Significance
Parkinson’s disease (PD) is an incurable neurodegenerative disorder characterized by motor and nonmotor deficits, including cognitive decline and dementia. The protein αSyn is strongly associated with PD pathogenesis, whereas αSyn mutations, such as p.A53T, cause familial forms of PD. Animal models are crucial for understanding PD pathogenesis, but there are limitations in the extent to which these models reproduce faithfully the human disease. Cell-reprogramming technologies allow the generation of human neurons from patients with PD, but it has proven difficult to identify cellular pathologies in induced pluripotent stem cell–derived neurons. In this study, we created a robust p.A53T patient–derived model of PD that captures disease-related phenotypes under basal conditions, thus providing a unique system for studies of disease mechanisms and development of therapeutics.
Keywords: α-synuclein, axonal degeneration, dystrophic neurites, Parkinson’s disease, small molecules
Abstract
α-Synuclein (αSyn) is the major gene linked to sporadic Parkinson’s disease (PD), whereas the G209A (p.A53T) αSyn mutation causes a familial form of PD characterized by early onset and a generally severe phenotype, including nonmotor manifestations. Here we generated de novo induced pluripotent stem cells (iPSCs) from patients harboring the p.A53T mutation and developed a robust model that captures PD pathogenic processes under basal conditions. iPSC-derived mutant neurons displayed novel disease-relevant phenotypes, including protein aggregation, compromised neuritic outgrowth, and contorted or fragmented axons with swollen varicosities containing αSyn and Tau. The identified neuropathological features closely resembled those in brains of p.A53T patients. Small molecules targeting αSyn reverted the degenerative phenotype under both basal and induced stress conditions, indicating a treatment strategy for PD and other synucleinopathies. Furthermore, mutant neurons showed disrupted synaptic connectivity and widespread transcriptional alterations in genes involved in synaptic signaling, a number of which have been previously linked to mental disorders, raising intriguing implications for potentially converging disease mechanisms.
Parkinson’s disease (PD) is the second most common neurodegenerative disease characterized by progressive loss of striatal-projecting dopaminergic neurons of the substantia nigra, resulting in debilitating motor deficits (1). Although motor symptoms are the obvious outward sign, the disease involves a more widespread neuronal dysfunction, leading to early and late nonmotor features such as hyposmia, depression, sleep disturbance, cognitive decline, and dementia (2, 3). The hallmark of PD pathology is the presence of neuronal inclusions, known as Lewy bodies or Lewy neurites, composed mainly of αSyn (4). These protein aggregates are found in various central nervous system areas, shifting the focus from a defect in dopamine neurons to a more widespread disruption that forms a basis for the nonmotor manifestations of PD (5).
Evidence from genetic, biochemical, and biophysical studies supports that αSyn monomers, oligomers (6), fibrils, and other conformers have central roles in the pathogenesis of PD and other synucleinopathies (7). αSyn is the major sporadic PD-linked gene (8), whereas point mutations (9) and multiplications (10) of the locus cause an autosomal dominant form of PD. The best-characterized mutation is p.A53T (G209A SNCA), first identified in families of Italian and Greek ancestry (11). A large number of p.A53T-based in vitro and in vivo animal models have been created for understanding the mechanisms of PD pathogenesis and progression and for assisting in drug development. However, an important limitation is the extent to which these experimental models recapitulate key neuropathological features of the human disease (12). Recent advances in cell-reprogramming technologies have allowed generation of induced pluripotent stem cells (iPSCs) from somatic cells of patients with sporadic or familial PD (13–19), offering the opportunity to elucidate disease phenotypes, investigate the underlying mechanisms, and screen for new drugs. However, it has been difficult to identify cellular pathologies in iPSC-derived PD neurons in the absence of oxidative or other cellular stress.
Here we generated de novo iPSC lines from two male patients with early disease onset who harbored the p.A53T αSyn mutation. By directed differentiation, we created a robust model containing dopaminergic, GABAergic, and glutamatergic neurons that displays disease-relevant phenotypes at basal conditions, including protein aggregation, compromised neurite outgrowth, and axonal neuropathology. Small molecules targeting αSyn reverted the degenerative phenotype under both basal and induced stress conditions, indicating that this strategy may be beneficial in individuals with PD and related disorders. Importantly, mutant neurons showed defective synaptic connectivity and remarkably dysregulated expression of genes involved in synaptic signaling, a number of which have been previously linked to schizophrenia, autism, and bipolar disorder. Our findings uncover disease-associated phenotypes, provide an unexpected link between PD and mental disorders that raises intriguing implications for potentially converging pathological mechanisms in psychiatric and neurologic diseases, and highlight a promising therapeutic strategy for PD and other synucleinopathies.
Results
Generation of iPSCs.
Skin fibroblasts from two p.A53T patients (SI Appendix, Table S1 A and B, for clinical case description) and from one unaffected individual (control) were reprogrammed to iPSCs (20). All generated iPSC lines showed human embryonic stem cell (HUES)-like morphology and expressed pluripotency markers (SI Appendix, Fig. S1A). Two clones from each individual were analyzed further. The differentiation capacity of iPSCs was confirmed by in vitro (SI Appendix, Fig. S1B) and in vivo (SI Appendix, Fig. S1C) germ-layer differentiation assays. Global gene-expression profiling (SI Appendix, Fig. S1D) and RT-PCR for selected pluripotency markers (SI Appendix, Fig. S1E) demonstrated that iPSC clones were distinctly different from the originating fibroblasts and similar to HUES (21). The G209A SNCA mutation was detected in PD fibroblasts and PD-iPSCs but not in control cells (SI Appendix, Fig. S1F). Patient-derived and control iPSC clones exhibited normal karyotype throughout serial passaging (SI Appendix, Fig. S1G).
Neuronal Differentiation of iPSCs.
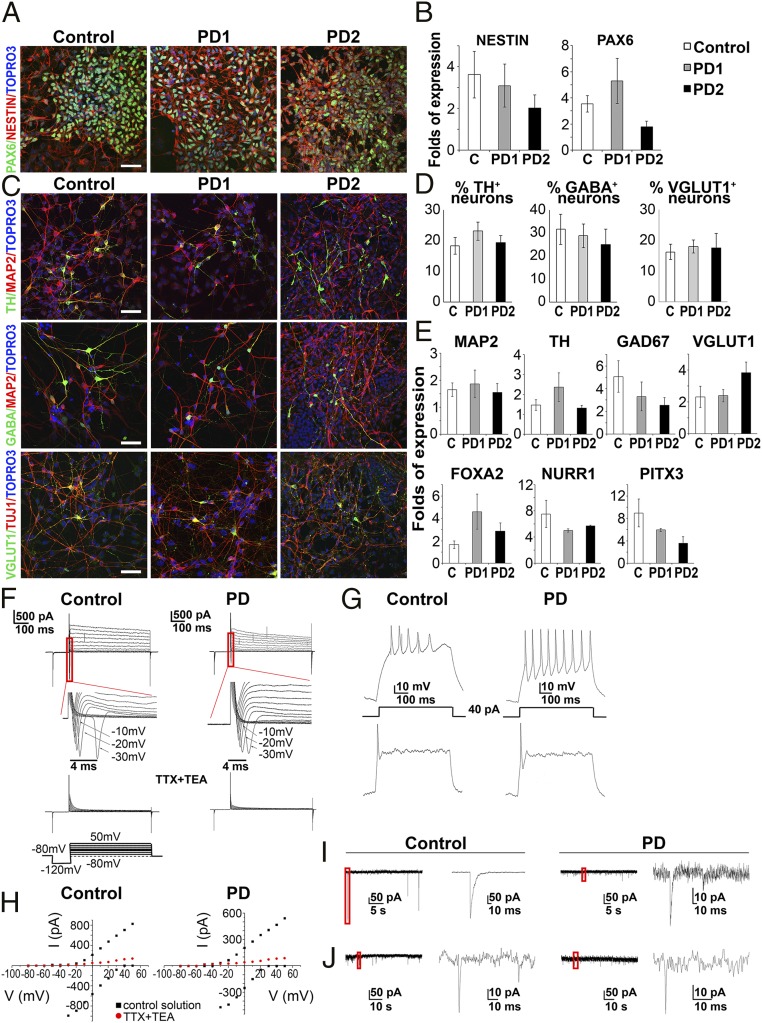
PD and control iPSC lines were differentiated to dopaminergic neurons following a dual SMAD inhibition protocol (22, 23) (SI Appendix, Fig. S2). Neural progenitor cells (NPCs) expressing Pax6 and Nestin were efficiently generated from control and PD iPSC lines (Fig. 1 A and B). Cells were further directed to differentiate into βΙΙΙ-tubulin+ and MAP2+ neurons (Fig. 1C). At 50–60 days in vitro (DIV), 18–23% MAP2+ neurons expressed tyrosine hydroxylase (TH; dopaminergic neurons: control—18.32 ± 2.7%; PD1—23.11 ± 2.9%; PD2—19.38 ± 2.3%; n = 5), 25–30% expressed the neurotransmitter GABA (GABAergic neuron marker: control—31.58 ± 6.5%; PD1—28.78 ± 5.2%; PD2—25 ± 6.6%; n = 5), and 16–18% expressed VGLUT1 (glutamatergic neuron marker: control—16.24 ± 2.4%; PD1—18.02 ± 2.1%; PD2—17.58 ± 4.7%; n = 3), as determined by immunofluorescence analysis (Fig. 1 C and D). RT-qPCR confirmed mRNA expression of the dopaminergic lineage markers FOXA2, NURR1, PITX3, and TH, as well as that of GAD67 and VGLUT1, respectively, characterizing GABAergic and glutamatergic neurons (Fig. 1E). In addition, next-generation transcriptome sequencing (RNA-seq) showed that both PD and control cultures expressed a number of immature and mature neuronal markers as well as neurotransmitter receptors (SI Appendix, Fig. S3A).
Fig. 1.
Directed neuronal differentiation of iPSCs. (A) Immunostaining of control, PD1, and PD2 iPSC-derived NPCs for Pax6 (green) and Nestin (red). Cell nuclei are counterstained with TOPRO3 (blue). (Scale bar, 40 µm.) (B) RT-qPCR analysis of Nestin and Pax6 mRNA expression normalized to GAPDH levels. Data represent mean ± SEM (n = 3–5 for each cell line). (C) Immunostaining of iPSC-derived neurons at 50 DIV. Cells were stained (red) for MAP2 or βIII-tubulin (TUJ1) and (green) for TH (dopaminergic neuron marker), GABA (GABAergic neuron marker), and VGLUT1 (glutamatergic neuron marker). TOPRO3+ nuclei are in blue. (Scale bar, 40 µm.) (D) Quantification of TH+, GABA+, and VGLUT1+ neurons as percentage of MAP2+ cells in control, PD1, and PD2 lines. Data represent mean ± SEM (n = 3–5 for each cell line). (E) RT-qPCR analysis of mRNA expression for MAP2, TH, GAD67 (GABAergic neuron marker), and VGLUT1, as well as for the dopaminergic lineage markers FOXA2, NURR1, and PITX3. Data represent mean ± SEM (n = 3–5 for each cell line). (F–J) Representative electrophysiological recordings from control and PD cells with typical neuronal morphology between 55 and 70 DIV. (F, Upper panels) Superimposed traces of currents evoked by depolarizing voltage steps (scheme of the protocol is shown in the Bottom Left panel). (Insets below Upper panels) Expanded from red lines rectangular traces of fast-activating, fast-inactivating inward Na+ currents evoked by depolarizing voltage steps. (Bottom panels) Superimposed traces of currents evoked by the same set of depolarizing voltage steps after 1-min preapplication of TTX (tetrodotoxin, 1 µM) + TEA (tetraethylammonium, 20 mM). Note the strong inhibition of both inward and outward components. (G) Examples of voltage deflections and different patterns of action potential generation induced by current injections (40 pA). Current-clamp recordings from two control cells (Left traces) and two PD-derived cells (Right traces). Protocol of current step is shown. (H) Current–voltage relations of outward K+ and inward Na+ currents (black) under control conditions and after 1 min application of 1 µM TTX + 20 mM TEA (red). (I and J) Spontaneous synaptic activity of the neurons measured at −70 mV. GABAergic (I) and glutamatergic (J) synaptic currents are depicted.
Functional maturation of iPSC-derived neurons was demonstrated by electrophysiology. Between 55 and 70 DIV, patch-clamp recordings showed that >70% of neurons exhibited transient inward sodium currents and sustained outward potassium currents (53 from 68 control cells and 52 from 64 PD cells) that could be blocked by specific pharmacological inhibitors (Fig. 1 F and H). Current-clamp recordings demonstrated that both control and PD cells developed the ability to fire action potentials in response to somatic current injections (Fig. 1G). Several output patterns were observed, including single bursts, repetitive firing, or trains of action potentials, indicating that iPSC-derived neurons had reached varying degrees of maturation. Additionally, cells responded to key neurotransmitters such as GABA, glutamate, glycine, and nicotine, confirming the presence of functional receptors (SI Appendix, Fig. S3B). Both PD and control neurons exhibited spontaneous synaptic activity, eliciting inhibitory (GABAergic) and excitatory (glutamatergic) postsynaptic currents (Fig. 1 I and J) that could be blocked by specific antagonists. Notably, expression of spontaneous synaptic activity was about twofold higher for control cells than for PD cells. Thus, in whole-cell recordings, 27.4% of control cells exhibited synaptic currents (n = 62), whereas only 16% (n = 30) and 12.5% (n = 40) of PD1 and PD2 cells, respectively, generated synaptic events.
Pathological Phenotypes in PD iPSC-Derived Neurons.
PD-associated dementia is a major nonmotor manifestation, most prevalent in patients carrying the highly penetrant p.A53T mutation (24–26). Because p.A53T pathology is not limited to dopaminergic neurons, we took advantage of our iPSC-based system containing a mixed neuronal population as a more comprehensive model for p.A53T synucleinopathy.
Because the p.A53T mutation induces pathological αSyn aggregation (27), we confirmed by RNA-seq that the mutant SNCA allele is expressed in patient neurons (SI Appendix, Fig. S4). Immunofluorescence revealed that αSyn protein was present in the soma and neurites of both PD and control neurons, albeit more cells were strongly positive for αSyn in PD cultures (Fig. 2A). An increase in αSyn was confirmed in PD neurons by Western blotting, although quantification of αSyn mRNA by RT-qPCR did not show statistically significant differences between control and PD neurons (Fig. 2C). Notably, the pathological form of αSyn that is phosphorylated on serine 129 was detected primarily in PD cultures, revealing the existence of Lewy-like neurites by immunofluorescence (Fig. 2B), and in sister cultures by immunoblot (Fig. 2C).
Fig. 2.
Pathological phenotypes of PD iPSC-derived neurons. (A) Immunostaining for αSyn (green) and TUJ1 (red) in control, PD1, and PD2 iPSC-derived neurons at 50 DIV. (Insets) The marked regions at higher magnification. (Scale bar, 40 µm.) (B) Immunostaining for Ser129-phosphorylated αSyn [p(Ser129)αSyn] (green) and TUJ1 (red) in control and PD iPSC-derived neurons at 50 DIV. (Scale bar, 40 µm.) pS129 staining in PD neurites is shown at higher magnification (Right). (C, Upper graph) Quantification of αSyn mRNA by RT-qPCR in control (C), PD1, and PD2 iPSC-derived neurons at 48 DIV. Data represent mean ± SEM (n = 3–5 for each cell line). (Lower panel) Detection of αSyn and p(Ser129)αSyn by Western blot (WB); GAPDH shows equal protein loading. (D) Thioflavin S staining shows protein aggregates in PD cultures at 50 DIV. Clearance of protein depositions by proteinase K. (Scale bar, 20 µm.) Costaining of aggregated proteins (aggresomes; arrowheads; Upper micrograph in red) and αSyn (green) inside inclusion bodies (merged picture, Lower micrograph). (Scale bar, 20 μm.) (E) Immunostaining for TUJ1 in control, PD1, and PD2 iPSC-derived neurons at 50 DIV. Higher magnification (Lower panels) shows neurites with swollen varicosities and spheroid inclusions (arrowheads in PD1 and PD2 neurons) that frequently end up in fragmented processes (arrow). (Scale bar, 10 µm.) (F) Coimmunostaining for αSyn (green) and TUJ1 (red) in PD iPSC-derived neurons shows αSyn+ swollen varicosities (arrowheads) in neurites with earlier (i) and more advanced (ii) signs of degeneration. (Scale bars, 10 µm.) (G) Coimmunostaining for TUJ1 (red) and the axonal protein TAU (green) in iPSC-derived neurons reveals colocalization of the two proteins in swollen varicosities and axonal fragments. Arrowheads and arrows indicate blebbed and fragmented axons, respectively. (Scale bar, 10 µm.)
Next, we examined potential aggregate formation by thioflavin S staining. Grain-like protein aggregates, also containing αSyn (SI Appendix, Fig. S5A), were detected in PD cultures at 50 DIV, whereas control neurons were completely devoid of thioflavin S-positive deposits (Fig. 2D). The protein nature of these aggregates was confirmed by treatment with proteinase K that efficiently cleared the majority of protein depositions in PD neurons (Fig. 2D). The presence of αSyn-positive protein aggregates inside inclusion bodies (aggresomes) was further validated using a fluorescence-based assay for detection of aggregated protein cargo (28) in combination with αSyn immunofluorescence (Fig. 2D and SI Appendix, Fig. S5B). Concomitant with aggregate formation, PD neurons started to exhibit distinct morphological features that distinguished them from control cells and were indicative of extensive neuritic pathology and degeneration. PD neuronal processes immunostained for βIII-tubulin (TUJ1) appeared more contorted with αSyn+ swollen varicosities and large spheroid inclusions (Fig. 2 E and F) similar to the dystrophic neurites identified in the brain of p.A53T patients (29, 30). TUJ1+/αSyn+ swellings could be detected in otherwise morphologically intact axons, most likely marking an early event in neuritic degeneration (Fig. 2F, i). Quite often the distorted neurites of PD neurons ended up in fragmented processes reminiscent of the thread-like pathology found in the brain of p.A53T patients (29, 30) (Fig. 2E, arrow). Interestingly, the pathological phenotype of neuronal processes was not evident in cells stained for the somatodendritic marker MAP2, suggesting an axonal neuropathology, which was confirmed by staining for the axonal protein Tau (Fig. 2G; for quantification of axonal degeneration index, see Fig. 6I).
Taken together, these data show that an iPSC-based model of p.A53T PD recapitulates closely the neuropathological features identified in the brain of patients carrying the mutation, simulating reliably the human disease.
Synaptic Defects in PD iPSC-Derived Neurons.
To gain molecular insight into the pathogenic mechanisms caused by the p.A53T mutation, we performed transcriptome-wide RNA-seq at specific stages of the differentiation procedure, corresponding to iPSCs, iPSC-derived NPCs (13 DIV) and neurons (48 DIV). Total RNA from two control lines (C1-1 and C1-2) and two PD lines (PD1-1 and PD1-2), the originating fibroblasts and from HUES, HUES-derived NPCs, and neurons, were used for cDNA library preparation. Following poly-A selection, RNA-seq was performed for global gene-expression profiling. Principal component analysis confirmed a reset in gene expression following fibroblast reprogramming and demonstrated that control and PD1 cells clustered primarily according to their differentiation stage, illustrating similar transcription profiles depending on cell state (Fig. 3 A and B). Nevertheless, a large number of differentially expressed mRNAs were identified between PD1 and control samples (P < 0.05; Fig. 3C and SI Appendix, Table S1C). In particular, 647 differentially expressed genes (379 down-regulated and 268 up-regulated) were identified between PD1-1/PD1-2 and C1-1/C1-2 neurons (P < 0.05; Fig. 3D). Of these, 34.62% (n = 224) corresponded to noncoding transcripts, including long noncoding RNAs (n = 93), antisense transcripts (n = 37), and pseudogenes (n = 94), and were excluded from further analysis.
Fig. 3.
Summary of RNA-seq analysis. (A) Graph depicting the first two principal components (PC1 and PC2) of all sequenced samples. Principal component analysis was performed on the 1,000 genes having the highest expression variance across all samples. All groups exhibit distinct expression patterns and within-group uniformity. Samples: fibroblasts (green)—1, fetal; 2, control (C1); and 3, PD1; HUES/iPSCs (magenta)—4, HUES; 5, C1-1 iPSCs; 6, C1-2 iPSCs; 7, PD1-1 iPSCs; and 8, PD1-2 iPSCs; iPSC-derived NPCs (purple)—9, HUES-NPCs; 10, C1-1 NPCs; 11, C1-2 NPCs; 12, PD1-1 NPCs; and 13, PD1-2 NPCs; iPSC-derived neurons (orange)—14, HUES-neurons; 15, C1-1 neurons; 16, C1-2 neurons; 17, PD1-1 neurons; and 18, PD1-2 neurons. (B) Sample gene expression distance heat map calculated using all expressed genes in all samples: fibroblasts (green), HUES/iPSCs (magenta), NPCs (purple), and iPSC-derived neurons (orange). (C) Heat map depicting the expression of the 500 genes with the highest mean expression across all sequenced samples. (D) Heat map of differentially expressed transcripts between PD1-1/PD1-2 and C1-1/C1-2 iPSC-derived neurons. A total of 647 differentially expressed transcripts (268 up-regulated and 379 down-regulated) were detected between PD and control neurons (P < 0.05). Higher expressions are in red, and lower expressions are in blue. (E) Enrichment analysis of the significantly (P < 0.05) altered genes in the RNA-seq analysis of PD versus control iPSC-derived neurons against GO terms.
Detailed bioinformatics analyses revealed several striking features of differentially expressed genes. Enrichment analysis based on Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and Reactome demonstrated significant perturbations in genes associated with metabolic function, cell cycle, extracellular matrix (ECM) and cytoskeletal organization, neuronal differentiation, maturation, and function (Fig. 3E and SI Appendix, Table S1D). Specifically, the alterations in GO categories of neurotransmitter receptor activity and binding, terminal button, nerve terminal, synapse, postsynaptic membrane, and ECM indicated that multiple neuronal pathways were compromised in PD neurons under basal culture conditions. Using Information Hyperlinked Over Proteins (iHOP) and PubMed–National Center for Biotechnology Information, we identified 15 genes associated with PD and another nine with other related neurodegenerative diseases, such as Alzheimer’s and Huntington’s (SI Appendix, Table S1E). Importantly, a significant portion of altered mRNA transcripts (29 genes) was associated with psychiatric diseases, such as autism, schizophrenia, and bipolar disorder (SI Appendix, Table S1E), where synaptic dysfunction and eventually synaptic loss comprise the most prominent features (31–33). This was a rather unexpected finding that prompted us to further analyze gene transcripts encoding proteins involved in presynaptic vesicle formation and trafficking, vesicular and plasma membrane neurotransmitter transporters, axonal guidance, postsynaptic organization, and synaptic cell adhesion. In total, 92 relevant genes were altered; 80 were significantly down-regulated and 12 were up-regulated (SI Appendix, Table S1 F–K). Of those, 20 encoded for presynaptic proteins (SI Appendix, Table S1F), 18 for postsynaptic molecules (SI Appendix, Table S1G), 18 for trans-synaptic adhesion molecules (SI Appendix, Table S1 H and I), and 14 for axon guidance proteins (SI Appendix, Table S1J and Fig. 4 A–E). Selected genes representative of the above categories were validated by RT-qPCR using independent samples of PD1-1, PD1-2, C1-1, and C1-2, as well as two clones of the second patient (PD2-1 and PD2-2). For all 16 genes tested, significant down-regulation was confirmed in both clones from PD1 and PD2 (Fig. 4G). Of these, SYN3, SV2C, RPH3A, and DOC2B are found in the presynaptic area, where they are involved in synaptogenesis and neurite extension, synaptic vesicle organization, spontaneous synaptic vesicle exocytosis, and regulation of neurotransmitter release, respectively (34–37). Three of six members of the human SLITRK family (SLITRK1, -2, and -4) located at the postsynaptic membrane to act as organizers of excitatory synapse formation (38) were also down-regulated in PD1 and PD2 neurons, with SLITRK2 and -4 being hardly detectable. DLGAP2, another synaptic organizer enriched in the postsynaptic density (PSD) (39), as well as GRIN2D and GRIP2, which encode the NMDA glutamate receptor subunit ε-4 and the glutamate receptor interacting protein 2, respectively, were all down-regulated in PD1 and PD2 neurons. Two genes of the cadherin/proto-cadherin family (SI Appendix, Table S1I), CDH13 and CDH15 (40), were dramatically down-regulated in PD1 and PD2 neurons, further enhancing our notion of defective synapse formation and function.
Fig. 4.
Gene expression analysis of iPSC-derived neurons. (A–F) Differential gene expression between control (clones C1-1 and C1-2) and PD1 (clones PD1-1 and PD1-2) iPSC-derived neurons at 48 DIV. Heat maps of genes encoding presynaptic (A) and postsynaptic proteins (B), trans-synaptic adhesion molecules (C), cadherins (D), axon guidance molecules (E), and calcium-associated proteins (F). High expressions are in red and low expressions are in blue. (G) RT-qPCR analysis of selected genes in control (C), PD1, and PD2 iPSC-derived neurons at 48 DIV: presynaptic SYN3, SV2C, RPH3A, and DOC2B; postsynaptic DLGAP2 and receptors GRIN2D and GRIP2; trans-synaptic adhesion SLITRK1, -2, and -4; cadherins CDH 13 and 15, genes associated with axon guidance FABP7 and ABLIM3; and calcium-associated RCN3 and HPCA. Gene expression normalized to GAPDH. Data represent mean ± SEM (one-way ANOVA, *P < 0.05, **P < 0.01, ***P < 0.001, n = 3–5 for each cell line).
Secreted glycoproteins belonging to the WNT family (41) are another class of molecules that promote synaptogenesis and regulate synaptic function. The mRNA expression of the family members WNT3A, WNT5A, WISP1, RSPO1, RSPO3, FRZB, and DKK2 was found differentially expressed (SI Appendix, Table S1K). Notably, a significant number of calcium-associated proteins (SI Appendix, Table S1L, and Fig. 4F), such as RCN3, HPCA, CCBE1, CACNA2D4, and CACNA1D, together with various neurotransmitter receptors and channels (SI Appendix, Table S1G) known to be involved in synaptic function and neurotransmission (42), were also significantly down-regulated at the mRNA level. Of these, RCN3, HPCA, GRI2ND, and GRIP2 were validated by RT-qPCR in both PD1 and PD2 neurons (Fig. 4G). Overall, our data show that the p.A53T mutation affects the expression of pre- and postsynaptic genes involved in different processes of synapse formation, maturation, and function. A number of genes associated with axon guidance were also perturbed in PD1 neurons (SI Appendix, Table S1J and Fig. 4E) with FABP7 and ABLIM3 verified in both PD1 and PD2 (Fig. 4G).
To investigate potential consequences of synaptic gene dysregulation, we assessed the ability of control and PD neurons to form synaptic connections. To this end, neurons were seeded on a feeder layer of mouse primary astrocytes to enhance their maturation for up to 100 DIV. In these cultures, the characteristic immunofluorescence puncta of the presynaptic protein synapsin1 (SYN1) were clearly detected on the neurites of control and PD neurons at 70 and 100 DIV (Fig. 5A). However, costaining of SYN1 with MAP2 revealed less complex neuronal networks with thinner or less fasciculated neuronal processes in PD neurons. Additionally, in many PD neurons, SYN1 was retained mainly in the soma rather than in the processes (Fig. 5A). Synapse formation was identified by coimmunostaining for SYN1 and the postsynaptic marker PSD95 (Fig. 5B). Quantification of the number of SYN1+/PSD95+ puncta pairs showed a 27% reduction in the number of synaptic contacts in PD neurons at 70 DIV (control 2.64 ± 0.18 pairs per 10 μm vs. PD 1.93 ± 0.15 pairs per 10 μm, P = 0.005; Fig. 5C) and a 22% reduction at 100 DIV (control 2.86 ± 0.26 pairs per 10 μm vs. PD 2.22 ± 0.13 pairs per 10 μm, P = 0.045; Fig. 5D), a phenotype strongly linked to the dysregulated expression of synaptic genes identified in p.A53TαSyn neurons.
Fig. 5.
Synaptic connections in iPSC-derived neurons. (A) Immunofluorescence puncta of the presynaptic protein synapsin 1 (SYN1, green) in control and PD MAP2-positive (red) neurons seeded on mouse astrocytes and maintained for 100 DIV. Arrowheads indicate that SYN1 remains in the soma of many PD neurons in contrast to control neurons. (Insets) The marked regions at higher magnification. (Scale bar, 40 µm.) (B) Maximum projection confocal images showing SYN1+ (red) and PSD95+ (green) synaptic puncta pairs in control and PD neurons. (Scale bar, 10 µm.) (C and D) Quantification of the number of SYN1+/PSD95+ puncta pairs per 10 µm at 70 DIV (C) and at 100 DIV (D) in control and PD neurons. Data represent mean ± SEM (Student’s t test, *P < 0.05, **P < 0.01).
Rescue of Neuropathological Phenotypes of PD Neurons by Small Molecules Targeting αSyn.
Because the molecular perturbations in PD neurons indicated dysregulation in neurostructural processes and network formation, we further examined the morphology of control and PD neurons 7 d after transduction with a lentiviral vector for expression of the red fluorescent protein DsRed under the control of the human synapsin 1 promoter (LV.SYN1.DsRed) to facilitate imaging of single neurons (Fig. 6A). Although at 50 DIV soma size was comparable (Fig. 6B), neurite length was significantly reduced in PD neurons (control: 293.91 ± 27.39 μm; PD1: 142.85 ± 19.80 μm; PD2: 102.45 ± 15.35 μm; control vs. PD1: P < 0.0001; control vs. PD2: P < 0.0001; Fig. 6C), as well as the total number of neurites extending from the soma (control: 3.97 ± 0.12; PD1: 3.42 ± 0.14; PD2: 3.15 ± 0.14; control vs. PD1: P = 0.011; control vs. PD2: P < 0.0001; Fig. 6 D and E). To check whether this phenotype is causally related to pathological p.A53T-αSyn, we used three de novo in silico-designed compounds—NPT100-18A (43), NPT100-14A (patent #8,450,481), and ELN484228 (44)—that all interact with and reduce αSyn toxicity by interfering with αSyn oligomer formation through distinct mechanisms. Their addition in the 1 to 20 nM range did not induce toxicity in control or PD neurons. All three compounds could quench the differences observed in neurite length between control and PD1 neurons when added at a final concentration of 2 nM throughout the neuronal differentiation period (Fig. 6F). Treatment with NPT100-18A that interacts with the C terminus of αSyn was most effective in restoring the number of neurites extending from the soma of PD neurons (DMSO: 3.72 ± 0.22; NPT100-18A: 4.83 ± 0.24; ELN484228: 4.23 ± 0.22; NPT100-14A: 3.8 ± 0.19; DMSO vs. NPT100-18A: P = 0.013; Fig. 6G), whereas it had no effect in control neurons (DMSO: 4.68 ± 0.23; NPT100-18A: 4.53 ± 0.26; ELN484228: 4.4 ± 0.28; NPT100-14A: 4.5 ± 0.34; Fig. 6G). Importantly, all three compounds rescued to a large extent the dramatic pathology observed in TUJ1+ PD neurons as they alleviated significantly the existence of distorted/degenerating axons, with NPT100-18A and ELN484228 being most effective (axon degeneration index: control–DMSO: 1 ± 0.13; PD1–DMSO: 8.83 ± 0.67; PD1–NPT100-18A: 2.89 ± 0.29; PD1–ELN484228: 3.34 ± 0.31; PD1–NPT100-14A: 5.59 ± 1.21; control–DMSO vs. PD1–DMSO: P < 0.0001; PD1–DMSO vs. PD1–NPT100-18A: P < 0.0001; PD1–DMSO vs. PD1–ELN484228: P < 0.0001; PD1–DMSO vs. PD1–NPT100-14A: P = 0.002; Fig. 6 H and I). Overall, these observations causally link the disease-related phenotypes of PD neurons to αSyn pathology.
Fig. 6.
Reversal of the neuropathological phenotype of PD iPSC-derived neurons by small molecules targeting αSyn. (A) Neurite analysis. Representative fluorescent images of iPSC-derived neurons at 50 DIV transduced with a lentiviral vector expressing red fluorescent protein DsRed under the control of the human synapsin 1 promoter (LV.SYN1.DsRed). (Scale bar, 40 µm.) Quantification of soma size (B), neurite length (C), and number of neurites extending from the soma (D and E) in SYN1.DsRed-positive cells. Data represent mean ± SEM (one-way ANOVA, *P < 0.05, ***P < 0.001, n = at least 100 single DsRed-labeled neurons for each cell line). (F and G) Quantification of neurite length (F) and the number of neurites extending from the soma (G) of SYN1.DsRed-positive cells in control and PD1 neurons without treatment (DMSO) and after exposure to NPT100-18A, ELN484228, and NPT100-14A (2 nM). Data represent mean ± SEM (Student’s t test for control–DMSO vs. PD1–DMSO, **P < 0.01, one-way ANOVA for control–DMSO vs. control–compounds and for PD1–DMSO vs. PD1–compounds, *P < 0.05, n = at least 100 single DsRed-labeled neurons for each condition). (H) Axonal pathology observed by TUJ1 immunostaining in PD1 cells is significantly improved by compound treatment. (Scale bar, 40 µm.) (I) Quantification of axonal degeneration by measuring the ratio of TUJ1+ spots over the total TUJ1+ area in untreated (DMSO) or compound-treated PD1 iPSC-derived neurons. Data represent mean ± SEM (one-way ANOVA, **P < 0.01, ***P < 0.001, n = 20 randomly selected fields for each condition).
Reversal of Induced-Stress Phenotypes of PD Neurons by Small Molecules Targeting αSyn.
To check whether the above-used small molecules were also effective under induced stress conditions, we accelerated neuronal degeneration and cell death by treatment with the proteasome inhibitors epoxomicin and MG-132 that interfere with αSyn clearance via the proteasome. Initial experiments confirmed a dose-dependent induction of cell death by both inhibitors as assessed by lactate dehydrogenase (LDH) release and an increased sensitivity of PD neurons to proteasome stress (SI Appendix, Fig. S6). In subsequent experiments, each inhibitor was added for 24 h at the concentration that induced the largest difference between control and PD cells. Epoxomicin (1 μM) and MG-132 (10 μM) treatment evoked a significant increase in cleaved caspase 3 immunoreactivity and a pronounced disruption of the MAP2+ network (Fig. 7A), consistent with the levels of LDH release in PD neurons (Fig. 7 B and C). Quantification of LDH release also revealed that untreated PD neurons were more susceptible to death (Fig. 7 B and C).
Fig. 7.
Rescue of the cytotoxic effect of proteasome inhibition on PD iPSC-derived neurons. (A) Representative images of control and PD iPSC-derived neurons (55 DIV) immunostained for active cleaved caspase 3 (green) and MAP2 (red) after 24 h incubation with or without the proteasome inhibitors epoxomicin (1 µM) and MG-132 (10 µM). (Scale bar, 40 µm.) (B and C) Quantification of LDH activity (490–630 nm) in the culture supernatant as a measure of cytotoxicity in cells treated with epoxomicin (B) or MG-132 (C) under the same conditions as above. Data represent mean ± SEM from LDH activity in supernatants derived from 4 to 32 wells of four to six independent experiments performed in neurons derived from two iPSC lines from each subject (one-way ANOVA or ANOVA in Ranks for between-group comparisons followed by Dunn’s test or Holm–Sidak for pairwise comparisons, *P < 0.05, ***P < 0.001). (D and E) Induced-cytotoxicity experiments in iPSC-derived neurons (55–57 DIV) untreated (DMSO) or treated with small-molecule inhibitors of αSyn aggregation NPT100-18A, NPT100-14A, and ELN484228 (2 µM). Representative fluorescent images show TUJ1-positive neuronal network in DMSO and compound-treated cells after (D) epoxomicin (1 µM) or (E) MG-132 (10 µΜ) addition for 24 h. (Scale bar in D, 40 µm.)
We then performed a series of induced stress experiments in neurons treated with NPT100-18A, NPT100-14A, or ELN484228. At 48 DIV, neurons were replated, and 7–9 d later, epoxomicin and MG-132 were added for 24 h. Epoxomicin-treated PD neurons showed an extensively degenerate MAP2+ network that was most effectively protected by NPT100-18A and to a lesser extent by NPT100-14A and ELN484228 (Fig. 7D). Similar results were obtained in MG-132–treated PD neurons, where NPT100-18A and NPT100-14A preserved the MAP2+ network (Fig. 7E).
Discussion
We report pathological phenotypes and protective effects of small-molecule inhibitors of αSyn aggregation in iPSC-derived neurons from patients with PD carrying the p.A53T mutation. Our data strongly support an iPSC-based model that faithfully simulates disease pathogenesis and uncovers disease-relevant phenotypes under basal conditions. These include protein aggregation, compromised neuritic outgrowth, and axonal αSyn/Tau-associated pathology, resulting in decreased synaptic connectivity. Accordingly, mutant neurons showed a profound dysregulation in the expression of genes involved in synaptic signaling, including genes associated with synapse formation, trans-synaptic adhesion, and postsynaptic organization. Importantly, small molecules targeting αSyn could correct the degenerative phenotype of PD neurons, thus providing a direct mechanistic link and a therapeutic strategy that may be beneficial for patients with PD and related disorders.
In addition to its involvement in rare familial PD cases, αSyn consists of the major sporadic PD-linked gene identified so far, underlying its importance in PD initiation and progression. Patients harboring the p.A53T mutation in αSyn manifest prominent motor and nonmotor symptoms, including autonomic dysfunction, cognitive decline, dementia, and psychotic features (25, 26) (SI Appendix, Table S1B). It is now recognized that a stronger focus on the nonmotor symptoms is essential for assessing and treating the disease-specific and drug-induced psychiatric symptoms. Additionally, increasing evidence suggests that the neuropsychological deficits seen early in the course of the disease might also be a powerful predictor of the overall progression of cognitive dysfunction to dementia, with implications for early pharmacological intervention (45). Our findings from human iPSC-derived neurons suggest that disruption of synaptic connections may form a basis for the nonmotor deficits in p.A53T patients with PD.
A striking finding in our study is that patient-derived neurons capture PD neuropathological processes over a relatively short period in culture and in the absence of induced stress. They exhibit thioflavin S-positive aggregates, αSyn-containing intracellular inclusion bodies, and extensive neuritic pathology with grain-like inclusions and knotted spheroids, similar to the structures detected in the neocortex, deep cortical areas, hippocampus, forebrain, and midbrain of p.A53T patients (29, 30). Interestingly, the appearance of swellings marked an early event in neuritic degeneration. In agreement, overexpression of mutant p.A53T-αSyn in rats induced dystrophic axons and alterations in axonal transport that preceded neuronal loss (46). Tau-positive inclusions were also prominent, indicating a severe axonal pathology consistent with the presence of extensive Tau lesions in the brains of p.A53T patients (29). Our data support the hypothesis that Tau and αSyn are involved in shared or converging pathways in the pathogenesis of PD, as well as in the development of cognitive impairment and dementia in patients with familial and possibly also idiopathic PD (47, 48). These findings have important implications for understanding the interface between Tau and αSyn pathways in neurodegenerative disorders.
The extensive axonal pathology and the degenerative phenotype of PD neurons could be rescued by small-molecule inhibitors that interfere specifically with αSyn aggregation (patent #8,450,481) (43, 44). This report demonstrates the therapeutic effect of antiaggregation compounds in patient iPSC-derived neurons that not only improved their basal neuropathological features but also restored the neuronal network after proteasome inhibition, suggesting a positive impact even under conditions of increased cellular stress. NPT100-18A, which is most effective in patient neurons (Figs. 6 and 7), has been recently shown by Masliah and coworkers (43) to reduce αSyn toxicity in transgenic rodent models through a mechanism that involves αSyn displacement from the membrane. Hence, in the absence of isogenic gene-corrected control lines, the protective effects of these small molecules provide a direct link between the disease-associated phenotypes identified here and pathological αSyn. Most important, our data on patient iPSC-derived neurons uniquely demonstrate that targeting αSyn is a feasible therapeutic approach for developing new disease-modifying treatments for PD and other synucleinopathies.
An important observation is the endogenous dysregulation in p.A53T PD neurons, most notably down-regulation, of genes involved in various neuronal processes such as axon growth and transport, differentiation and maturation, and synaptic signaling. The presynaptic molecules altered included synapsin III (SYN3), a high-affinity αSyn interactor that has been found to colocalize with αSyn in the caudate-putamen of patients with PD (49) and to work cooperatively with αSyn to regulate synaptic function in dopaminergic neurons. SV2C, a molecule that also colocalizes with αSyn in synaptic puncta (50) and is involved in synaptic vesicle recycling (51), was also misrepresented. Additionally, genes such as DOC2B, a Ca2+-dependent protein involved in vesicle trafficking, and RPH3A, a synaptic vesicle fusion molecule, were found diminished in PD neurons. The majority of presynaptic genes in our study exhibited decreased expression, a finding that confirms the loss of critical presynaptic proteins and the deficits in neurotransmitter release previously described in transgenic mice overexpressing human αSyn (52). Within the same context, Scott et al. (52) have shown that overexpression of αSyn in cultured hippocampal neurons promotes a reduction in the levels of synaptic proteins at presynaptic terminals, a phenomenon termed “vacant synapses.” Moreover, studies in sporadic and experimental Parkinson’s disease suggest abnormalities in axonal transport proteins and alterations in synaptic activity (53, 54). Future experiments should associate the altered gene expression demonstrated here with disturbances in protein levels.
The postsynaptic side of the synapse and its complex molecular composition largely depend on signals received from the presynaptic terminal. Correspondingly, PD neurons exhibited significant changes in the expression of various postsynaptic molecules, including DLGAP2, GRIND2, and GRIP2. DLGAP2 is a membrane-bound synapse organizer the rare mutations of which are associated with autism (55), and GRIN2D and GRIP2 are components of the excitatory synapse. Furthermore, p.A53T-αSyn expression in iPSC-derived neurons affected greatly synaptic cell-adhesion molecules, required to mediate synaptic contact and alignment for proper synaptogenesis and maturation. From those cell-adhesion molecules, a striking number of the cadherin/proto-cadherin family members (40) had reduced expression, including CDH13 and CDH15, which are strongly linked to autism (56). Other autism-associated genes identified with diminished expression in PD neurons are the three members of the postsynaptic Slit- and Trk-like protein family, SLITRK1, -2, and -4, all promoting excitatory synapse formation through binding to presynaptic protein tyrosine phosphatases (38, 57). Because neuronal communication depends on the formation of trans-synaptic adhesion complexes, their misrepresentation in PD neurons points to defective synaptogenesis. Indeed, PD neurons transduced with LV.SYN1.DsRed showed impaired neuritic growth, whereas PD neurons left to mature up to 100 d on an astrocytic feeder layer had significantly reduced synaptic contacts. The molecular and cellular phenotypes recognized in our study were corroborated by initial electrophysiological observations indicating changes in functional synaptic connectivity that deserve further investigation.
Defects in synaptogenesis and dysfunction in neuronal communication form the basis for neurodevelopmental disorders and a common feature of neurological diseases (58). Our data support the hypothesis that common mechanisms may operate in neurons in these diverse pathologies that may be activated by the presence of pathological αSyn and/or other aggregated proteins (59–61). This is an intriguing hypothesis, especially in the light of recent epidemiological findings that high rates of Parkinsonism are diagnosed in adults with autism (62).
There are currently no effective treatments for PD. Here, we have used iPSC technology to generate a cellular model that simulates key neuropathological features of the human disease with robust and reproducible phenotypes in patient-derived neurons. We reveal previously unrecognized impaired synaptic connectivity in p.A53T neurons and axonal neuropathology that could be reverted by small molecules targeting αSyn. Given the urgent need for effective drug development, our approach provides a basis for attempting such strategies to treat PD and other synucleinopathies. Furthermore, our cellular model, which has uncovered mechanistic insights into disease pathophysiology, is a powerful tool for functional analyses and can serve as a platform for identification and testing of innovative disease-modifying compounds.
Materials and Methods
Extended experimental procedures are described in SI Appendix, SI Materials and Methods.
Study Approval.
All procedures for generation of human iPSCs were approved by the Scientific Council and Ethics Committee of Attikon University Hospital (Athens, Greece), which is one of the Mendelian forms of Parkinson’s Disease clinical centers, and by the Hellenic Pasteur Institute Ethics Committee overlooking stem cell research. Informed consent was obtained from all donors before skin biopsy.
Data and Materials Availability.
RNA-seq data have been deposited in the Gene Expression Omnibus database under accession code GSE84684.
Supplementary Material
Acknowledgments
We thank Prof. Fred Gage (Salk Institute for Biological Studies) for providing the plasmids for production of the lentiviral vector LV.SYN1.DsRed; Prof. Socrates Tzartos (Hellenic Pasteur Institute) for electrophysiology support; and Lina Florentin and Franci Sachinidi (Alpha Lab) for karyotype analysis. This work was supported by the following grants to R.M.: European Union FP7 REGPOT NEUROSIGN Project 264083; the Hellenic General Secretariat for Research and Technology Grants SYNERGASIA Noiseplus 09SΥΝ-21-969 and ARISTEIA 2272 ParkinsonTransMed; the Fondation BNP Paribas; Empeirikion Foundation; the Fondation Santé; and the Institut Pasteur Grants PTR 417 and 523.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: RNA-seq data have been deposited in Gene Expression Omnibus (accession no. GSE84684).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1617259114/-/DCSupplemental.
References
- 1.Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 2.Obeso JA, et al. Missing pieces in the Parkinson’s disease puzzle. Nat Med. 2010;16:653–661. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- 3.Schapira AH, Tolosa E. Molecular and clinical prodrome of Parkinson disease: Implications for treatment. Nat Rev Neurol. 2010;6:309–317. doi: 10.1038/nrneurol.2010.52. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, Braak E. Pathoanatomy of Parkinson’s disease. J Neurol. 2000;247:II3–II10. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- 5.Bendor JT, Logan TP, Edwards RH. The function of α-synuclein. Neuron. 2013;79:1044–1066. doi: 10.1016/j.neuron.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dettmer U, et al. Corrigendum: Parkinson-causing α-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation. Nat Commun. 2015;6:8008. doi: 10.1038/ncomms9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14:38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simón-Sánchez J, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrucci S, Ginevrino M, Valente EM. Phenotypic spectrum of alpha-synuclein mutations: New insights from patients and cellular models. Parkinsonism Relat Disord. 2016;22:S16–S20. doi: 10.1016/j.parkreldis.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Chartier-Harlin MC, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 11.Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 12.Tieu K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb Perspect Med. 2011;1:a009316. doi: 10.1101/cshperspect.a009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devine MJ, et al. Parkinson’s disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nat Commun. 2011;2:440. doi: 10.1038/ncomms1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan SD, et al. Isogenic human iPSC Parkinson’s model shows nitrosative stress-induced dysfunction in MEF2-PGC1α transcription. Cell. 2013;155:1351–1364. doi: 10.1016/j.cell.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung CY, et al. Identification and rescue of α-synuclein toxicity in Parkinson patient-derived neurons. Science. 2013;342:983–987. doi: 10.1126/science.1245296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schöndorf DC, et al. iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat Commun. 2014;5:4028. doi: 10.1038/ncomms5028. [DOI] [PubMed] [Google Scholar]
- 17.Jiang H, et al. Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nat Commun. 2012;3:668. doi: 10.1038/ncomms1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper O, et al. Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson’s disease. Sci Transl Med. 2012;4:141ra90. doi: 10.1126/scitranslmed.3003985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nabi R, Serajee FJ, Chugani DC, Zhong H, Huq AH. Association of tryptophan 2,3 dioxygenase gene polymorphism with autism. Am J Med Genet B Neuropsychiatr Genet. 2004;125B:63–68. doi: 10.1002/ajmg.b.20147. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Cowan CA, et al. Derivation of embryonic stem-cell lines from human blastocysts. N Engl J Med. 2004;350:1353–1356. doi: 10.1056/NEJMsr040330. [DOI] [PubMed] [Google Scholar]
- 22.Chambers SM, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soldner F, et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spira PJ, Sharpe DM, Halliday G, Cavanagh J, Nicholson GA. Clinical and pathological features of a Parkinsonian syndrome in a family with an Ala53Thr alpha-synuclein mutation. Ann Neurol. 2001;49:313–319. [PubMed] [Google Scholar]
- 25.Markopoulou K, et al. Clinical, neuropathological and genotypic variability in SNCA A53T familial Parkinson’s disease. Variability in familial Parkinson’s disease. Acta Neuropathol. 2008;116:25–35. doi: 10.1007/s00401-008-0372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papadimitriou D, et al. Motor and nonmotor features of carriers of the p.A53T alpha-synuclein mutation: A longitudinal study. Mov Disord. 2016;31:1226–1230. doi: 10.1002/mds.26615. [DOI] [PubMed] [Google Scholar]
- 27.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 28.Shen D, et al. Novel cell- and tissue-based assays for detecting misfolded and aggregated protein accumulation within aggresomes and inclusion bodies. Cell Biochem Biophys. 2011;60:173–185. doi: 10.1007/s12013-010-9138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotzbauer PT, et al. Fibrillization of alpha-synuclein and tau in familial Parkinson’s disease caused by the A53T alpha-synuclein mutation. Exp Neurol. 2004;187:279–288. doi: 10.1016/j.expneurol.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Duda JE, et al. Concurrence of alpha-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol. 2002;104:7–11. doi: 10.1007/s00401-002-0563-3. [DOI] [PubMed] [Google Scholar]
- 31.Zoghbi HY, Bear MF. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb Perspect Biol. 2012;4:a009886. doi: 10.1101/cshperspect.a009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mirnics K. Schizophrenia. Special issue introduction. Int J Dev Neurosci. 2011;29:189–191. doi: 10.1016/j.ijdevneu.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Schloesser RJ, Huang J, Klein PS, Manji HK. Cellular plasticity cascades in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33:110–133. doi: 10.1038/sj.npp.1301575. [DOI] [PubMed] [Google Scholar]
- 34.Porton B, Wetsel WC, Kao HT. Synapsin III: Role in neuronal plasticity and disease. Semin Cell Dev Biol. 2011;22:416–424. doi: 10.1016/j.semcdb.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janz R, Südhof TC. SV2C is a synaptic vesicle protein with an unusually restricted localization: Anatomy of a synaptic vesicle protein family. Neuroscience. 1999;94:1279–1290. doi: 10.1016/s0306-4522(99)00370-x. [DOI] [PubMed] [Google Scholar]
- 36.Burns ME, Sasaki T, Takai Y, Augustine GJ. Rabphilin-3A: A multifunctional regulator of synaptic vesicle traffic. J Gen Physiol. 1998;111:243–255. doi: 10.1085/jgp.111.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedrich R, et al. DOC2B acts as a calcium switch and enhances vesicle fusion. J Neurosci. 2008;28:6794–6806. doi: 10.1523/JNEUROSCI.0538-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yim YS, et al. Slitrks control excitatory and inhibitory synapse formation with LAR receptor protein tyrosine phosphatases. Proc Natl Acad Sci USA. 2013;110:4057–4062. doi: 10.1073/pnas.1209881110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheng M, Kim E. The postsynaptic organization of synapses. Cold Spring Harb Perspect Biol. 2011;3:a005678. doi: 10.1101/cshperspect.a005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arikkath J, Reichardt LF. Cadherins and catenins at synapses: Roles in synaptogenesis and synaptic plasticity. Trends Neurosci. 2008;31:487–494. doi: 10.1016/j.tins.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Budnik V, Salinas PC. Wnt signaling during synaptic development and plasticity. Curr Opin Neurobiol. 2011;21:151–159. doi: 10.1016/j.conb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Südhof TC. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron. 2013;80:675–690. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wrasidlo W, et al. A de novo compound targeting α-synuclein improves deficits in models of Parkinson’s disease. Brain. 2016;139:3217–3236. doi: 10.1093/brain/aww238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tóth G, et al. Targeting the intrinsically disordered structural ensemble of α-synuclein by small molecules as a potential therapeutic strategy for Parkinson’s disease. PLoS One. 2014;9:e87133. doi: 10.1371/journal.pone.0087133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010;9:1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- 46.Chung CY, Koprich JB, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci. 2009;29:3365–3373. doi: 10.1523/JNEUROSCI.5427-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goedert M. The significance of tau and alpha-synuclein inclusions in neurodegenerative diseases. Curr Opin Genet Dev. 2001;11:343–351. doi: 10.1016/s0959-437x(00)00200-8. [DOI] [PubMed] [Google Scholar]
- 49.Zaltieri M, et al. α-Synuclein and synapsin III cooperatively regulate synaptic function in dopamine neurons. J Cell Sci. 2015;128:2231–2243. doi: 10.1242/jcs.157867. [DOI] [PubMed] [Google Scholar]
- 50.Busch DJ, et al. Acute increase of α-synuclein inhibits synaptic vesicle recycling evoked during intense stimulation. Mol Biol Cell. 2014;25:3926–3941. doi: 10.1091/mbc.E14-02-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nemani VM, et al. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott DA, et al. A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J Neurosci. 2010;30:8083–8095. doi: 10.1523/JNEUROSCI.1091-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chu Y, et al. Alterations in axonal transport motor proteins in sporadic and experimental Parkinson’s disease. Brain. 2012;135:2058–2073. doi: 10.1093/brain/aws133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simunovic F, et al. Gene expression profiling of substantia nigra dopamine neurons: Further insights into Parkinson’s disease pathology. Brain. 2009;132:1795–1809. doi: 10.1093/brain/awn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chien WH, et al. Deep exon resequencing of DLGAP2 as a candidate gene of autism spectrum disorders. Mol Autism. 2013;4:26. doi: 10.1186/2040-2392-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Redies C, Hertel N, Hübner CA. Cadherins and neuropsychiatric disorders. Brain Res. 2012;1470:130–144. doi: 10.1016/j.brainres.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 57.Um JW, et al. Structural basis for LAR-RPTP/Slitrk complex-mediated synaptic adhesion. Nat Commun. 2014;5:5423. doi: 10.1038/ncomms6423. [DOI] [PubMed] [Google Scholar]
- 58.Habela CW, Song H, Ming GL. Modeling synaptogenesis in schizophrenia and autism using human iPSC derived neurons. Mol Cell Neurosci. 2016;73:52–62. doi: 10.1016/j.mcn.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jellinger KA. Lewy body/alpha-synucleinopathy in schizophrenia and depression: A preliminary neuropathological study. Acta Neuropathol. 2009;117:423–427. doi: 10.1007/s00401-009-0492-5. [DOI] [PubMed] [Google Scholar]
- 60.Korth C. Aggregated proteins in schizophrenia and other chronic mental diseases: DISC1opathies. Prion. 2012;6:134–141. doi: 10.4161/pri.18989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Atkin TA, Brandon NJ, Kittler JT. Disrupted in Schizophrenia 1 forms pathological aggresomes that disrupt its function in intracellular transport. Hum Mol Genet. 2012;21:2017–2028. doi: 10.1093/hmg/dds018. [DOI] [PubMed] [Google Scholar]
- 62.Starkstein S, Gellar S, Parlier M, Payne L, Piven J. High rates of parkinsonism in adults with autism. J Neurodev Disord. 2015;7:29. doi: 10.1186/s11689-015-9125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.