Significance
The nature of overactive Ca2+ release in malignant hyperthermia (MH) and the mechanism of action of the drug dantrolene that arrests MH events are poorly understood. Here, we show that dantrolene stops overactive Ca2+ release by increasing the affinity of the ryanodine receptor (RyR) to Mg2+. In particular, Ca2+ waves induced by MH triggers in human muscle are not affected by dantrolene unless Mg2+ increases above resting levels, a condition met by the increase in MgATP hydrolysis during an MH episode. We suggest that only the combination of dantrolene and increased Mg2+ can depress overactive Ca2+ release and the resulting excessive heat production to arrest MH.
Keywords: malignant hyperthermia, dantrolene, ryanodine receptor, magnesium, skeletal muscle fiber
Abstract
Malignant hyperthermia (MH) is a clinical syndrome of skeletal muscle that presents as a hypermetabolic response to volatile anesthetic gases, where susceptible persons may develop lethally high body temperatures. Genetic predisposition mainly arises from mutations on the skeletal muscle ryanodine receptor (RyR). Dantrolene is administered to alleviate MH symptoms, but its mechanism of action and its influence on the Ca2+ transients elicited by MH triggers are unknown. Here, we show that Ca2+ release in the absence of Mg2+ is unaffected by the presence of dantrolene but that dantrolene becomes increasingly effective as cytoplasmic-free [Mg2+] (free [Mg2+]cyto) passes mM levels. Furthermore, we found in human muscle susceptible to MH that dantrolene was ineffective at reducing halothane-induced repetitive Ca2+ waves in the presence of resting levels of free [Mg2+]cyto (1 mM). However, an increase of free [Mg2+]cyto to 1.5 mM could increase the period between Ca2+ waves. These results reconcile previous contradictory reports in muscle fibers and isolated RyRs, where Mg2+ is present or absent, respectively, and define the mechanism of action of dantrolene is to increase the Mg2+ affinity of the RyR (or “stabilize” the resting state of the channel) and suggest that the accumulation of the metabolite Mg2+ from MgATP hydrolysis is required to make dantrolene administration effective in arresting an MH episode.
Ryanodine receptors (RyRs) are essential regulators of cytoplasmic Ca2+ in muscle, heart, and brain (1–3). Congenital or acquired mishandling of Ca2+ by RyRs is associated with organ dysfunction, myopathy, and the increased risk of sudden death (1, 4–7). Consequently, the search for drugs to modulate RyR function is an area of intense research (8–10). An example of a successful drug controlling the Ca2+ mishandling of the RyR is the muscle relaxant dantrolene (11). It is primarily used to treat malignant hyperthermia (MH), a life-threatening condition in which genetically predisposed individuals adversely react to the exposure of volatile anesthetics (5, 12). Since its approval, the drug has cut the mortality rate associated with MH from more than 80% to below 2% (11). Despite this success, the exact mechanism of how dantrolene antagonizes MH episodes and depresses overactive Ca2+ release during MH episodes remains unknown.
Susceptibility to MH most commonly arises from mutations in the RyR1 gene, the Ca2+ release channel of skeletal muscle. Mg2+ is present in the muscle at ∼1 mM and exerts an inhibitory action over the RyR1, which needs to be overcome for normal voltage-controlled Ca2+ release (13–16). RyR1 variants have a lowered affinity for Mg2+ (13, 17), making them more prone to opening and resulting in increased sensitivity to RyR agonist. The ensuing abnormal Ca2+ release in RyR variants (18–24) during an MH event leads to excessive heat production as the muscle attempts to clear the persistently high cytoplasmic Ca2+.
Functional studies on muscle fibers suggested the RyR as the potential target for the action of dantrolene (25, 26), and respective binding sites were mapped to the protein (27–29). However, no action of dantrolene was found in most studies on single RyRs incorporated into lipid bilayers (26, 30, 31). A major difference between the experiments examining the effect of dantrolene in bilayers and intact fibers is the ionic conditions; the bilayer studies significantly diverge from physiological ionic conditions, in particular with the exclusion of Mg2+ from the cytoplasmic solution (see, e.g., ref. 13). This is necessary in bilayer studies to remove the resting inhibition on the RyR and induce a measureable opening probability in the isolated channel.
We hypothesized that the lack of dantrolene action on single RyRs was due to the fact that the experiments were carried out either in the absence of or in low free [Mg2+]cyto (26, 30, 31). In contrast, the intact fiber experiments, where dantrolene inhibited the RyR, maintained the endogenous level of free [Mg2+]cyto (26). Using mechanically skinned fibers, where the cytoplasmic environment can be rapidly manipulated (14, 15), we tested the effectiveness of dantrolene across the relevant range of free [Mg2+]cyto. Thus, we used skinned fibers to mimic the ionic conditions of both bilayer experiments and intact fiber experiments in the same preparation. Additionally, because the skinned fiber is bathed in, effectively, an infinite volume of internal solution, any metabolites generated during experiments diffuse from the preparation in less than a second so that the ionic conditions set in the bath are maintained inside the fiber. Using this approach, we identify that dantrolene shifts the affinity of the RyR for Mg2+ (alternatively termed “stabilizing the closed state of the channel”), and therefore, the drug requires the presence of Mg2+ to properly close the channel. Furthermore, we imaged Ca2+ transients in the presence of halothane (volatile anesthetic) in human MH-susceptible muscle and observed that the metabolite Mg2+ is required to supplement the resting free [Mg2+]cyto for dantrolene to depress halothane-induced overactive Ca2+ release.
Results
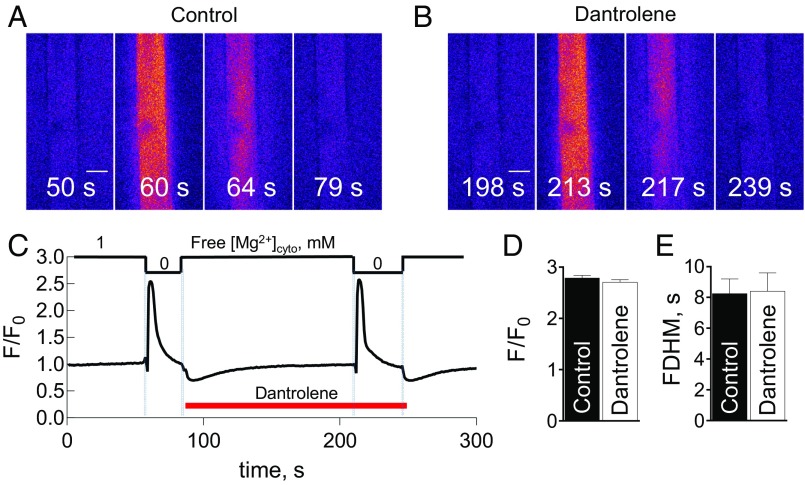
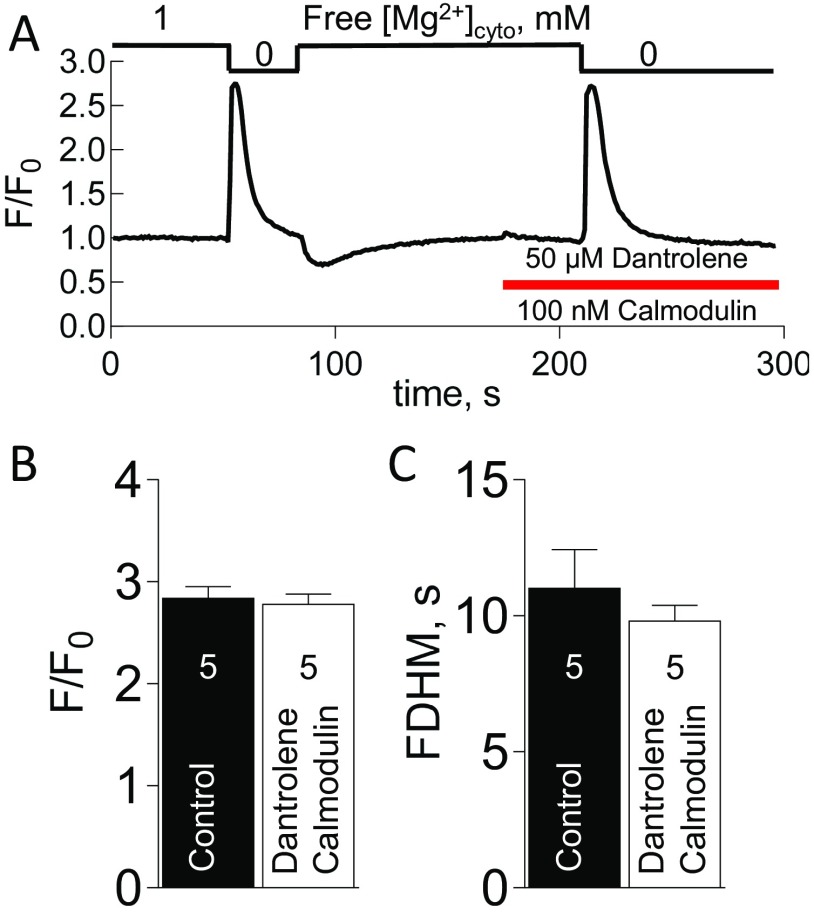
To simulate cytoplasmic ionic conditions during Ca2+ release occurring in bilayer experiments (zero or low free [Mg2+]cyto) (26, 30, 31), we directly released Ca2+ through the RyRs of skinned fibers by lowering free [Mg2+]cyto (14, 15, 32) (note that our notation of [Mg2+]cyto always indicates the free [Mg2+]cyto, which has been calculated taking into the account the cytoplasmic buffers, which include ATP, creatine phosphate, and EGTA; see Methods). The lowering of free [Mg2+]cyto removes the resting inhibition on the RyRs and allows Ca2+ release from the sarcoplasmic reticulum (SR), as reported by the Ca2+-dependent fluorescence of rhod-2 in the internal bathing solution (24, 32). This form of Ca2+ release was not inhibited upon the introduction of 50 µM dantrolene (Fig. 1). Profiles of the spatially averaged fluorescence showed that the amplitude and full duration at half magnitude (FDHM) were not affected by the presence of dantrolene (Fig. 1). The addition of exogenous calmodulin (100 nM) did not affect this result (Fig. S1).
Fig. 1.
Ca2+ transients evoked by the removal of Mg2+ are not inhibited by dantrolene. Selected images of cytoplasmic rhod-2 fluorescence during continuous xyt recordings (at 0.8 s·frame−1) in rat skinned fibers acquired while applying Ca2+ release inducing 0 Mg2+ solution either in the absence (A) or presence of dantrolene (B) and 1 mM EGTA and 300 nM Ca2+ (Table S1). (Scale bar: 50 μm.) (C) Spatially averaged values of normalized fluorescence intensity (F/F0) versus elapsed time from the full experiment that is represented in A and B. Applied free [Mg2+]cyto is given at the top, and exchange of solutions is indicated by pale blue vertical bars. A 2-min time interval was allowed for after each low Mg2+ transient to recover SR Ca2+. The applied free [Ca2+]cyto was kept constant throughout the recording. The presence of 50 µM dantrolene is indicated by the horizontal bar. Mean normalized peak amplitude values (F/F0) (D) and FDHM (E) in the absence and presence of dantrolene (n = 5 fibers). A paired Student’s t test revealed no significant difference (P > 0.05) in both D and E.
Fig. S1.
Ca2+ transients evoked by the removal of Mg2+ are not inhibited by dantrolene in the presence of exogenous calmodulin. (A) Spatially averaged profile of xyt recordings of Ca2+ transients in rat skinned fibers evoked by the nominal removal of Mg2+ either in the absence (first Ca2+ release) or presence of dantrolene and exogenous calmodulin (second Ca2+ release) and the presence of 1 mM EGTA and 300 nM Ca2+ (see Table S1). Applied free [Mg2+]cyto is given at the top. A 2-min time interval was allowed for after each low Mg2+ transient to recover SR Ca2+ levels in a solution with 1 mM free Mg2+ and 300 nM Ca2+. The presence of 50 µM dantrolene and 100 nM calmodulin is indicated by the horizontal bar. Mean normalized peak amplitude values (F/F0) (B) and FDHM (C) in the absence and presence of dantrolene (n = 5 fibers). A paired Student’s t test revealed no significant difference (P > 0.05) in both B and C.
Next, we wished to test the possibility that the inhibitory action of dantrolene on RyR Ca2+ release required the presence of physiologically relevant free [Mg2+]cyto, consistent with the suppression of Ca2+ release by dantrolene in intact and skinned fiber preparations (21, 26). To release Ca2+ in the presence of mM levels of free [Mg2+]cyto, we used electrical field stimulation at 1 Hz and recorded Ca2+ release by confocal line-scanning of Ca2+-dependent rhod-2 fluorescence in rat skinned fibers (33). The activation of the t-system voltage-sensor via action potential stimulation causes the temporary removal of Mg2+ inhibition exerted on the RyR to allow rapid release of Ca2+ through the transition of the RyR from the closed to the open state (14, 15). Note that the rate of Ca2+ release evoked by action potential in skinned fibers is not distinguishable from that occurring in intact fibers (33, 34).
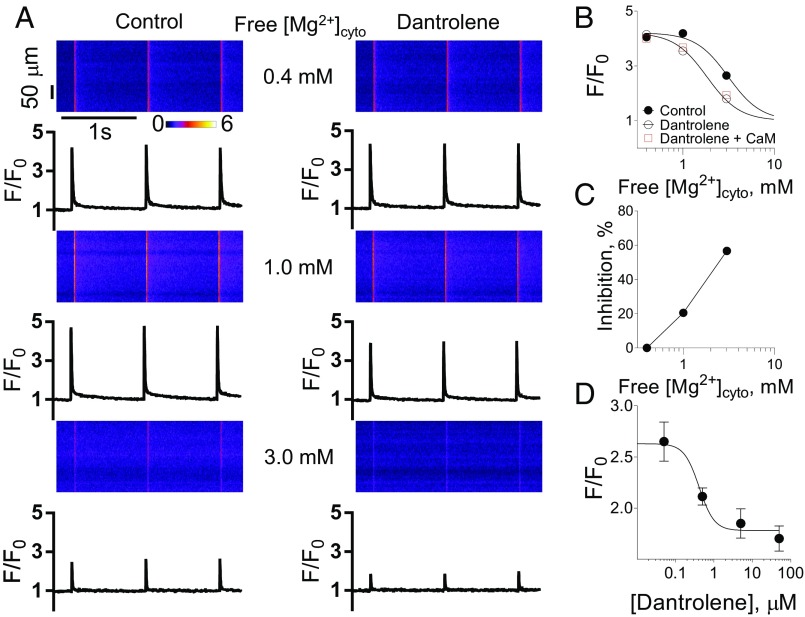
Fig. 2A, Left shows examples of Ca2+ release in the presence of 0.4, 1, and 3 mM free [Mg2+]cyto. The increase of free [Mg2+]cyto from 0.4 to 1 mM did not affect the shape or amplitude of the Ca2+ transients. However, increasing free [Mg2+]cyto to 3 mM significantly reduced the amplitude of the Ca2+ transient. Field stimulation failed to elicit Ca2+ transients in the presence of 10 mM free [Mg2+]cyto. This effect of increasing free [Mg2+]cyto suppressing Ca2+ release is consistent with the results of others (14, 15) and provides further verification of the free [Mg2+]cyto present in the internal bathing solutions.
Fig. 2.
Inhibition of electrically evoked Ca2+ transients by dantrolene is dependent on the free [Mg2+]cyto. (A) Original recordings in rat skinned fibers as obtained by confocal line scans parallel to the fiber long axis (Top), with corresponding line-averaged and normalized rhod-2 fluorescence signals (F/F0, Bottom). Cytosolic Ca2+ transients were elicited by electrical field stimulation at 1 Hz in the absence (Left) or presence (Right) of 50 µM dantrolene at free [Mg2+]cyto of 0.4 (Top), 1 (Middle), or 3 mM (Bottom). All solutions contained 1 mM EGTA and 100 nM free Ca2+ (Table S1). Note that rhod-2 fluorescence was recorded at 2 ms·line−1. (B) Effect of dantrolene on Ca2+ transient peak amplitudes for the [Mg2+]cyto as shown in A. Best fit of the data to a Hill equation in the absence and presence of 50 µM dantrolene yielded IC50 values of 2.95 ± 1.42 and 1.73 ± 1.63 mM, respectively. Curves are significantly different from each other (Extrasum of square F test). The red data points represent the Ca2+ transient amplitudes in the presence of 100 nM exogenous calmodulin (CaM) in the presence of dantrolene. Note that these points are not different from the responses in the presence of dantrolene with only the endogenous calmodulin. (C) % inhibition of Ca2+ transient by dantrolene at indicated [Mg2+]cyto. (D) Concentration-dependent inhibition of electrically evoked Ca2+ transients by dantrolene at 3 mM free [Mg2+]cyto. Best fit Hill curve had an IC50 value of 0.41 ± 2.61 µM. All data are derived from 5 to 18 fibers and presented as mean ± SEM.
Ca2+ transients in the presence of 50 µM dantrolene are shown in Fig. 2A, Right. In the presence of 0.4 mM free [Mg2+]cyto, 50 µM dantrolene did not affect the amplitude of the Ca2+ transient (Fig. 2A, Top). However, when the free [Mg2+]cyto was increased from 0.4 to 1 and 3 mM, 50 µM dantrolene reduced the Ca2+ transient amplitude (Fig. 2A, Middle and Bottom). We also tested the effect of exogenous calmodulin (100 nM) at each free [Mg2+]cyto with dantrolene present on Ca2+ release evoked by action potential stimulation. No change in Ca2+ release amplitude was observed (Fig. 2B, red data points).
The effect of dantrolene on suppressing electrically evoked Ca2+ transients across free [Mg2+]cyto of 0.4–3 mM is summarized in Fig. 2B. This figure shows the inhibitory effect of free [Mg2+]cyto on suppressing RyR Ca2+ release (14, 15) is shifted to the left by dantrolene, and Fig. 2C shows that the % inhibition of 50 µM dantrolene on Ca2+ release increased with increasing free [Mg2+]cyto. These results indicate that mM levels of free [Mg2+]cyto are required for dantrolene to affect RyR Ca2+ release. We also determined the concentration response of dantrolene on electrically evoked Ca2+ transients in the presence of 3 mM free [Mg2+]cyto, where the inhibitory effect of dantrolene was greatest. A Hill curve fitted these data (Fig. 2D).
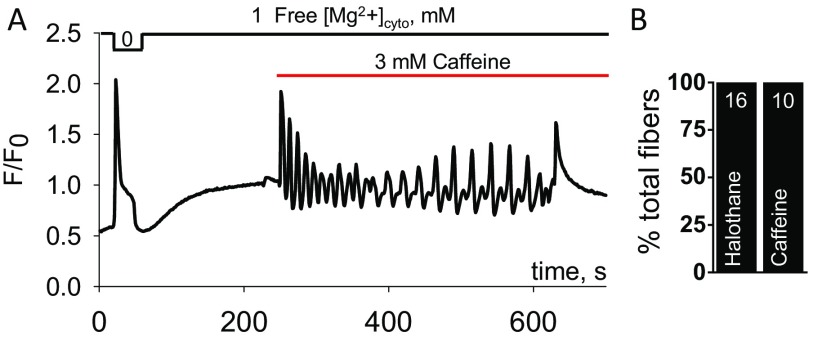
Next, we wished to determine the action of dantrolene at various free [Mg2+]cyto against halothane-induced Ca2+ release in MHS human muscle. We used skinned fibers isolated from needle biopsies obtained from unrelated subjects. As it was possible to isolate many fibers from each biopsy, a significant number of repeat measures, with appropriate controls, could be conducted on each biopsy (35). The collection of repeat measures from individual biopsies allowed for assessment of the subject’s sensitivity to halothane and dantrolene. Subject A carried RyR1 variant in exon 36, pArg1976Cys. The sensitivity of these mutant fibers to relatively low concentrations of RyR agonists (1 mM halothane and 3 mM caffeine; Fig. 3 and Fig. S2) was consistent with MHS status, as reported previously from a calibration of Ca2+ release in human skinned fibers and the in vitro contracture test (20). Subject B had previously submitted to MH diagnosis at The Royal Melbourne Hospital, Melbourne, Australia. A diagnosis of MHS (halothane) was returned following the in vitro contracture test (with responses of 0.07 g at 2 mM caffeine and 0.26 g at 2% halothane) (36).
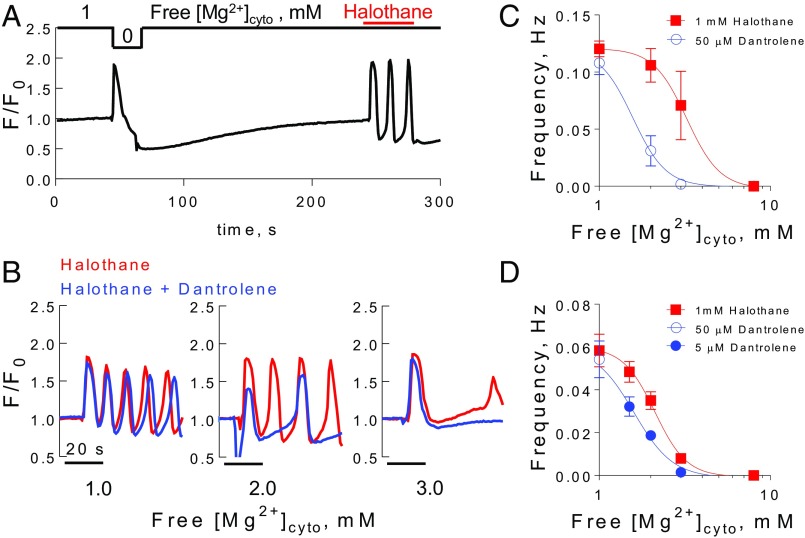
Fig. 3.
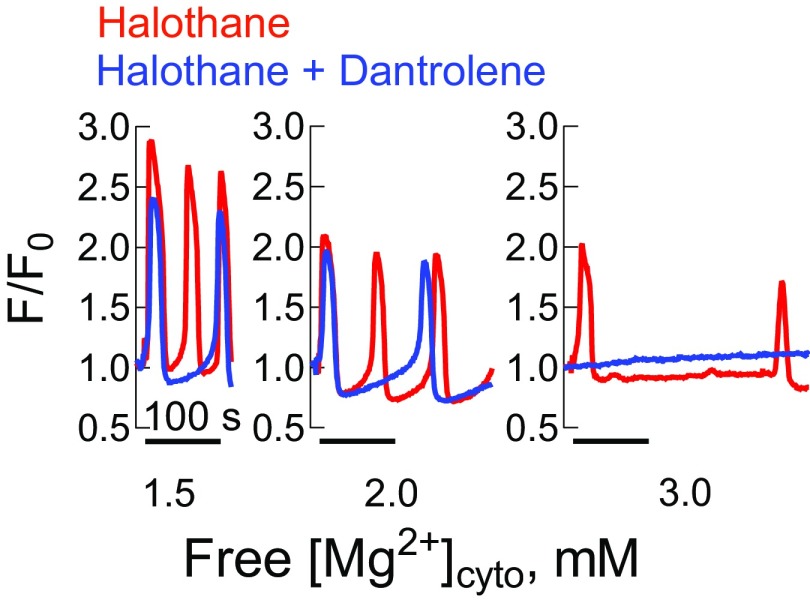
Halothane-induced Ca2+ waves in human MHS fibers require increases in free [Mg2+]cyto to be affected by dantrolene. (A) Spatially averaged cytoplasmic rhod-2 fluorescence in a fiber from subject A during changes in the internal bathing solution from one containing 1 mM free [Mg2+]cyto to 0 Mg2+ followed by reintroduction of 1 mM free [Mg2+]cyto and subsequent exposure to 1 mM halothane. Note that halothane induced repetitive Ca2+ waves in the presence of 1 mM free [Mg2+]cyto and that data were acquired at 0.8 s·frame−1. [Ca2+] was 100 nM in all solutions. (B) Examples of MHS fibers from subject A exposed to 1 mM halothane and 1, 2, and 3 mM free [Mg2+]cyto in the presence or absence of 50 µM dantrolene. Frequency of halothane-induced Ca2+ waves in the presence of 1–3 mM free [Mg2+]cyto and the presence and absence of dantrolene in fibers isolated from the biopsies of subject A (C) and subject B (D). Data in C and D were fit with a Hill equation. IC50 values amounted to 3.2 ± 1.05 and 1.6 ± 1.06 mM (C) and 2.1 ± 1.05 and 1.6 ± 1.06 mM (D) in the presence of halothane and halothane plus dantrolene, respectively. Curves in the presence and absence of 5 (D) and 50 µM dantrolene (C) were significantly different from each other (extra sum of squares F test). All data points are derived from four to five fibers and presented as mean ± SEM.
Fig. S2.
The effect of low caffeine on human muscle fiber from subject A. (A) Spatially averaged profile of cytoplasmic rhod-2 fluorescence of human skinned fiber during the reduction of [Mg2+]cyto from 1 to nominally 0, and the exposure to 3 mM caffeine and 1 mM Mg2+. The fiber is in the constant presence of 0.1 mM EGTA and 100 nM Ca2+. Note that regenerative Ca2+ waves were observed through the period in the presence of 3 mM caffeine. (B) Summary of the number of fibers from the biopsy used responding with regenerative Ca2+ waves to 1 mM halothane or 3 mM caffeine in the presence of 1 mM Mg2+. The numbers in the bars represent the number of fibers. The response to low concentrations of halothane and caffeine are consistent with the muscle being MH-susceptible (20).
Fig. 3A shows an isolated fiber initially exposed to nominally zero free [Mg2+]cyto to directly stimulate Ca2+ release through the RyR from the biopsy of subject A. Following reloading the SR with Ca2+ (2 min in 100 nM Ca2+), exposure to 1 mM halothane in the presence of 1 mM free [Mg2+]cyto induced repetitive Ca2+ waves (Fig. 3A). Incrementally increasing free [Mg2+]cyto from 1 up to 3 mM had a progressive slowing effect on Ca2+ wave frequency in the presence of 1 mM halothane, which provided some variance between the subjects (Fig. 3B and Fig. S3) that is fully expected (36). Note that free [Ca2+]cyto was held at 100 nM throughout these experiments. Clinically relevant plasma levels of dantrolene [1.6 µg/mL (5 µM)] (37) and raised concentrations of dantrolene [16 µg/mL (50 µM)] were then tested on the frequency of halothane-induced Ca2+ waves in fibers from both subjects. Note that dantrolene was introduced in the internal bathing solution ∼15 s before the administration of halothane. The addition of the relatively high level of dantrolene (50 µM) had no effect on the halothane-induced Ca2+ waves in the presence of the resting level of free [Mg2+]cyto (1 mM) in fibers from subject A (Fig. 3C) or subject B (Fig. 3D). At 5 µM dantrolene, Ca2+ wave frequency was slowed when free [Mg2+]cyto was equal to or greater than 1.5 mM (Fig. 3D). Consistent with this, 50 µM dantrolene caused a significant impediment to halothane-induced Ca2+ waves when free [Mg2+]cyto was 2 mM or greater (Fig. 3C).
Fig. S3.
Halothane-induced Ca2+ waves in MHS fibers from subject B. Examples of MHS fibers from subject B exposed to 1 mM halothane and 1.5, 2, and 3 mM Mg2+ in the presence or absence of 5 µM dantrolene.
Discussion
Our results indicate that dantrolene increases the affinity of the RyR for Mg2+, to increase the ability of free [Mg2+]cyto to suppress Ca2+ release through the RyR (Figs. 1 and 2) (18–20, 22, 23). Critically, endogenous levels of resting free [Mg2+]cyto were found to be insufficient to effect the repetitive releases of Ca2+ in human MHS muscle in the presence of halothane, and it required an increase to at least 1.5 mM free [Mg2+]cyto for dantrolene to antagonize RyR activity (Fig. 3), conditions that likely arise inside a muscle fiber during an MH episode.
The apparent contradictory results in regard to the inhibitory effect of dantrolene on the RyR previously obtained with intact muscle fibers (26) and with isolated RyRs incorporated into bilayers (26, 30, 31) can now be reconciled. The key difference between the experimental approaches was the presence of mM levels of free [Mg2+]cyto (Figs. 1 and 2). Zero or very low Mg2+ in bilayer experiments and 1 mM Mg2+ in intact fibers is consistent with the presence and absence of the effect of dantrolene on Ca2+ flux through RyRs, respectively (Figs. 1 and 2). We have been able to directly assess the function of dantrolene across this broad range of free [Mg2+]cyto by using skinned fibers, where we could rapidly and accurately manipulate the ionic environment of the cytoplasm in conjunction with assessing RyR activity by directly imaging the Ca2+-dependent fluorescence that arises from RyR-mediated Ca2+ transients (14, 15, 24).
Oo et al. (38) previously reported that calmodulin is an important factor in the action of dantrolene in muscle by examining the activity of RyRs in isolated bilayers. However, the effect of dantrolene on RyRs in bilayers in the presence of calmodulin was only to lower open probability (Po) to levels that would be equivalent to that with excessively leaky RyRs in a muscle fiber. That is, if channel Po is 1.0 during normal Ca2+ release in a muscle fiber, a Ca2+ flux close to 200 mM·s−1, and the normal resting RyR Ca2+ leak is about 5 nM·s−1 (34, 39) (which hence equates to a Po of ∼2.5 × 10−8), then the Po of 0.009 reported by Oo et al. under conditions of dantrolene and calmodulin still corresponds to a very large leak flux through the RyRs of about 0.18 mM∙s−1. Those results with isolated RyRs, however, were found under ionic conditions far from those pertaining in vivo, particularly in the absence of Mg2+. Importantly, it has been shown that in skinned fibers calmodulin remains bound to the RyR (40). Thus, both free [Mg2+]cyto and calmodulin are present at normal physiological levels in our experiments (40, 41). By manipulating free [Mg2+]cyto, we could clearly show that mM levels of free [Mg2+]cyto are required for the inhibitory action of dantrolene on action potential-induced or halothane-induced Ca2+ release (Figs. 2 and 3). We further demonstrated that in the absence of free [Mg2+]cyto, dantrolene fails to significantly slow the release of Ca2+ through the RyR in the presence of endogenous or exogenous calmodulin (Figs. 1 and 2 and Fig. S1).
Dantrolene, even at a high concentrations (50 µM), did not affect halothane-induced Ca2+ release in the presence of resting levels of free [Mg2+]cyto (Fig. 3). Ca2+ waves induced by the action of halothane could only be suppressed by dantrolene when the free [Mg2+]cyto was increased above its normal resting levels (Fig. 3). Preadministration of dantrolene before halothane did not affect this requirement for raised free [Mg2+]cyto (Fig. 3). These results suggest that in a clinical setting, even if dantrolene was preadministered to an MHS person undergoing a general anesthesia, free [Mg2+]cyto would still need to rise above its normal resting level of 1 mM for dantrolene to be effective. A rise in muscle metabolites must precede any clinical symptoms of MH such as increasing body temperature and other changes, as they are a result of MgATP hydrolysis, which includes the defining heat production of the condition. Evidence for the rise of free [Mg2+]cyto during extensive muscle use is best documented during metabolic fatigue, which can be used as a model of metabolic changes inside the muscle during overactive Ca2+ release under an MH trigger. It has been shown in human type II fibers that ATP is depleted in the order of seconds during maximal activity (42). Similarly, we can expect that muscle contractures in an MH episode lead to major [ATP] decline. This decline of [ATP] significantly retards its capacity as the major cytoplasmic buffer of Mg2+, causing a parallel rise in free [Mg2+]cyto. Indeed, increases in free [Mg2+]cyto to levels in the order of 1.5–3 mM are reached during metabolic fatigue (43–45).
In summary, we have shown that (i) dantrolene acts by increasing the affinity of the RyR for Mg2+, (ii) repetitive Ca2+ waves generated by halothane can occur even with free [Mg2+]cyto increased above resting levels, and (iii) dantrolene requires the metabolite Mg2+ to adequately close the RyR and inhibit overactive Ca2+ release. Overall these results suggest that dantrolene is a poor antagonist of RyR activity but one that is good enough to arrest MH, where the susceptibility to this condition arises from only a relatively minor decrease in RyR affinity for the endogenous inhibitory stabilizer Mg2+ (16, 17).
Methods
All experimental methods using rodents were approved by the Animal Ethics Committee at The University of Queensland, and the use of human muscle was approved by The University of Queensland Human Ethics Committee. Subjects signed informed consent forms before their involvement in this study. Wistar rats (University of Queensland Biological Resources, Brisbane) were killed by cervical dislocation, and the extensor digitorum longus (EDL) muscles were rapidly excised. Muscles were placed in a Petri dish under paraffin oil above a layer of Sylgard. Muscle biopsies were collected under local anesthesia (Xylocaine, 10 mg·mL−1) from the midportion of the Vastus Lateralis muscle, using a 6-mm Bergstrom biopsy needle modified for manual suction. Biopsy was taken from a 30-y-old female with a RyR1 variant (subject A) and a 45-y-old male (subject B). Muscle tissue collected from the biopsy needle was blotted on filter paper (Whatman no. 1) to remove blood and extracellular fluid. With both rat and human muscle, segments of individual fibers were isolated and mechanically skinned to completely remove the surface membrane. Skinned fibers were transferred to a custom-built experimental chamber with a coverslip bottom, where they were bathed in an “internal solution,” containing (in mM) K+, 126; Na+, 36; free Mg2+, 1 (total Mg, 8.5); MgATP, 7 (total ATP, 8); Ca2+, 0.0001; rhod-2, 0.01; creatine phosphate, 10; EGTA, 1; and HDTA, 49 with pH adjusted to 7.1 ± 0.1 with KOH. To load Ca2+ into the SR, Ca2+ was increased to 300 nM (in rat skinned fiber experiments), and to release Ca2+, a nominally Mg2+-free solution was used (24, 32). In human muscle fiber experiments, Ca2+ was kept at 100 nM in all solutions. Free Mg2+ was lowered and raised to 0.4 and 10 mM for experiments involving electrical stimulation of skinned fibers without changing total [ATP]. Solution composition was calculated using MaxChelator software. BTS was added to all solutions to inhibit contraction. Dantrolene was added to internal solutions from a 10-mM stock dissolved in DMSO (38). At the highest [dantrolene] used (nominally 50 µM), it was possible that dantrolene partially precipitated upon dilution in aqueous solution, reducing its concentration to a value not lower than 20 µM (37). However, we did not observe any precipitate under the conditions we used to prepare dantrolene and consider the concentration of dantrolene to be close to the calculated value. Halothane was administered to fibers during imaging from a syringe to minimize exposure to air and reduce evaporation. In experiments using halothane to release Ca2+ from human muscle fibers, [EGTA] was lowered from 1 to 0.1 mM so that transients could be observed. Calmodulin derived from bovine testes was used in experiments. Table S1 provides the full details of the internal solutions composition used in this study. All chemicals were from Sigma, unless otherwise stated.
Table S1.
Internal solution compositions
| Solution | [EGTA], mM | Total [Ca], mM | Free [Ca2+], nM | Total [Mg], mM | Free [Mg2+], mM |
| 1 mM Mg2+, 300 nM Ca2+ (R) | 1 | 0.485 | 300 | 8.5 | 1 |
| 0 Mg2+, 271 nM Ca2+ (R) | 1 | 0.485 | 271 | 0 | 0 |
| 0.4 mM Mg2+, 100 nM Ca2+ (R) | 1 | 0.247 | 104 | 7 | 0.4 |
| 1 mM Mg2+, 100 nM Ca2+ (R) | 1 | 0.247 | 107 | 8.5 | 1 |
| 3 mM Mg2+, 100 nM Ca2+ (R) | 1 | 0.247 | 119 | 11.9 | 3 |
| 10 mM Mg2+, 100 nM Ca2+ (R) | 1 | 0.247 | 161 | 22 | 10.8 |
| 1 mM Mg2+, 100 nM Ca2+ (H) | 0.1 | 0.024 | 100 | 8.5 | 0.92 |
| 1.5 mM Mg2+, 100 nM Ca2+ (H) | 0.1 | 0.024 | 103 | 9.6 | 1.5 |
| 2 mM Mg2+, 100 nM Ca2+ (H) | 0.1 | 0.024 | 106 | 10.3 | 1.96 |
| 3 mM Mg2+, 100 nM Ca2+ (H) | 0.1 | 0.024 | 112 | 11.8 | 2.98 |
Each solution contains 8 mM total ATP, 10 mM creatine phosphate, 90 mM Hepes, 126 mM K+, 36 mM Na+, and 49 (R) or 49.9 (H) mM HDTA. Final pH was 7.1. The solution name in the left column represents the rounded value of free [Mg2+] and free [Ca2+] in solutions, and the values in the associated row represent the calculated values. The letters in parentheses, (R) and (H), refer to solutions used for rat and human fiber experiments, respectively. Dantrolene and halothane were added to these solutions as indicated in the text.
The experimental chamber containing a skinned fiber was placed above a water immersion objective (40×, N.A. 0.9) of the confocal laser scanning system (FV1000, Olympus). During low Mg2+-induced Ca2+ release, the fiber was imaged in xyt mode at 0.8 s·frame−1 (Figs. 1 and 3). Images were analyzed to determine fluorescence amplitude and FDHM as described previously (24). For fibers exposed to electrical stimulation, field pulses at 1 Hz were applied across platinum electrodes parallel to the long axis of the fiber as previously described (33). Imaging for electrical stimulation experiments was in xt mode, at a rate of 2 ms·line−1 (Fig. 2). All imaging was performed at 21–24 °C. Statistical analysis and nonlinear curve fitting was performed with GraphPad Prism. IC50 values were derived from fits to Hill equations. All data are given as mean ± SEM.
Acknowledgments
We thank Andrew Bjorksten and Robyn Gillies (Malignant Hyperthermia Diagnostic Unit, Royal Melbourne Hospital) for providing IVCT and sequencing results; Graham Lamb (La Trobe University) for helpful comments on the manuscript; and Robert Fassett and Jeff Coombes (University of Queensland) for biopsy of human muscle. B.S.L. was a Future Fellow of the Australian Research Council (FT140101309).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 4576.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1619835114/-/DCSupplemental.
References
- 1.Liu X, et al. Role of leaky neuronal ryanodine receptors in stress-induced cognitive dysfunction. Cell. 2012;150:1055–1067. doi: 10.1016/j.cell.2012.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 3.Melzer W, Herrmann-Frank A, Lüttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- 4.Oda T, et al. Oxidation of ryanodine receptor (RyR) and calmodulin enhance Ca release and pathologically alter RyR structure and calmodulin affinity. J Mol Cell Cardiol. 2015;85:240–248. doi: 10.1016/j.yjmcc.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowling JJ, Lawlor MW, Dirksen RT. Triadopathies: An emerging class of skeletal muscle diseases. Neurotherapeutics. 2014;11:773–785. doi: 10.1007/s13311-014-0300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santulli G, Marks AR. Essential roles of intracellular calcium release channels in muscle, brain, metabolism, and aging. Curr Mol Pharmacol. 2015;8:206–222. doi: 10.2174/1874467208666150507105105. [DOI] [PubMed] [Google Scholar]
- 7.Snoeck M, et al. RYR1-related myopathies: A wide spectrum of phenotypes throughout life. Eur J Neurol. 2015;22:1094–1112. doi: 10.1111/ene.12713. [DOI] [PubMed] [Google Scholar]
- 8.Rebbeck RT, et al. High-throughput screens to discover small-molecule modulators of ryanodine receptor calcium release channels. SLAS Discov. 2017;22:176–186. doi: 10.1177/1087057116674312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kushnir A, Marks AR. Ryanodine receptor patents. Recent Pat Biotechnol. 2012;6:157–166. doi: 10.2174/1872208311206030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulhunty AF, Casarotto MG, Beard NA. The ryanodine receptor: A pivotal Ca2+ regulatory protein and potential therapeutic drug target. Curr Drug Targets. 2011;12:709–723. doi: 10.2174/138945011795378595. [DOI] [PubMed] [Google Scholar]
- 11.Larach MG, Brandom BW, Allen GC, Gronert GA, Lehman EB. Cardiac arrests and deaths associated with malignant hyperthermia in North America from 1987 to 2006: A report from the North American Malignant Hyperthermia Registry of the Malignant Hyperthermia Association of the United States. Anesthesiology. 2008;108:603–611. doi: 10.1097/ALN.0b013e318167aee2. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg H, Pollock N, Schiemann A, Bulger T, Stowell K. Malignant hyperthermia: A review. Orphanet J Rare Dis. 2015;10:93. doi: 10.1186/s13023-015-0310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laver DR, et al. Reduced inhibitory effect of Mg2+ on ryanodine receptor-Ca2+ release channels in malignant hyperthermia. Biophys J. 1997;73:1913–1924. doi: 10.1016/S0006-3495(97)78222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamb GD, Stephenson DG. Effects of intracellular pH and [Mg2+] on excitation-contraction coupling in skeletal muscle fibres of the rat. J Physiol. 1994;478:331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamb GD, Stephenson DG. Effect of Mg2+ on the control of Ca2+ release in skeletal muscle fibres of the toad. J Physiol. 1991;434:507–528. doi: 10.1113/jphysiol.1991.sp018483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamb GD. Ca2+ inactivation, Mg2+ inhibition and malignant hyperthermia. J Muscle Res Cell Motil. 1993;14:554–556. doi: 10.1007/BF00141551. [DOI] [PubMed] [Google Scholar]
- 17.Steele DS, Duke AM. Defective Mg2+ regulation of RyR1 as a causal factor in malignant hyperthermia. Arch Biochem Biophys. 2007;458:57–64. doi: 10.1016/j.abb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Duke AM, Hopkins PM, Steele DS. Effects of Mg2+ and SR luminal Ca2+ on caffeine-induced Ca2+ release in skeletal muscle from humans susceptible to malignant hyperthermia. J Physiol. 2002;544:85–95. doi: 10.1113/jphysiol.2002.022749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duke AM, Hopkins PM, Steele DS. Mg2+ dependence of halothane-induced Ca2+ release from the sarcoplasmic reticulum in rat skeletal muscle. J Physiol. 2003;551:447–454. doi: 10.1113/jphysiol.2003.046623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duke AM, Hopkins PM, Halsal JP, Steele DS. Mg2+ dependence of halothane-induced Ca2+ release from the sarcoplasmic reticulum in skeletal muscle from humans susceptible to malignant hyperthermia. Anesthesiology. 2004;101:1339–1346. doi: 10.1097/00000542-200412000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Owen VJ, Taske NL, Lamb GD. Reduced Mg2+ inhibition of Ca2+ release in muscle fibers of pigs susceptible to malignant hyperthermia. Am J Physiol. 1997;272:C203–C211. doi: 10.1152/ajpcell.1997.272.1.C203. [DOI] [PubMed] [Google Scholar]
- 22.Duke AM, Hopkins PM, Halsall PJ, Steele DS. Mg2+ dependence of Ca2+ release from the sarcoplasmic reticulum induced by sevoflurane or halothane in skeletal muscle from humans susceptible to malignant hyperthermia. Br J Anaesth. 2006;97:320–328. doi: 10.1093/bja/ael179. [DOI] [PubMed] [Google Scholar]
- 23.Duke AM, Hopkins PM, Calaghan SC, Halsall JP, Steele DS. Store-operated Ca2+ entry in malignant hyperthermia-susceptible human skeletal muscle. J Biol Chem. 2010;285:25645–25653. doi: 10.1074/jbc.M110.104976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cully TR, Edwards JN, Launikonis BS. Activation and propagation of Ca2+ release from inside the sarcoplasmic reticulum network of mammalian skeletal muscle. J Physiol. 2014;592:3727–3746. doi: 10.1113/jphysiol.2014.274274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leslie GC, Part NJ. The action of dantrolene sodium on rat fast and slow muscle in vivo. Br J Pharmacol. 1981;72:665–672. doi: 10.1111/j.1476-5381.1981.tb09147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szentesi P, et al. Effects of dantrolene on steps of excitation-contraction coupling in mammalian skeletal muscle fibers. J Gen Physiol. 2001;118:355–375. doi: 10.1085/jgp.118.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul-Pletzer K, Palnitkar SS, Jimenez LS, Morimoto H, Parness J. The skeletal muscle ryanodine receptor identified as a molecular target of [3H]azidodantrolene by photoaffinity labeling. Biochemistry. 2001;40:531–542. doi: 10.1021/bi001502s. [DOI] [PubMed] [Google Scholar]
- 28.Paul-Pletzer K, et al. Identification of a dantrolene-binding sequence on the skeletal muscle ryanodine receptor. J Biol Chem. 2002;277:34918–34923. doi: 10.1074/jbc.M205487200. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi S, et al. Dantrolene stabilizes domain interactions within the ryanodine receptor. J Biol Chem. 2005;280:6580–6587. doi: 10.1074/jbc.M408375200. [DOI] [PubMed] [Google Scholar]
- 30.Diaz-Sylvester PL, Porta M, Copello JA. Halothane modulation of skeletal muscle ryanodine receptors: Dependence on Ca2+, Mg2+, and ATP. Am J Physiol Cell Physiol. 2008;294:C1103–C1112. doi: 10.1152/ajpcell.90642.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner LE, 2nd, Groom LA, Dirksen RT, Yule DI. Characterization of ryanodine receptor type 1 single channel activity using “on-nucleus” patch clamp. Cell Calcium. 2014;56:96–107. doi: 10.1016/j.ceca.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards JN, et al. Ultra-rapid activation and deactivation of store-operated Ca2+ entry in skeletal muscle. Cell Calcium. 2010;47:458–467. doi: 10.1016/j.ceca.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Edwards JN, Cully TR, Shannon TR, Stephenson DG, Launikonis BS. Longitudinal and transversal propagation of excitation along the tubular system of rat fast-twitch muscle fibres studied by high speed confocal microscopy. J Physiol. 2012;590:475–492. doi: 10.1113/jphysiol.2011.221796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bakker AJ, Cully TR, Wingate CD, Barclay CJ, Launikonis BS. Doublet stimulation increases Ca2+ binding to troponin C to ensure rapid force development in skeletal muscle. J Gen Physiol. 2017;149:323–334. doi: 10.1085/jgp.201611727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cully TR, et al. Human skeletal muscle plasmalemma alters its structure to change its Ca2+-handling following heavy-load resistance exercise. Nat Commun. 2017;8:14266. doi: 10.1038/ncomms14266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gillies RL, Bjorksten AR, Du Sart D, Hockey BM. Analysis of the entire ryanodine receptor type 1 and alpha 1 subunit of the dihydropyridine receptor (CACNA1S) coding regions for variants associated with malignant hyperthermia in Australian families. Anaesth Intensive Care. 2015;43:157–166. doi: 10.1177/0310057X1504300204. [DOI] [PubMed] [Google Scholar]
- 37.Podranski T, et al. Compartmental pharmacokinetics of dantrolene in adults: Do malignant hyperthermia association dosing guidelines work? Anesth Analg. 2005;101:1695–1699. doi: 10.1213/01.ANE.0000184184.40504.F3. [DOI] [PubMed] [Google Scholar]
- 38.Oo YW, et al. Essential role of calmodulin in RyR inhibition by dantrolene. Mol Pharmacol. 2015;88:57–63. doi: 10.1124/mol.115.097691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baylor SM, Hollingworth S. Intracellular calcium movements during excitation-contraction coupling in mammalian slow-twitch and fast-twitch muscle fibers. J Gen Physiol. 2012;139:261–272. doi: 10.1085/jgp.201210773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodney GG, Schneider MF. Calmodulin modulates initiation but not termination of spontaneous Ca2+ sparks in frog skeletal muscle. Biophys J. 2003;85:921–932. doi: 10.1016/S0006-3495(03)74531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cully TR, Edwards JN, Murphy RM, Launikonis BS. A quantitative description of tubular system Ca(2+) handling in fast- and slow-twitch muscle fibres. J Physiol. 2016;594:2795–2810. doi: 10.1113/JP271658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karatzaferi C, de Haan A, Ferguson RA, van Mechelen W, Sargeant AJ. Phosphocreatine and ATP content in human single muscle fibres before and after maximum dynamic exercise. Pflugers Arch. 2001;442:467–474. doi: 10.1007/s004240100552. [DOI] [PubMed] [Google Scholar]
- 43.Westerblad H, Allen DG. Myoplasmic free Mg2+ concentration during repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol. 1992;453:413–434. doi: 10.1113/jphysiol.1992.sp019236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westerblad H, Allen DG. Myoplasmic Mg2+ concentration in Xenopus muscle fibres at rest, during fatigue and during metabolic blockade. Exp Physiol. 1992;77:733–740. doi: 10.1113/expphysiol.1992.sp003639. [DOI] [PubMed] [Google Scholar]
- 45.Allen DG, Lamb GD, Westerblad H. Impaired calcium release during fatigue. J Appl Physiol. 2008;104:296–305. doi: 10.1152/japplphysiol.00908.2007. [DOI] [PubMed] [Google Scholar]