words (48), haptic stimulation (49, 50), or sensory-substitution–based audition (51), and that they be independent from low-level effector-bound motor properties and experience, such that they develop normally in individuals with atypical bodies, as in the dysplasics.
It is important to note that the view presented here does not preclude a role for sensorimotor experience in the fine details of the organization of the occipito-temporal cortex. In fact, a finding of this study—namely, that in addition to the HTO two dysplasics also displayed a conjoined foot and tool selectivity, functionally connected to the dorsal, action-processing areas and sensorimotor cortex—seems to encourage this possibility. Curiously, the two dysplasics who show this effect had never used prostheses. However, it is not easy to know how to best interpret this observation as not only these two dysplasics but also one typically developed participant showed a FTO (but dissimilar functional connectivity patterns). Although potentially indicative of some experience-based modulation of occipito-temporal cortex organization, current results do not allow clear conclusions about such modulation.
The results reported here have indirect but clear implications for claims regarding the role of motor simulation in action recognition. On such theories, recognition of a hand action is claimed to depend on the simulation of the viewed action in the observer’s own motor system (52). Individuals born without upper limbs recognize hand actions without difficulty and show the same patterns of behavior as typically developed individuals in various hand movement and action recognition tasks, suggesting that motor simulation is not causally involved in action recognition (53). However, it has been proposed that, in the impossibility of hand motor simulation, a cross-limb matching of the viewed action in such individuals would be performed implicitly with the feet, based on experiencing their synchronous co-occurrence (54). However, the HTO of the dysplasics does not show functional connectivity to their sensorimotor foot region, which could have suggested the existence of such implicit imitation (Fig. 2B). Furthermore, in additional control experiments, no positive activation was found in the HTO of the dysplasics when performing unseen grasping or reaching movements with their feet (β = 0.13, t = 1.79, P > 0.21; see Fig. S4 and Materials and Methods for detail). This stands in contrast to finding motor responses for unseen hand (but not foot) movements in the controls’ HTO (Fig. S4). Therefore, the current data do not support an alternative motor explanation for the existence of the HTO in the absence of hands and do not support the hypothesis that hand action perception involves motor simulation with the feet.
In conclusion, the clear preservation of functional organization of hand- and tool-sensitive regions in the occipito-temporal cortex in people born without hands suggests that sensorimotor ontogenetic experience is not required for the specialization of the occipito-temporal cortex. Instead, it points to an evolutionarily driven functional selectivity, which can develop based on inherited connectivity constraints (18).
Materials and Methods
Participants.
Five individuals born with severely shortened or completely absent upper limbs (individuals with upper limb dysplasia; dysplasics 1–5), and 10 typically developed control subjects, matched for age (no group difference; P < 0.29), participated in the experiment. The causes of dysplasia were genetic, ototoxic medications (thalidomide), or unknown. See Table S1 for the summary of the characteristics of the dysplasics, as well as images of their residual limbs. None of the dysplasics had a history of phantom limb sensations or movements, and all were adept at performing everyday actions and tool use with their feet. Dysplasic subject D1 had three residual fingers attached to the shoulder (Table S1). Dysplasic subjects D2 and D3 had bilateral dysplasic malformations with totally missing upper limbs on both sides (a complete absence of arm, forearm, hand, and fingers). Dysplasic subject D4 had a shortened right arm (±10-cm humerus). Dysplasic subject D5 had one residual finger attached to the shoulder.
Dysplasic subjects D1 and D3 report no history of prostheses use. For full detail of participants D2, D4, and D5 prosthetic use see SI Materials and Methods. Importantly, the prostheses used by D4 and D5 did not include cosmetic hands and all of them report having used these prostheses mainly, if not uniquely, to pull, maintain in place, or push objects but not to manipulate and use objects for their functional use (e.g., eating with a fork) with their feet.
All participants had normal or corrected-to-normal vision, had no history of psychiatric or neurological disorder, and gave written informed consent in accordance with the institutional review board of Harvard University.
Experimental Design.
Grayscale pictures depicting a hand, a foot, a tool, or a nonmanipulable artifact were displayed in a block design. All epochs lasted 8 s and were followed by an 8-s rest interval. Eight images of different objects from the same category were presented in each epoch; each image was presented for 800 ms and was followed by a 200-ms blank screen. A central red fixation point was presented throughout the experiment. The experiment had four runs, and each condition was repeated eight times in a pseudorandom order in each run. The subjects were instructed to fixate and respond (by foot response) to catch trials, in which an image was repeated twice consecutively. There were two catch trials in each run of the experiment, and the data from these trials were excluded from further analysis.
The tools and nonmanipulable artifacts categories included eight different objects, with 16 different exemplars each. The hands and legs included 16 different exemplars each. Objects were matched across categories for familiarity (P < 0.82) and differed significantly on manipulability (tools and nonmanipulable artifacts’ manipulability average, 6.87 and 1.25, respectively; P < 0.00001) based on the ratings (scores of 1–7) of independent control groups using Amazon Mechanical Turk. Tools were chosen from a list of items that all dysplasics reported using with their feet (SI Materials and Methods, Stimuli for the full list all dysplasics reported to have used with their feet).
For full detail of the ratings and other stimulus parameters, see SI Materials and Methods. Stimuli images were matched in number of pixels, vertical size, horizontal size, and vertical–horizontal ratio, to eliminate any low-level visual confound.
Functional Imaging.
The blood oxygen level-dependent (BOLD) fMRI measurements were obtained in a Siemens Trio 3-T scanner at the Center for Brain Science at Harvard University. For acquisition detail, see SI Materials and Methods. The main experiment had four runs of 287 whole-brain images each collected in one functional scan. Data analysis was performed using the BrainVoyager QX 2.8 software package (Brain Innovation) using standard preprocessing procedures (SI Materials and Methods). Functional and anatomical datasets for each subject were aligned and fit to standardized Talairach space (55). Single-subject data were spatially smoothed with a 3D 6-mm full-width at half-maximum Gaussian to reduce intersubject anatomical variability, and then grouped using a general linear model (GLM). Group analyses in the control group were conducted in a hierarchical random effects analysis (RFX) (56) at a surface representation level, due to the variability of the lateral occipito-temporal cortex location in volumetric space. Anatomical cortical reconstruction procedures included the segmentation of the white matter using a grow-region function embedded in BrainVoyager. The Talairach normalized cortical surface was then inflated, and the obtained activation maps were superimposed onto it. Surface-based alignment was conducted across the subjects according to their cortical curvature (sulci and gyri) patterns, and RFX GLM analysis (Fig. 1A) was then conducted.
Due to the small sample size of the unique dysplasic group, analyses were based on single subject (Fig. 1 C and E) and probabilistic mapping of the overlap of significant single-subject activation (Fig. 2B), to enable an assessment of the consistency of the findings. The minimum significance level of the results presented in this study was set to P < 0.05, corrected for multiple comparisons, using the spatial extent method based on the theory of Gaussian random fields (57, 58) (a set-level statistical inference correction). Individual HTO activation (a conjunction of two conservative contrasts) was corrected for multiple comparisons using the spatial extent method within the visually active occipital and occipitotemporal cortex [all image types vs. baseline, in both groups, P < 0.05, false-discovery rate (FDR) (59) corrected]. This was done based on the Monte Carlo stimulation approach, extended to 3D datasets using the threshold size plug-in for BrainVoyager QX.
Comparison between the two groups in peak location of the HTO was applied using the BF approach (60), appropriate for testing small samples of unique populations and patients. The BF is the probability of the data under one hypothesis relative to the probability of the data given another (H0/H1) and, therefore, allows evaluating the strength of the evidence for both alternatives. We calculated and compared the mean Euclidean distance between each of the dysplasics HTO peak and each of the controls (between-group distance, mean = 12.6 mm, SD = 6) and the mean distance of each of the controls to the other controls (within-group distance, mean = 9.7 mm, SD = 2.38). The BF for the comparison between the groups’ average distance was calculated twice, once without the two control subjects whose HTO appeared in the right hemisphere (BF = 0.64, P > 0.41) and once when their peaks were included but reversed in laterality (BF = 0.76, P > 0.25). BF analysis was also used to compare HTO volume between the groups (BF = 0.56, P > 0.53). In all cases, BF was below 1, favoring the absence of difference between the groups.
MVPA was conducted in spheres centered at the individual HTO peaks (4-mm radius). MVPA was performed using a linear support vector machine classifier as implemented by BrainVoyager. Within each individual region of interest (ROI), z-normalized β weights were estimated based on eight trials per condition (hands, feet) and run, resulting in 32 β values per condition. Classification accuracies were computed using leave-five-out cross-validation, that is, the classifier was trained using the data of 27 patterns and tested on its accuracy at classifying the unseen data from the remaining 5 patterns. The average classification was tested against classification with a random permutation of trial labels (1,000 iterations), averaged across the cross-validation procedures.
Functional Localizers and Control Experiments.
Functional localizers for sensorimotor cortex hand and foot regions, grasping control experiment, and a visual motion selectivity experiment were also conducted. For details, see SI Materials and Methods.
Functional Connectivity Data Analysis and MRI Acquisition.
A dataset of spontaneous BOLD fluctuations for the investigation of intrinsic [rest state (61)] functional connectivity was collected while the subjects lay supine in the scanner without any external stimulation or task. A total of 400 whole-brain images was collected in one functional scan. For details of the acquisition and preprocessing parameters, see SI Materials and Methods. Single-subject data were spatially smoothed with a 3D 6-mm half-width Gaussian. Seed ROIs were defined as spheres (4-mm radius) around each subject’s peak of hand–tool selectivity overlap in the occipito-temporal cortex (Fig. 1D), to avoid confounds related to seed size. Individual time courses from this seed ROI were sampled from each of the participants, z-normalized, and used as individual predictors in single-subject GLM analyses. Probability overlap across the individual subjects (Fig. 2 A and B) were computed from individual maps, each at P < 0.05, FDR corrected for multiple comparisons. The maps were overlaid, and the percentage of subjects showing activation at each voxel was calculated. Functional connectivity group comparison (Fig. 2C) was conducted using Bayesian standardized difference test appropriate for comparing a single case to a control/normative sample (38, 62). Individual maps of functional connectivity from the HTO seeds of each dysplasic subject were compared with the maps of the control subjects in a Crawford modified t test (38). A probabilistic mapping of the overlap of significant individual-subjects t test results was computed (Fig. 2B) to enable an assessment of the consistency of the findings, reflecting the percentage of subjects showing this pattern. The probabilistic mapping of the functional connectivity differences is presented at a relatively permissive threshold, such that only two (of four) dysplasic subjects need to have a difference from the controls, at P < 0.005 uncorrected, for a voxel to be shown. Individual functional connectivity maps were also computed from seed ROIs defined as spheres (4-mm radius) around the subjects’ peak of foot–tool selectivity overlap in the occipito-temporal cortex (Fig. 2D).
SI Materials and Methods
Participants Prosthetic Use.
Dysplasic subjects D1 and D3 report no history of prostheses use. D2 occasionally used a wood composite prosthesis with locking elbow and hooks controlled by cables attached to leg straps from 3 to 7 y old, a wood composite prosthesis with electronic elbow and three pronged hooks controlled by micro switches in shoulder harness from 7 to 11 y old, and a composite prosthesis with myoelectric elbows and cosmetic hands from 11 to 15 y old. D4 used switch-based right and left arms prosthesis as a child and still uses occasionally a switch-based right arm prosthesis as an adult. D5 used myoelectric and manual prostheses 5 h a day between 3 and 14 y old. Importantly, however, the prostheses used by D4 and D5 did not include cosmetic hands and all of them report having used these prostheses mainly, if not uniquely, to pull, maintain in place, or push objects but not to manipulate and use objects for their functional use (e.g., eating with a fork) with their feet.
Stimuli.
The stimuli set consisted of grayscale pictures depicting a hand, a foot, a tool, or a nonmanipulable artifact. The hands and feet categories included each 16 different exemplars depicting a right or a left limb in different orientations and configurations. The tool category included 16 different exemplars of eight different object types (16 combs, 16 hair brushes, 16 bowl scrapers, 16 hand fans, 16 scissors, 16 computer mouse, 16 vegetable peelers, and 16 can openers). The nonmanipulable artifact category also included 16 different exemplars of eight different object types (16 fences, 16 chimneys, 16 anchors, 16 bathtubs, 16 trains, 16 couches, 16 tables, and 16 traffic lights).
The tools and the nonmanipulable artifacts were selected among a larger sample of 154 objects for which ratings (scales from 1 to 7) of conceptual familiarity (i.e., the degree to which you come into contact, see, hear, or think about that object in everyday life), manipulability (i.e., how easy it is to grasp and use the object with one hand; see ref. 64), body control (i.e., the degree to which your hand/arm is directly in control of each object when you use them), body extension (i.e., the degree to which each object “feels like” a physical extension of your hand and arm during its typical use), and charade (i.e., how easily you could mime the use of each object so that a person looking at you could recognize which object you are miming; see ref. 65) were obtained with six different independent groups of 30 participants (one group for each dimension) using Amazon Mechanical Turk. The tools and the nonmanipulable artifacts were selected to be matched for conceptual familiarity [t(10) < 1] but as different as possible according to the other dimensions [all values of t(10) > 3.8, all values of P < 0.001]. Tools were chosen from a list of items that all dysplasics reported using with their feet. The dysplasics were provided, over a month before the scan, with a list of 187 tools and small graspable objects and noted for each which body part they use it with, or if they have never used it before.
The following is a list of tools all five dysplasic subjects reported to have already used to achieve their typical function with their lower limbs, and with them only:
bowl scraper, calculator, can opener, cards, chess pawn, comb, computer mouse, cooking strainer, correction pen, elastic band, erasing gum, file, frisbee, garlic press, glue stick, hair brush, hair dryer, hand fan, hole punch, iron, kettle, kitchen sponge, match, nail, nail polish, paper clip, pencil sharpener, protractor, razor, rolling pin, scissors, screw, sewing needle, spinning top, stapler, syringe, tambourine, thermometer, toaster, toothbrush, vegetable peeler, and yo-yo.
The instructions that the dysplasic subjects were given are the following: Please indicate your experience in using the listed objects by putting an X in the appropriate column (columns were “I use it with my upper limb(s)”; “I use it with my lower limb(s)”; “I use it with my mouth”; “I have never used it to achieve its typical function”; “I would be able to use it to achieve its typical function, if I had the opportunity to try”; “?”). If you have already used the object to achieve its typical function (e.g., using a hammer to put on a nail, using a sword to sword fight, and so on), please indicate whether you used your upper limbs, lower limbs, or mouth to use it. If you use an object with a combination of several body parts or if you use indifferently different body parts to use it, please put an X in all of the appropriate columns (for instance, lower limbs and mouth). If you have never used a given tool to achieve its typical function, that is, if you have never touched it or if you have only transported it, then put an X in the column “I have never used it to achieve its typical function” and, then, indicate whether you estimate that you would nevertheless be able to use it to achieve its typical function if you were given the opportunity by putting an X in the last column, or not, by letting the last column empty. If you don’t know the object, or if you are not sure of what it refers to, or if you are not sure of your response, put an X in the column “?”.
Once the object categories were selected, the 16 exemplars of every category were matched in number of pixels, vertical size, horizontal size, and vertical–horizontal ratio, to eliminate any low-level visual confound.
Functional Imaging Acquisition and Preprocessing.
Acquisition parameters were the following. The pulse sequence used was gradient-echo echo-planar imaging (GE-EPI) that employed multiband RF pulses and Simultaneous Multi-Slice (SMS) acquisition (factor of 3). We used 69 slices of 2-mm thickness. The data in-plane matrix size was 108 × 108; field of view (FOV), 21.6 cm × 21.6 cm; time to repetition (TR), 2,000 ms; flip angle, 80°; and time to echo (TE), 28 ms. The first two images of each scan (during the first baseline rest condition) were excluded from the analysis because of non–steady-state magnetization. Data analysis was performed using the BrainVoyager QX 2.8 software package (Brain Innovation) using standard preprocessing procedures. fMRI data preprocessing included head motion correction, slice scan time correction, and high-pass filtering (cutoff frequency: 3 cycles/scan) using temporal smoothing in the frequency domain to remove drifts and to improve the signal-to-noise ratio. No data included in the study showed translational motion exceeding 2 mm in any given axis, or had spike-like motion of more than 1 mm in any direction.
Separate 3D recordings were used for coregistration and surface reconstruction. Three-dimensional anatomical volumes were collected using T1-weighted images using a magnetization-prepared rapid gradient-echo T1-weighted sequence. Typical parameters were as follows: FOV, 25.6 cm × 25.6 cm; data matrix, 256 × 256 × 256 (1-mm isovoxel); TR, 2,530 ms; TE, 1.64, 3.5, 5.36, 7.22 ms; and flip angle, 7°.
A dataset of spontaneous BOLD fluctuations for the investigation of intrinsic [rest state (61)] functional connectivity was collected while the subjects lay supine in the scanner without any external stimulation or task. Functional connectivity MRI uses slow (<0.1 Hz) spontaneous (not task-related, e.g., during rest) fluctuations in the BOLD signal, which were discovered nearly two decades ago (61), and which have since been convincingly and repeatedly shown to correlate between areas that are parts of the same functional network (66–69) [although dynamic alterations are also present (70, 71)], closely mimicking anatomical [although not necessarily monosynaptic (72)] connectivity. The pulse sequence used was gradient-echo EPI with parallel imaging (factor of 4). The data in-plane matrix size was 108 × 108; FOV, 21.6 cm × 21.6 cm; TR, 1,500 ms; flip angle, 75°; and TE, 28 ms. A total of 68 slices of 2-mm thickness (with 0.2-mm spacing) was used to obtain full coverage of the subject’s brain, and 400 whole-brain images were collected in one functional scan. The first two images of each scan were excluded from the analysis because of non–steady-state magnetization. Ventricles and white-matter signal were sampled using a grow-region function embedded in the BrainVoyager from a seed in each individual brain. Using MATLAB (MathWorks), ventricle and white-matter time courses were regressed out of the data, and the resulting time course was filtered to the frequency bandwidth of 0.1–0.01 Hz (in which typical spontaneous BOLD fluctuations occur). The resulting data were then imported back onto BrainVoyager for further analyses.
Functional Localizers and Control Experiments.
Functional localizers for sensorimotor cortex hand and foot regions, grasping control experiment, and a visual motion selectivity experiment were conducted as follows:
-
i)
An independent group sensorimotor cortex mapping in a typically developed control group (other than the subjects of the main experiment) was carried out in a block design fMRI experiment (73). Seven healthy, right-handed subjects participated in the experiment. Tongue, hands, and feet were moved in separate blocks (9-s bilateral movement and 9-s rest) according to an auditory cue. Nine movements were performed in each block at a frequency of 1 Hz. Hand-selective sensorimotor cortex is marked (Fig. 2 A and B) based on significant (P < 0.05, FDR corrected) preference for hand movement over movement of the other body parts.
-
ii)
An individual-based sensorimotor cortex mapping was conducted in the subjects participating in the main experiment. The motor localizer was carried out in a block design fMRI experiment, with three runs of 186 TRs, whose acquisition and preprocessing parameters were identical to those of the main experiment. Mouth, abdomen, and either-side hands (for the control subjects), shoulders, and feet were moved in separate blocks (6-s movement and 6-s rest) according to an auditory cue (metronome). Four flex and relax movements were performed in each block at a frequency of 0.66 Hz. Hand-selective or foot-selective sensorimotor cortex is marked (Fig. 2D) based on significant (P < 0.05, FDR corrected) preference for bilateral hand/foot movement over movement of the other body parts, per individual subject. Maps of hand and foot motor selectivity for the control and dysplasic groups (P < 0.05, FDR corrected; RFX for the control group) can be found in Fig. S4, showing that the HTO is selectively activated by hand movements in the control group but not by foot movements in either group.
-
iii)
A control experiment for foot motor involvement in HTO was carried out in four of the five dysplasics who participated in the main experiment (ID3 did not participate due to technical reasons). The motor control experiment was carried out in a block design fMRI experiment, with two runs of 255 TRs, whose acquisition and preprocessing parameters were identical to those of the main experiment. Unseen foot movements of grasping a foam bar (between the two largest toes) or reaching and touching the bar were conducted with either the right or left foot (6-s movement and 8-s rest) according to an auditory cue (metronome). Two movements were performed in each block at a frequency of 0.33 Hz. The control subjects conducted also a hand motor version of this experiment, grasping between the thumb and index finger, in different runs for hands and feet. Activation (β values) for foot movement (vs. baseline) was sampled in the HTO of dysplasics 1, 2, and 5, and showed no significant activity above baseline. Activation maps for both groups’ limb movements (P < 0.05, FDR corrected) can be found in Fig. S4, showing that the HTO is selectively activated by hand movements in the control group but not by foot movements in either group.
-
iv)
A visual-motion selectivity localizer was carried out in three of the five dysplasics who participated in the main experiment as well as in all control participants. This experiment was carried out in a block design fMRI experiment, with one run of 220 TRs, whose acquisition and preprocessing parameters were identical to those of the main experiment. Stationary or moving (contracting and expanding; similar to ref. 74) low-contrast rings were presented (8-s visual stimuli and 8-s rest) around a central fixation point. Single-subject and group motion-selective regions (Figs. S1 and S2) were identified contrasting moving > stationary rings, at P < 0.05 corrected (spatial extent method corrected for individual data, FDR corrected for group data).
Acknowledgments
We are thankful to the dysplasic subjects who participated in our experiments. We thank Tamar Makin for helpful discussions. This work was supported by Società Scienze Mente Cervello–Fondazione Cassa di Risparmio di Trento e Rovereto, by a grant from the Provincia Autonoma di Trento, and by a Harvard Provostial postdoctoral fund (to A.C.); and by the European Union’s Horizon 2020 Research and Innovation Programme under Marie Sklodowska-Curie Grant Agreement 654837 and the Israel National Postdoctoral Award Program for Advancing Women in Science (to E.S.-A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. H.O.d.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620289114/-/DCSupplemental.
References
- 1.Kanwisher N. Functional specificity in the human brain: A window into the functional architecture of the mind. Proc Natl Acad Sci USA. 2010;107:11163–11170. doi: 10.1073/pnas.1005062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caramazza A, Shelton JR. Domain-specific knowledge systems in the brain: The animate-inanimate distinction. J Cogn Neurosci. 1998;10:1–34. doi: 10.1162/089892998563752. [DOI] [PubMed] [Google Scholar]
- 3.Blundo C, Ricci M, Miller L. Category-specific knowledge deficit for animals in a patient with herpes simplex encephalitis. Cogn Neuropsychol. 2006;23:1248–1268. doi: 10.1080/02643290600896449. [DOI] [PubMed] [Google Scholar]
- 4.Mahon BZ, Caramazza A. Concepts and categories: A cognitive neuropsychological perspective. Annu Rev Psychol. 2009;60:27–51. doi: 10.1146/annurev.psych.60.110707.163532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Proklova D, Kaiser D, Peelen MV. Disentangling representations of object shape and object category in human visual cortex: The animate-inanimate distinction. J Cogn Neurosci. 2016;28:680–692. doi: 10.1162/jocn_a_00924. [DOI] [PubMed] [Google Scholar]
- 6.Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- 7.Kriegeskorte N, et al. Matching categorical object representations in inferior temporal cortex of man and monkey. Neuron. 2008;60:1126–1141. doi: 10.1016/j.neuron.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stigliani A, Weiner KS, Grill-Spector K. Temporal processing capacity in high-level visual cortex is domain specific. J Neurosci. 2015;35:12412–12424. doi: 10.1523/JNEUROSCI.4822-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konkle T, Caramazza A. Tripartite organization of the ventral stream by animacy and object size. J Neurosci. 2013;33:10235–10242. doi: 10.1523/JNEUROSCI.0983-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullally SL, Maguire EA. A new role for the parahippocampal cortex in representing space. J Neurosci. 2011;31:7441–7449. doi: 10.1523/JNEUROSCI.0267-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troiani V, Stigliani A, Smith ME, Epstein RA. Multiple object properties drive scene-selective regions. Cereb Cortex. 2014;24:883–897. doi: 10.1093/cercor/bhs364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- 13.Srihasam K, Vincent JL, Livingstone MS. Novel domain formation reveals proto-architecture in inferotemporal cortex. Nat Neurosci. 2014;17:1776–1783. doi: 10.1038/nn.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bukach CM, Gauthier I, Tarr MJ. Beyond faces and modularity: The power of an expertise framework. Trends Cogn Sci. 2006;10:159–166. doi: 10.1016/j.tics.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Gainotti G. Inborn and experience-dependent models of categorical brain organization. A position paper. Front Hum Neurosci. 2015;9:2. doi: 10.3389/fnhum.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golarai G, Liberman A, Grill-Spector K. Experience shapes the development of neural substrates of face processing in human ventral temporal cortex. Cereb Cortex. 2017;27:1229–1244. doi: 10.1093/cercor/bhv314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Röder B, Ley P, Shenoy BH, Kekunnaya R, Bottari D. Sensitive periods for the functional specialization of the neural system for human face processing. Proc Natl Acad Sci USA. 2013;110:16760–16765. doi: 10.1073/pnas.1309963110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahon BZ, Caramazza A. What drives the organization of object knowledge in the brain? Trends Cogn Sci. 2011;15:97–103. doi: 10.1016/j.tics.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannagan T, Amedi A, Cohen L, Dehaene-Lambertz G, Dehaene S. Origins of the specialization for letters and numbers in ventral occipitotemporal cortex. Trends Cogn Sci. 2015;19:374–382. doi: 10.1016/j.tics.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Heimler B, Striem-Amit E, Amedi A. Origins of task-specific sensory-independent organization in the visual and auditory brain: Neuroscience evidence, open questions and clinical implications. Curr Opin Neurobiol. 2015;35:169–177. doi: 10.1016/j.conb.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Behrmann M, Plaut DC. A vision of graded hemispheric specialization. Ann N Y Acad Sci. 2015;1359:30–46. doi: 10.1111/nyas.12833. [DOI] [PubMed] [Google Scholar]
- 22.Bracci S, Cavina-Pratesi C, Ietswaart M, Caramazza A, Peelen MV. Closely overlapping responses to tools and hands in left lateral occipitotemporal cortex. J Neurophysiol. 2012;107:1443–1456. doi: 10.1152/jn.00619.2011. [DOI] [PubMed] [Google Scholar]
- 23.Bracci S, Peelen MV. Body and object effectors: The organization of object representations in high-level visual cortex reflects body-object interactions. J Neurosci. 2013;33:18247–18258. doi: 10.1523/JNEUROSCI.1322-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downing PE, Chan AW-Y, Peelen MV, Dodds CM, Kanwisher N. Domain specificity in visual cortex. Cereb Cortex. 2006;16:1453–1461. doi: 10.1093/cercor/bhj086. [DOI] [PubMed] [Google Scholar]
- 25.Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- 26.Wiggett AJ, Pritchard IC, Downing PE. Animate and inanimate objects in human visual cortex: Evidence for task-independent category effects. Neuropsychologia. 2009;47:3111–3117. doi: 10.1016/j.neuropsychologia.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Simmons WK, Martin A. Spontaneous resting-state BOLD fluctuations reveal persistent domain-specific neural networks. Soc Cogn Affect Neurosci. 2012;7:467–475. doi: 10.1093/scan/nsr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarhan LY, Watson CE, Buxbaum LJ. Shared and distinct neuroanatomic regions critical for tool-related action production and recognition: Evidence from 131 left-hemisphere stroke patients. J Cogn Neurosci. 2015;27:2491–2511. doi: 10.1162/jocn_a_00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ricciardi E, Handjaras G, Pietrini P. The blind brain: How (lack of) vision shapes the morphological and functional architecture of the human brain. Exp Biol Med (Maywood) 2014;239:1414–1420. doi: 10.1177/1535370214538740. [DOI] [PubMed] [Google Scholar]
- 30.Renier L, De Volder AG, Rauschecker JP. Cortical plasticity and preserved function in early blindness. Neurosci Biobehav Rev. 2014;41:53–63. doi: 10.1016/j.neubiorev.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bi Y, Wang X, Caramazza A. Object domain and modality in the ventral visual pathway. Trends Cogn Sci. 2016;20:282–290. doi: 10.1016/j.tics.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Peelen MV, et al. Tool selectivity in left occipitotemporal cortex develops without vision. J Cogn Neurosci. 2013;25:1225–1234. doi: 10.1162/jocn_a_00411. [DOI] [PubMed] [Google Scholar]
- 33.Striem-Amit E, Amedi A. Visual cortex extrastriate body-selective area activation in congenitally blind people “seeing” by using sounds. Curr Biol. 2014;24:687–692. doi: 10.1016/j.cub.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Konkle T, Caramazza A. The large-scale organization of object-responsive cortex is reflected in resting-state network architecture. Cereb Cortex. 2016;2016:1–13. doi: 10.1093/cercor/bhw287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stansbury DE, Naselaris T, Gallant Jack L. Natural scene statistics account for the representation of scene categories in human visual cortex. Neuron. 2013;79:1025–1034. doi: 10.1016/j.neuron.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nasr S, Echavarria CE, Tootell RB. Thinking outside the box: Rectilinear shapes selectively activate scene-selective cortex. J Neurosci. 2014;34:6721–6735. doi: 10.1523/JNEUROSCI.4802-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yue X, Pourladian IS, Tootell RB, Ungerleider LG. Curvature-processing network in macaque visual cortex. Proc Natl Acad Sci USA. 2014;111:E3467–E3475. doi: 10.1073/pnas.1412616111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crawford JR, Howell DC. Comparing an individual’s test score against norms derived from small samples. Clin Neuropsychol. 1998;12:482–486. [Google Scholar]
- 39.Orlov T, Makin TR, Zohary E. Topographic representation of the human body in the occipitotemporal cortex. Neuron. 2010;68:586–600. doi: 10.1016/j.neuron.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 40.Frey SH. Tool use, communicative gesture and cerebral asymmetries in the modern human brain. Philos Trans R Soc Lond B Biol Sci. 2008;363:1951–1957. doi: 10.1098/rstb.2008.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davare M, Rothwell JC, Lemon RN. Causal connectivity between the human anterior intraparietal area and premotor cortex during grasp. Curr Biol. 2010;20:176–181. doi: 10.1016/j.cub.2009.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Binkofski F, et al. Human anterior intraparietal area subserves prehension: A combined lesion and functional MRI activation study. Neurology. 1998;50:1253–1259. doi: 10.1212/wnl.50.5.1253. [DOI] [PubMed] [Google Scholar]
- 43.Tunik E, Rice NJ, Hamilton A, Grafton ST. Beyond grasping: Representation of action in human anterior intraparietal sulcus. Neuroimage. 2007;36:T77–T86. doi: 10.1016/j.neuroimage.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson-Frey SH, Newman-Norlund R, Grafton ST. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb Cortex. 2005;15:681–695. doi: 10.1093/cercor/bhh169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Króliczak G, Frey SH. A common network in the left cerebral hemisphere represents planning of tool use pantomimes and familiar intransitive gestures at the hand-independent level. Cereb Cortex. 2009;19:2396–2410. doi: 10.1093/cercor/bhn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brandi M-L, Wohlschläger A, Sorg C, Hermsdörfer J. The neural correlates of planning and executing actual tool use. J Neurosci. 2014;34:13183–13194. doi: 10.1523/JNEUROSCI.0597-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitada R, et al. The brain network underlying the recognition of hand gestures in the blind: The supramodal role of the extrastriate body area. J Neurosci. 2014;34:10096–10108. doi: 10.1523/JNEUROSCI.0500-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, et al. How visual is the visual cortex? Comparing connectional and functional fingerprints between congenitally blind and sighted individuals. J Neurosci. 2015;35:12545–12559. doi: 10.1523/JNEUROSCI.3914-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pietrini P, et al. Beyond sensory images: Object-based representation in the human ventral pathway. Proc Natl Acad Sci USA. 2004;101:5658–5663. doi: 10.1073/pnas.0400707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolbers T, Klatzky RL, Loomis JM, Wutte MG, Giudice NA. Modality-independent coding of spatial layout in the human brain. Curr Biol. 2011;21:984–989. doi: 10.1016/j.cub.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Striem-Amit E, Cohen L, Dehaene S, Amedi A. Reading with sounds: Sensory substitution selectively activates the visual word form area in the blind. Neuron. 2012;76:640–652. doi: 10.1016/j.neuron.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 52.Rizzolatti G, Sinigaglia C. The mirror mechanism: A basic principle of brain function. Nat Rev Neurosci. 2016;17:757–765. doi: 10.1038/nrn.2016.135. [DOI] [PubMed] [Google Scholar]
- 53.Vannuscorps G, Caramazza A. Typical action perception and interpretation without motor simulation. Proc Natl Acad Sci USA. 2016;113:86–91. doi: 10.1073/pnas.1516978112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gazzola V, et al. Aplasics born without hands mirror the goal of hand actions with their feet. Curr Biol. 2007;17:1235–1240. doi: 10.1016/j.cub.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 55.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme; New York: 1988. [Google Scholar]
- 56.Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- 57.Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 58.Forman SD, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 59.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 60.Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon Bull Rev. 2009;16:225–237. doi: 10.3758/PBR.16.2.225. [DOI] [PubMed] [Google Scholar]
- 61.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 62.Crawford JR, Garthwaite PH. Comparison of a single case to a control or normative sample in neuropsychology: Development of a Bayesian approach. Cogn Neuropsychol. 2007;24:343–372. doi: 10.1080/02643290701290146. [DOI] [PubMed] [Google Scholar]
- 63.Stoeckel MC, Seitz RJ, Buetefisch CM. Congenitally altered motor experience alters somatotopic organization of human primary motor cortex. Proc Natl Acad Sci USA. 2009;106:2395–2400. doi: 10.1073/pnas.0803733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saccuman MC, et al. The impact of semantic reference on word class: An fMRI study of action and object naming. Neuroimage. 2006;32:1865–1878. doi: 10.1016/j.neuroimage.2006.04.179. [DOI] [PubMed] [Google Scholar]
- 65.Magnié MN, Besson M, Poncet M, Dolisi C. The Snodgrass and Vanderwart set revisited: Norms for object manipulability and for pictorial ambiguity of objects, chimeric objects, and nonobjects. J Clin Exp Neuropsychol. 2003;25:521–560. doi: 10.1076/jcen.25.4.521.13873. [DOI] [PubMed] [Google Scholar]
- 66.Smith SM, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Auer DP. Spontaneous low-frequency blood oxygenation level-dependent fluctuations and functional connectivity analysis of the “resting” brain. Magn Reson Imaging. 2008;26:1055–1064. doi: 10.1016/j.mri.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 68.Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- 69.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 70.Di X, Gohel S, Kim EH, Biswal BB. Task vs. rest-different network configurations between the coactivation and the resting-state brain networks. Front Hum Neurosci. 2013;7:493. doi: 10.3389/fnhum.2013.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mennes M, Kelly C, Colcombe S, Castellanos FX, Milham MP. The extrinsic and intrinsic functional architectures of the human brain are not equivalent. Cereb Cortex. 2013;23:223–229. doi: 10.1093/cercor/bhs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 73.Zeharia N, Hertz U, Flash T, Amedi A. Negative blood oxygenation level dependent homunculus and somatotopic information in primary motor cortex and supplementary motor area. Proc Natl Acad Sci USA. 2012;109:18565–18570. doi: 10.1073/pnas.1119125109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hasson U, Harel M, Levy I, Malach R. Large-scale mirror-symmetry organization of human occipito-temporal object areas. Neuron. 2003;37:1027–1041. doi: 10.1016/s0896-6273(03)00144-2. [DOI] [PubMed] [Google Scholar]