Significance
Previously, we showed that the virus that persists in human immunodeficiency virus (HIV)-infected individuals on antiretroviral therapy (ART) is derived from cells infected prior to initiating treatment. We also showed that HIV-infected cells can undergo cellular proliferation during ART. However, it is not known what fraction of infected cells that persist during ART are latent and what fraction are actively producing HIV RNA. The method described here was developed to determine the fraction of infected cells that produce HIV RNA and the levels of HIV RNA in single cells, including cells that have undergone cellular proliferation. Additionally, the method can be used to identify the sources of rebound virus after stopping ART and the efficacy of experimental interventions designed to cure HIV infection.
Keywords: HIV expression, proviral expression, persistence on ART, single-cell analysis, CARD-SGS
Abstract
Little is known about the fraction of human immunodeficiency virus type 1 (HIV-1) proviruses that express unspliced viral RNA in vivo or about the levels of HIV RNA expression within single infected cells. We developed a sensitive cell-associated HIV RNA and DNA single-genome sequencing (CARD-SGS) method to investigate fractional proviral expression of HIV RNA (1.3-kb fragment of p6, protease, and reverse transcriptase) and the levels of HIV RNA in single HIV-infected cells from blood samples obtained from individuals with viremia or individuals on long-term suppressive antiretroviral therapy (ART). Spiking experiments show that the CARD-SGS method can detect a single cell expressing HIV RNA. Applying CARD-SGS to blood mononuclear cells in six samples from four HIV-infected donors (one with viremia and not on ART and three with viremia suppressed on ART) revealed that an average of 7% of proviruses (range: 2–18%) expressed HIV RNA. Levels of expression varied from one to 62 HIV RNA molecules per cell (median of 1). CARD-SGS also revealed the frequent expression of identical HIV RNA sequences across multiple single cells and across multiple time points in donors on suppressive ART consistent with constitutive expression of HIV RNA in infected cell clones. Defective proviruses were found to express HIV RNA at levels similar to those proviruses that had no obvious defects. CARD-SGS is a useful tool to characterize fractional proviral expression in single infected cells that persist despite ART and to assess the impact of experimental interventions on proviral populations and their expression.
Several methods have been developed to extract human immunodeficiency virus (HIV) RNA from blood mononuclear cells to quantify and sequence it as a population (1–4),* but this approach cannot be used to profile RNA expression in single infected cells. As a consequence, it is not known what fraction of infected blood cells express HIV RNA at a given point in time, to what levels HIV RNA is expressed in single cells, or if expression levels and profiles change with antiretroviral therapy (ART) or remain the same. Determining the fraction of infected cells that express HIV RNA and the levels of expression in single cells both before and during ART can provide a better understanding of HIV persistence during ART and its rebound after stopping ART. The importance of profiling HIV RNA expression at the single-cell level has recently been highlighted by the finding that the reservoir of HIV can persist through clonal expansion of single infected cells (5, 6). In one well-characterized individual, a large clonal cell population of CD4+ T cells carrying an intact provirus produced low levels of infectious viremia that could not be suppressed by ART even though the virus was sensitive to each of the drugs in the ART regimen (7). In another recent study, expanded clones that persisted during long-term ART were found to express HIV RNA that matched some of the HIV RNA sequences in the viremia that rebounded after ART was interrupted (1). These observations suggest that infected cells that have undergone clonal expansion can be an important reservoir for HIV and that proviral expression during ART in at least some clonal cell populations could constitute an “active” form of the reservoir that must be eliminated to cure HIV infection. Although all of the infected cells that persist on ART are often described as the HIV reservoir, the actual reservoir only includes proviruses that can produce infectious virus, and such proviruses are a minor fraction (∼2%) of all viral DNA that persists (8).
Collectively, these observations suggest a model of the HIV reservoir that comprises populations of clonally expanded, infected cells with some that have latent proviruses and some that have transcriptionally active proviruses. Only a very small fraction contain intact proviruses capable of producing infectious virus, and only a small fraction of these proviruses produce infectious virus particles at any one time. Nonetheless, with very few possible exceptions, there are enough of these cells in all individuals on suppressive long-term ART to lead to rapid rebound viremia when ART is interrupted.
To test the validity of this model, quantification of in vivo HIV RNA expression levels is needed in single cells and across populations of infected cells. Accordingly, we developed the HIV cell-associated RNA and DNA single-genome sequencing (CARD-SGS) assay, a method that can determine (i) the fraction of all proviruses that express HIV RNA, (ii) the levels of HIV RNA expression from proviruses in single cells, and (iii) the fractional proviral expression and levels of HIV RNA in infected cell clones. This assay combines improved isolation of intracellular RNA from peripheral blood mononuclear cells (PBMCs) with end-point dilution analysis of HIV RNA-expressing cells. This method can be used to identify cellular and proviral sources of persistent and rebound viremia, as well as the impact of ART and experimental approaches to cure HIV infection on proviral expression at the single-cell level.
Results
CARD-SGS Assay Development.
The CARD-SGS assay was developed by optimizing methods for the extraction of total nucleic acid from PBMCs (9–11) (Table S1), efficient degradation of genomic DNA (gDNA) (11) for RNA purification (Tables S1–S3), efficient synthesis of cDNA from cell-associated HIV RNA (Table S2), and amplification and sequencing of cell-associated unspliced HIV RNA (HIV RNA) molecules from small numbers of cells with expressed proviruses (Fig. S1 and Tables S2 and S3).
Table S1.
Optimizing recovery of gDNA and HIV RNA for CARD-SGS
| Recovery of gDNA measured by CCR5 copies | Digestion of gDNA from ACH-2 cells by DNase I | Digestion of HIV DNA from donor PBMCs at an end point for CARD-SGS by DNase I | ||||
| No. of PBMCs or ACH-2 cells extracted | Recovery of gDNA, % | No. of HIV proviruses before treatment with DNase I | No. of HIV proviruses detected in no RT control after DNase I digestion* | No. of HIV proviruses in patient sample before treatment with DNase I† | No. of HIV proviruses detected in no RT controls after DNase I digestion† | No. of HIV RNA copies after digestion with DNase I* |
| 2,000,000 | 36‡ | 9,382,973 | 145 | 280 | 0 | 5 |
| 1,000,000 | 52‡ | 2,734,645 | 9 | 235 | 0 | 5 |
| 600,000 | 145§ | 1,218,179 | 9 | 140 | 0 | 13 |
| 200,000 | 59 | 233,347 | 0 | 42 | 0 | 27 |
| 66,667 | 70 | 133,357 | 0 | |||
| 22,222 | 60 | 36,848 | 0 | |||
| 7,407 | 48 | 5,493 | 0 | |||
| 2,469 | 62 | |||||
HIV copies were measured by qPCR (11).
HIV copies were measured by end-point dilution PCR of the P6-PR-RT target used for the other studies presented here. Gray shading indicates the highest level of recovery of gDNA was from 600,000 cells.
Average of triplicates measured by qPCR for CCR5 copy number. PBMCs were stored at −80 °C for 3–12 mo before extraction.
Twofold deviation of qPCR measurements suggests that recovery was likely 100%.
Table S3.
Sensitivity of CARD-SGS to detect HIV RNA
| No. of ACH-2 cells spiked into HIV-negative PBMCs (no. of replicates) | No. of negative replicates for HIV RNA by CARD-SGS | Median no. of HIV RNA-positive wells per 96-well plate (range) by CARD-SGS* |
| 10 (13 replicates) | 0 | 58 (12–92) |
| 3 (10 replicates) | 0 | 38 (6–78) |
| 1(20 replicates) | 6 | 1.5 (0–40)† |
| 1,000, 100, 10 without RT‡ (12 replicates) | 12 | 0 (0–0) |
HIV-negative PBMCs spiked with one to 10 ACH-2 cells were assayed by CARD-SGS. The resulting number of positive PCR reactions for the 1.3-kb product by SGS (Fig. S1) was used to determine the sensitivity of CARD-SGS to detect HIV RNA molecules.
Thirty percent of the single-cell spikes were negative (0 RNA copies), consistent with expectation from a Poisson distribution.
No RT controls were performed to ensure that all bands were from HIV RNA.
Table S2.
Optimizing cDNA synthesis for CARD-SGS
| RT conditions and PCR efficiency | Efficiency of cDNA synthesis using different primers* | Efficiency of cDNA synthesis using different sources for RT* | Efficiency with and without RNaseH* | Efficiency after freezing-thawing cell pellets* | Control for contaminating DNA* | ||||
| Conditions used in RT reaction | Gene-specific primer 3500−† | Random hexamers | SuperScript III (Invitrogen) | AffinityScript (Agilent) | Without RNaseH | With RNaseH | No freeze-thaw | With freeze-thaw‡ | No RT control |
| No. of positive PCR reactions/no. of PCR wells (% efficiency of cDNA synthesis) | 12/96§ (100%) | 5/96§ (50%) | 12/96§ (100%) | 15/96§ (100%) | 5/48§ (100%) | 5/48§ (100%) | 21/192 (100%) | 19/192 (100%) | 0/96§ |
Cell-associated RNA was extracted from 1 million PBMCs from a patient with 11 HIV RNA copies per 1 million PMBCs measured by integrase cell-associated RNA (11).
Primer 3500− is used for standard SGS of P6-PR-RT (14).
Extracted PBMCs after freezing the cell pellet overnight at −80 °C. cDNA was synthesized with a gene-specific primer using AffinityScript RT.
Efficiency of CARD-SGS was determined by spreading the full contents of the cDNA reaction across a 96-well PCR plate and amplifying single molecules by SGS (14) to determine the number of cDNA molecules present in the RT reaction by the number of 1.3-kb bands on the plate. One hundred percent efficiency would be ∼11 of 96 positive wells with some variation because the number of expressing cells can vary slightly across PBMC aliquots used for the extractions.
Fig. S1.
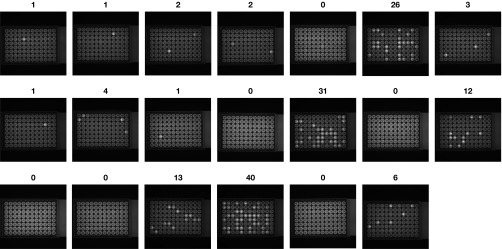
PCR results from CARD-SGS performed on 20 replicates of an average of one ACH-2 cell spiked into 500,000 PBMCs from an HIV-negative donor. The highlighted wells contain amplification products from one or more HIV cDNA molecules. The dark wells are negative for HIV cDNA. The number above each plate shows the number of single HIV RNA molecules detected in each aliquot of spiked cells.
To optimize recoveries of gDNA and intracellular RNA, extractions were performed on various quantities of HIV-infected PBMCs (Tables S1 and S2) and on uninduced ACH-2 cells [which contain a single clonal HIV provirus per cell and express very low levels of unspliced HIV RNA (12, 13)] tested directly (Table S1) or spiked at low frequencies into 500,000 PBMCs from an HIV-negative donor (Fig. S1 and Table S3). The method for extracting gDNA was modified slightly from the method of Besson et al. (9) and the method for extracting HIV cell-associated RNA was modified slightly from the method of Hong et al. (11) by omitting the sonication step in each method to keep the HIV DNA or RNA as intact as possible for amplification of longer (1.3 kb) HIV sequences. Recovery of gDNA was assessed by quantitative PCR (qPCR) for CCR5 gene copy number and recovery of HIV RNA was measured by end-point dilution or by qPCR (11) using in vitro-synthesized RNA transcripts as absolute standards. We found that optimal recovery of gDNA was achieved starting with 1 million or fewer PBMCs or ACH-2 cells, with recoveries ranging from 100% (600,000 cells) to 52% (1 million cells). Recovery declined when gDNA was extracted from replicates of 2 million cells (Table S1, columns 1 and 2). To test for complete digestion of HIV proviral DNA by DNase I, total nucleic acid was extracted from ∼9 million to 5,500 ACH-2 cells (Table S1, columns 3 and 4), from PBMCs collected from four HIV-infected individuals (donor characteristics are shown in Table 1, and results are shown in Table S1, columns 5–7), and from ACH-2 cells spiked into PBMCs from an HIV-negative donor (Table S3). We found that the DNase I digestion of HIV DNA was complete up to at least ∼230,000 ACH-2 genomes but that a small amount of HIV DNA (nine copies) was still detectable when 1.2 million or more ACH-2 cells were extracted. ACH-2 cells each contain one HIV provirus (12). By contrast, in HIV-infected individuals, a small minority of PBMCs carry HIV proviruses [about 200 proviruses per million PBMCs, on average (9)]. Therefore, the DNase I digestion step used here can remove all detectable proviruses from 1 million PBMCs from an HIV-infected donor. To confirm this expectation, we assayed for undigested proviral DNA after DNase I treatment by end-point dilution of four donor samples containing 42–280 HIV proviruses and replicates of ACH-2 cells (1,000, 100, or 10 replicates) spiked into a background of HIV-negative PBMCs (Table S1, columns 5–7 and Table S3, row 6). In each case, no HIV DNA was detected in the no reverse transcriptase (NRT) controls, whereas positive PCR reactions were obtained when reverse transcriptase (RT) was added, indicating that all amplification observed was from cDNA and none was from contaminating cell-associated HIV DNA.
Table 1.
Donor characteristics and cell-associated HIV DNA and RNA quantification
| PID | Gender | Ethnicity | Duration of ART, years | ART regimen | Plasma HIV RNA copies/mL* | Cell-associated HIV DNA (copies/1 million PBMCs)† | Cell-associated HIV RNA (copies/1 million PBMCs)† |
| 001 | Male | African American | Untreated | NA | 17,340 | 505 | 743 |
| 002 | Female | African American | 2 | TNV, FTC, DRV/r | 8 | 227 | 21 |
| 003 | Male | African American | >4 | TNV, FTC, ATV/r | 1.9 | 205 | 11 |
| <0.6‡ | 223‡ | 13‡ | |||||
| 004 | Male | Caucasian | 9 | TNV, FTC, EFV | 0.8 | 984 | 242 |
| <0.6‡ | 665‡ | 117‡ |
Experiments were performed to determine the accuracy and sensitivity of the assay for cell-associated RNA. First, 500,000 HIV-negative PBMCs were spiked with uninduced ACH-2 cells, at an average of either 10 cells (13 replicates), three cells (10 replicates), or one cell (20 replicates) and assayed by CARD-SGS, amplifying the 1.3-kb p6-protease (PR)-RT fragment (14) (Fig. S1 and Table S3). Uninduced ACH-2 cells are known to express HIV RNA at low levels (13). All PBMCs spiked with an average of 10 ACH-2 cells and three ACH-2 cells were positive for HIV RNA (ranging from six to 92 positive wells per 96-well PCR plate), whereas only 70% of the PBMCs spiked with an average of one ACH-2 cell were positive (near the 63% expected from the Poisson distribution) (Fig. S1). The number of HIV RNA molecules detected in the spikes containing an average of one ACH-2 cell ranged from 0 to 40, indicating that these cells express HIV RNA at varying levels (Fig. S1). The fact that 70% of the single ACH-2 cell–spiked samples were positive for HIV RNA implies that CARD-SGS was capable of detecting single cells containing very low levels of HIV RNA molecules, because 37% of the PCR reactions would be expected to have no ACH-2 cell present at all.
To compare the relative sensitivity of CARD-SGS with a qPCR assay for intracellular HIV pol RNA (11), we compared the levels of HIV RNA measured by the two assays in three controls of 10 ACH-2 cells spiked into 500,000 HIV-negative PBMCs and in six donor PBMC samples (Table 2). As described above for the single ACH-2 cell–spiked samples, we detected 0–40 HIV RNA-positive wells per 96-well plate by CARD-SGS. From this result, we would expect to measure between ∼7 and 520 HIV RNA molecules in each of the 10 ACH-2 cell–spiked samples by sensitive qPCR (11). The qPCR assay measured 112, 165, and 615 HIV RNA copies in each of the three 10 ACH-2 cell–spiked samples, similar to the expected range, suggesting that the CARD-SGS assay has comparable sensitivity for HIV RNA as qPCR. For the clinical samples, we found that the fold differences in the levels of HIV RNA detected by the two assays ranged from twofold to 13-fold, with four of the six samples showing threefold or lower differences. The two samples with higher fold differences could be due to primer or probe mismatches used in either assay, to differences in the number of HIV RNA-expressing cells and levels of expression across the different aliquots tested (discussed below), or to differences in the frequency of proviruses with deletions or hypermutation in the two regions targeted by the assays [gag-RT for CARD-SGS and 3′ integrase (IN) for qPCR].
Table 2.
Varying fraction of HIV-expressing cells per donor
| PID | Years on cART | No. of PBMC genomes recovered | No. of infected cells analyzed | Estimated no. of infected cells expressing HIV RNA | Infected PBMCs expressing HIV RNA, % | Average no. of HIV RNA molecules detected per aliquot | Fold difference in HIV RNA copies compared with qPCR |
| 001 | 0 | 270,000 | 136 | 5 | 4 | 19 | 10 (qPCR higher) |
| 002 | 2 | 350,000 | 79 | 14 | 18 | 13 | 2 (CARD-SGS higher) |
| 003 | >4 | 400,000 | 82 | 5 | 6 | 9 | 2 (CARD-SGS higher) |
| 230,000 | 51 | 5 | 10 | 9 | 3 (CARD-SGS higher) | ||
| 004 | 9 | 1,110,000 | 1,092 | 17 | 2 | 20 | 13 (qPCR higher) |
| 280,000 | 186 | 8 | 4 | 13 | 3 (qPCR higher) | ||
| Average | 440,000 | 271 | 9 | 7 | 14 | 5.5 |
cART, combination ART.
The cDNA synthesis efficiencies were determined using different primer types, different RT enzymes, and different storage conditions of cell pellets (Table S2). We found that cDNA synthesis was optimal when a gene-specific primer was used rather than random hexamers, and that there was no significant difference in the number of cDNA molecules amplified when RNase H was used to remove template RNA following the RT step compared with when it was not (Table S2).
Optimized CARD-SGS Assay.
An overview of the optimized methods for CARD-SGS is shown in Fig. 1. In brief, total nucleic acid is extracted from multiple aliquots (typically five to 10) of about 30,000 to 1 million PBMCs per donor sample depending on the frequency of HIV RNA-expressing cells. In donors who have low genetic diversity by standard SGS, the end point for cells expressing HIV RNA is determined by serially diluting the cells over a 96-well plate and performing cDNA synthesis and PCR in each well until less than 30% of the PCR reactions are positive for amplified cDNA. In chronically infected donors with high genetic diversity, CARD-SGS can be performed on aliquots near, but not at, the end point, containing as many as 12 HIV RNA-expressing cells because proviral expression in multiple single cells can be identified by the presence of genetically distinct HIV RNA variants [neighbor-joining (NJ) analyses are performed as described below to link HIV RNA to individual proviruses]. Once the appropriate dilution end point is determined, extracts from aliquots diluted to the end point are treated with DNase I and used for cDNA synthesis as described in Methods (except for one aliquot that is reserved for SGS on proviral DNA and not treated with DNase I). The total cDNA from each aliquot is diluted 1:2 and spread across a 96-well plate for PCR amplification of a 1.3-kb p6-PR-RT amplicon (14). If <30% of the PCR reactions from the majority of aliquots are positive after PCR, then amplicons are sequenced as single HIV RNA molecules (removing any mixed sequences). If aliquots result in >30% amplicon-positive wells, then the assay is repeated at a higher dilution of PBMCs. The PCR product from the positive wells is sequenced by Sanger sequencing using standard SGS methods (15). The extracted gDNA from the aliquot that was not DNase-treated is diluted to an end point for HIV DNA and used for proviral SGS as described in Methods. A fraction of the extracted gDNA is retained for CCR5 copy quantification to determine the percent recovery of gDNA, and a separate aliquot of PBMCs is retained for qPCR to quantify the levels of pol-containing proviruses per sample (11). The resulting sequences are used for NJ tree analysis. Identical sequences from the same aliquot (shown as rakes of the same color in Fig. 1) are interpreted to arise from multiple HIV RNA molecules within the same infected cell. Identical sequences across multiple aliquots result either from expression in clonally expanded cells or from multiple cells infected with the same viral variant. By estimating the number of different HIV variants in each aliquot of PBMCs, one can estimate the fraction of infected cells that express HIV RNA [keeping in mind that variants differing by only 1-nt or 2-nt changes from rakes of multiple identical sequences are not above the background error rates for RT of the identical templates (ca. 10−4 errors per nucleotide copied, or about one error in one of seven single sequences) (14), and therefore are not considered different variants]. Because of this background error rate, it is possible that the reported fraction of infected cells expressing HIV RNA is slightly underestimated. We note that all published and future assays that make use of RT for this purpose are subject to the same error. Additionally, the fraction of expressing cells is based on the total number of infected cells that are present in each aliquot. We quantified the number of infected cells using qPCR with primers and probe in integrase (11). Because HIV proviruses are known to contain many deletions, as well as frequent hypermutation, it is possible that the number of proviruses per aliquot will vary depending on the placement of primers and probes, affecting the calculations for the fraction of expressing cells somewhat.
Fig. 1.
Flow chart of CARD-SGS methods. PBMCs from HIV-infected donors are initially collected and divided into separate aliquots each containing ca. 30,000–1 million cells (represented by differently colored tubes), including few infected cells from which RNA is extracted and used to synthesize cDNA, which is then distributed over a 96-well plate and PCR-amplified. Two rounds of PCR result in 1.3-kb p6-PR-RT amplicons. Analysis of distance trees distinguishes identical HIV RNA sequences from the same aliquot (indicating that they were derived from different single infected cells) or from different aliquots (suggesting that they were derived from clonally expanded cells).
Participants and HIV Quantification.
PBMC samples were analyzed by CARD-SGS from four donors recruited by the University of Pittsburgh Clinical Trials Unit (Table 1). All donors provided written informed consent, and the study was approved by the University of Pittsburgh Institutional Review Board. One individual was not on ART, and three were virologically suppressed on ART for 2, 4, or 9 years. One of the virologically suppressed donors had a history of lymphoma and began ART 1 year after effective treatment for the cancer. The demographics for each participant are shown in Table 1. Table 1 also shows the levels of plasma HIV RNA measured by the single-copy assay (16) and the levels of HIV DNA and cell-associated RNA measured by qPCR (11).
Genetics of HIV Proviruses in Each Participant.
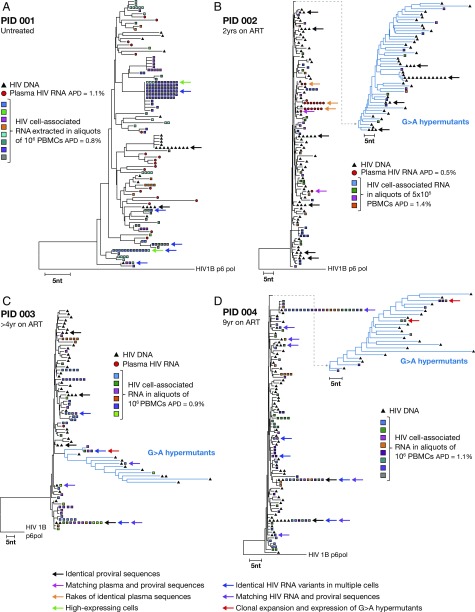
We investigated the structure of the HIV DNA population in the PBMCs from each participant (Fig. 2). NJ analysis and measurements of average pairwise distance (APD) showed diverse proviral populations in both the untreated and treated individuals. However, populations of identical proviruses were also found in all individuals (indicated with black arrows in Fig. 2). The two populations of identical proviruses in the untreated individual [participant identification (PID) 001; Fig. 2A] may have resulted from identical viral variants infecting multiple cells, which is typical of early infection (17), or from infection of single infected cells that underwent clonal expansion. To confirm that this participant’s sample was not collected during early infection, we performed SGS on the plasma virus population (red circles in Fig. 3A) and found that every sequence was unique, with an APD of 1.1%, which is typical of a chronically infected individual (18, 19). None of the plasma virus sequences detected in PID 001 matched the rakes of identical proviruses found in the PBMC DNA, suggesting that the identical proviruses could be defective, and thus more likely to represent expanded clones than spreading infection. Because previous studies have shown a lack of ongoing viral replication in the periphery of participants on suppressive ART (20), and because the ART-treated individuals in this study carried diverse proviral populations (Fig. 2 B–D), the identical proviruses detected in these individuals (PID 002–004) are more likely to have arisen from the clonal expansion of cells that were infected before initiating ART than from cells independently infected with the same variants before initiating ART, although one cannot be certain without sequencing the integration sites of specific proviral variants, a technology that is needed but has yet to be developed. G→A hypermutants were detected in HIV DNA populations in all treated subjects (shown in blue in Fig. 2 B–D) but not in the untreated individual (Fig. 2A). Two populations of identical proviruses in PID 002 and one in PID 003 (Fig. 2 B and C) are G→A hypermutants containing stop codons. Hence, these identical populations must be the result of clonal expansion of a single infected cell, because they cannot generate infectious virus.
Fig. 2.
NJ analyses of HIV DNA amplified from PBMCs. The four distance trees are shown for individual participants PID001 (A, untreated and viremic), PID002 (B, 2 years on ART), PID003 (C, >4 years on ART), and PID004 (D, 9 years on ART). The black triangles represent HIV DNA, and hypermutant DNA sequences are indicated by the blue triangles. The black arrows indicate groups of identical proviruses in each tree.
Fig. 3.
NJ analyses of HIV DNA from PBMCs and intracellular RNA. The four distance trees from participants PID001 (A), PID002 (B), PID003 (C), and PID004 (D) show HIV DNA sequences as black triangles and plasma RNA sequences as red circles (no plasma RNA sequences were obtained for PID004). Differently colored squares represent sequences obtained from individual extractions of cell-associated HIV RNA from PBMCs. Green arrows show high HIV RNA-expressing cells, blue arrows show identical HIV RNA variants in multiple cells, black arrows show groups of identical proviruses, pink arrows show matching plasma and proviral sequences, orange arrows show identical plasma sequences present during ART, purple arrows show matching HIV DNA and intracellular HIV RNA in different single cells, and red arrows show HIV RNA expression in clonally expanded populations carrying G>A hypermutant proviruses.
It is important to note that SGS amplifies all HIV DNA molecules, integrated or not. Although studies have shown that most of the HIV DNA is integrated in individuals on ART (21–23), untreated patients or those patients recently initiating ART containing an integrase inhibitor may harbor a higher frequency of unintegrated DNA, with some eventually becoming integrated as part of the replication cycle and some as dead-end products, including one- and two-LTR circles (21). None of the donors studied here was on integrase inhibitor-containing ART (Table 1). Sequencing and quantifying unintegrated genomes may affect the calculations of the number of “genomes” expressing RNA transcripts. With time on ART, the contribution of two-LTR circles to total HIV DNA becomes negligible (<0.6%) (9).
NJ Profiles from CARD-SGS.
CARD-SGS analysis was first performed on PBMCs obtained from one time point for each participant (Fig. 3). Four to eight aliquots of 500,000 to 1 million PBMCs were extracted from each PBMC sample. Single-genome sequences of plasma HIV RNA from the untreated subject (PID 001) and from two of the treated subjects (PID 002 and PID 003) were included in the genetic analysis (red circles in Fig. 3 A–C). Levels of viremia were too low (less than two copies per milliliter) in the other treated donor (PID 004) to obtain HIV RNA sequences from the plasma. In untreated PID 001 (Fig. 3A), most plasma HIV RNA sequences differed from CARD-SGS sequences, suggesting that most blood cells expressing HIV RNA are not contributing to plasma viremia before ART or that the extent of diversity in the two compartments is so great that the level of sampling is too limited to find matches between them. In treated subject PID 002, we detected clonal plasma virus populations (orange arrows in Fig. 3B), as has been previously reported in ART-treated individuals (24, 25). The identical plasma sequences did not match exactly any sequences detected in cell-associated HIV RNA or DNA by CARD-SGS (although several were only different by a few nucleotides), suggesting, again, that many of the transcriptionally active cells in blood do not produce readily detectable virions in plasma (this possibility is supported by the expression of hypermutants), and that the failure to detect frequent matches between cellular and plasma HIV RNA sequences is due to the small fraction of the diverse population of total infected cells sampled. Only a single plasma sequence was recovered in PID 003, and it did not match variants detected in the sampled cells.
The level of cell-associated HIV RNA expression varied across single infected cells in all donors. Of the ∼43 HIV-expressing cells detected in untreated participant PID 001 (Fig. 3A), 56% had more than one HIV RNA molecule, but only 2% had more than 10 HIV RNA molecules (indicated by green arrows in Fig. 3A). We refer to cells that contain >10 HIV RNA molecules as “high expressers.” In the three treated individuals, a median of 29% of the HIV RNA-expressing cells had more than one HIV RNA molecule detected, but none contained more than 10 molecules. Whether the observed differences in the levels of HIV RNA expression reflect the different treatment status of the donors will require a much larger study to determine. The levels of HIV RNA expression are shown in detail for each donor in Fig. S2. In three of four donors studied, we detected expression of identical HIV variants across multiple aliquots of PBMCs (indicated with blue arrows in Fig. 3 A, C, and D). As discussed above, the finding of different cells expressing identical HIV RNA molecules could arise either from infection of multiple cells by the same HIV variant before ART or from single infected cells undergoing clonal expansion before and/or during ART, but the weight of evidence against ongoing viral replication in the periphery during ART (20, 26–28) favors clonal expansion as the mechanism. In several instances, we found matches between proviruses in one aliquot and HIV RNA in at least one other aliquot, likely from clonally expanded infected cell populations expressing HIV RNA (purple arrows in Fig. 3 C and D). Several matches across aliquots between proviral DNA or between cellular HIV RNA sequences were G→A hypermutants (indicated with red arrows in Fig. 3 C and D), which could only have arisen through clonal expansion of infected cells because defective proviruses cannot cause cell-to-cell transmission of virus. This finding also indicates that hypermutated proviruses can express HIV RNA, as previously shown in humans (1, 29) and macaques (4).
Fig. S2.
Bar graph showing the proportion of cells with one detected HIV RNA molecule, two to 10 HIV RNA molecules, and >10 HIV RNA molecules for each donor. Primers are in pro-pol; therefore, the number of HIV RNA molecules detected only reflects those HIV RNA molecules that are unspliced and not deleted in this region. High-expressing cells (with >10 HIV RNA molecules per cell) were only detected in the untreated donor. The majority of cells in both untreated and treated individuals had only a single HIV RNA molecule detected by CARD-SGS.
Longitudinal Expression of HIV During ART.
To investigate the longitudinal expression of HIV RNA during ART, we performed CARD-SGS on PBMC samples from treated participants PID 003 and 004 collected 2 and 4 weeks apart (Fig. 4). NJ analysis revealed that viral expression in clonally expanded cells persisted over time on ART in both participants (indicated by brown arrows in Fig. 4 A and B). PID 004 exhibited a particularly high level of sustained proviral expression containing seven distinct populations of clonally expanded cells expressing HIV RNA over the 2-week period of sampling. These results indicate that clonally expanded populations of HIV-infected cells can persistently express HIV RNA during ART.
Fig. 4.
NJ analysis of intracellular RNA sequences in longitudinal samples from two ART-treated individuals. CARD-SGS was performed at two time points on PBMCs collected from two ART-treated individuals: PID003 (A) and PID004 (B). Identical RNA variants were detected at both time points (time 1 in squares and time 2 in circles) in both individuals. Persistent expression of clonally expanded populations of infected cells is indicated with brown arrows. Branch lengths on G>A hypermutants were removed in B for purposes of the diagram.
Fraction of HIV Infected Cells That Express Viral RNA.
Beyond analyzing the genetics of the HIV RNA populations, CARD-SGS can be used in combination with quantitation of proviral DNA by qPCR (9) and gDNA measurements to estimate both the fraction of all p6-PR-RT–containing proviruses that express HIV RNA in single-cell samples (Table 2) and the frequency and level of HIV RNA expression of identical proviruses in different cells. Cellular DNA (CCR5 copies) and proviral quantification are used to determine the number of cellular genomes and proviruses recovered, respectively, in the extractions. HIV RNA and DNA sequences are subjected to NJ analyses and used to determine the number of different proviral and HIV RNA variants detected, the number of different HIV RNA variants per aliquot (which is equivalent to the number of different expressing proviruses per aliquot), and the levels of HIV RNA expression per provirus. Applying these analyses, we determined that the average fraction of HIV-infected cells expressing HIV RNA across all donors was 7% (range: 2–18%) (Table 2, column 6). The median expressing fraction was 8% in donors on ART, which was somewhat higher than the 4% expressing fraction in the one untreated donor, although there was considerable overlap and the small number of observations precluded meaningful statistical analyses. This preliminary analysis highlights the potential value of using CARD-SGS for quantifying the fraction of proviruses that express HIV RNA across individuals with varying levels of viremia, either untreated or on ART.
Discussion
Characterizing and quantifying the reservoirs of HIV that persist despite clinically effective ART, and that are the source of viral rebound if ART is stopped, are critical objectives for HIV cure research. The method described here, CARD-SGS, can assess the genetics of HIV RNA in single cells collected from both viremic and virologically suppressed individuals; in combination with proviral quantification (9) and end-point dilution of proviruses, it can also be used to estimate the fraction of all proviruses that express HIV RNA. Although other methods, such as florescence in situ hybridization, are capable of detecting HIV RNA in single infected cells (30, 31), such techniques cannot be used to assess the genetics of the expressed proviruses (or the latent ones), and therefore cannot be used determine the fraction of expressed proviruses that are in expanded clones or that are constitutively expressed (i.e., without ex vivo stimulation). The essential features of CARD-SGS are dilution of cells to the end point, or near the end point, for HIV RNA expression before nucleic acid extraction and use of very efficient methods for the extraction of cell-associated HIV DNA and HIV RNA, for cDNA synthesis, and for PCR of single DNA or cDNA templates (less than 30% of PCR reactions positive for 1.3-kb p6-PR-RT amplicon and informatic removal of the few remaining mixtures). HIV DNA and HIV RNA sequences are then analyzed to assess the proviral origin of the HIV RNA within and across cell aliquots. The method has single-cell sensitivity and is capable of interrogating 30–100 HIV RNA-expressing cells in a sample of 5–20 million PBMCs without prior culturing or stimulation of the cells, as is required for other detection methods (30, 31).
Mixtures of ACH-2 cells and PBMCs were used to confirm complete DNA digestion by DNase I and to demonstrate the sensitivity of CARD-SGS to quantify and sequence HIV RNA in single HIV-infected cells. Recovery of HIV RNA was compared with qRT-PCR for pol sequences standardized to HIV RNA transcripts, and was found to be as efficient as this well-characterized assay (11). Primers for qRT-PCR and CARD-SGS are in the HIV gag and pol genes; therefore, proviruses with deletions or extensive mutation in the same region of gag and pol are not detected. It is important to note that the fraction of expressing cells reported here includes only those cells that express proviruses with intact pro-pol sequences; the fraction of expressing cells is likely to differ if another proviral region or transcript type is analyzed, especially the many different types of splice variants. In the development of this method, we used pro-pol primers because we are familiar with the efficiency of these primers and with the genetic diversity of this region of the genome. However, the method can be readily modified to include the evaluation of any gene fragment or spliced variant by using different sets of primers. The assay was optimized to allow determination of the fraction of infected cells that express HIV RNA [assuming one provirus per infected cell as shown by prior work (32)], the levels of HIV RNA expression across single cells, and the levels of HIV RNA expression in infected cells that have expanded into clones after infection.
To demonstrate its utility, we applied CARD-SGS to PBMC samples from HIV-infected donors both on and not on ART. Although the number of individuals analyzed in this pilot study was small, some unique and interesting observations were made. First, only a small fraction of infected cells contain HIV RNA in both viremic and virologically suppressed donors, but the expressed proviral population is highly genetically diverse. The observation that the fraction of proviruses expressing HIV RNA is small, averaging ∼7%, indicates that the large majority of infected cells, even in a viremic individual, are not contributing to viremia at any one time. In the virologically suppressed donors, CARD-SGS revealed that infected cells that persist are primarily in the form of transcriptionally silent (latent) proviruses, with only 2–18% of infected cells transcribing HIV RNA. Whether a provirus is expressed may depend upon the cell cycle; the site of proviral DNA integration; the level of DNA methylation, histone modification, or other epigenetic effects; or the genetics of the provirus itself (e.g., mutations in tat or rev, or in the LTR, that affect expression of HIV RNA) (33). In this initial study, the fraction of proviruses expressing HIV RNA was not obviously different between donors on and off ART. Possibly, the low levels of expression occur for two different reasons: selection for cells containing inactive proviruses in donors on ART (33–38) and large amounts of unintegrated and nontranscribed viral DNA in donors not on ART (39–41). In either case, establishing the generality of this unexpected finding will require confirmation and further investigation in larger numbers of individuals both on and off ART.
Second, we showed that expressed HIV RNA includes defective sequences (i.e., hypermutants that contain stop codons), indicating that at least some defective proviruses are expressed during ART, consistent with recent reports (1, 4, 29). We also found expressed HIV RNAs with intact p6-PR-RT sequences. The large majority (98% or more) of proviruses that persist on ART have been reported to be defective with extensive deletion and hypermutation (33).† It remains to be determined whether intact proviruses capable of producing infectious virus are among those proviruses expressing p6-PR-RT RNA in virologically suppressed individuals on ART.
Third, when CARD-SGS was applied to longitudinal PBMC samples collected from virologically suppressed donors, we found that the same proviral variants, presumably amplified by clonal expansion, were detected in different cells over a time period of at least 2–4 weeks, revealing that clonal cell populations expressing HIV RNA can persist over time. If cells containing intact proviruses are similarly found, then the HIV reservoir could include expanded infected cell populations that constitutively express HIV RNA during ART. One previous study claimed that clonally expanded cells contain only defective proviruses (38), although the same group has recently reported a contrary conclusion (42), whereas another study clearly showed, in one individual, the persistence of viremia in longitudinal samples produced by an intact provirus in a highly expanded clone that was present in blood and in multiple tissues (7). The latter report shows that at least some cells in clonal populations can express HIV RNA and infectious virions during ART, which could be a source of rapid rebound viremia if ART is interrupted. Importantly however, the expression of HIV RNA, or even viral proteins, is not an indication of ongoing viral replication because the majority of these transcripts and proteins are likely to be defective, consistent with their proviral templates.
Finally, it is noteworthy that all of the HIV RNA-expressing cells in virologically suppressed individuals, and most of the ones in the viremic individual, contain very small numbers (one to five copies detected) of HIV RNA molecules. That these results accurately represent the number of transcripts per cell in vivo is suggested by the data derived from the ACH-2 spiking experiments and comparison of CARD-SGS with qPCR assays having single HIV RNA copy sensitivity. It is thus likely that the proviral expression level in the large majority of infected cells is not sufficient to support virus production, which requires two RNA genomes to be packaged and thousands of protein molecules per virion. The only exception to the low number of HIV RNA molecules was seen in the sample from the viremic individual, in whom two “high-expressing” cells were detected. We speculate that high-expressing cells may represent those cells in the process of virus replication. Obviously, further studies are needed to test this concept.
Moving forward, the CARD-SGS assay will facilitate translational studies to understand HIV pathogenesis better, to define persistent HIV reservoirs more clearly, and to determine the efficacy at the single-cell level of experimental interventions designed to eliminate HIV reservoirs.
Methods
Participants and HIV Quantification.
PBMC samples were obtained from four donors recruited at the University of Pittsburgh Clinical Trials Unit (Table 1). All donors provided written informed consent, and the study was approved by the University of Pittsburgh Institutional Review Board. Several aliquots of PBMCs containing 10–20 million cells were stored in liquid nitrogen at the University of Pittsburgh until used for quantification of HIV DNA (9) and RNA (11). Other aliquots were shipped to the National Cancer Institute (NCI)-Frederick on dry ice to be assayed by CARD-SGS. Cells were stored in liquid nitrogen at the NCI until CARD-SGS was performed.
PBMC Preparation.
RPMI medium, warmed to 37 °C, was added drop-wise to thaw cells for HIV DNA and HIV RNA extraction. The thawed cells were divided into many replicate aliquots, ranging from 1 million cells to 30,000 cells, spun at 500 × g for 5 min in a 1.5-mL centrifuge tube, and the supernatant was removed with a small transfer pipette. The pelleted cells were used immediately or were frozen on dry ice and stored in liquid nitrogen until ready for use. The end point for HIV RNA-expressing cells was determined empirically by performing CARD-SGS on varying quantities of cells from the cell pellets. The end point was reached when no more than 30% of the PCR reactions in a 96-well plate were positive when the entire cDNA contents were spread across the plate.
Nucleic Acid Extraction.
Extraction of HIV DNA and intracellular HIV RNA was modified from the protocols for qPCR assays (11) and optimized for CARD-SGS. Recoveries of gDNA were determined by extracting known quantities of HIV-negative PBMCs and measuring CCR5 DNA levels by qPCR using plasmid DNA as standards. HIV RNA recoveries were determined by extracting negative PBMCs spiked with varying quantities of ACH-2 cells, which are known to express low levels of HIV RNA without induction of transcription, and quantifying HIV RNA by limiting dilution PCR (Fig. S1 and Tables S1–S3). In one assay, duplicate sets of pellets were stored overnight at −80 °C to answer the question of whether freezing/thawing cell pellets affects the yield of gDNA and RNA.
Total nucleic acid was extracted by adding 100 μL of 3 M guanidine HCl and 5 μL of 20 mg/mL Proteinase K to each cell pellet, vortexing, and incubating on a heat block at 42 °C for 1 h. Addition of 400 μL of 6 M guanidine isothiocyanate (GuSCN) and 9 μL of glycogen (20 mg/mL) to each sample was followed by mixing and incubation at 42 °C for an additional 10 min. Five hundred microliters of 100% isopropanol was added with mixing, followed by centrifugation at 21,000 × g at room temperature for 10 min to precipitate nucleic acids (11). The supernatant was removed without disturbing the pellet, and the pellet was washed with 750 μL of 70% ethanol. Precipitated nucleic acid was air-dried. Recovery of gDNA was determined by qPCR assay for CCR5 DNA (catalog no. 4312660; PE Biosystems). Additional RNA isolation included resuspending the extracted nucleic acid in 38 μL of DNase buffer solution and 2 μL of 10 units/μL DNase I (catalog no. 04716728001; Roche) and incubating for 20 min at 37 °C. Two hundred microliters of 6 M GuSCN was added and mixed well, and 250 μL of 100% isopropanol was then added and again mixed. Samples were centrifuged at 21,000 × g for 10 min, supernatants were removed, pellets were washed with 750 μL of 70% ethanol, and the RNA pellet was air-dried. RNA was resuspended in 20 μL of 5 M Tris⋅HCl (pH 8.0) and used for cDNA synthesis.
SGS.
SGS was performed as previously described (19). The cDNA was synthesized by adding 2.5 μL of 10 mM dNTPs and 2.5 μL of 2 μM gene-specific primer (3500− for p6-PR-RT) to each 20-μL RNA sample in a 96-well PCR plate. The plates were sealed (catalog no. MSA-5001, Microseal A film; MJ Research, Inc.), and the RNA was denatured at 65 °C for 10 min and placed in an Eppendorf 96-well cold block at −20 °C. In a separate 1.5-mL tube, master mix was made by combining the following from a SuperScript III Reverse Transcriptase kit (catalog no. 18080-044; Invitrogen): 10 μL of 5× first strand buffer, 0.5 μL of 0.1 M DTT, 13.5 μL of RNase-free water, 0.5 μL of 40 U/μL RNaseOUT recombinant ribonuclease inhibitor (catalog no. 10777-019; Invitrogen), and 0.5 μL of 200 U/μL SuperScript III Reverse Transcriptase. After cooling the RNA for 1 min on the −20 °C block, master mix was added, followed by incubation at 50 °C for 50 min and then inactivation at 85 °C for 10 min. The cDNA was cooled to 4 °C and used immediately or stored at −80 °C after dilution with 150 μL of 5 mM Tris⋅HCl (pH 8.0). Two hundred microliters of diluted cDNA was added to 800 μL of Platinum Taq master mix containing the following: 100 μL of 10× PCR buffer, 40 μL of 50 mM MgCl2, 20 μL of 10 mM dNTPs, 4 μL of each primer (1849+ and 3500−), 624 μL of molecular-grade water, and 8 μL of Platinum Taq polymerase (catalog no. 10966-083; Invitrogen). The total volume of cDNA and master mix was spread across each well of a 96-well plate (10 μL per well). PCR cycling was performed as follows: 94 °C for 2 min and 45 cycles of 94 °C for 30 s, 50 °C for 30 s, and 72 °C for 1 min and 30 s, followed by a final extension at 72 °C for 3 min. Eighty microliters of 5 mM Tris⋅HCl (pH 8.0) was added to every well in the PCR plate. After PCR, 2 μL was transferred from each well to a 96-well plate containing 8 μL of Platinum Taq master mix (contents listed above), but with primers 1870+ and 3410−. Nested PCR cycling was performed as follows: 94 °C for 2 min and 45 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min and 30 s, followed by a final extension at 72 °C for 3 min. Positive wells were identified using GelRed to illuminate amplified DNA (catalog no. 41003; Biotium). Positive PCR reactions were sequenced by Sanger sequencing.
Sequence Analysis.
The sequencing data were analyzed using MEGA (43) to generate NJ trees to identify identical sequences present within the same extraction. Identical sequences obtained from the same extraction were inferred to result from a single cell (as discussed above). Diversity was calculated by overall APD also using MEGA.
Acknowledgments
We thank the individuals who donated samples for this study. We also thank Connie Kinna, Valerie Turnquist, Anne Arthur, and Susan Toms for administrative support; Frank Maldarelli, Shawn Hill, Sherimay Alban, Jianbo Chen, Guillaume Besson, and Beth Fyne for providing valuable reagents and cell lines; Joseph Meyer for help in generating the figures; and Valerie Boltz for helpful scientific discussions. This work was supported by intramural NIH funding to the HIV Dynamics and Replication Program; by NIH Grants 1U01CA200441-01 (to M.F.K.), B11-A-14 (to M.F.K.), NRC-15007 (IATAP FY15/16) (to M.F.K.), and NRC14005 (Bench-to-Bedside) (to M.F.K.); and by Leidos Biomedical Research, Inc. Subcontracts 12XS547 (to J.W.M.) and 13SX110 (to J.M.C.). J.M.C. is a Research Professor of the American Cancer Society. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute (NCI) or the NIH.
Footnotes
The authors declare no conflict of interest.
*Hong F, et al., Conference on Retroviruses and Opportunistic Infections, February 22–25, 2016, Boston, MA, abstr 330.
†Bruner KM, et al., Conference on Retroviruses and Opportunistic Infections, February 22–25, 2016, Boston, MA, abstr 83.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. KY819144–KY820040).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1617961114/-/DCSupplemental.
References
- 1.Kearney MF, et al. Origin of rebound plasma HIV includes cells with identical proviruses that are transcriptionally active before stopping of antiretroviral therapy. J Virol. 2015;90:1369–1376. doi: 10.1128/JVI.02139-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaiser P, et al. Assays for precise quantification of total (including short) and elongated HIV-1 transcripts. J Virol Methods. 2017;242:1–8. doi: 10.1016/j.jviromet.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiselinova M, et al. HIV-1 RNA and HIV-1 DNA persistence during suppressive ART with PI-based or nevirapine-based regimens. J Antimicrob Chemother. 2015;70:3311–3316. doi: 10.1093/jac/dkv250. [DOI] [PubMed] [Google Scholar]
- 4.Kearney MF, et al. Well-mixed plasma and tissue viral populations in RT-SHIV-infected macaques implies a lack of viral replication in the tissues during antiretroviral therapy. Retrovirology. 2015;12:93. doi: 10.1186/s12977-015-0212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maldarelli F, et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science. 2014;345:179–183. doi: 10.1126/science.1254194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner TA, et al. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science. 2014;345:570–573. doi: 10.1126/science.1256304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonetti FR, et al. Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc Natl Acad Sci USA. 2016;113:1883–1888. doi: 10.1073/pnas.1522675113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruner KM, et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat Med. 2016;22:1043–1049. doi: 10.1038/nm.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besson GJ, et al. HIV-1 DNA decay dynamics in blood during more than a decade of suppressive antiretroviral therapy. Clin Infect Dis. 2014;59:1312–1321. doi: 10.1093/cid/ciu585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cillo AR, et al. Impact of chemotherapy for HIV-1 related lymphoma on residual viremia and cellular HIV-1 DNA in patients on suppressive antiretroviral therapy. PLoS One. 2014;9:e92118. doi: 10.1371/journal.pone.0092118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong F, et al. Novel assays for measurement of total cell-associated HIV-1 DNA and RNA. J Clin Microbiol. 2016;54:902–11. doi: 10.1128/JCM.02904-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folks TM, et al. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci USA. 1989;86:2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pomerantz RJ, Trono D, Feinberg MB, Baltimore D. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: A molecular model for latency. Cell. 1990;61:1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- 14.Palmer S, et al. Multiple, linked human immunodeficiency virus type 1 drug resistance mutations in treatment-experienced patients are missed by standard genotype analysis. J Clin Microbiol. 2005;43:406–413. doi: 10.1128/JCM.43.1.406-413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kearney M, et al. Frequent polymorphism at drug resistance sites in HIV-1 protease and reverse transcriptase. AIDS. 2008;22:497–501. doi: 10.1097/QAD.0b013e3282f29478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cillo AR, et al. Improved single-copy assays for quantification of persistent HIV-1 viremia in patients on suppressive antiretroviral therapy. J Clin Microbiol. 2014;52:3944–3951. doi: 10.1128/JCM.02060-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keele BF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kearney M, et al. Human immunodeficiency virus type 1 population genetics and adaptation in newly infected individuals. J Virol. 2009;83:2715–2727. doi: 10.1128/JVI.01960-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maldarelli F, et al. HIV populations are large and accumulate high genetic diversity in a nonlinear fashion. J Virol. 2013;87:10313–10323. doi: 10.1128/JVI.01225-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kearney MF, et al. Lack of detectable HIV-1 molecular evolution during suppressive antiretroviral therapy. PLoS Pathog. 2014;10:e1004010. doi: 10.1371/journal.ppat.1004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDermott JL, et al. Decay of human immunodeficiency virus type 1 unintegrated DNA containing two long terminal repeats in infected individuals after 3 to 8 years of sustained control of viremia. J Clin Microbiol. 2005;43:5272–5274. doi: 10.1128/JCM.43.10.5272-5274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharkey M. Tracking episomal HIV DNA: Implications for viral persistence and eradication of HIV. Curr Opin HIV AIDS. 2013;8:93–99. doi: 10.1097/COH.0b013e32835d08c2. [DOI] [PubMed] [Google Scholar]
- 23.Sloan RD, Wainberg MA. The role of unintegrated DNA in HIV infection. Retrovirology. 2011;8:52. doi: 10.1186/1742-4690-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meteer JD, Schinazi RF, Mellors JW, Sluis-Cremer N. Molecular mechanism of HIV-1 resistance to 3′-azido-2′,3′-dideoxyguanosine. Antiviral Res. 2014;101:62–67. doi: 10.1016/j.antiviral.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey JR, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol. 2006;80:6441–6457. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinoso JB, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci USA. 2009;106:9403–9408. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gandhi RT, et al. AIDS Clinical Trials Group (ACTG) A5244 Team No effect of raltegravir intensification on viral replication markers in the blood of HIV-1-infected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;59:229–235. doi: 10.1097/QAI.0b013e31823fd1f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandhi RT, et al. AIDS Clinical Trials Group A5244 team The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7:7. doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imamichi H, et al. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc Natl Acad Sci USA. 2016;113:8783–8788. doi: 10.1073/pnas.1609057113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baxter AE, et al. Single-cell characterization of viral translation-competent reservoirs in HIV-infected individuals. Cell Host Microbe. 2016;20:368–380. doi: 10.1016/j.chom.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deleage C, et al. Defining HIV and SIV reservoirs in lymphoid tissues. Pathog Immun. 2016;1:68–106. doi: 10.20411/pai.v1i1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Josefsson L, et al. Single cell analysis of lymph node tissue from HIV-1 infected patients reveals that the majority of CD4+ T-cells contain one HIV-1 DNA molecule. PLoS Pathog. 2013;9:e1003432. doi: 10.1371/journal.ppat.1003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho YC, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Althaus CL, Joos B, Perelson AS, Günthard HF. Quantifying the turnover of transcriptional subclasses of HIV-1-infected cells. PLOS Comput Biol. 2014;10:e1003871. doi: 10.1371/journal.pcbi.1003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Archin NM, et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci USA. 2012;109:9523–9528. doi: 10.1073/pnas.1120248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan CN, Dietrich I, Hosie MJ, Willett BJ. Recent developments in human immunodeficiency virus-1 latency research. J Gen Virol. 2013;94:917–932. doi: 10.1099/vir.0.049296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothenberger MK, et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci USA. 2015;112:E1126–E1134. doi: 10.1073/pnas.1414926112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohn LB, et al. HIV-1 integration landscape during latent and active infection. Cell. 2015;160:420–432. doi: 10.1016/j.cell.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bell P, Montaner LJ, Maul GG. Accumulation and intranuclear distribution of unintegrated human immunodeficiency virus type 1 DNA. J Virol. 2001;75:7683–7691. doi: 10.1128/JVI.75.16.7683-7691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lau JW, Levy DN, Wodarz D. Contribution of HIV-1 genomes that do not integrate to the basic reproductive ratio of the virus. J Theor Biol. 2015;367:222–229. doi: 10.1016/j.jtbi.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panther LA, Coombs RW, Zeh JE, Collier AC, Corey L.(1998Unintegrated circular HIV-1 DNA in the peripheral mononuclear cells of HIV-1-infected subjects: Association with high levels of plasma HIV-1 RNA, rapid decline in CD4 count, and clinical progression to AIDS J Acquir Immune Defic Syndr Hum Retrovirol 17303–313. [DOI] [PubMed] [Google Scholar]
- 42.Lorenzi JC, et al. Paired quantitative and qualitative assessment of the replication-competent HIV-1 reservoir and comparison with integrated proviral DNA. Proc Natl Acad Sci USA. 2016;113:E7908–E7916. doi: 10.1073/pnas.1617789113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]