Significance
Dysfunctional uterine receptivity is responsible for approximately 70% of implantation failures. This periimplantation event is dominated by ovarian hormones progesterone and estrogen functioning through their corresponding receptors, PR and ER, coupled with locally produced signaling molecules. However, the hierarchical landscape of the molecular pathways that govern this process remains largely unexplored. Here we show that Shp2, a classic cytoplasmic protein, is mainly located in the nuclei in uterine cells at periimplantation. Further investigation revealed that nuclear Shp2 enhances the Src kinase-mediated ERα tyrosine phosphorylation, facilitates ERα binding to Pgr promoter, and thus promotes the ERα transcription activity in periimplantation uteri. Our findings have high clinical significance in identifying novel therapeutic targets for the aberrant uterine receptivity and implantation failure.
Keywords: Shp2, ERK signaling, Src kinase, estrogen receptor, uterine receptivity
Abstract
Estrogen and progesterone coupled with locally produced signaling molecules are essential for embryo implantation. However, the hierarchical landscape of the molecular pathways that governs this process remains largely unexplored. Here we show that the protein tyrosine phosphatase Shp2, a positive transducer of RTK signaling, is predominately localized in the nuclei in the periimplantation mouse uterus. Uterine-specific deletion of Shp2 exhibits reduced progesterone receptor (PR) expression and progesterone resistance, which derails normal uterine receptivity, leading to complete implantation failure in mice. Notably, the PR expression defects are attributed to the limited estrogen receptor α (ERα) activation in uterine stroma. Further analysis reveals that nuclear Shp2, rather than cytosolic Shp2, promotes the ERα transcription activity. This function is achieved by enhancing the Src kinase-mediated ERα tyrosine phosphorylation, which facilitates ERα binding to Pgr promoter in an ERK-independent manner in periimplantation uteri. Besides uncovering a regulatory mechanism, this study could be clinically relevant to dysfunctional ERα-caused endometrial disorders in women.
Successful implantation requires synchronization between an implantation-competent blastocyst and a receptive uterus. In humans, natural conception per cycle is poor (∼30%), and ∼75% of failed pregnancies are considered to be due to implantation failure (1). One-third of implantation failure is attributed to the embryo itself, whereas the remaining two-thirds appear to result from inadequate uterine receptivity (2). The endometrium enters into a receptive stage for blastocyst implantation only in a restricted time period termed “implantation window,” which is dominated by precisely regulated proliferation and differentiation of endometrial epithelium and stroma under the influence of progesterone and estrogen (3).
Estrogen and progesterone bind to the estrogen receptor (ER) and progesterone receptor (PR), respectively, coupled with specific cofactors to endure the optimal functions. The activity of these nuclear receptors is also regulated at the posttranslational level by various modifications, such as phosphorylation, which can influence the protein stability, interaction with the cofactors and DNA binding affinity. So far, a wide range of nuclear receptor cofactors have been identified to ensure the normal ER transcriptional activation, such as the well-known steroid receptor coactivator (SRC) family members, which bind with ERα in the chromatin to recruit the histone acetyltransferase p300 (P300) for transcriptional activation (4–6). It is conceivable that ordered and combinatorial recruitment of cofactors at a specific target promoter after ERα binding to DNA sequence was essential to ensure target gene transcription. Recent evidence shows that nuclear receptor coactivator 6 is essential for embryo implantation by maintaining the appropriate level and activity of uterine ERα. Its deficiency induces aberrantly increased ERα protein level and thus hyperactivation of estrogen–ER signaling (7). Because either hyper- or hypoactivation of estrogen–ER signaling is associated with pregnancy disorders, such as recurrent implantation failure and endometriosis in women, it is extremely important to further explore the underlying mechanism governing normal ER transcriptional activation in the uterus during early pregnancy.
Src homology 2 domain containing protein tyrosine phosphatase (SHP2), encode by the gene Ptpn11, is a ubiquitously expressed protein tyrosine phosphatase, and its dysregulation is associated with malignant neoplasms and developmental disorders (8, 9). Structurally, Shp2 contains two N-terminally located Src-homology 2 domains (N-SH2 and C-SH2), a central phosphotyrosine phosphatase (PTP) domain and a C-terminal tail with tyrosyl phosphorylation sites and a proline-rich motif. Shp2 has been well defined as a positive cytoplasmic regulator for receptor protein tyrosine kinases (RTKs) in mediating cellular response to hormones, growth factors, and cytokines. Systemic deletion of Shp2 in mice results in embryonic lethality shortly after implantation, due to defective extracellular signal-regulated kinase (ERK) activation and the resulted death of trophoblast stem cells (10). However, it remains largely unknown whether uterine Shp2 is physiologically involved in regulating early pregnancy events. Because various RTK-coupled cytokines and growth factors, such as leukemia inhibitory factor (11), Interleukin 11 (12), and EGF family growth factors (13, 14), are essential for normal embryo implantation, we speculated that Shp2 could be an important player in directing normal early pregnancy events.
In the current study, using multiple genetic and molecular approaches, we provided strong evidence that Shp2 is essential for normal uterine receptivity and embryo implantation in an unexpected ERK-independent manner. Uterine-specific deletion of Shp2 dramatically limits estrogen receptor ERα transcriptional activity, leading to impaired uterine receptivity and complete implantation failure. In searching for the underlying molecular causes, we were surprised to find that nuclear Shp2 promotes ERα transcription activity by enhancing the Src kinase-mediated ERα tyrosine phosphorylation, which facilitates ERα binding to Pgr promoter in the periimplantation uteri.
Results
Shp2 Exhibits Nuclear Localization in Mouse Uteri at Periimplantation.
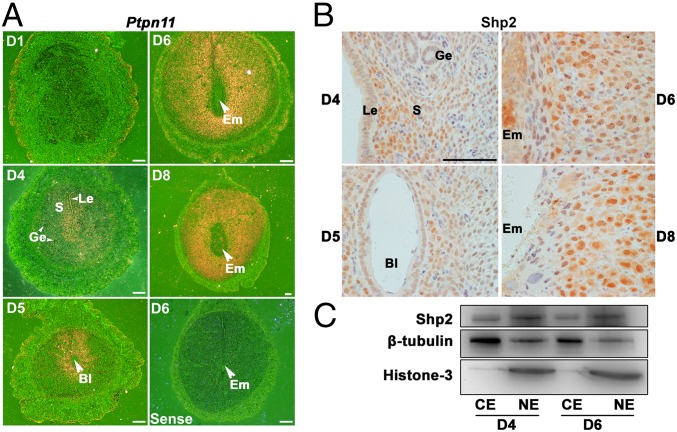
To address the pathophysiological significance of Shp2 during early pregnancy, we first examined mouse uterine expression pattern of Shp2 at periimplantation stage. As revealed by in situ hybridization, whereas Ptpn11 was nearly undetectable in the uterus on day 1 of pregnancy (D1, day 1 = vaginal plug positive), its expression was intensely observed in subepithelial stromal cells on D4 (Fig. 1A). With the onset of embryo implantation on D5, Ptpn11 was highly up-regulated in both the epithelial and stromal cells around the implanting blastocyst (Fig. 1A). With the initiation and progression of decidualization on D6–8, Ptpn11 expression expanded into the entire decidual bed (Fig. 1A). This dynamic expression pattern suggests that Shp2 could be an important player during the periimplantation stage.
Fig. 1.
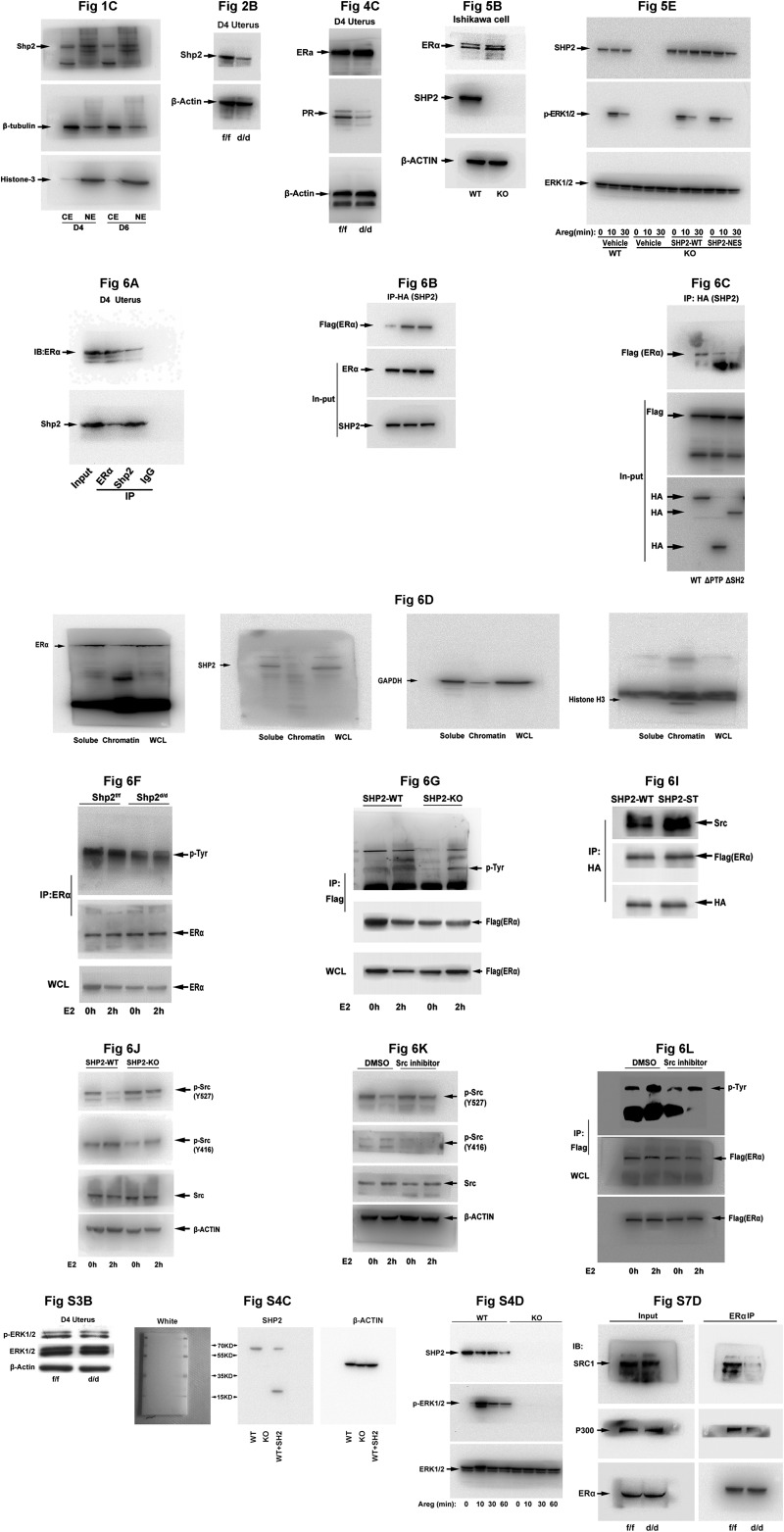
Shp2 is spatiotemporally expressed in periimplantation mouse uteri exhibiting a nuclear localization. (A) In situ hybridization of Ptpn11 mRNA in D1–4 uteri and D5–8 implantation sites. Shp2 mRNA is visible as the brown color. The white arrowhead indicates the embryo. (White scale bars, 250 μm.) (B) Immunohistochemical staining of Shp2 protein in D4 uteri and D5–8 implantation sites. The brown color indicates positive staining for Shp2. Note the dominant nuclear location of Shp2. (Black scale bar, 100 μm.) (C) Immunoblotting analysis of Shp2 protein in different cellular fractions from the D4 uteri and D6 implantation sites. β-Tubulin serves as a cytoplasmic loading control, whereas histone 3 serves as a nuclear loading control. Bl, blastocyst; CE, cytoplasmic extraction; Em, embryo; Ge, glandular epithelium; Le, luminal epithelium; NE, nuclear extraction; S, stroma. Data shown are representative of at least three independent experiments.
We next analyzed Shp2 protein distribution in periimplantation uteri by immunohistochemistry analysis. Consistent with its RNA expression pattern, Shp2 protein was highly expressed in uterine stroma (Fig. 1B). It was worthy to note that Shp2 was also localized to nuclei in proliferating and decidualizing stromal cells on D4–8 of pregnancy (Fig. 1B). This finding was further confirmed by the Western blot analysis showing a higher Shp2 expression in nuclear extracts (Fig. 1C). These observations point toward a potential nuclear-related function of Shp2 in the uterus during early pregnancy.
Uterine Shp2 Deficiency Completely Blocks Embryo Implantation Resulting in Female Infertility.
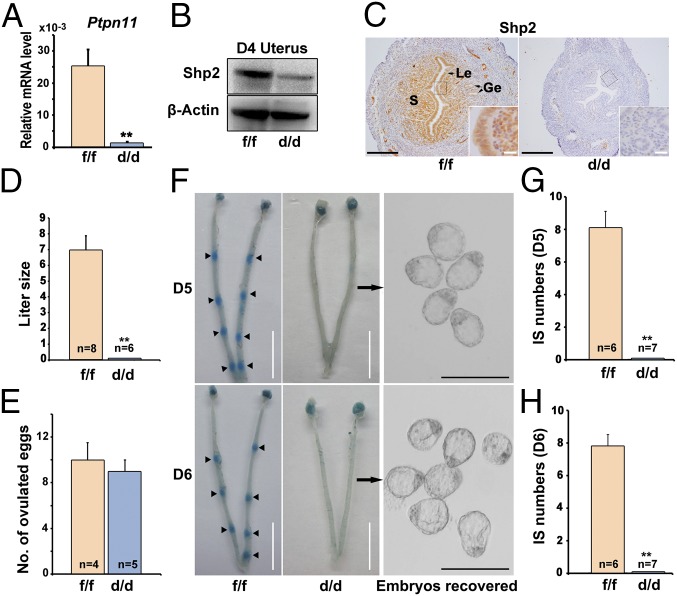
To explore the uterine function of Shp2 at periimplantation, a mouse model harboring a uterine-specific deletion of Shp2 (Shp2d/d) was generated by crossing Shp2-loxp mice (Shp2f/f) with PR-Cre mice (PgrCre/+). As illustrated in Fig. 2 A–C, Shp2 was efficiently deleted in PR-expressing uterine cells of Shp2d/d females. No pups were found in vaginal plug-positive Shp2d/d mice after mating with fertile wild-type (WT) males (Fig. 2D), demonstrating that the uterine depletion of Shp2 caused a complete infertile phenotype. To identify the stage-specific defects through pregnancy in Shp2d/d females, early pregnancy events, including ovulation and implantation, were examined accordingly. On D1, all Shp2d/d mice ovulated comparable numbers of oocytes as Shp2f/f females (Fig. 2E). However, on D5–6, whereas Shp2f/f mice displayed normal attachment reaction as visible by blue dye injection, none of Shp2d/d mice showed signs of implantation and unimplanted blastocysts with normal morphology can be recovered from the Shp2d/d uteri (Fig. 2 F–H). These results clearly indicate that uterine Shp2 is indispensable for normal embryo implantation.
Fig. 2.
Uterine Shp2 deficiency induces complete implantation failure resulting in female infertility. (A) Real-time PCR analysis of Shp2 mRNA levels in D4 uteri from wild-type (Shp2f/f) and knockout (Shp2d/d) mice. The values are normalized to the Gapdh expression level and indicated as the mean ± SEM, n = 3. **P < 0.01. (B) Immunoblotting analysis of Shp2 protein levels in D4 Shp2f/f and Shp2d/d uteri. β-Actin is used as loading control. (C) Immunohistochemistry analysis of Shp2 protein in D4 Shp2f/f and Shp2d/d uteri. Ge, glandular epithelium; Le, luminal epithelium; S, stroma. (White scale bar, 25 μm; black scale bar, 250 μm.) (D) Average litter sizes in Shp2f/f and Shp2d/d female mice. Number within the bar indicated the number of mice tested. **P < 0.01. (E) Number of ovulated oocytes in Shp2f/f and Shp2d/d mice. Number within the bar indicates the number of mice tested. (F) Implantation status of Shp2f/f and Shp2d/d mice on D5–6. Implantation sites are visualized by blue dye method and the unimplanted embryos are recovered from Shp2d/d uteri (Right image). Black arrowheads indicate the implantation sites. Black arrows indicate the embryos in the right panels are recovered from the corresponding uteri. (White scale bar, 1 cm; black scale bar, 250 μm.) (G and H) Average number of implantation sites in Shp2f/f and Shp2d/d mice on D5 (G) and D6 (H) of pregnancy. Number within the bar indicates the number of mice tested. Mice that failed to recover any embryos are excluded in statistical analysis. IS, implantation site; **P < 0.01. Data shown are representative of at least four independent experiments.
Shp2 Depletion Derails Normal Uterine Receptivity Depicting Progesterone Resistance.
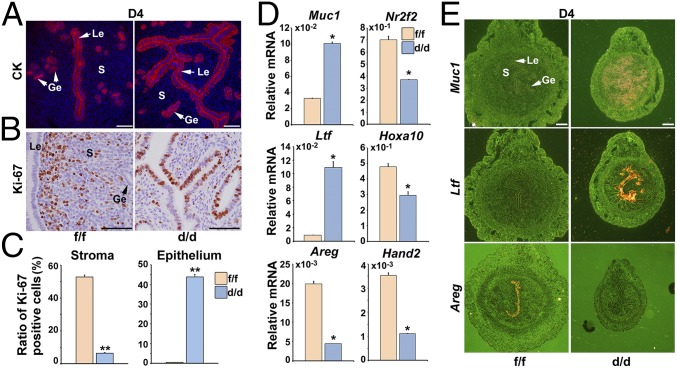
To portray the uterine lumen morphology at periimplantation, we performed immunofluorescence staining of cytokeratin (an epithelial marker) and Ki-67 (a cell proliferation marker). We observed that Shp2d/d uteri exhibited a defective luminal closure characterized by increased uterine epithelial branches (Fig. 3A). Moreover, Ki-67 staining analysis revealed an aberrant epithelial proliferation accompanied by a dramatically decreased stromal cell division in Shp2d/d mice (Fig. 3 B and C). These cellular defects indicate an abnormal uterine receptivity upon Shp2 deficiency.
Fig. 3.
Shp2 depletion derails normal uterine receptivity depicting progesterone resistance. (A) Immunofluorescence staining of cytokeratin (CK) in D4 Shp2f/f and Shp2d/d uteri. Cy3-labeled antigen is showed in red, whereas Hoechst 33342 labeled the nuclei as blue. (Scale bars, 200 μm.) (B) Immunohistochemical staining of Ki-67 in D4 Shp2f/f and Shp2d/d uteri. The brown color indicates the positive signal. (Scale bars, 100 μm.) (C) Quantitative analysis of Ki-67 positive cells in D4 Shp2f/f and Shp2d/d uteri. **P < 0.01. (D) Real-time PCR analysis of the implantation-related marker gene expression in D4 Shp2f/f and Shp2d/d uteri. The values are normalized to the Gapdh expression level and indicated as the mean ± SEM, n = 3. *P < 0.05. (E) In situ hybridization of Ltf, Muc1, and Areg in D4 Shp2f/f and Shp2d/d uteri. The brown color indicates the positive signal. Ge, glandular epithelium; Le, luminal epithelium; S, stroma. (Scale bars, 250 μm.) Data shown are representative of at least three independent experiments.
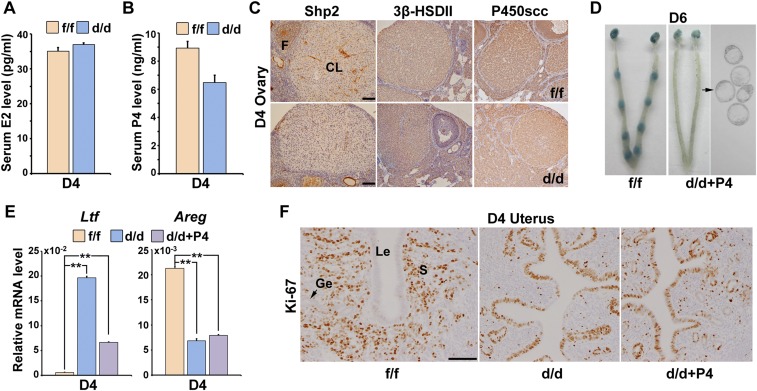
Because progesterone and estrogen are the principle determinants of uterine receptivity in mice, we next measured serum levels of progesterone (P4) and estradiol-17β (E2) in D4 Shp2f/f and Shp2d/d female mice. As shown in Fig. S1 A and B, we observed comparable circulating levels of E2 and a slight decreased level of P4. Immunohistochemistry staining revealed that the corpus luteum expressed lower levels of Shp2 in comparison with the developing follicles (Fig. S1C). However, depletion of luteal Shp2 exerted no apparent influence on the expression of progesterone biosynthetic enzymes, cytochrome P450 cholesterol side-chain cleavage enzyme, and 3β-hydroxysteroid dehydrogenase II (Fig. S1C). To further test whether this slightly reduced progesterone secretion was the main cause for implantation failure in Shp2d/d females, we supplied P4 in the null mice for 3 consecutive days starting on D3. However, P4 supplementation failed to restore embryo implantation in Shp2d/d mice (Fig. S1D). This result indicates that implantation failure in Shp2d/d mice is intrinsic to uterine dysfunctions.
Fig. S1.
Progesterone supplementation failed to restore embryo implantation in Shp2d/d mice. (A) Serum estradiol-17β (E2) level in Shp2f/f and Shp2d/d mice on day 4 (D4) of pregnancy. (B) Serum progesterone (P4) level in Shp2f/f and Shp2d/d mice on D4 of pregnancy. Data are means ± SEM (*P < 0.05; Student’s t test). Numbers within bars indicate numbers of mice examined. (C) Immunohistochemistry staining of 3β-hydroxysteroid dehydrogenase II (3β-HSDII) and P450 cholesterol side-chain cleavage enzyme (P450scc) in Shp2f/f and Shp2d/d D4 ovaries. CL, corpus luteum; F, follicle. (Scale bars, 100 μm.) (D) Embryo implantation status of Shp2d/d mice as revealed by blue dye method on D6 of pregnancy after receiving daily P4 supplementation (2 mg per mouse) from D3–5. Black arrow indicates the embryos in the right panel are recovered from the corresponding uteri. (E) Real-time PCR analysis of the uterine receptivity marker genes on D4 after P4 supplementation. The values are normalized to the Gapdh expression level and indicated as the mean ± SEM, n = 3. **P < 0.01. (F) Immunohistochemical staining of Ki-67 in Shp2f/f and Shp2d/d in D4 uteri. (Scale bar, 100 μm.) Ge, glandular epithelium; Le, luminal epithelium; S, stroma.
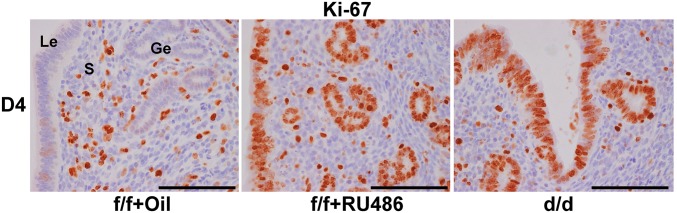
To reveal the molecular basis of phenotypic defects in the absence of uterine Shp2, we then analyzed the expression of implantation-related genes in D4 uteri by qPCR and in situ hybridization. As shown in Fig. 3 D and E, whereas the epithelial estrogen-regulated genes including mucin 1 (Muc1) and lactotransferrin (Ltf) were aberrantly induced in Shp2d/d mice, all progesterone-targeting genes, such as amphiregulin (Areg), homeobox A10 (Hoxa10), heart and neural crest derivatives expressed 2 (Hand2), and chicken ovalbumin upstream promoter transcription factor 2 (Coup-tfII, also known as Nr2f2) were down-regulated in Shp2d/d uteri. This abnormal expression profile of implantation-related genes was well coincident with abnormal epithelial versus stromal proliferation (Fig. 3 B and C), indicating an impaired progesterone activity and loss of antagonistic influence of progesterone–PR signaling on estrogen-stimulated epithelial proliferation in Shp2d/d uteri. However, this molecular and cellular abnormality was not due to above-mentioned reduced circulating progesterone level, because progesterone supplementation failed to restore normal uterine receptivity marker gene expression (Fig. S1E) and uterine epithelial versus stromal proliferation in Shp2d/d uteri (Fig. S1F). Similar observation of enhanced uterine epithelial proliferation with reduced stromal proliferation can be mimicked in Shp2f/f mice by the treatment of progesterone antagonist RU486 (Fig. S2), phenocopying the progesterone resistance in mice lacking uterine Shp2.
Fig. S2.
RU486 treatment in wild-type (Shp2f/f) mice induced aberrant uterine luminal epithelial proliferation. Immunostaining of Ki-67 in Shp2f/f (f/f) uteri treated with oil or RU486 on day 4 (D4) of pregnancy. d/d indicated D4 Shp2d/d uteri. The brown color indicated the positive signal. (Scale bars, 100 μm.) Ge, glandular epithelium; Le, luminal epithelium; S, stroma.
Loss of Shp2 Compromises Uterine Estrogen Responsiveness and thus PR Expression Independent of ERK Pathway.
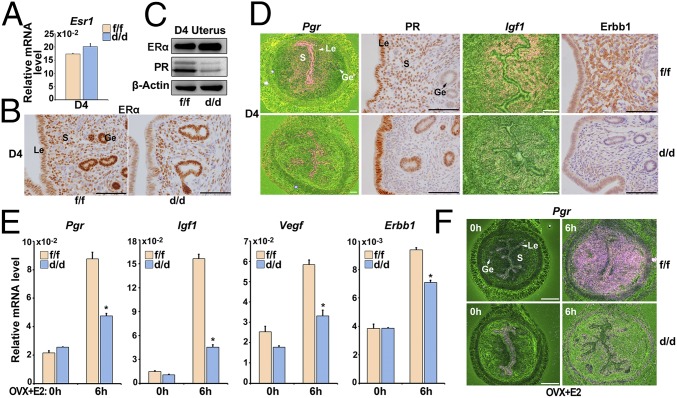
To reveal the underlying causes of progesterone resistance in Shp2d/d mice, we next analyzed ERα and PR expression in D4 Shp2f/f and Shp2d/d uteri. As shown in Fig. 4 A–C, ERα was comparably expressed even in the absence of uterine Shp2. In contrast, the expression of PR (encoded by Pgr) was significantly decreased in Shp2d/d uteri. Further localization analysis revealed that the stroma PR was severely decreased in Shp2d/d uteri (Fig. 4D). As the stroma Pgr is induced by the estrogen–ER signaling, to explore whether this decreased Pgr expression is due to hampered estrogen activities, we further analyzed some other ER target genes, including insulin-like growth factor 1 (Igf1) and erythroblastosis oncogene B 1 (Erbb1). They also displayed decreased expression in the Shp2d/d uteri (Fig. 4D). This interesting finding suggests that progesterone resistance from Shp2 deficiency is secondary to impaired uterine responsiveness to estrogen, regardless of normal circulating estrogen level and uterine ERα expression in null mice.
Fig. 4.
Loss of Shp2 compromises uterine estrogen responsiveness and thus PR expression. (A) Real-time PCR detection of the mRNA levels of Esr1 in D4 Shp2f/f and Shp2d/d uteri. The values are normalized to the Gapdh expression level and indicated as the mean ± SEM, n = 3. (B) Immunohistochemical staining of ERα in D4 Shp2f/f and Shp2d/d uteri. The brown color indicates the positive signal. (C) Immunoblotting analysis of ERα and PR in D4 Shp2f/f and Shp2d/d uteri. β-Actin is used as loading control. (D) In situ hybridization and immunohistochemical analysis of estrogen–ERα target genes in D4 Shp2f/f and Shp2d/d uteri. The brown color indicates the positive signal. (E) Real-time PCR detection of estrogen–ERα target gene expression levels in Shp2f/f and Shp2d/d ovariectomized mouse uteri in response to E2 treatment. The values are normalized to the Gapdh expression level and indicated as the mean ± SEM, n = 3. *P < 0.05. (F) In situ hybridization of Pgr in Shp2f/f and Shp2d/d ovariectomized mouse uteri after E2 treatment. The brown color indicates the positive signal. Ge, glandular epithelium; Le, luminal epithelium; S, stroma. (White scale bar, 250 μm; black scale bar, 100 μm.) Data shown are representative of at least three independent experiments.
To further confirm that this reduced PR expression in uterine stroma was due to hampered estrogen activities upon Shp2 deletion, we used an ovariectomized mouse model. As illustrated in Fig. 4E, all tested ER target genes, including Pgr, Igf1, vascular endothelial growth factor (Vegf), and Erbb1 were expressed at significantly lower levels at 6 h after estrogen challenge in ovariectomized Shp2d/d uteri compared with that in Shp2f/f females. In situ hybridization analysis further revealed that the induction of ER-targeting gene-like Pgr was not noted in Shp2d/d stroma after estrogen stimulation (Fig. 4F). These results reinforce the notion that Shp2 deficiency compromises uterine estrogen responsiveness and thus its target gene expression including Pgr in uterine stroma.
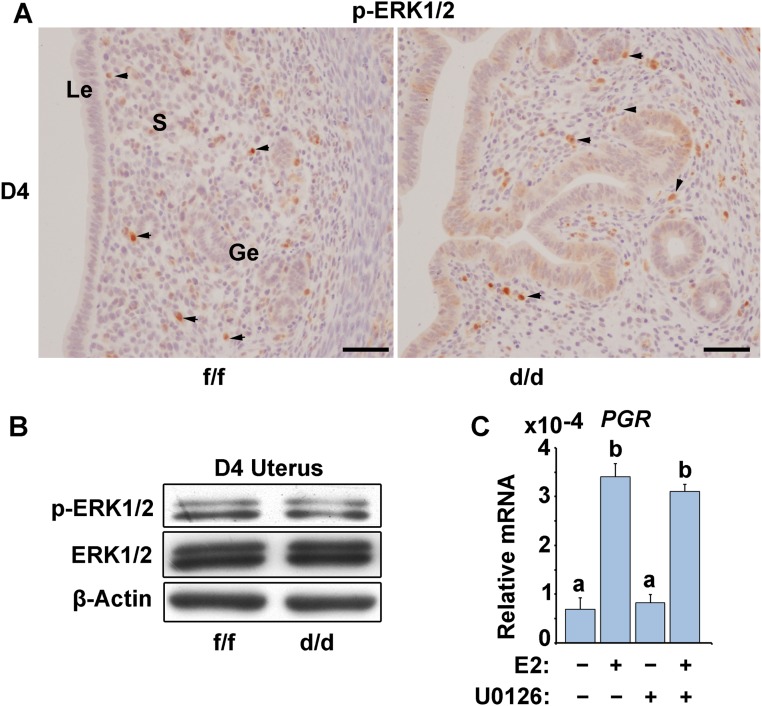
Because Shp2 is known as a positive signal transducer in ERK pathway, to test whether Shp2 would function through ERK pathway to regulate ERα activity in the uterus, we next examined the expression of p-ERK1/2 (activated form of ERK1/2) in D4 Shp2f/f and Shp2d/d uteri. Unexpectedly, p-ERK1/2 levels remained unaltered in Shp2d/d uteri (Fig. S3 A and B), suggesting that Shp2 may function through an ERK-independent manner to ensure ER activation during uterine receptivity establishment. To further exclude the possibility that Shp2 ensure the ERα activity via ERK pathway, we treated the human endometrial Ishikawa cells with a specific MEK1/2 inhibitor, U0126. The data demonstrated that ERK inhibition exerts apparently no influence on ERα transcriptional activation, because E2 can significantly induce ER-targeting gene PGR expression even in the presence of U0126 (Fig. S3C).
Fig. S3.
ERK1/2 expression and phosphorylation remain at comparable levels in D4 Shp2f/f (f/f) and Shp2d/d (d/d) uteri. (A) Immunostaining of phospho-ERK1/2 (p-ERK1/2) in Shp2f/f and Shp2d/d uteri on D4 of pregnancy. (Scale bars, 100 μm.) Black arrowheads indicate the positive cells. Ge, glandular epithelium; Le, luminal epithelium; S, stroma. (B) Immunoblotting analysis of p-ERK1/2 and total ERK1/2 expression in Shp2f/f and Shp2d/d uteri on D4 uteri. β-Actin serves as a control. (C) Real-time PCR analysis of PGR expression in human endometrial Ishikawa cells treated with the ERK1/2 selective inhibitor U0126. The values are normalized to the GAPDH expression level and indicated as the mean ± SEM, n = 3. The different letters (a and b) indicate significant differences between groups (a vs. b: P < 0.01).
Nuclear Shp2 Rather than Cytosolic Shp2 Is Required for Normal ERα Activation.
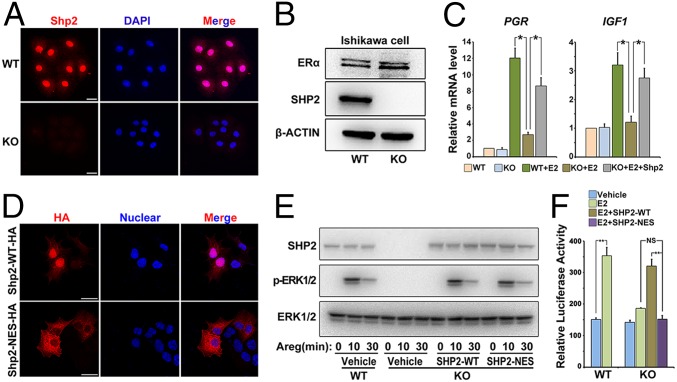
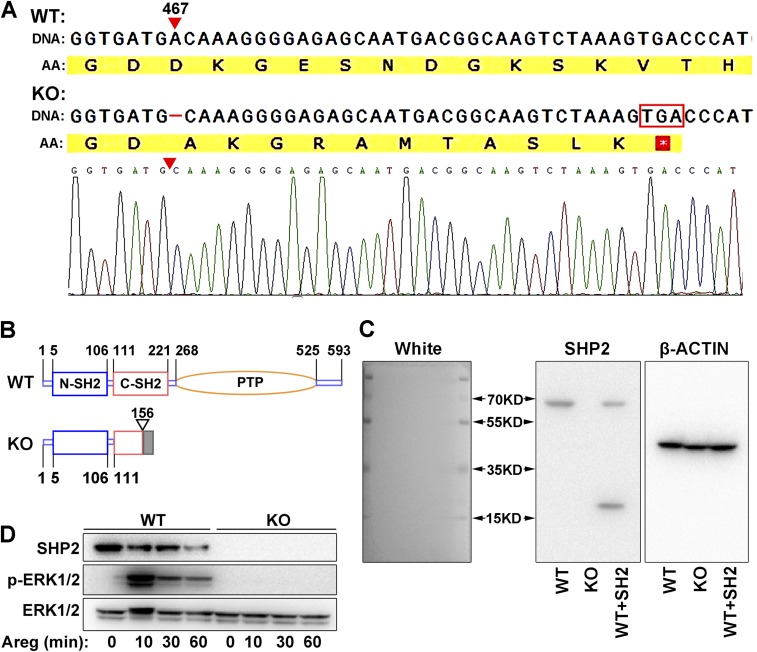
To further delineate the molecular basis of Shp2 on ERα, we next used a human endometrial Ishikawa cell line for in vitro study. Because Ishikawa cells expressed high levels of endogenous SHP2 (Fig. 5 A and B), we generated a SHP2 null mutant Ishikawa cell line by CRISPR/Cas9 strategy (Fig. S4 A–C). As shown in Fig. 5 A and B, we found the expression of SHP2 was completely deleted in SHP2 null cells. Besides, the ERK1/2 activation in response to amphiregulin (Areg) was also abolished in the knockout cells (Fig. S4D). Most importantly, SHP2 deficiency in Ishikawa cells significantly hampered estrogen-induced target gene expression, such as PGR and IGF1, whereas transfection with the full-length wild-type SHP2 (SHP2-WT) can rescue this defect (Fig. 5C). These observations confirm the findings in Shp2d/d mouse uteri, proving that SHP2 is essential for normal ER transcriptional activity.
Fig. 5.
Nuclear SHP2 is essential for normal ERα activation. (A) Immunofluorescence staining of SHP2 in WT and SHP2 knockout (KO) Ishikawa cells. Cy3-labeled antigen is showed in red; DAPI labeled the nuclei as blue. (Scale bars, 25 μm.) (B) Immunoblotting analysis of SHP2 and ERα in WT and KO Ishikawa cell line. β-Actin serves as a control. (C) Real-time PCR detection of estrogen–ERα target genes PGR and IGF1 in SHP2 KO Ishikawa cells. The values are normalized to the GAPDH expression level and shown as the mean ± SEM, *P < 0.05, n = 3. (D) Immunofluorescence staining of HA-tagged SHP2 in SHP2 knockout Ishikawa cell line transfected with the WT-SHP2 or NES-SHP2. The DAPI is used for nuclear staining. (Scale bars, 150 μm.) (E) Western blot analysis of p-ERK1/2 and total ERK1/2 after amphiregulin (Areg) treatment in SHP2 WT and KO Ishikawa cell line transfected with different vectors as indicated. (F) ERE-luciferase reporter assay to evaluate the ERα activation in SHP2 WT and KO cells transfected with HA-tagged wild-type or SHP2-NES vectors. The values are shown as the mean ± SEM, *P < 0.05, n = 3. Data shown are representative of at least three independent experiments. **P < 0.01. NS, no significance.
Fig. S4.
SHP2 knockout human endometrial Ishikawa cell line was established by CRISPR/Cas9 strategy. (A) Sanger sequence of SHP2 gene in the knockout cells. A base of nucleic acid was deleted at 467 of coding sequence resulting in frameshift mutation and the translation termination by a stop codon soon after the mutant site. AA, amino acid sequence. (B) Schematic diagram of the structure of wild-type (WT) SHP2 and its mutant form (KO) by CRISPR/Cas9 strategy. (C) Immunoblotting analysis SHP2 protein in WT and KO Ishikawa cells by specific antibody (BD Transduction Laboratories, 610621) that identifies the N-terminal of SHP2. WT cell line transfected with ΔPTP mutant containing only the SH2 domains serves as positive control (WT+SH2). β-Actin is used as loading control. (D) Induction of ERK1/2 phosphorylation in response to amphiregulin (Areg) treatment in WT and KO cells. ERK and its phosphorylation status were analyzed by immunoblotting, showing the functional loss of SHP2 in KO cells.
Because we observed a high level of Shp2 within the nucleus, to test whether the facilitating effect of Shp2 on ERα activation would depend on its nuclear localization, we next introduced a nucleus-excluded form of HA-tagged SHP2 (SHP2-NES) that contained a potent nuclear export sequence fused to the carboxyl terminus of original SHP2 (15). As expected, whereas the SHP2 mutant Ishikawa cell line transfected with HA-labeled SHP2-WT exhibited both cytosolic and nuclear localization, the null mutant cells transfected with SHP2-NES showed prominent cytosolic accumulation of SHP2 (Fig. 5D). Furthermore, whereas ERK1/2 activation in response to amphiregulin (Areg) could be largely restored by both SHP2-NES and SHP2-WT in knockout Ishikawa cells (Fig. 5E), SHP2-NES, unlike SHP2-WT, failed to rescue E2-induced ERE (estrogen-responsive element) response in mutant cells (Fig. 5F). These findings indicate that nuclear Shp2 rather than cytosolic Shp2 is required for normal ERα activity.
Nuclear Shp2 Enhances ERα Phosphorylation via Src Kinase Activation, Facilitating Its Binding to Pgr Promoter.
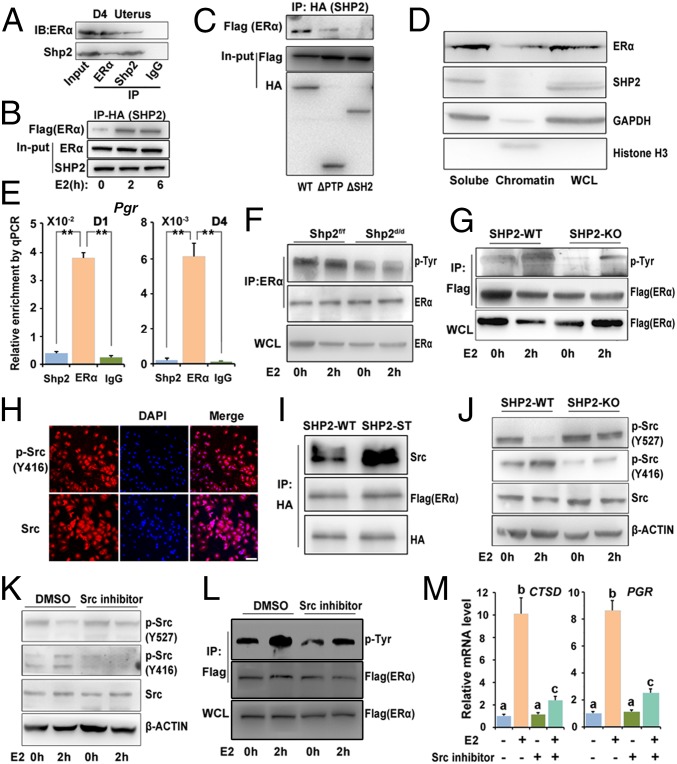
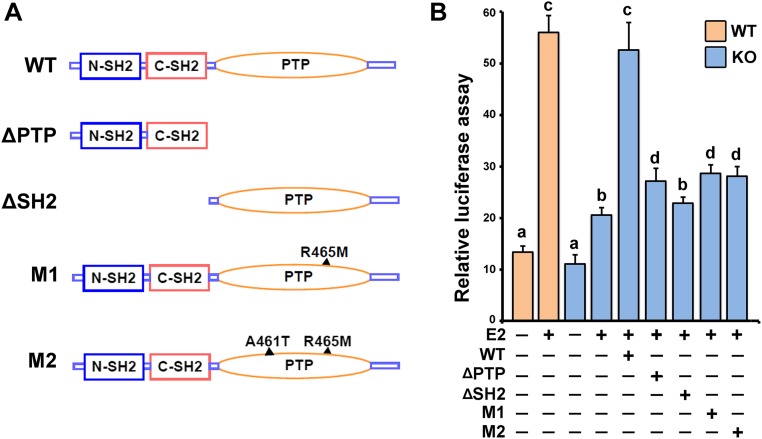
Because ERα and Shp2 both exhibited nuclear localization in the uterus, we tested the possibility of whether Shp2 could interact with ERα. Coimmunoprecipitation analysis revealed that Shp2 can physically interact with ERα in D4 receptive uteri as well as in Ishikawa cells (Fig. 6 A and B). To further define which part of SHP2 mediated the interaction with ERα, we generated truncation mutants of SHP2 harboring PTP domain deletion (ΔPTP) or SH2 domain deletions (ΔSH2). Using coimmunoprecipitation analysis, we found, whereas the ΔSH2 mutant containing PTP domain only exhibited barely detectable binding affinity with ERα, ΔPTP mutant with intact SH2 domains showed significantly higher binding affinity (Fig. 6C). These findings suggest that SH2 domains are the key regions physically interacting with ERα. However, neither the ΔSH2 nor ΔPTP could reverse the defective E2–ER response in the absence of intact SHP2 (Fig. S5). The inability of inactive SHP2 with phosphatase point mutation to restore the E2–ER response further suggests that the phosphatase activity of SHP2 is also critical for promoting the ER activity.
Fig. 6.
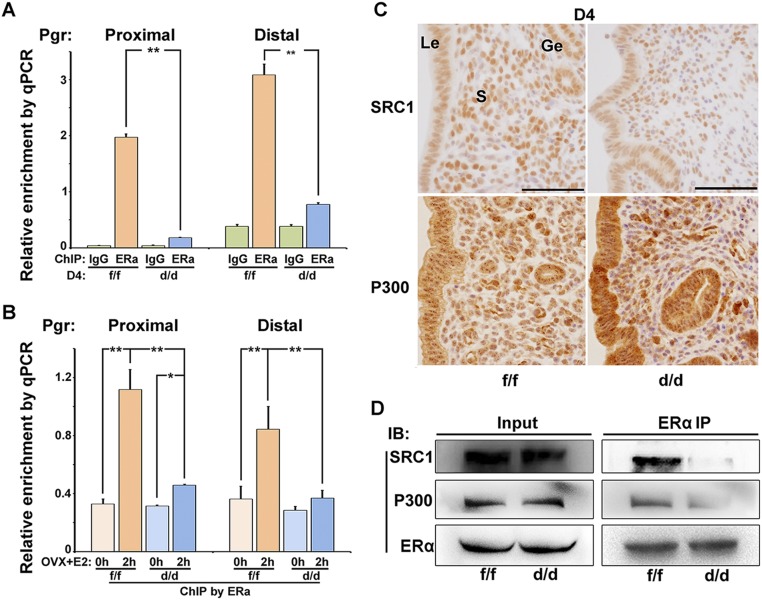
Nuclear Shp2 enhances ERα phosphorylation via Src kinase activation. (A) Immunoprecipitation analysis to detect the interaction of endogenous Shp2 and ERα. D4 uterine tissue lysates were subjected to immunoprecipitation using an anti-ERα, anti-Shp2 antibody, or rabbit IgG, followed by immunoblotting with respective antibodies. (B) Immunoprecipitation analysis for detecting the association of exogenously expressed SHP2 (HA tagged) and ERα (Flag tagged). Cell lysis with or without E2 treatment is immunoprecipitated by HA (SHP2) antibody and immunoblotted with the antibodies against Flag (ERα). (C) Immunoprecipitation analysis of the physical interaction of ERα with the different SHP2 mutants. Ishikawa cell lysates (cotransfection with Flag-tagged ERα vectors and HA-tagged wild-type or truncated SHP2 vectors) are subjected to immunoprecipitation by HA antibody, and the immunoprecipitates are then immunoblotted against the Flag antibody. (D) Immunoblot analysis of Shp2 and ER proteins in uterine cellular soluble or insoluble (chromatin) fraction. Whole cell lysate (WCL) was used as control. GAPDH is used as soluble fraction control and histone H3 as the chromatin fraction control. (E) ChIP–qPCR analysis for enrichment of Shp2 and ERα in the Pgr promotor in D1 and D4 uteri. The values are shown as the mean ± SEM, n = 3. **P < 0.01. (F and G) Immunoblot analysis of ER Tyr phosphorylation in anti-ER immunoprecipitates by p-Tyr antibody. The cellular lyses are from the E2-challenged ovariectomized mice (F) or in vitro cultured Ishikawa cells (G). (H) Immunofluorescence staining of total and active form of Src in Ishikawa cells. DAPI was used to stain the nuclear DNA. (Scale bar, 100 μm.) (I) Immunoblot analysis of indicated proteins in anti-HA (SHP2) immunoprecipitates. The cellular lyses for immunoprecipitation are from the Ishikawa cells transfected with the HA-tagged WT or substrate trapping (ST) Shp2 and Flag-tagged ERα. (J) Immunoblot analysis for detecting the phosphorylation level of Src in SHP2 WT or KO Ishikawa cells with or without E2 treatment; β-actin was used as loading control. (K) Immunoblot analysis for detecting phosphorylated Src in WT Ishikawa cells treated with Src inhibitor saracatinib or control DMSO for 2 h before the E2 challenge. (L) Immunoblot analysis of ER Tyr phosphorylation in anti-Flag (ERα) immunoprecipitates following the Src inhibitor treatment. (M) Real-time PCR analysis of E2-induced gene in Ishikawa cells pretreated with Src inhibitor saracatinib. The values are normalized to the GAPDH expression level and shown as the mean ± SEM, n = 4. The different letters (a, b, and c) indicate significant differences between groups (a vs. b and b vs. c: P < 0.01; a vs. c: P < 0.05). Data shown are representative of at least three independent experiments. IP, immunoprecipitation.
Fig. S5.
The phosphatase activity of Shp2 was indispensable for enhancing the ER activity. (A) Schematic diagram of Shp2 mutants. ΔPTP: SHP2 harboring PTP domain deletion; ΔSH2: SHP2 harboring SH2 domain deletions; M1, M2: SHP2 harboring point mutation in PTP domain (phosphatase inactivation). (B) ERE-luciferase reporter assay to evaluate the ERα activation in SHP2 WT and KO cells (transfection with HA-tagged wild-type or mutant SHP2). The values are shown as the mean ± SEM, n = 4. The different letters (a, b, c, and d) indicate significant differences between groups (a vs. b and b vs. d: P < 0.05; a vs. c: P < 0.01).
As a transcription factor, nuclear-located ERα would bind to the DNA of target genes, we wonder whether Shp2 would translocate to the chromatin with ERα. The whole cell lysates were fractionated into chromatin and soluble fractions. As shown in Fig. 6D, Shp2 was detected in the soluble compartment, but not in the chromatin fraction, whereas the ERα was positive both in the soluble and chromatin fractions. The ChIP–qPCR analysis further confirmed the absence of Shp2 in the chromatin fraction. There is no significant enrichment of Shp2 on the Pgr promotor compared with the IgG, whereas ERα indeed can bind within this element (Fig. 6E).
Next, we asked whether the Shp2 could influence the ER protein modification before the ERα recruitment to the DNA, particularly the ERα Tyr phosphorylation that has been reported to regulate the ERα activity (16). Because Shp2 is a protein tyrosine phosphatase, we speculated that Shp2 deficiency would lead to an increased Tyr phosphorylation of ERα in case ERα is a direct substrate of Shp2. By contrast, after E2 challenge, a decreased Tyr phosphorylation level of ERα was observed both in vivo and in vitro in the absence of Shp2 (Fig. 6 F and G and Fig. S6 A and B), implying that ERα is not a direct substrate of Shp2, and there is another upstream kinase for ERα phosphorylation modification.
Fig. S6.
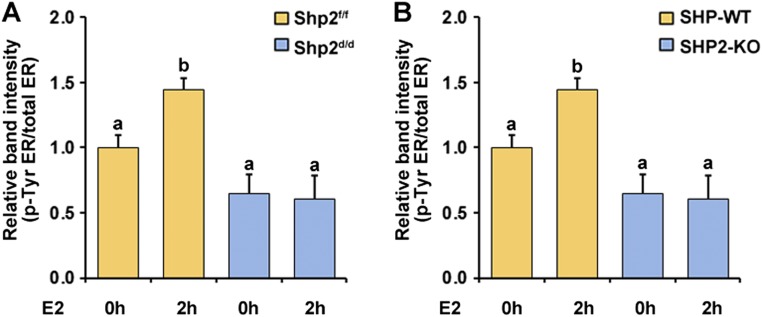
ER Tyr phosphorylation level in response to E2 treatment was reduced in the absence of Shp2. (A) The relative band intensity corresponds to data shown in Fig. 6F. (B) The relative band intensity corresponds to data shown in Fig. 6G. The different letters (a and b) indicate significant differences between groups (a vs. b: P < 0.01).
The phosphorylation of ERα has been reported to influence the ER binding to DNA and interaction with the cofactors such as SRC and P300. Indeed, the ChIP–qPCR analysis revealed that binding of ERα to Pgr promotor was severely dampened in the absence of Shp2 both in D4 uteri and E2-challenged ovariectomized mouse uteri (Fig. S7 A and B). Moreover, even the cofactors SRC1 and P300 were normally expressed in the Shp2 knockout uteri (Fig. S7C), the interaction between ERα and these cofactors was markedly reduced in the Shp2 null uteri (Fig. S7D). These findings suggest that uterine Shp2 deficiency attenuates ERα Tyr phosphorylation, and decreases its recruitment to target genes and interaction with the cofactors, leading to impaired uterine estrogen responsiveness.
Fig. S7.
Shp2 promoted the ERα recruitment to the Pgr promotor and interaction with the cofactors SRC1 and P300. (A) ChIP–qPCR analysis for enrichment of ERα to the promoter region of Pgr in D4 Shp2f/f (f/f) and Shp2d/d (d/d) uteri. **P < 0.01, n = 7. (B) ChIP–qPCR analysis for enrichment of ERα to the promoter region of Pgr in Shp2f/f and Shp2d/d uteri in response to E2 challenge. *P < 0.05; **P < 0.01, n = 5. (C) Immunohistochemistry analysis of ERα coactivators SRC1 and P300 in D4 Shp2f/f and Shp2d/d uteri. (Scale bars, 100 μm.) Ge, glandular epithelium; Le, luminal epithelium; S, stroma. (D) Immunoprecipitation analysis for the association of cofactors SRC1 and P300 with ERα in D4 uteri. IB, immune blot; IP, immunoprecipitation.
Because Src kinase was reported to influence the ERα Tyr phosphorylation, we thus examined the expression of Src and its activated phosphorylated form (p-Y416). As shown in Fig. 6H, the protein exhibited both the cytoplasmic and nuclear localization. Previous studies suggest that Shp2 promotes c-Src activation by directly or indirectly controlling phosphorylation of C-terminal inhibitory phosphorylation site Tyr527. We then used the substrate trapping strategy to define whether the Src kinase is a direct substrate of Shp2. Unlike ERα, the Src kinase preferentially interacted with the trapping mutant Shp2, suggesting the Src rather than ERα is a potential substrate of SHP2 phosphatase (Fig. 6I). Moreover, the inhibitory Y527 phosphorylation form of Src was more prominent in the SHP2 knockout cells (Fig. 6J).
We then analyzed the consequence of Src kinase inhibition on ER activation. As shown in Fig. 6K, pretreatment with SRC inhibitor saracatinib abolished estrogen-evoked Src activation, as demonstrated by decreased level of pY416 and abnormal high level of inhibitory Y527 phosphorylation. This inhibitor pretreatment also substantially impaired the ERα Tyr phosphorylation (Fig. 6L). Moreover, Src kinase inhibition also abolished the E2-induced target gene expression (Fig. 6M), faithfully mimicking the Shp2 knockout phenotype. Together, these data support the notion that Shp2 promotes the ERα transcriptional activity by enhancing the Src kinase-mediated ERα tyrosine phosphorylation.
Discussion
In our study, we observed a dramatically decreased PR expression in the stroma in the absence of uterine Shp2, associated with attenuated stromal growth and aberrant epithelial overproliferation, indicating that Shp2 is essential for normal stromal PR expression and epithelial–stromal interactions during uterine transformation for receptivity. This observation is consistent with previous observations that insufficient PR signaling hampers the intimate stromal–epithelial crosstalk and thus uterine receptivity (17, 18). However, it was a meandering path for us to realize and confirm that this functional progesterone resistance in the absence of uterine Shp2 is seeded by the significantly decreased level of stromal PR protein, which results from an impaired estrogen–ERα activity in stroma. It is also worthy to note that the epithelial estrogen–ER signaling seems normal, which is consistent with the low expression level of Shp2 in epithelium. This finding suggests a cell context-specific function of Shp2 in ensuring the ERα activity and/or the disappearance of stromal PR antagonistic influence. Meanwhile, there is no apparent decrease of epithelial PR expression in Shp2d/d uteri at periimplantation, confirming the hampered ER activity in the absence of Shp2, because it is the stroma estrogen–ERα signaling that suppresses the epithelial PR expression (19).
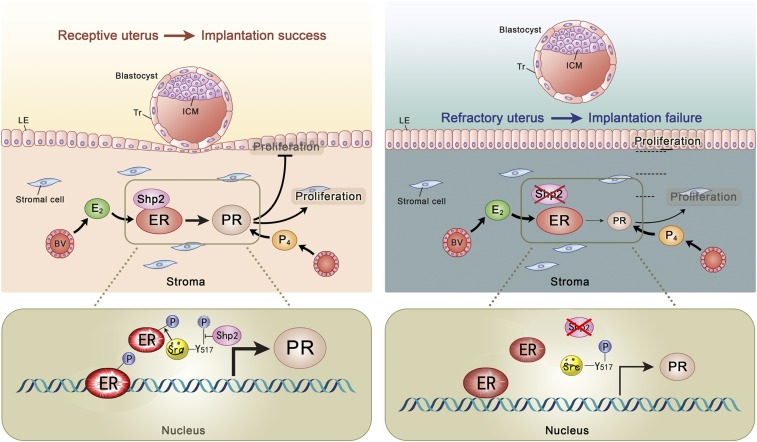
In searching for the underlying molecular mechanisms, we further demonstrate that Shp2 in the nucleus can enhance the Src kinase-mediated ERα tyrosine phosphorylation, facilitate ERα binding to target gene promoter, and thus promote the ERα transcription activity in periimplantation uteri (Fig. S8). In fact, there was evidence showing that Shp2 can translocate into the nucleus to participate in DNA damage-induced apoptotic responses (20, 21), forming a complex with STAT5 for regulation of target gene transcription (22) or interacting with telomerase reverse transcriptase involved in replicative senescence (23). Most recent studies also showed that nuclear Shp2 can dephosphorylate parafibromin influencing Wnt targeting-gene activation (24, 25). In our study, we found that Shp2 mainly functions in the nucleus in an enzyme activity-dependent way and interacts with the ERα mainly through SH2 domain. However, this interaction is not equivalent to enzyme–substrate interaction and therefore does not necessarily suggest that ER is a substrate candidate of Shp2. Indeed, the Shp2 enhances the Tyr phosphorylation of ER by facilitating the Src kinase activity through dephosphorylation of an inhibitory Tyr in Src kinase. Whereas the Src kinase is broadly recognized as a cytoplasmic protein, recent reports also observed the nuclear function of this kinase that was activated by Shp2 through dephosphorization of the inhibitory p-Tyr site for DNA damage response (26).
Fig. S8.
Illustrative model of uterine Shp2 function during uterine receptivity establishment for embryo implantation. In a normal situation, the process of uterine receptivity is dominated by tightly regulated proliferation and differentiation of uterine epithelial and stromal cells in response to ovarian progesterone (P4) and estradiol-17β (E2). Upon E2 stimulation, nuclear Shp2 physically interacts with ERα, facilitating ERα Tyr phosphorylation by Src kinase activation through dephosphorylation of the inhibitory p-Tyr-527 site on Src kinase, and promoting ERα binding to its target genes, and enhancing target gene transcription. The Shp2–ERα signaling directs normal PR expression in uterine stroma to ensure normal uterine receptivity, thus successful embryo implantation. In uteri harboring a specific deletion of Shp2, the ERα Tyr phosphorylation was dampened as the Src kinase activity was inhibited by high-level p-Tyr-527; thus, binding activity of ERα on its target gene is decreased. Eventually, Shp2 deficiency leads to a reduced Pgr expression, resulting in aberrant uterine receptivity and embryo implantation failure. BV, blood vessel; ICM, inner cell mass; LE, luminal epithelium; Tr, trophoectoderm.
Nonetheless, besides uncovering a function and mechanism of nuclear Shp2 in ensuring normal ERα transcriptional activation conducive to uterine receptivity and implantation, our findings have high clinical significance, because dysfunctional ERα activity is often associated with multiple endometrial disorders.
Materials and Methods
Animals and Treatments.
Shp2flox/flox mice were generated as previously described (27). Uterine-specific knockout mice were generated by crossing Shp2flox/flox mice with PgrCre/+ mice. All mice were housed in the animal care facility of Xiamen University, in accordance with the guidelines for the care and use of laboratory animals. Experimental procedures to treat mice and analyze implantation sites are provided in SI Materials and Methods.
In Situ Hybridization.
In situ hybridization was performed as previously described (28). Sections hybridized with the sense probes served as negative controls. Mouse-specific cRNA probes for Ptpn11, Ltf, Muc1, Areg, Pgr, and Igf1 were used for hybridization.
Immunostaining.
Immunohistochemistry and immunofluorescence analysis were performed as described previously (29).
Western Blot.
Protein extraction and Western blot analysis were performed as described previously (28). Insoluble chromatin fraction and soluble fraction were extracted according to the previous methods (30). Uncropped images of immunoblots are provided in Fig. S9.
Fig. S9.
Uncropped images of immunoblots experiments. Uncropped photos of images for all immunoblotting experiments are shown. IB, immune blot; IP, immunoprecipitation; WCL, whole cell lysis.
Real-Time RT-PCR Analysis.
Quantitative RT-PCR was performed as described (31). All primers for real-time PCR are listed in Table S1. All real-time PCR experiments were repeated at least three times.
Table S1.
Primers for real‐time PCR
| Genes | Primers |
| IGF1-HOMO-F | 5′-TGATTACACCTACAGTGAAGA-3′ |
| IGF1-HOMO-R | 5′-GTGGGCTTGTTGAAATAAAAG-3′ |
| PGR-HOMO-F | 5′-TGGACAAGGAGACAAGTAAT-3′ |
| PGR-HOMO-R | 5′-TTAGTGAAGTAAGGATAAGCAAT-3′ |
| GAPDH-HOMO-F | 5′-GTCGCCAGCCGAGCCACATC-3′ |
| GAPDH-HOMO-R | 5′-CCAGGCGCCCAATACGACCA-3′ |
| Nr2f2-MUS-F | 5′-CTTTGGAAGAGTACGTTAGG-3′ |
| Nr2f2-MUS-R | 5′-AACAATTGCTCTATGACTGA-3′ |
| Pgr-MUS-F | 5′-ACCTGATCTAATCCTAAATGA-3′ |
| Pgr-MUS-R | 5′-ATTGTGTTAAGAAGTAGTAAGAC-3′ |
| Hand2-MUS-F | 5′-TCGGTTATCTAGTGCTGTC-3′ |
| Hand2-MUS-R | 5′-ATACTTACAATGTTTACACCTTCA-3′ |
| Igf1-MUS-F | 5′-CTTGAAGATAAAGATACACATCA-3′ |
| Igf1-MUS-R | 5′-TGGGCTTGTTGAAGTAAA-3′ |
| Muc1-MUS-F | 5′-AGCCCCTATGAGGAGGTTTCG-3′ |
| Muc1-MUS-R | 5′-AAGTGGTCACCACAGCTGGG-3′ |
| Ltf-MUS-F | 5′-GGGCAAGTGCGGTTTAGTT-3′ |
| Ltf-MUS-R | 5′-CCATTGCTTTGGAGGATTT-3′ |
| Areg-MUS-F | 5′-GACAAGAAAATGGGACTGTGC-3′ |
| Areg-MUS-R | 5′-GGCTTGGCAATGATTCAACT-3′ |
| Gapdh-MUS-F | 5′-TGGCAAAGTGGAGATTGTTGCC-3′ |
| Gapdh-MUS-R | 5′-AAGATGGTGATGGGCTTCCCG-3′ |
| Shp2-MUS-F | 5′-CTGAAAGAGAAGAATGGAGATG-3′ |
| Shp2-MUS-R | 5′-CTTTTCCAGACAAGTGACC-3′ |
Generation of SHP2 Knockout Human Ishikawa Cell Line.
Ishikawa cells were maintained at 37 °C in an atmosphere of 5% CO2/95% air in DMEM supplemented with 10% (vol/vol) FBS. SHP2-deficient Ishikawa cells were generated by CRISPR/Cas9-mediated genome engineering.
Coimmunoprecipitation and Chromatin Immunoprecipitation.
Coimmunoprecipitation (co-IP) and chromatin immunoprecipitation (ChIP) analysis were performed as previously described (31). Immunoprecipitated proteins were separated by SDS/PAGE and detected by immunoblotting using the respective antibodies. Specific primers were used to detect immunoprecipitated chromatin fragments, as well as input chromatin (Table S1).
Statistical Analysis.
All data are presented as mean ± SEM. Each experiment included at least three independent samples. Comparison between two groups was made by unpaired Student’s two-tailed t test, and comparisons among three groups or more were made by one-way ANOVA test. P < 0.05 was considered to indicate a significant result.
SI Materials and Methods
Animals and Treatments.
Female mice were mated with fertile WT males to induce pregnancy. Implantation sites were visualized by an i.v. injection of Chicago blue dye (1%). Mice failing to recover any embryos were excluded in statistical analysis. For P4 treatment, each mouse received a daily s.c. injection of 2 mg in 0.1 mL of sesame oil at 10 AM starting on D3 until mice were killed for analysis. For RU486 treatment, each mouse was intraperitoneally injected with 100 mg in 0.1 mL of sesame oil at 10 AM on D3. Mouse blood samples were collected on D4 morning. Serum steroid hormone levels were measured by radioimmunoassay.
Immunostaining.
Tissue sections from control and experimental groups were processed onto the same slide for immunohistochemistry and immunofluorescence to detect the expression of Shp2 (Santa Cruz Biotechnology, sc-280), CK (DAKO, Z0622), Ki-67 (Epitomics, 4203-1), ERα (DAKO M7047), PR (DAKO, A0098), ErbB1 (Epitomics, 1902-1), Src (CST, 2123), p-Src (Y416, CST 6943). The numbers of Ki-67 positive cells in epithelium and stroma were counted, respectively, to estimate the ratio of positive cells in the total numbers of epithelial and stromal cells in each section.
Western Blot.
Cytoplasmic and nuclear proteins were extracted using a nuclear extraction kit according to the manufacturer’s instructions (Millipore). Insoluble chromatin fraction and soluble fraction were extracted according to previous methods. The antibodies used were the same as the immunostaining and IP experiment. p-Src (Y527) were purchased from the CST (Cell Signaling Technology, 2105). Immunoblot images were captured by the Bio-Rad ChemiDoc XRS System, and all uncropped images of immunoblots are provided in Fig. S9.
Ishikawa Cell Culture, Plasmid Transfection, Luciferase Assay, and SHP2 Knockout Cell Generation.
Ishikawa cells were maintained at 37 °C in an atmosphere of 5% CO2/95% air in DMEM supplemented with 10% (vol/vol) FBS. Cells were plated in six-well plates and transfected with plasmid DNA using Lipofectamine 2000 (Life Technologies) the next day at a cell confluence of about 80%. Treatment with 0.1% (vol/vol) DMSO (vehicle) or 100 nM E2 in medium was performed 12 h after transfection and cells were collected after another 12 h for RNA and protein extraction. For luciferase assay, Ishikawa cells were kept in DMEM plus 10% FBS. The cDNA-derived ERα and deletion or full-length SHP2 proteins were cloned into pFlag-CMV-3 (Sigma) and pCMV-HA-N (Clontech) vector, respectively. All constructs were transiently transfected into Ishikawa cells using Lipofectamine 2000. pRL-TK, internal control plasmid expressing Renilla (Promega), was cotransfected into the cells to normalize firefly luciferase activity of the reporter plasmids. Cells were collected 48 h after transfection and luciferase activities were measured using the Dual-Luciferase Reporter Assay System. Assays were performed at least three times with each in duplicate.
To generate SHP2 knockout human Ishikawa cell line, a target sequence in the second exon of human SHP2 (GTGCGCACTGGTGATGACAAAGG) was designed at the website crispr.mit.edu. The oligonucleotides were cloned into the BbsI site of pSpCas9 (BB)-2A-GFP (PX458) (Addgene). Ishikawa cells were transfected with the SHP2 targeting PX458 vector using Lipofectamine 2000. Twenty-four hours posttransfection, GFP-positive cells were sorted by FACS and cultured at limiting dilution to pick individual colonies. The absence of SHP2 was identified by DNA sequencing and Western blot detection.
Co-IP.
Protein samples (1 mg) from uterine tissues or Ishikawa cells lysated by mild buffer (25 mM Tris, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 5% glycerol; pH 7.4) were used for co-IP experiments. The strong buffer (25 mM Tris, 150 mM NaCl, 1 mM EDTA, 0.1% SDS; pH 7.4) was used for the ERα p-Tyr detection. Antibodies to ERα (1:1,000; Santa Cruz Biotechnology), Shp2 (1:1,000; Santa Cruz Biotechnology), Flag tag (1:2,000, Sigma) and HA tag (1:2,000, Abclonal) were used. Protein A agarose beads (Thermo) were washed and incubated with protein lysates overnight at 4 °C. Immunoprecipitated proteins were separated by SDS/PAGE and detected by immunoblotting using the respective antibodies.
ChIP.
ChIP analysis was performed according to the instructions of the ChIP Assay Kit (Millipore). In brief, uteri were cut into small pieces and then suspended in 1% formaldehyde (Sigma)-PBS solution for cross-linking. After the cross-linking was terminated, cell pellets were lysed and sheared by sonication until the average length of DNA was ∼500 bp as evaluated by agarose gel electrophoresis. The sheared chromatin fragments were incubated with the indicated antibodies overnight at 4 °C. Normal rabbit IgG (Santa Cruz Biotechnology) was used as a negative control for nonspecific immunoprecipitation. After washing, the beads were suspended in elution buffer and the precipitated protein/DNA complexes were eluted from the antibodies/beads. Then the chromatin was subjected to cross-link reversal and DNA was purified by phenol/chloroform extraction and ethanol precipitation. Specific primers were used to detect immunoprecipitated chromatin fragments, as well as input chromatin (Table S1).
Acknowledgments
We thank Xiaofang Tang for her assistance in immunoblot analysis. This work was supported in parts by the National Natural Science Foundation (81130009, 81330017, and 81490744 to H.W.; 81601285 to S.K.; and 31471106 to S.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700978114/-/DCSupplemental.
References
- 1.Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–1408. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 2.Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12:731–746. doi: 10.1093/humupd/dml004. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Dey SK. Roadmap to embryo implantation: Clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 4.Xu J, et al. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 5.Suen CS, et al. A transcriptional coactivator, steroid receptor coactivator-3, selectively augments steroid receptor transcriptional activity. J Biol Chem. 1998;273:27645–27653. doi: 10.1074/jbc.273.42.27645. [DOI] [PubMed] [Google Scholar]
- 6.Xu J, et al. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci USA. 2000;97:6379–6384. doi: 10.1073/pnas.120166297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawagoe J, et al. Nuclear receptor coactivator-6 attenuates uterine estrogen sensitivity to permit embryo implantation. Dev Cell. 2012;23:858–865. doi: 10.1016/j.devcel.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohi MG, Neel BG. The role of Shp2 (PTPN11) in cancer. Curr Opin Genet Dev. 2007;17:23–30. doi: 10.1016/j.gde.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Grossmann KS, Rosário M, Birchmeier C, Birchmeier W. The tyrosine phosphatase Shp2 in development and cancer. Adv Cancer Res. 2010;106:53–89. doi: 10.1016/S0065-230X(10)06002-1. [DOI] [PubMed] [Google Scholar]
- 10.Yang W, et al. An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival. Dev Cell. 2006;10:317–327. doi: 10.1016/j.devcel.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Stewart CL, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 12.Robb L, et al. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med. 1998;4:303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- 13.Xie H, et al. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci USA. 2007;104:18315–18320. doi: 10.1073/pnas.0707909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Large MJ, et al. The epidermal growth factor receptor critically regulates endometrial function during early pregnancy. PLoS Genet. 2014;10:e1004451. doi: 10.1371/journal.pgen.1004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li B, et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature. 2014;513:251–255. doi: 10.1038/nature13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J, Zhou W, Kaliappan K, Nawaz Z, Slingerland JM. ERα phosphorylation at Y537 by Src triggers E6-AP-ERα binding, ERα ubiquitylation, promoter occupancy, and target gene expression. Mol Endocrinol. 2012;26:1567–1577. doi: 10.1210/me.2012-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee K, et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38:1204–1209. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- 18.Tranguch S, et al. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci USA. 2005;102:14326–14331. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurita T, et al. Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod. 2000;62:821–830. doi: 10.1093/biolreprod/62.4.821. [DOI] [PubMed] [Google Scholar]
- 20.Yuan L, Yu WM, Yuan Z, Haudenschild CC, Qu CK. Role of SHP-2 tyrosine phosphatase in the DNA damage-induced cell death response. J Biol Chem. 2003;278:15208–15216. doi: 10.1074/jbc.M211327200. [DOI] [PubMed] [Google Scholar]
- 21.Yuan L, Yu WM, Xu M, Qu CK. SHP-2 phosphatase regulates DNA damage-induced apoptosis and G2/M arrest in catalytically dependent and independent manners, respectively. J Biol Chem. 2005;280:42701–42706. doi: 10.1074/jbc.M506768200. [DOI] [PubMed] [Google Scholar]
- 22.Chughtai N, Schimchowitsch S, Lebrun JJ, Ali S. Prolactin induces SHP-2 association with Stat5, nuclear translocation, and binding to the beta-casein gene promoter in mammary cells. J Biol Chem. 2002;277:31107–31114. doi: 10.1074/jbc.M200156200. [DOI] [PubMed] [Google Scholar]
- 23.Jakob S, et al. Nuclear protein tyrosine phosphatase Shp-2 is one important negative regulator of nuclear export of telomerase reverse transcriptase. J Biol Chem. 2008;283:33155–33161. doi: 10.1074/jbc.M805138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi A, et al. SHP2 tyrosine phosphatase converts parafibromin/Cdc73 from a tumor suppressor to an oncogenic driver. Mol Cell. 2011;43:45–56. doi: 10.1016/j.molcel.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsutsumi R, et al. YAP and TAZ, Hippo signaling targets, act as a rheostat for nuclear SHP2 function. Dev Cell. 2013;26:658–665. doi: 10.1016/j.devcel.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, et al. Gain-of-function mutations of Ptpn11 (Shp2) cause aberrant mitosis and increase susceptibility to DNA damage-induced malignancies. Proc Natl Acad Sci USA. 2016;113:984–989. doi: 10.1073/pnas.1508535113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang EE, Chapeau E, Hagihara K, Feng GS. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc Natl Acad Sci USA. 2004;101:16064–16069. doi: 10.1073/pnas.0405041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, et al. Wnt6 is essential for stromal cell proliferation during decidualization in mice. Biol Reprod. 2013;88:5. doi: 10.1095/biolreprod.112.104687. [DOI] [PubMed] [Google Scholar]
- 29.Lu J, et al. A positive feedback loop involving Gcm1 and Fzd5 directs chorionic branching morphogenesis in the placenta. PLoS Biol. 2013;11:e1001536. doi: 10.1371/journal.pbio.1001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu L, Li L, Zhou B, Qin Z, Dou Y. H2B ubiquitylation promotes RNA Pol II processivity via PAF1 and pTEFb. Mol Cell. 2014;54:920–931. doi: 10.1016/j.molcel.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S, et al. Uterine Rbpj is required for embryonic-uterine orientation and decidual remodeling via Notch pathway-independent and -dependent mechanisms. Cell Res. 2014;24:925–942. doi: 10.1038/cr.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]