ACOX2 (acyl-CoA oxidase 2) is a peroxisomal enzyme that plays a key role in the metabolism of bile acid intermediates and the oxidation of branched-chain fatty acids. A recent PNAS paper by Vilarinho et al. (1) on ACOX2 deficiency in an 8-y-old male with elevated transaminase levels identifies a homozygous nonsense mutation (p.Y69*) that generates a truncated protein. Inheritance of this mutation was shown to be responsible for a significant elevation in bile acid intermediates in this child. In addition, a study by Monte et al. (2) identified a homozygous ACOX2 (R225W) mutation in an adolescent presenting with persistent hypertransaminasemia; C24 bile acids were low, but levels of C27 bile acid pathway intermediates were elevated.
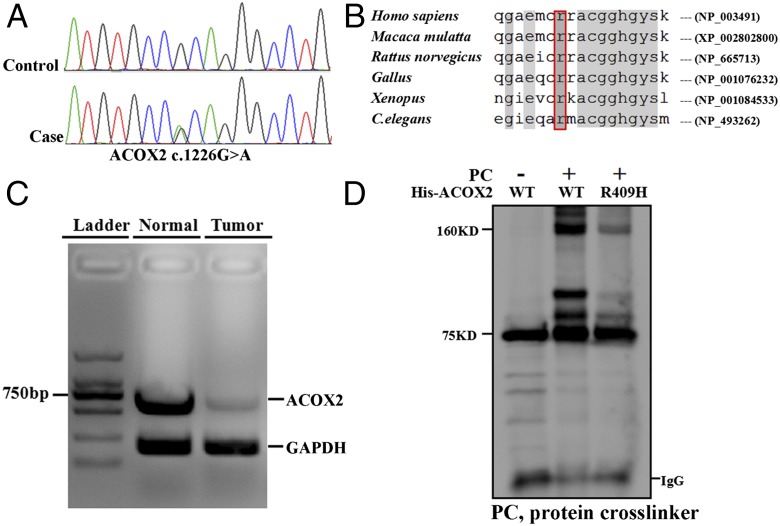
We performed whole-transcriptome analyses on two patients with primary malignant cardiac tumors. Patient 1 was a 40-y-old male diagnosed with cardiac primary malignant fibrous histiocytoma. Patient 2 was a 44-y-old female with primary cardiac angiosarcomas. In this study, all procedures were approved by the Medical Ethics Committee of Fudan University, and written consent was obtained before commencement. Unexpectedly, many of the genes responsible for peroxisomal and mitochondrial β-oxidation of fatty acids were uniformly down-regulated in the cardiac tumors of both patients. To further explore this gene expression pattern, genetic analyses of genes related to β-oxidation were performed. In patient 1 we identified a heterozygous mutation in ACOX2 (c.1226G > A, p.Arg409His) that is located in a binding site required for ACOX2 to dimerize (Fig. 1A). The R409 residue of ACOX2 is conserved in different species (Fig. 1B). Based on data from the 1000 Genomes Project, this variant is novel and the minor allele frequency in the ExAC database was found to be 0.0001484 (18/121314). ACOX2 (R409H) is predicted to disrupt protein function based on five different software-based algorithms including SIFT, Poly-phen2, Mutation-taster, Mutation-assessor, and Fathmm. Subsequent biochemical assays indicated that the R409H mutation, at least in part, reduced the ability of His-tagged ACOX2 recombinant protein to dimerize (Fig. 1C). Furthermore, RT-PCR analysis indicated that expression of ACOX2 was reduced in cardiac tumor tissue from patient 1 (Fig. 1D). Unfortunately, we could not evaluate the accumulation of C27 intermediates in patient 1 in vivo owing to the patient’s death.
Fig. 1.
ACOX2 deficiency in a patient with a primary malignant cardiac tumor. (A) Sanger sequencing of ACOX2 (c.1226G > A, p.Arg409His) using blood samples from patient 1. (B) Sequence alignment of ACOX2 p.Arg409His (National Center for Biotechnology Information reference sequences are indicated). C. elegans, Caenorhabditis elegans. (C) ACOX2 and GAPDH were amplified from the cDNA of adjacent normal and tumor tissue from patient 1 as indicated. GAPDH served as a positive control to demonstrate the reduction of ACOX2 expression in tumor tissues. (D) ACOX2 (R409H) affected normal dimer formation. HEK293T cells were transfected with His-tagged wild-type or (R409H) ACOX2 for recombinant overexpression. A 1:100 dilution of a protein cross-linker (PC) was added to the RIPA buffer before cell lysis. Subsequently, anti-His immunoprecipitation and anti-ACOX2 immunoblotting were performed following standard procedures. A 75-KDa band was indicative of ACOX2-His recombinant protein. After PC treatment, a 160-KDa band was generated, corresponding to an ACOX2 dimer. This band was significantly decreased in cells transfected with mutant ACOX2.
Unlike most organs, heart tissue uses fatty acids instead of glucose as a primary energy source to fulfill its high energy demand. Previous studies identified variants of ACOX2 that are associated with cardiovascular disease and breast cancer (3, 4). Here, we postulate that ACOX2 (R409H) may provide the initial driving force to switch the primary energy source of the heart from fatty acids to glucose, thus increasing dependence on glucose uptake and mimicking the Warburg effect, a hallmark of cancer metabolism.
Acknowledgments
We thank Richard H. Finnell for helpful discussions, and Bin Qiao, Wenyuan Duan, and Huasheng Xiao for specimen collection. This work was supported by 973 Program Grant 2013CB945403 and National Natural Science Foundation of China Grants 81430005 and 81601283.
Footnotes
The authors declare no conflict of interest.
References
- 1.Vilarinho S, et al. ACOX2 deficiency: A disorder of bile acid synthesis with transaminase elevation, liver fibrosis, ataxia, and cognitive impairment. Proc Natl Acad Sci USA. 2016;113:11289–11293. doi: 10.1073/pnas.1613228113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monte MJ, et al. ACOX2 deficiency: An inborn error of bile acid synthesis identified in an adolescent with persistent hypertransaminasemia. J Hepatol. 2017;66:581–588. doi: 10.1016/j.jhep.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Johansson A, et al. Identification of ACOX2 as a shared genetic risk factor for preeclampsia and cardiovascular disease. Eur J Hum Genet. 2011;19:796–800. doi: 10.1038/ejhg.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjørklund SS, et al. Expression of an estrogen-regulated variant transcript of the peroxisomal branched chain fatty acid oxidase ACOX2 in breast carcinomas. BMC Cancer. 2015;15:524. doi: 10.1186/s12885-015-1510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]