Significance
The Venus flytrap has been in the focus of scientists since Darwin’s time. Carnivorous plants, with their specialized lifestyle, including insect capture, as well as digestion and absorption of prey, developed unique tools to gain scarce nutrients. In this study, we describe mechanistic insights into the cascade of events following the capture of insect prey. Action potentials evoked by the struggling prey are translated into touch-inducible hormone signals that promote the formation of secretory vesicles. Different varieties of digestive compounds are released sequentially into the flytrap’s “green stomach” and break down the captured animal. Amperometry provides insight into the kinetics and chemistry of the stimulus-coupled glandular secretion process.
Keywords: amperometry, exocytosis, Dionaea muscipula, secretion, plant digestion
Abstract
The Venus flytrap Dionaea muscipula captures insects and consumes their flesh. Prey contacting touch-sensitive hairs trigger traveling electrical waves. These action potentials (APs) cause rapid closure of the trap and activate secretory functions of glands, which cover its inner surface. Such prey-induced haptoelectric stimulation activates the touch hormone jasmonate (JA) signaling pathway, which initiates secretion of an acidic hydrolase mixture to decompose the victim and acquire the animal nutrients. Although postulated since Darwin’s pioneering studies, these secretory events have not been recorded so far. Using advanced analytical and imaging techniques, such as vibrating ion-selective electrodes, carbon fiber amperometry, and magnetic resonance imaging, we monitored stimulus-coupled glandular secretion into the flytrap. Trigger-hair bending or direct application of JA caused a quantal release of oxidizable material from gland cells monitored as distinct amperometric spikes. Spikes reminiscent of exocytotic events in secretory animal cells progressively increased in frequency, reaching steady state 1 d after stimulation. Our data indicate that trigger-hair mechanical stimulation evokes APs. Gland cells translate APs into touch-inducible JA signaling that promotes the formation of secretory vesicles. Early vesicles loaded with H+ and Cl− fuse with the plasma membrane, hyperacidifying the “green stomach”-like digestive organ, whereas subsequent ones carry hydrolases and nutrient transporters, together with a glutathione redox moiety, which is likely to act as the major detected compound in amperometry. Hence, when glands perceive the haptoelectrical stimulation, secretory vesicles are tailored to be released in a sequence that optimizes digestion of the captured animal.
Certain plants have turned the sword; they capture and consume animals, including potential herbivores (1, 2). Growing on mineral-deficient soils, the carnivorous Venus flytrap (Dionaea muscipula) lures, captures, and digests small arthropods (3–8) to feed on the nutrients extracted from their flesh (9–12). Closure of the bilobed snap trap is initiated by mechanical stimulation of trigger hairs located at the inner trap surface. Each trigger-hair bending elicits the firing of an action potential (AP). With the first AP, the trap stays open, but memorizes the initial strike. If a second one fires within 20 s, it triggers rapid trap closure. In case an insect is trapped and struggles to escape, two and more haptoelectric stimuli activate jasmonate (JA) signaling and biosynthesis (3, 6, 7). From the fifth strike on, glands raise their expression levels of hydrolase and nutrient transporter genes. When mechanostimulation is replaced by application of coronatine (COR), a mimic of the biologically active JA hormone JA-Ile, it can substitute for the mechanoelectric stimulation of the flytrap (7). Haptoelectric signaling and touch hormone activation turn the closed trap into a “green stomach,” flooding the entrapped prey with an acidic digestive fluid (3, 6, 13). Although prey capture and consumption of the Venus flytrap has been known since Darwin’s time (2), the molecular mechanisms of fluid phase secretion underlying animal consumption have remained unknown (14). In this study, amperometric carbon fibers were used in the plant field to monitor the dynamics and kinetics of mechanoelectric and JA stimulation of the secretory events, providing insight into exocytosis-dependent liquid filling of the digestive organ.
Results
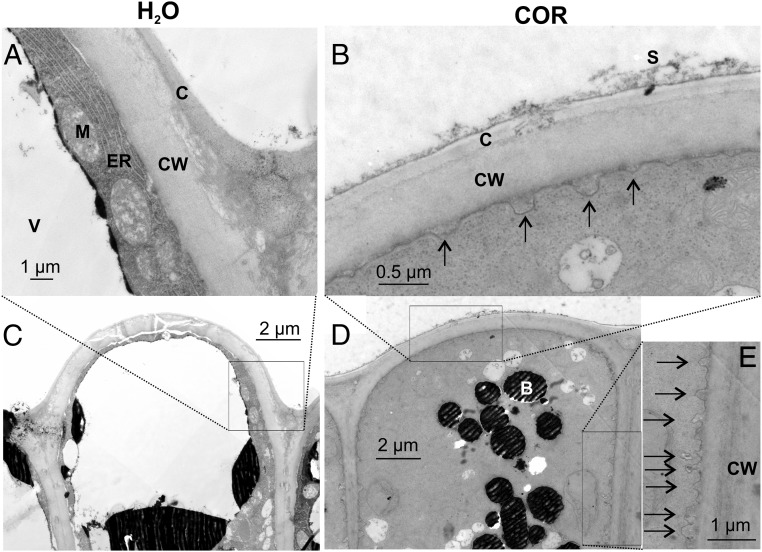
Upon haptoelectric trap activation, the surface area of the multicellular gland cell complex increases by 30% and an acidic protein moiety is released into the green stomach formed by the hermetically sealed lobes of the trap (3, 5–7, 13). In search of the membrane reservoir responsible for the surface increase of stimulated glands, we exposed traps to the JA-Ile mimic COR. Forty-eight hours after stimulus onset, membrane pits observed in electron micrographs (EMs) of glands suggested that secretory vesicle fusion had taken place predominantly at the apical end of head cells (outermost cell layer or L1) (Fig. 1 and Fig. S1B). Head cells of nonstimulated glands only occasionally showed exocytotic vesicles (1.5 ± 0.4 per cell; Fig. 1 A and C), but in the outermost layer of JA-stimulated glands’ cells, we detected a pronounced increase of pits associated with the more apical plasma membrane sections (16.5 ± 1.5 per cell, ∼0.18 μm in diameter; Fig. 1 B, D, and E). These results indicate that the secretory stimulation causes granule docking and membrane fusion.
Fig. 1.
Exocytotic vesicle fusion is stimulated in activated gland complexes. EMs of the outer layer of resting (A and C) and COR-stimulated (B, D, and E) Dionaea gland complexes are shown. A detailed view (A, B, and E) and overview (C and D) are shown. Whereas resting glands only exhibit a few exocytotic events, a massive rise in exocytotic vesicle fusion with the plasma membrane (black arrows) could be detected 48 h after COR stimulation. B, dark-stained body; C, cuticle; CW, cell wall; ER, endoplasmic reticulum; M, mitochondria; S, secreted fluid; V, vacuole. Slight shadow lines are due to carrier film handling during TEM sample preparation; all images are noncomposite originals.
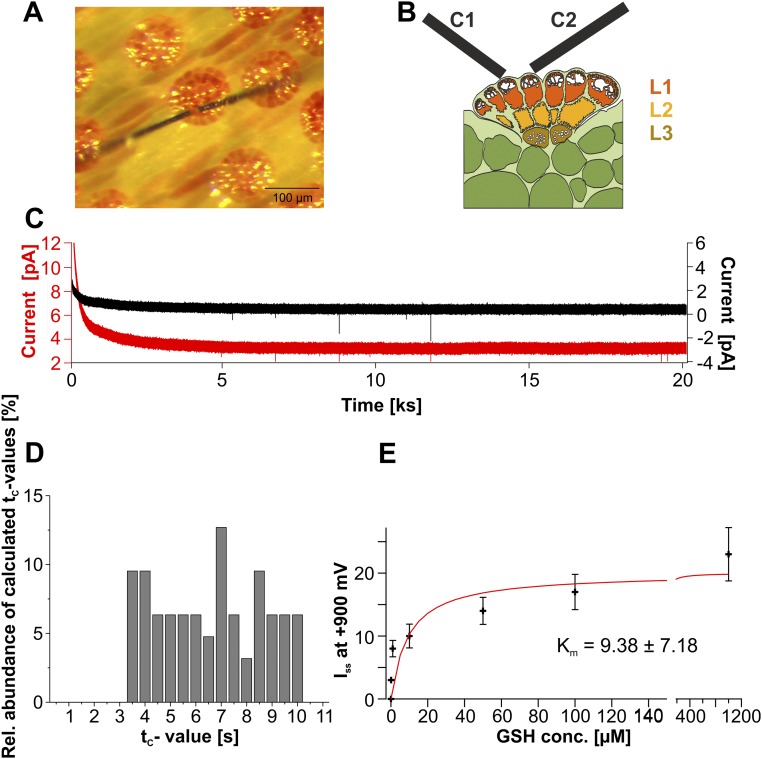
Fig. S1.
Amperometric detection of secretion. (A) Overview of the inner Dionaea trap surface. Two amperometric carbon fibers were clamped to +900 mV and placed on top of a gland complex to follow exocytotic secretion. (B) Model of two carbon fibers (C1 and C2) attached to the upper layer (L1) of a gland complex as shown in A. The three functional layers (L1–L3) are indicated: upper secretory layer L1 (red), inner layer L2 (yellow), and endodermoid layer L3 (brown). (C) Long-time amperometric response measured with two separate carbon fiber electrodes clamped to +900 mV. Electrodes were placed on top of an unstimulated Venus flytrap’s gland. After initial settling, currents resulted in a steady-state value at about 30 min. Under these conditions, artificial spiking of the electrodes was characterized as very fast spiking mostly pointing toward negative values. (D) Calculated tc values derived from Eq. 1 plotted against the relative (Rel.) signal abundance. In 63 analyzed spikes the tc values were distributed broadly homogeneously between 3.15 and 10.0 s. (E) Michaelis–Menten fit of a dose–response curve of amperometric current detected at carbon fibers held at +900 mV in solutions containing different concentrations (conc.) of reduced GSH (n = 5, mean ± SD).
Microelectrode Ion Flux Measuring Resolves Early Secretion of Acidic Vesicles.
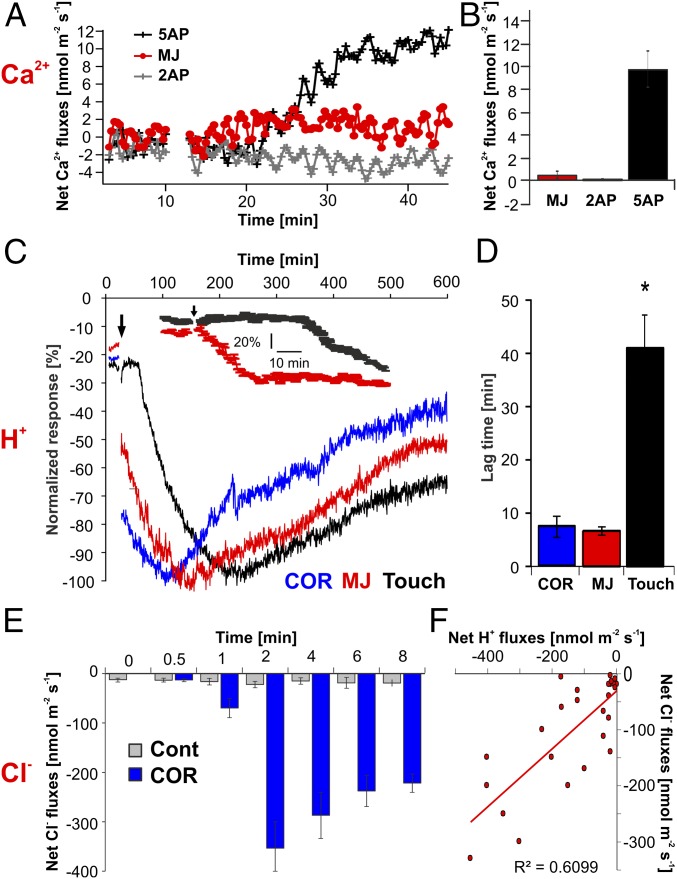
In a previous study, we compared the transcriptomic profile of nonstimulated glands with the transcriptomic profile of glands stimulated by either insects or COR. Before stimulation, the transcription profile of resting glands is already dominated by secretory processes (7). Dionaea secretion is directly coupled to acidification; H+ and chloride, Cl−, are released into the digestive fluid of the tightly sealed trap (15). To test whether touch stimulation of the flytrap’s trigger hairs is translated into ion fluxes across the gland plasma membrane, we used Ca2+-, Cl−-, and H+-sensitive microelectrode ion flux measuring (MIFE) microelectrodes (3, 16), which measure fluxes by recording local concentration gradients. After five to 10 consecutive trigger-hair stimulations and a lag time of about 10 min, a rapid shift in the net ion fluxes toward net Ca2+ uptake into the gland cells was observed (Fig. 2A). The mean net Ca2+ flux after mechanical stimulation (five APs) of the Venus flytrap was about 9.9 ± 1.8 nmol⋅m−2⋅s−1 (Fig. 2B; mean ± SE, n = 6). Within the first hour following stimulation, the ion fluxes were dominated by Ca2+ fluxes. Upon Ca2+ entry, the intracellular Ca2+ level rises (6) and JA signaling is activated (3, 7). Either consecutive trigger-hair stimulation alone or a direct application of JAs or COR induces secretion. With JAs, secretion in traps is initiated before they close (6). Following application of JAs, however, Ca2+-sensitive MIFE electrodes did not record net Ca2+ flux into glands (Fig. 2 A and B). Hormone stimulation triggered proton release, however, which appeared within 5–10 min following stimulation onset (Fig. 2 C and D). Net H+ efflux reached its peak between 1 and 2.5 h after stimulus application and then gradually recovered (Fig. 2C). When comparing the time that glands required to reach peak proton extrusion in response to mechanical or chemical stimulation, JAs were the fastest (Fig. 2C). Thus, JA-induced proton release was significantly faster than proton release elicited by mechanical stimulation (Fig. 2C, Inset), which reached peak currents of 54 ± 7 nmol⋅m−2⋅s−1 (mean ± SE, n = 6). Also the lag time of H+ efflux resulting from the different stimulations was longest in response to mechanical stimulation (Fig. 2D). This time dependence fits the notion that the rise in gland JA is downstream of haptoelectrics and gland calcium entry.
Fig. 2.
Net ion fluxes measured from stimulated Dionaea glands via the MIFE technique. (A) Net Ca2+ flux in response to mechanical (touched either two or five times within 10 s) and chemical [1 mM methyl jasmonate (MJ)] stimulation. (B) Peak Ca2+ flux response values for data shown in A (mean ± SE, n ≥ 5). (C) H+ flux kinetics in response to touch and JA stimulation. Each flux was normalized to its maximum flux (100%) to illustrate the difference in the peak time (mean ± SE, n ≥ 5). (Inset) Comparison of touch and MJ treatments at high temporal resolution. (D) Lag time in H+ flux responses between treatments shown in C. JA-induced proton release was significantly faster compared with mechanical induction (P ≤ 0.01, one-way ANOVA). (E) Net Cl− fluxes measured in COR-stimulated (blue bars) and nonstimulated (gray bars) glands at various time points after stimulation (mean ± SE, n ≥ 4). Cont, control. (F) Correlation of net H+ and Cl− fluxes measured from COR-stimulated glands at different time points illustrated in E. Each point represents a separate measurement. For all MIFE flux data, the sign convention is “influx-positive.”
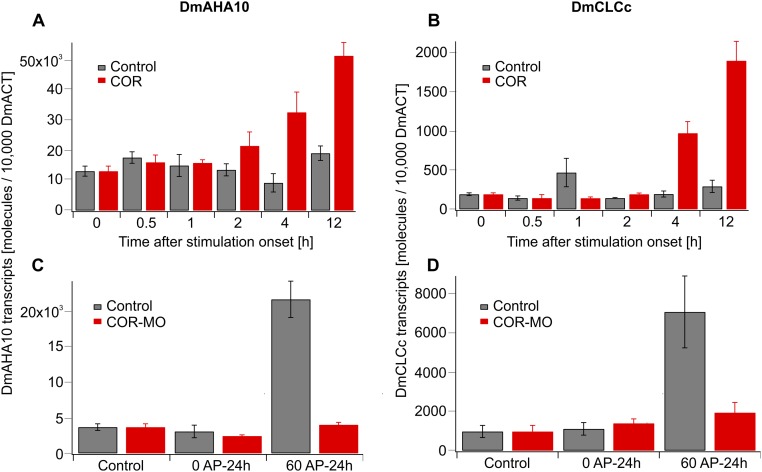
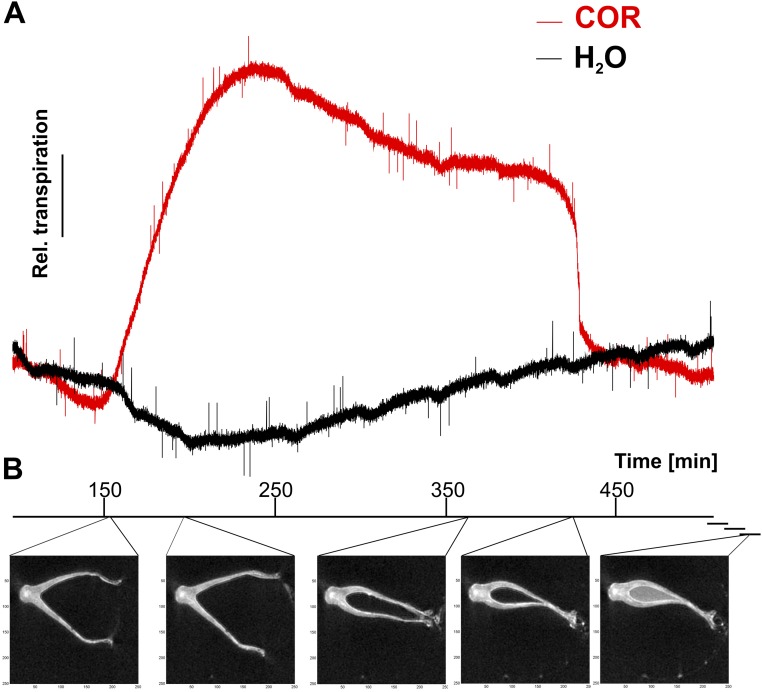
Regardless of whether it was stimulated or not, the resting membrane potential of glands remained in the range of −120 to −140 mV (12). This finding might indicate that trap acidification results from electroneutral exocytotic H+ release rather than the massive activation of plasma membrane proton pumps. This notion is supported by the COR-induced increase in vacuolar AHA10-type proton pump transcripts (17), together with the proton pump transcripts of a ClC-type proton-chloride antiporter (18), two components required for hyperacidification of secretory vesicles (SI Text and Fig. S2). To test whether H+ fluxes are accompanied by Cl− fluxes, we used chloride-sensitive MIFE electrodes side-by-side with the pH microelectrodes. Confirming our working model, we monitored pronounced Cl− net efflux from glands in COR-stimulated traps (Fig. 2E; blue), but not in resting (Fig. 2E; gray) traps. COR-induced chloride currents appeared with a similar time dependence and amplitude as the proton fluxes (Fig. 2 C and E). Both fluxes were correlated with each other (R2 = 0.61, P < 0.01), exhibiting a stoichiometry between H+ and Cl− close to 1:1 (Fig. 2F). The electrochemistry-based MIFE experiments illustrated above can only be conducted in an aqueous environment. In such a wet scenario, we monitored initial secretion-associated proton extrusion in response to COR about 9 min after stimulation (Fig. 2D). To resolve the onset of gross gland fluid secretion in the initially dry Dionaea trap, we followed the fluid production after COR stimulation by infrared gas analysis (IRGA) and magnetic resonance imaging (MRI). First, fluid phase secretion-associated trap water vapor emission was detected in IRGA recordings 151 ± 13 min (n = 3, mean ± SD) following trap stimulation with COR (Fig. S2A). After reaching peak humidity, trap water emission slowly decreased and suddenly dropped after 445 ± 84 min (n = 3, mean ± SD) to the basal level of evaporation before COR application and in nonstimulated controls (Fig. S3A). This rapid drop in water emission reflects hermetical sealing of the trap lobes (6). Filling of the closed trap with digestive fluid was visualized by MRI (Fig. S3B and Movie S1).
Fig. S2.
Expression of DmAHA10 and DmCLCc is induced in activated Dionaea gland complexes in a JA-dependent manner. (A and B) Traps were sprayed with water (control, gray) or 100 μM COR (red), and gland complexes were harvested after the time points indicated. (C and D) Effect of the JA antagonist COR-MO on electromechanical induction of DmAHA10 and DmCLCc expression. Traps were pretreated 4 h before application of zero or 60 APs with H2O (gray) or 100 μM COR-MO (red). RNA was sampled 24 h after onset of mechanostimulation. Transcript numbers are given relative to 10,000 molecules of DmACT1. Data are given as mean ± SE (n = 6).
Fig. S3.
COR induces exocytosis-based fluid phase secretion. (A) COR-stimulated kinetics of relative transpiration. The trap was sprayed with 100 μM COR and placed in the IRGA gas exchange chamber. The increase in relative humidity reflects the start of secretion. The drop in relative humidity was paralleled by hermetical sealing during trap closure. A representative experiment is shown. (B) MRI imaging of a COR-stimulated flytrap. Representative photographs are shown according to the different phases of Dionaea stimulation. (Left to Right) Unstimulated trap; phase 1–2, the trap transiently opens up wider and starts to produce fluid film; phase 3, the trap closes slowly to reach a position similar to mechanically induced fast trap closure; and phase 4, the trap lobes seal hermetically and the trap is completely filled by a fluid, acidic hydrolase moiety.
Detection of Digestive Vesicles via Amperometry.
With animal cells, exocytotic events can be monitored noninvasively via amperometry, detecting redox currents when electrodes are placed near the membrane surface of secretory cells (19, 20). Given that amperometry detects oxidizable substances, such as neurotransmitters, neuropeptides, and hormones released from secretory vesicles, we adopted this electrochemical approach to probe for exocytotic events in active flytrap glands. Aiming to detect spikes associated with secretory cargo release from the inner trap surface, we placed carbon fiber microelectrodes in contact with the apical face of the glands’ upper head cells (Fig. S1 A and B). Under these experimental conditions, no amperometric signals were detectable in nonstimulated glands (Fig. S1C). However, with glands stimulated by five to 20 trigger-hair displacements, signals similar to the signals measured with secretory animal cells could be monitored (21, 22) (Fig. 3A), albeit with a much slower time course due to cell wall geometry. When placing two electrodes next to each other, both electrodes recorded characteristic increases in amperometric current in a close temporal relationship, as shown in Fig. 3 A and B, excluding the possibility that such discrete events were artifacts generated in one or the other electrode. In these experiments, we had to use strong pH buffering to preserve the sensitivity of the amperometric electrodes (Materials and Methods).
Fig. 3.
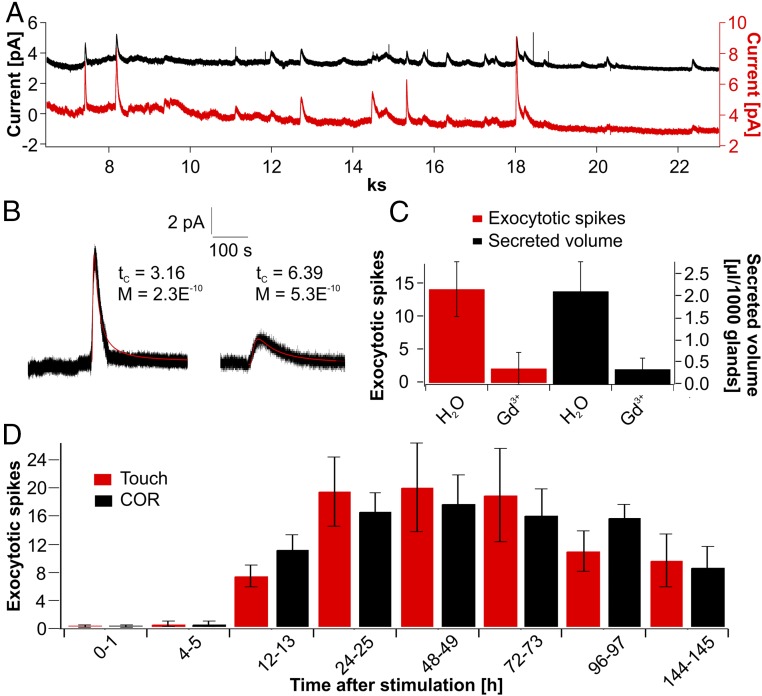
Amperometric detection of exocytotic events in Dionaea glands. (A) Long-term spiking response of stimulated glands. Current spikes resulting from the exocytosis of individual vesicles were detected with two electrodes simultaneously clamped to +900 mV. (B) Two examples of analyzed exocytotic current spikes are shown. Fitting these events with Eq. 1 [f(x) = M/(t−t0)1.5 * exp(−tc/(t−t0)] (red line) reveals characteristics of release quantified by M and tc that reflect the amount and distance of fusing vesicle to carbon fiber. (C) Gadolinium (Gd3+; 10 mM) was sprayed 24 h before mechanical stimulation of the Venus flytraps. Twenty-four hours after stimulation, the number of amperometrically detected events within 1 h (red) and the secreted volume (black) were calculated. Compared with control traps, gadolinium inhibits secretion as well as amperometrically detectable exocytotic spiking. Data are mean ± SD (n ≥ 25). (D) Time course of exocytosis-related spiking in response to touch (red) and COR (black). For the given time points, the number of exocytotic events was calculated. Both stimuli lead to the same long-term spiking response in flytrap glands. Data represent mean ± SD (n ≥ 54).
The amperometrically detected chemical species is released to the apoplast at the point of exocytosis. From that point source, the released substance diffuses to the electroactive tip of the carbon fiber, where it is oxidized. It has been shown that placing electrodes more than several microns away from the cell surface results in a significant decrease in signal and spatiotemporal resolution (23, 24). Therefore, the best scenario for detecting exocytotic events without diffusional dilution is to touch the cell surface with the electrode. This limitation by diffusion can be described by Fick’s law. Thus, we fitted the amperometrically detected spikes with a 3D diffusion equation according to Eq. 1 (Materials and Methods). From this calculation, we gained a parameter, tc, which is a characteristic diffusion time, depending on the distance between the point source of secretion and the carbon fiber tip, given a certain diffusion coefficient, D. Fitting sharp secretory events observed when electrodes were placed directly on the Dionaea gland surface with a high spatiotemporal resolution resulted in tc values of about 4–5 s. Plotting the relative signal abundance against the calculated tc values of detected secretory events, a broadly homogeneous distribution was obtained (Fig. S1D). In other words, the amperometric approach we used detects secretory events originating from various distances to the tip of the carbon fiber or else implies a range of diffusion coefficients. Interestingly, we did not obtain any tc values ≤3.15 s in 63 analyzed spikes. Assuming a constant D value in the performed experiments and tc ≥ 3.15 s, we can calculate a lower bound for the geometrical distance between the point of secretion and the carbon fiber (Eq. 2) and the diffusion constant, D, of the secreted substance in the medium (Materials and Methods). In contrast to animal cells, the plasma membrane of plant cells is covered with an extra layer of cellulose-based cell wall and a lipid-based cuticle. Thus, the minimal tc value obtained for Dionaea glands very likely results from the cell wall-cuticle shell that keeps the fiber electrode at some distance (r) from exocytotic vesicles fusing with the gland cell plasma membrane. From EMs similar to the EMs shown in Fig. 1, we calculated a minimal distance between the electrode and secreted vesicle fusing with the head gland cell plasma membrane of ∼0.5 μm (Fig. 1B). Introducing this value in the Eq. 2, we are able to calculate the diffusion constant of the fluid secreted from Dionaea in its diffusion medium (containing the cell wall and cuticle). The calculated value of D = 1.92 × 10−10 cm2⋅s−1 indicates a high diffusional resistance of the gland cell wall. For comparison, the diffusion coefficient of dopamine in water was reported at 6.0 × 10−6 cm2⋅s−1 (25). Also, in the animal system, diffusion in tissue or in solutions containing biological macromolecules is known to be hindered by the cellular matrix. Hafez et al. (26) have reported that the diffusion coefficient of dopamine at the surface of an adrenal cell is one-tenth compared with its diffusion in water. The small diffusion constant reported here for Dionaea also illustrates the slow time characteristics of the detected amperometric spikes with a half-life (t1/2) time constant of 87.82 ± 12.14 s (mean ± SE, n = 92). Compared with the free aqueous diffusion of catecholamine release in neuronal cells, the t1/2 of Dionaea plant secretory events is enlarged by a factor of ∼10,000 (24, 26).
To determine emergence and manifestation of gland cell exocytosis, we monitored the frequency of secretory events for up to 145 h. Traps were stimulated either mechanically by a series of 20 consecutive trigger-hair bendings or by spraying COR onto the traps’ inner surface. Within the first 4–5 h after stimulation onset, no significant signals could be monitored. The first exocytosis-type spiking was observed after about 6 h (Fig. 3D). Thereafter, exocytotic events occurred more frequently, reaching about half-maximum spiking after 12–13 h. Maximal spiking rates were detected after 24 h and remained high for another 2 d before slowly declining at days 4 and 6 (Fig. 3D). Interestingly, COR stimulation and trigger-hair bendings resulted in a similar time dependence of spiking frequency. This finding indicates that JA induction of secretory vesicle formation, loading, and membrane fusion, rather than touch induction of JA biosynthesis, represents the rate-limiting step during Dionaea gland cell exocytosis.
We also found that the Ca2+ channel blocker gadolinium strongly reduced the volume of secreted fluid (Movie S2). To investigate the inhibitory effect of Gd3+ on trap secretory fluid production further, traps were sprayed with 10 mM Gd3+ (∼2.5 μmol) 24 h before mechanical stimulation. This Gd3+ challenge did not affect the traps’ naturally fast closure in response to two trigger-hair strikes, however. When traps were mechanically stimulated for secretion by five to 20 trigger-hair displacements, gadolinium-sprayed traps were found to be strongly reduced in extruded fluid volume. Compared with control traps, which secreted 2.12 ± 0.67 μL per 1,000 glands within 48 h, Gd3+-pretreated traps released only 0.35 ± 0.25 μL per 1,000 glands (Fig. 3C, black bars). At the same time, exocytotic events amperometrically determined with single gland cells dropped from 14.3 ± 4.17 events per hour in controls to 2.1 ± 2.45 events per hour in the Gd3+-exposed traps (Fig. 3C, red bars). The pronounced Gd3+ block of secretion seen by amperometry and MRI suggests that JA and calcium signaling is required for haptoelectric and JA stimulation of Dionaea gland cell secretion.
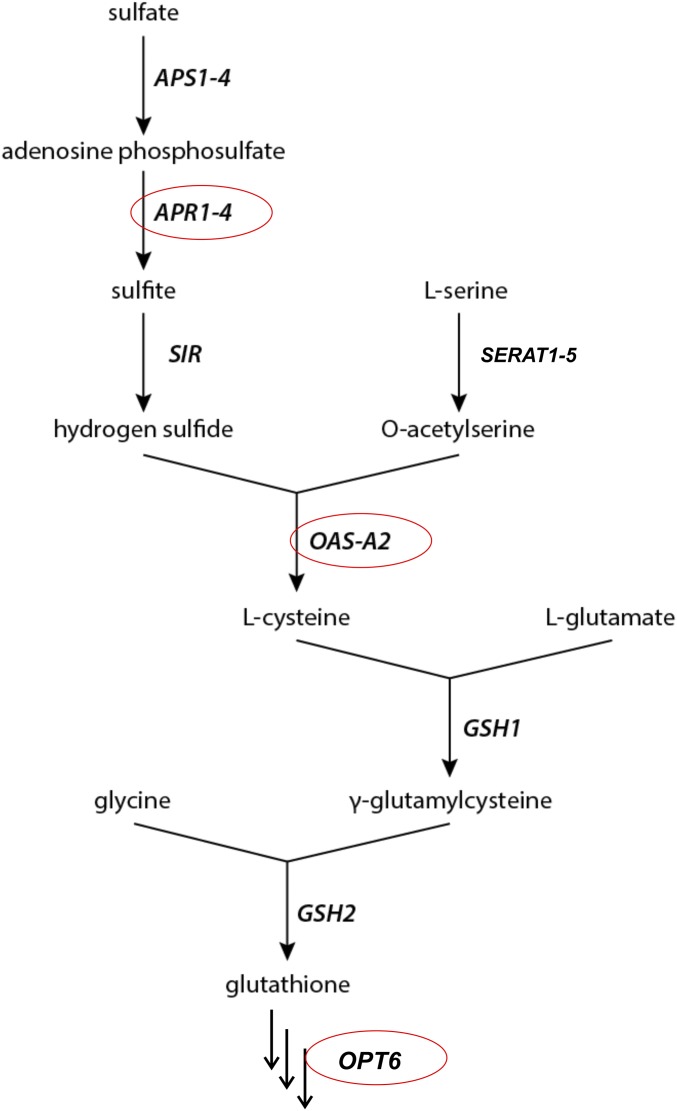
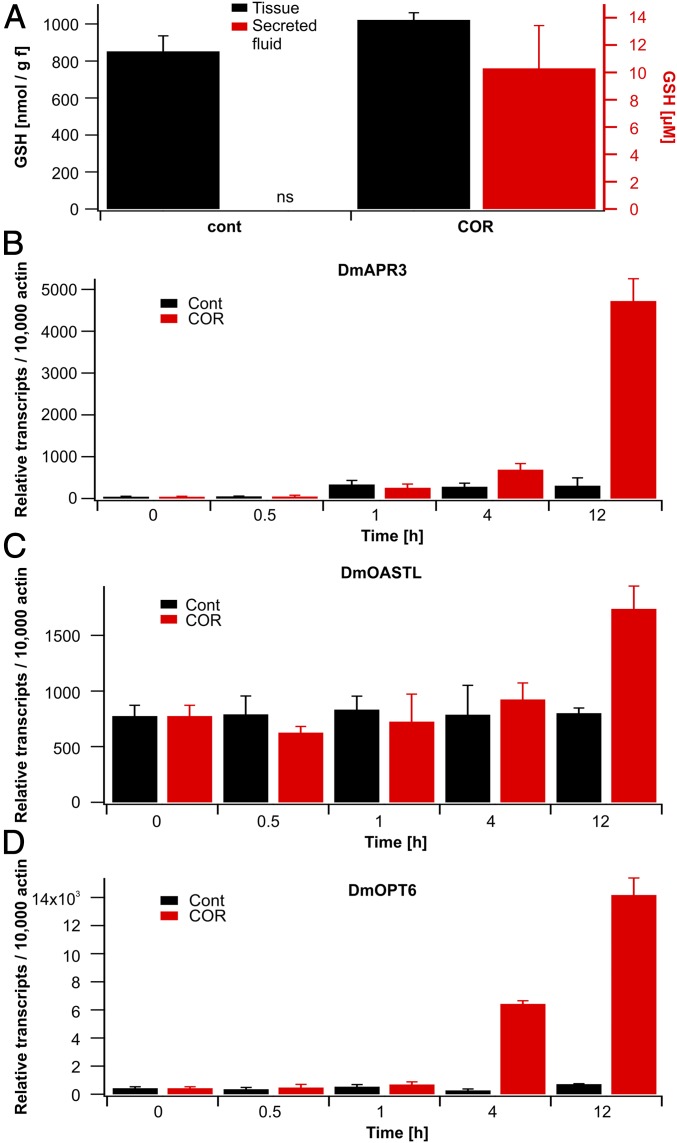
What kind of redox moiety does Dionaea’s secretory gland cells release? To gain and maintain functional integrity of cysteine-rich hydrolytic enzymes exuded into the digestive fluid (13, 27, 28), a defined redox status in the extracellular bioreactor is required. Glutathione (GSH) represents an important redox regulator of enzyme functions in plant cells (29, 30). GSH can be derived from activated sulfate [adenosine 5′-phosphosulfate (APS)] via a well-known enzymatic pathway (Fig. S4). Gene expression analysis based on RNA-sequencing data (available at tbro.carnivorom.com; cf. ref. 7) indicated that COR might induce genes involved in GSH production and transport. These analyses were further confirmed by quantitative real-time PCR. Among these genes, the APS reductase (DmAPR3) is strongly up-regulated 12 h after COR stimulation (Fig. 4B). APS reductase represents the most important regulatory enzyme of the pathway that determines the flux of sulfate into organic sulfur compounds in plants (31, reviewed in ref. 32). In addition, the availability of C-N skeletons for cysteine synthesis is promoted in response to the JA mimic through enhanced serine O-acetyltransferase (DmSERAT2) expression and through cysteine synthesis by itself via elevated O-acetylserine(thiol)lyase (DmOASTL) expression (33) (Fig. 4C). Moreover, the putative GSH transporter, oligopeptide transporter 6 (DmOPT6), is transcriptionally induced after COR treatment as well. Interestingly, all four transcripts are induced by both COR or prey capture in a similar fashion (Fig. 4 B–D and tbro.carnivorom.com). Therefore, enhanced sulfate reduction and assimilation seems to be required for both the synthesis of cysteine-rich hydrolytic enzymes and additional synthesis of GSH, which can be detected in the secreted fluid.
Fig. S4.
Biosynthetic pathway of GSH. APS1–4, ATP sulfurylases 1–4; APR1-4, adenosine phosphosulfate reductases 1–4; GSH1 & 2, GSH synthetase 1 & 2; OASTL, O-acetylserine (thiol) lyase; OPT6, oligopeptide transporter 6; SERAT, serine acetyl transferase; SIR, sulfite reductase.
Fig. 4.
Synthesis of the reactive oxygen species scavenger GSH is induced in stimulated Dionaea traps. (A) GSH levels in traps (black) or secreted fluid (red) under nonstimulated conditions (cont) or 24 h after spray application of 100 μM COR. Note that resting traps do not secrete (ns) digestive fluid. Data represent mean ± SD (n ≥ 4). (B–D) COR induces key genes involved in GSH biosynthesis. Expression of the APS reductase (DmAPR3), O-acetylserine(thiol)lyase (DmOASTL), and oligopeptide transporter 6 (DmOPT6) in Dionaea gland complexes is shown. Traps were sprayed with water (Cont, black) or 100 μM COR (red), and gland complexes were harvested at the time points indicated. Transcript numbers are given relative to 10,000 molecules of actin (DmACT1) (mean ± SE, n = 6).
To test whether GSH is released into the extracellular compartment, we sampled digestive fluids from stimulated flytraps and analyzed the samples for the presence of antioxidants (34–36). Indeed, we could detect GSH in Dionaea’s extracellular fluid (Fig. 4A). In contrast to GSH, however, ascorbate was not detectable by state-of-the-art methods (36, 37). Although the GSH concentration in whole Dionaea traps was not significantly altered by COR treatment, the stomach GSH concentration was on the order of 10 μM 48 h after stimulation onset (Fig. 4A, red bars). To test the sensitivity of the carbon fibers used in our amperometric analysis toward this reactive oxygen species (ROS) scavenger, we performed experiments with defined GSH concentrations (Fig. S1E). In these experiments, the reduced GSH was oxidized at the positively charged carbon fiber, resulting in a positive current. Interestingly, the amperometric current, detected with a constant potential of +900 mV in solutions of defined GSH concentrations, saturated with a half-maximal concentration of 10 μM (Fig. S1E), which corresponds well with the actual GSH concentration in the secreted fluid. Thus, it is likely that under our conditions, secreted GSH is detected in the amperometric analysis. Nevertheless, we expect the amperometry to detect additional electroactive substances besides GSH released in the secreted fluid of stimulated Venus flytraps.
SI Text
JA signaling stimulates the expression of vesicle acidification-associated transcripts in Dionaea. To accumulate high vacuolar H+ concentrations, lemon fruits engage unique P-type ATPase (48) at the tonoplast. The orthologous ATPase in Dionaea DmAHA10 is expressed in resting Dionaea glands and is up-regulated upon insect, mechanical, and COR stimulation (7) (Fig. S2 and tbro.carnivorom.com). Whereas protons are accompanied by the citrate anion in the lemon system, chloride is the counter anion in Dionaea (15) (Fig. 2F). In bacteria and plants, ClC-type anion channels operate as H+/anion antiporters (49–51). Dionaea glands express a ClC-type anion channel already in the resting state, which is transcriptionally further induced upon trap stimulation (7) (DmCLCc; Fig. S2 and tbro.carnivorom.com). Interestingly, the JA-signaling inhibitor coronatine-methyloxime (COR-MO) suppressed mechanostimulation-induced transcription (Fig. S2 C and D). The fact that gland DmCLC expression and chloride release (Fig. 2E and Fig. S2) are triggered by touch and that the touch hormone mimics COR complements early findings that stimulated head cells of the gland accumulate chloride and disperse it after vacuolar fragmentation (2, 15). In these studies, the appearance of Cl− in the outer walls of stimulated Dionaea glands was correlated to vacuolar fragmentation into vesicles and their fusion with the plasma membrane (15). This assumption is supported by our ion-selective MIFE studies monitoring the release of Cl− and H+ about 1 h after gland stimulation (Fig. 2).
Discussion
The molecular machinery underlying secretory vesicle fusion with the plasma membrane in animal cells is known in great detail (38–40). Upon chemical or electrical stimulation of secretory animal cells, exocytotic events can be detected within milliseconds (41–44). In these fast-responding cells, certain pools of preformed cargo-loaded vesicles are released immediately after stimulus onset. Following haptoelectric calcium entry in Dionaea glands, JA signaling triggers vesicle acidification and de novo synthesis of secretory proteins. The fact that carbon fiber electrodes detect amperometric signals no earlier than about 6 h after mechanical and JA stimulation (Fig. 3D) may indicate that the oxidizable compound, most likely the tripeptide GSH, is contained only in those vesicles equipped with hydrolases. In the acidic extracellular digestive fluid, GSH is very stable, providing for a proper redox state for sustained hydrolase activity (45).
Dionaea’s secretion events occur on a slow time scale. The apparent diffusion constant of released substances, as calculated from the waveform of the amperometric signal, was D = 1.92 × 10−10 cm2⋅s−1, which indicates a high diffusional resistance of the gland cell wall. For comparison, the diffusion constant of catecholamines in aqueous solution is 1 × 10−6 to 8 × 10−7 cm2⋅s−1 (19, 46). Thus, diffusion in the cell wall of Dionaea glands is about four orders of magnitude slower than diffusion of small molecules in aqueous solution. In contrast to fast synaptic signaling in the nervous system of animals, this slow diffusion, as well as the slow time course of release, reflects the biology of the insect-processing flytrap: Once Dionaea captures prey via its fast haptoelectric sensing system, exocytotic release and slow diffusion of a tailored hydrolase mixture into the digestive fluid perfectly serves the long-term nutrient needs of the plant.
Materials and Methods
To access the inner trap surface for amperometric recordings (even in stimulated plants), unstimulated traps in the open position were fixed in a chamber and mechanically locked to prevent trap closure upon stimulation. For inhibitor pretreatments, plants were sprayed with 10 mM GdCl3, or H2O as a control. Twenty-four hours after pretreatments, traps were stimulated for secretion either mechanically (touch of trigger hairs five to 10 times within 1 min) or by hormone spraying (100 μM COR). At the given time points after stimulation, amperometric measurements were performed with open-fixed traps still attached to the plant. The chamber was filled with standard bath solution [1 mM KCl, 1 mM CaCl2, 50 mM Hepes/NaOH (pH 7)] and placed on a microscope stage (Zeiss Axioscope 2 FS). A three-electrode configuration was used, where an Ag/AgCl electrode served as the reference electrode grounding the bath solution. Two sensory carbon fiber electrodes with a diameter of 5 μm (ALA Scientific Instruments) were used for amperometric detection. Carbon fibers were gently placed on top of the gland head cells if not stated otherwise. During amperometric recordings, electrodes were held at +900 mV with two VA-10X amperometry amplifiers (ALA Scientific Instruments). Oxidative current was acquired via VA-10X amplifiers and digitized at 20 kHz through an ITC-18 digital-to-analog convertor (InstruTECH). Data were acquired using Patch master (HEKA Elektronik) and analyzed with a custom-written fit running under Igor 6. Detected events were described by following equations (47):
| [1] |
Here, t0 is the time of signal onset (a free-fitting parameter) and M depends on the amount of secreted substance as well as on the diffusion coefficient, D. The parameter tc depends on D and the distance, r, between the point source of secretion and the carbon fiber tip according to:
| [2] |
Further details on materials and methods can be found in SI Materials and Methods.
SI Materials and Methods
Plant Growth and Harvesting.
Dionaea plants were grown as described previously (12). Briefly, Dionaea muscipula plants were purchased from CRESCO Carnivora and grown in plastic pots at 22 °C in a 16-h/8-h light/dark photoperiod. For COR treatments, traps were directly sprayed with a 100 μM COR solution (Sigma–Aldrich). Isolation of secretory gland complexes was achieved by gently abrading the inner trap surface using a sharp razor blade. For mechanical induction of gene expression, trigger hairs were stimulated 60 times (frequency of one time per minute), and samples were collected 24 h after the first stimulus. In inhibitor tests, 100 μM COR-MO (3) was sprayed 4 h before mechanical stimulation.
Electron Microscopy.
Traps from intact Dionaea plants were either stimulated with COR solution (100 μM) or remained unstimulated. After 48 h, intact leaves were fixed with 2% glutaraldehyde in cacodylate buffer (75 mM, pH 7.0) for 1 h, cut into small trap sections with a razor blade, and further incubated for 24 h in total. Subsequently, samples were postfixed with 1% osmium tetroxide at 4 °C overnight. After being dehydrated through a series of graded acetone concentrations (30–100%), samples were finally embedded in plastic according to the technique of Spurr et al. (52). Ultrathin sections were obtained with an ultramicrotome (Ultracut E; Leica Reichert–Jung), transferred onto copper grids coated with Mowital, and stained with uranyl acetate followed by lead citrate (53). Sections were viewed with a LEO 906 E transmission electron microscope at 100 kV equipped with a MultiScan CCD Camera (model 794; Gatan) using Digital Micrograph 3.3 software (Gatan) to acquire, visualize, analyze, and process image data.
MIFE Studies.
Plant material handling.
A single lobe containing a midrib was cut and immobilized around a hollow plastic cylinder with a diameter of 8 mm using medical adhesive (VH355; Ulrich AG) and Parafilm. The immobilized lobe was mounted in a Petri dish containing basic salt medium [BSM; 0.5 mM KCl, 0.1 mM CaCl2, 0.1 mM NaCl (pH 5.8)]. Following recovery for 1 h, net Ca2+ and H+ fluxes were measured in response to mechanical or chemical stimulation. Mechanical stimulation was achieved by touching a trigger hair five to 10 times with a pipette tip. Chemical stimulation was performed by applying either 1 mM methyl JA or 100 μM COR to the glands. Net ion fluxes were monitored for 5 min in BSM, followed by stimulation and continuous recordings for about 10 h.
Ion flux measurements.
Net Ca2+, Cl−, and H+ fluxes were measured using the noninvasive MIFE technique (University of Tasmania) (16). Briefly, microelectrodes with an external tip diameter of ∼2 μm were pulled, silanized, and filled with selective mixture [H+ (catalog no. 95297), Cl− (catalog no. 99408), or Ca2+ (catalog no.99310; all from Sigma–Aldrich)]. Electrodes were mounted on a 3D micromanipulator (MMT-5; Narishige) and calibrated with an appropriate set of standards. A measuring chamber with the immobilized trap lobe was placed into a Faraday cage. Ion-selective microelectrodes were positioned, with their tips aligned ∼50 μm above the lobe’s surface using a 3D hydraulic micromanipulator. During measurements, a computer-controlled stepper motor moved microelectrodes in a slow 6 s/6 s square-wave cycle between two positions 100 μm apart in distance. The potential difference between two positions was recorded by the MIFE CHART software (54) and converted into an electrochemical potential difference using the calibrated Nernst slopes of the electrodes. Net ion fluxes were calculated using MIFEFLUX software for cylindrical diffusion geometry.
RNA Extraction, Sequencing, and Quantitative Real-Time PCR.
RNA was isolated separately from each flytrap sample using a modified cetyltrimethylammonium bromide (CTAB)-based protocol. In brief, 0.1 g of Dionaea plant material powdered in liquid nitrogen was thoroughly mixed with 0.7 mL of hot (65 °C) RNA extraction buffer [2% CTAB, 2% polyvinylpyrrolidone K 25, 100 mM Tris⋅HCl at pH 8.0, 25 mM Na-EDTA at pH 8.0, and 2 M NaCl, with 2.5% (vol/vol) 2-mercaptoethanol added immediately before use]. Following a 10-min incubation at 65 °C and extraction with 1 vol of chloroform/isoamyl alcohol [24:1 (vol/vol)], RNA was precipitated from the supernatant by adding 175 μL of 8 M LiCl overnight (4 °C). RNA was collected by centrifugation, resuspended in diethyl pyrocarbonate (DEPC)-treated H2O, and precipitated in the presence of 0.1 vol of 3 M Na acetate (pH 5.2) and 2.5 vol of 96% ethanol (EtOH). After a washing step with 70% EtOH, RNA was dissolved in 40 μL of DEPC H2O. DNA contamination was removed by DNase I treatment on a microcolumn (Roche). RNA quantity and quality were determined by capillary electrophoresis (Experion automated electrophoresis system and Experion RNA high-sense analysis kit; Bio-Rad Laboratories). Individual transcript levels were analyzed by quantitative real-time PCR (qPCR). qPCR was performed using a Realplex Mastercycler (Eppendorf), 1:20 diluted cDNA, and Absolute QPCR SYBR green capillary mix (Thermo Scientific). Expression levels were quantified using a standard for each primer pair and normalized to 10,000 molecules of actin (DmACT) cDNA transcripts. Gene-specific primers were designed using the software LightCycler Probe Design 2.0 (Roche Life Science) based on the transcriptomic information available at tbro.carnivorom.com (7). Individual transcripts deposited there are given in parentheses. The following primers were used: DmACT (comp226979_c1_seq1), DmACTLC forward (fw): 5′-TCTTTGATTGGGATGGAAGC-3′, DmACTLC reverse (rev): 5′-GCAATGCCAGGGAACATAGT-3′; DmAHA10 (comp234095_c1.1_seq9), DmAHA10LCfw: 5′-GACTTTACATGGGCTG-3′, DmAHA10LCrev: 5′-GCCCGAAAACTATTTATC-3′; DmCLCc (comp214625_c0.0_seq1), DmCLCcLCfw: 5′-ATATACGGTTGTTGAGAC-3′, DmCLCcLCrev: 5′-AATCTTCAGATCCCAC-3′; O-acetylserine(thiol)lyase (DmOASTL; comp199845_c0.0_seq1), DmOASTLLCfw: 5′-AAGTTATCACCGTGTC-3′, DmOASTLLCrev: 5′-AGAGTGCAAGGTAAATC-3′; DmAPR3 (comp215379_c1.0_seq1), APS reductase (DmAPR) (DmAPR3LCfw: 5′-GGAACTGGCTGACAAG-3′, DmAPR3LCrev: 5′-TGGATTACACTTAAAAG-3′; oligopeptide transporter 6 (DmOPT6; comp225114_c0_seq7), DmOPT6LCfw: 5′-GCCCGCTACAACTATA-3′, DmOPT6LCrev: 5′-CTTGCTTTGGAACTCTTAC-3′.
Ultra High-Performance Liquid Chromatography Measurements.
GSH and its metabolic precursors, γ-glutamylcysteine and cysteine, were quantified as monobromobimane (mBBr) derivatives (34, 35) by a modification of the ultra high-performance liquid chromatography (UPLC) method previously described (55). For this purpose, digestive fluids of stimulated flytraps were collected, immediately frozen in liquid N2, and stored at −80 °C until analyses. For derivatization, digestive fluids were diluted with 0.1 HCl as required (1:5–1:20) and aliquots of 50 μL were treated with mBBr (55). Stimulated flytraps were cut off, washed in ddH2O, immediately frozen in liquid N2, ground with a mortar and pestle to a homogeneous powder, and stored at −80 °C until analyses. Thiols were determined in aliquots of 50 mg of frozen powder after derivatization with mBBr (55). Thiol derivatives in samples from digestive fluids and trap tissues were separated by UPLC, detected by fluorescence at 380 nm/480 nm, and quantified by comparison with external standards (55). Ascorbic acid was determined in aliquots of 50 μL of digestive fluids using the colorimetric method previously described (36).
Gas Exchange Measurements.
Fluid phase secretion of COR-stimulated Venus flytraps was recorded in a setup of two whole-plant cuvettes. Two customized IRGA (HCM-1000; Walz; www.walz.com) were used to measure the increase of water vapor concentration in the air stream passing the flytraps. To avoid transpiration from the soil, the surface and the plant, except for one trap, were sprayed with a 100 μM COR solution (Sigma–Aldrich) and covered with water-tight foil. The composition of the gas stream of 1 L⋅min−1 through each cuvette was adjusted by mass flow meters (red-y smart series; Vögtlin; https://www.voegtlin.com/) and set to 24 °C, 47% relative humidity, and 350 ppm CO2. Illumination was provided by three LEDs, providing light at 655 nm at a photon fluence rate of 100 μmol⋅m2⋅s−1 (WEPDR3-S1 Power LED Star tiefrot 3W; Winger), at 455 nm at 8 μmol⋅m2⋅s−1 (Luxeon, Royal Blue; Philips) and at 395 nm (WEPUV3-S1 UV Power LED Star 395 nm; Winger). The three light beams were collected by dichroic mirrors (Q525 LPXR and DCLP 425; Chroma; https://www.chroma.com/) and guided through fiber optics to the cuvettes (Fiber Illuminator FL-460; Walz; www.walz.com).
MRI.
For sample preparation, plants, including roots, were prepared to carry a single flytrap only and were placed in a 15-mL Eppendorf tube filled with tap water or water with a concentration of 50 mM of GdCl3 and mounted in the MRI tube. COR was applied to trigger the flytrap’s slow closure and secretion (6).
MRI experiments were performed on a vertical 11.7-T superconducting magnet (Bruker BioSpin GmbH). We used an actively shielded Micro 2.5 gradient system (40-mm i.d., maximum strength of 660 mT/m). A custom-built birdcage-coil (15-mm i.d.) was used for the measurements. MRI measurements were conducted using an adjusted custom ultra-short echo time sequence. The repetition time was set to 50 ms, and for frequency encoding, 128 points were acquired at a bandwidth of 200 kHz. The echo-delay between the excitation pulse and start of the acquisition was 10 μs. A total of 59,157 (digestion) or 61,995 (GdCl3) spokes were acquired. The measurements were averaged (number of averages = 2) for signal-to-noise ratio optimization. The experiment was performed over a course of 12 d (digestion) or 3 d (GdCl3).
Reconstruction was based on the method proposed by Duyn et al. (56) using in-house written programs in MATLAB (The Mathworks, Inc.).
In the GdCl3 experiment, the Dionaea was placed in the tube with GdCl3 applied to the root medium and COR to the trap surface. Trigger hairs were touched to cause fast trap closure before MRI measurement was started. The Dionaea plant absorbed the gadolinium ions, and closed traps started to seal slowly (phase 3). Due to the presence of gadolinium, the longitudinal relaxation parameter in the stem decreased significantly, showing the distribution of the contrast agent within the plant due to the increase of signal within the plant tissue. When the Dionaea reached phase 4, no droplets of the digestive fluid could be detected on the trap’s surface.
Supplementary Material
Acknowledgments
We thank B. Neumann and P. Winter for technical assistance. This work was supported by the German Plant Phenotyping Network and by the European Research Council (ERC) under the European Union’s Seventh Framework Programme (FP/20010-2015)/ERC Grant Agreement (250194-Carnivorom). This work was also supported by the International Research Group Program (IRG14-08), Deanship of Scientific Research, King Saud University (to R.H., E.N., and K.A.S.A.-R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701860114/-/DCSupplemental.
References
- 1.Ellison AM, Gotelli NJ. Energetics and the evolution of carnivorous plants--Darwin’s ‘most wonderful plants in the world’. J Exp Bot. 2009;60:19–42. doi: 10.1093/jxb/ern179. [DOI] [PubMed] [Google Scholar]
- 2.Darwin C. Insectivorous Plants. Murray; London, UK: 1875. [Google Scholar]
- 3.Böhm J, et al. The Venus flytrap Dionaea muscipula counts prey-induced action potentials to induce sodium uptake. Curr Biol. 2016;26:286–295. doi: 10.1016/j.cub.2015.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robins RJ, Juniper BE. The secretory cycle of Dionaea-muscipula Ellis. 3. The mechanism of release of digestive secretion. New Phytol. 1980;86:313–327. [Google Scholar]
- 5.Libiaková M, Floková K, Novák O, Slováková L, Pavlovič A. Abundance of cysteine endopeptidase dionain in digestive fluid of Venus flytrap (Dionaea muscipula Ellis) is regulated by different stimuli from prey through jasmonates. PLoS One. 2014;9:e104424. doi: 10.1371/journal.pone.0104424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escalante-Pérez M, et al. A special pair of phytohormones controls excitability, slow closure, and external stomach formation in the Venus flytrap. Proc Natl Acad Sci USA. 2011;108:15492–15497. doi: 10.1073/pnas.1112535108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bemm F, et al. Venus flytrap carnivorous lifestyle builds on herbivore defense strategies. Genome Res. 2016;26:812–825. doi: 10.1101/gr.202200.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreuzwieser J, et al. The Venus flytrap attracts insects by the release of volatile organic compounds. J Exp Bot. 2014;65:755–766. doi: 10.1093/jxb/ert455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamec L. Mineral nutrition of carnivorous plants: A review. Bot Rev. 1997;63:273–299. [Google Scholar]
- 10.Böhm J, et al. Venus flytrap HKT1-type channel provides for prey sodium uptake into carnivorous plant without conflicting with electrical excitability. Mol Plant. 2016;9:428–436. doi: 10.1016/j.molp.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scherzer S, et al. Calcium sensor kinase activates potassium uptake systems in gland cells of Venus flytraps. Proc Natl Acad Sci USA. 2015;112:7309–7314. doi: 10.1073/pnas.1507810112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scherzer S, et al. The Dionaea muscipula ammonium channel DmAMT1 provides NH4+ uptake associated with Venus flytrap’s prey digestion. Curr Biol. 2013;23:1649–1657. doi: 10.1016/j.cub.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Schulze WX, et al. The protein composition of the digestive fluid from the venus flytrap sheds light on prey digestion mechanisms. Mol Cell Proteomics. 2012;11:1306–1319. doi: 10.1074/mcp.M112.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robins RJ, Juniper BE. The secretory cycle of Dionaea muscipula Ellis. 1. The fine-structure and the effect of stimulation on the fine-structure of the digestive gland-cells. New Phytol. 1980;86:279–296. [Google Scholar]
- 15.Rea PA, Joel DM, Juniper BE. Secretion and redistribution of chloride in the digestive glands of Dionaea muscipula Ellis (Venus flytrap) upon secretion stimulation. New Phytol. 1983;94:359–366. [Google Scholar]
- 16.Shabala L, Ross T, McMeekin T, Shabala S. Non-invasive microelectrode ion flux measurements to study adaptive responses of microorganisms to the environment. FEMS Microbiol Rev. 2006;30:472–486. doi: 10.1111/j.1574-6976.2006.00019.x. [DOI] [PubMed] [Google Scholar]
- 17.Aprile A, et al. Expression of the H+-ATPase AHA10 proton pump is associated with citric acid accumulation in lemon juice sac cells. Funct Integr Genomics. 2011;11:551–563. doi: 10.1007/s10142-011-0226-3. [DOI] [PubMed] [Google Scholar]
- 18.Ahnert-Hilger G, Jahn R. CLC-3 spices up GABAergic synaptic vesicles. Nat Neurosci. 2011;14:405–407. doi: 10.1038/nn.2786. [DOI] [PubMed] [Google Scholar]
- 19.Wightman RM, et al. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci USA. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow RH, von Rüden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- 21.Chow RH, Klingauf J, Heinemann C, Zucker RS, Neher E. Mechanisms determining the time course of secretion in neuroendocrine cells. Neuron. 1996;16:369–376. doi: 10.1016/s0896-6273(00)80054-9. [DOI] [PubMed] [Google Scholar]
- 22.Mosharov EV. Analysis of single-vesicle exocytotic events recorded by amperometry. Methods Mol Biol. 2008;440:315–327. doi: 10.1007/978-1-59745-178-9_24. [DOI] [PubMed] [Google Scholar]
- 23.Jankowski JA, Schroeder TJ, Ciolkowski EL, Wightman RM. Temporal characteristics of quantal secretion of catecholamines from adrenal medullary cells. J Biol Chem. 1993;268:14694–14700. [PubMed] [Google Scholar]
- 24.Wightman RM, Schroeder TJ, Finnegan JM, Ciolkowski EL, Pihel K. Time course of release of catecholamines from individual vesicles during exocytosis at adrenal medullary cells. Biophys J. 1995;68:383–390. doi: 10.1016/S0006-3495(95)80199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerhardt G, Adams RN. Determination of diffusion-coefficients by flow-injection analysis. Anal Chem. 1982;54:2618–2620. [Google Scholar]
- 26.Hafez I, et al. Electrochemical imaging of fusion pore openings by electrochemical detector arrays. Proc Natl Acad Sci USA. 2005;102:13879–13884. doi: 10.1073/pnas.0504098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paszota P, et al. Secreted major Venus flytrap chitinase enables digestion of Arthropod prey. Biochim Biophys Acta. 2014;1844:374–383. doi: 10.1016/j.bbapap.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi K, et al. A cysteine endopeptidase (“dionain”) is involved in the digestive fluid of Dionaea muscipula (Venus’s fly-trap) Biosci Biotechnol Biochem. 2011;75:346–348. doi: 10.1271/bbb.100546. [DOI] [PubMed] [Google Scholar]
- 29.Scheibe R, Dietz KJ. Reduction-oxidation network for flexible adjustment of cellular metabolism in photoautotrophic cells. Plant Cell Environ. 2012;35:202–216. doi: 10.1111/j.1365-3040.2011.02319.x. [DOI] [PubMed] [Google Scholar]
- 30.Noctor G, et al. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012;35:454–484. doi: 10.1111/j.1365-3040.2011.02400.x. [DOI] [PubMed] [Google Scholar]
- 31.Scheerer U, et al. Sulphur flux through the sulphate assimilation pathway is differently controlled by adenosine 5′-phosphosulphate reductase under stress and in transgenic poplar plants overexpressing gamma-ECS, SO, or APR. J Exp Bot. 2010;61:609–622. doi: 10.1093/jxb/erp327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rennenberg H, Herschbach C. A detailed view on sulphur metabolism at the cellular and whole-plant level illustrates challenges in metabolite flux analyses. J Exp Bot. 2014;65:5711–5724. doi: 10.1093/jxb/eru315. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi H, Kopriva S, Giordano M, Saito K, Hell R. Sulfur assimilation in photosynthetic organisms: Molecular functions and regulations of transporters and assimilatory enzymes. Annu Rev Plant Biol. 2011;62:157–184. doi: 10.1146/annurev-arplant-042110-103921. [DOI] [PubMed] [Google Scholar]
- 34.Schupp R, Rennenberg H. Diurnal changes in the glutathione content of spruce needles (Picea abies L) Plant Sci. 1988;57:113–117. [Google Scholar]
- 35.Strohm M, et al. Regulation of glutathione synthesis in leaves of transgenic poplar (Populus tremula × Populus alba) overexpressing glutathione synthetase. Plant J. 1995;7:141–145. [Google Scholar]
- 36.Arab L, et al. Acclimation to heat and drought Lessons to learn from the date palm (Phoenix dactylifera) Environ Exp Bot. 2016;125:20–30. [Google Scholar]
- 37.Herschbach C, Scheerer U, Rennenberg H. Redox states of glutathione and ascorbate in root tips of poplar (Populus tremula × P. alba) depend on phloem transport from the shoot to the roots. J Exp Bot. 2010;61:1065–1074. doi: 10.1093/jxb/erp371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leszczyszyn DJ, et al. Secretion of catecholamines from individual adrenal medullary chromaffin cells. J Neurochem. 1991;56:1855–1863. doi: 10.1111/j.1471-4159.1991.tb03441.x. [DOI] [PubMed] [Google Scholar]
- 39.Chow RH, Klingauf J, Neher E. Time course of Ca2+ concentration triggering exocytosis in neuroendocrine cells. Proc Natl Acad Sci USA. 1994;91:12765–12769. doi: 10.1073/pnas.91.26.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vukašinović N, Žárský V. Tethering complexes in the Arabidopsis endomembrane system. Front Cell Dev Biol. 2016;4:46. doi: 10.3389/fcell.2016.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tse A, Lee AK. Voltage-gated Ca2+ channels and intracellular Ca2+ release regulate exocytosis in identified rat corticotrophs. J Physiol. 2000;528:79–90. doi: 10.1111/j.1469-7793.2000.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klingauf J, Neher E. Modeling buffered Ca2+ diffusion near the membrane: Implications for secretion in neuroendocrine cells. Biophys J. 1997;72:674–690. doi: 10.1016/s0006-3495(97)78704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005;6:57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- 44.Koh DS, Hille B. Modulation by neurotransmitters of catecholamine secretion from sympathetic ganglion neurons detected by amperometry. Proc Natl Acad Sci USA. 1997;94:1506–1511. doi: 10.1073/pnas.94.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jocelyn PC. Biochemistry of the SH Group; The Occurrence, Chemical Properties, Metabolism and Biological Function of Thiols and Disulphides. Academic; London: 1972. [Google Scholar]
- 46.Rice ME, Gerhardt GA, Hierl PM, Nagy G, Adams RN. Diffusion coefficients of neurotransmitters and their metabolites in brain extracellular fluid space. Neuroscience. 1985;15:891–902. doi: 10.1016/0306-4522(85)90087-9. [DOI] [PubMed] [Google Scholar]
- 47.Jackson MB. Molecular and Cellular Biophysics. Cambridge Univ Press; Cambridge, UK: 2006. [Google Scholar]
- 48.Shi CY, et al. Citrus PH5-like H(+)-ATPase genes: Identification and transcript analysis to investigate their possible relationship with citrate accumulation in fruits. Front Plant Sci. 2015;6:135. doi: 10.3389/fpls.2015.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Picollo A, Pusch M. Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature. 2005;436:420–423. doi: 10.1038/nature03720. [DOI] [PubMed] [Google Scholar]
- 50.Robertson JL, Kolmakova-Partensky L, Miller C. Design, function and structure of a monomeric ClC transporter. Nature. 2010;468:844–847. doi: 10.1038/nature09556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheel O, Zdebik AA, Lourdel S, Jentsch TJ. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature. 2005;436:424–427. doi: 10.1038/nature03860. [DOI] [PubMed] [Google Scholar]
- 52.Spurr HW, Holcomb GE, Hildebrandt AC, Riker AJ. Distinguishing tissue of normal + pathological origin on complex media. Phytopathology. 1964;54:339–343. [Google Scholar]
- 53.Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shabala SN, Newman IA, Morris J. Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol. 1997;113:111–118. doi: 10.1104/pp.113.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samuilov S, Lang F, Djukic M, Djunisijevic-Bojovic D, Rennenberg H. Lead uptake increases drought tolerance of wild type and transgenic poplar (Populus tremula x P. alba) overexpressing gsh 1. Environ Pollut. 2016;216:773–785. doi: 10.1016/j.envpol.2016.06.047. [DOI] [PubMed] [Google Scholar]
- 56.Duyn JH, Yang Y, Frank JA, van der Veen JW. Simple correction method for k-space trajectory deviations in MRI. J Magn Reson. 1998;132:150–153. doi: 10.1006/jmre.1998.1396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.