Significance
Cytotoxic therapy is still the backbone of effective chemotherapy, although most current pharmaceutical interest is in targeted therapy. Our findings concern a general adaptive response of cells that causes resistance to gemcitabine and 15 other FDA-approved cytotoxic drugs when cells are grown at high density. Although on the surface cell confluence seems like it could be an irrelevant property of cells in culture, our work shows that it is very relevant to tumors in mice and retrospectively to the success of chemotherapy in humans. On a fundamental cell biological level, these studies identify a previously unappreciated function of the enigmatic Hippo pathway, which controls this response. “Switching-off” this pathway could present an opportunity to overcome drug resistance in pancreatic cancer.
Keywords: drug resistance, Hippo pathway, gemcitabine, cell density, cancer
Abstract
Chemotherapy is widely used for cancer treatment, but its effectiveness is limited by drug resistance. Here, we report a mechanism by which cell density activates the Hippo pathway, which in turn inactivates YAP, leading to changes in the regulation of genes that control the intracellular concentrations of gemcitabine and several other US Food and Drug Administration (FDA)-approved oncology drugs. Hippo inactivation sensitizes a diverse panel of cell lines and human tumors to gemcitabine in 3D spheroid, mouse xenografts, and patient-derived xenograft models. Nuclear YAP enhances gemcitabine effectiveness by down-regulating multidrug transporters as well by converting gemcitabine to a less active form, both leading to its increased intracellular availability. Cancer cell lines carrying genetic aberrations that impair the Hippo signaling pathway showed heightened sensitivity to gemcitabine. These findings suggest that “switching off” of the Hippo–YAP pathway could help to prevent or reverse resistance to some cancer therapies.
Despite the recent excitement surrounding targeted therapy, cytotoxic chemotherapy remains the bedrock of cancer treatment. Ultimately, the efficacy of cytotoxic therapy, like targeted therapy, is limited by drug resistance. Many studies have focused on genetic mechanisms, both intrinsic and acquired, that confer resistance to chemotherapy, as well as targeted therapy. Acquired resistance can occur by genetic mutation during treatment or by selection of preexisting genetic variants in the population. Adaptive (nongenetic or regulatory) responses, such as increased expression of the therapeutic target or activation of compensatory pathways, can also influence drug efficacy over time (1). Despite the widespread prevalence of tumor resistance, which in many cases may be due to drug resistance, many oncologists have noted occasional dramatic responses in patients, whom they referred to informally as “exceptional responders” (2). However, despite the many potential biomarkers and our increasingly sophisticated understanding of the molecular phenotype of the tumor cell, we cannot predict exceptional responders. Instead, clinical regimens are still based largely on prognostic clinicopathological parameters, such as tumor size, presence of lymph node metastases, and histological grade (3). This state of affairs has produced a growing conviction that the study of drug response and, in particular, the exceptional responders, could lead to improvements based on personalizing the choice of targeted and perhaps even cytotoxic chemotherapies.
We began our study of resistance with the nucleoside analog, gemcitabine, the first-line treatment for locally advanced and metastatic pancreatic cancer (4). Regrettably, most pancreatic ductal carcinoma (PDAC) patients treated with gemcitabine do not respond well to treatment. The 1- and 5-y survival rates for pancreatic cancers are about 10% and 4.6%, respectively, which are the lowest survival rates of all major cancers (4, 5). In trying to understand the resistance to gemcitabine and the variable response of patients, we unexpectedly found culture conditions for pancreatic tumor cells that affected their sensitivity to the drug. In each of 15 pancreatic cancer cell lines that we tested, resistance to gemcitabine very strongly depended on cell density. Each cell line was resistant at high density, but each was immediately sensitive when replated at low density, indicating that the resistance was not due to a preexisting or acquired genetic alteration, and this led us to describe a physiological means of drug resistance. The basis for this resistance turns out to be the activation of the Yes-associated protein (YAP) pathway, and this occurs by means of the down-regulation of several multidrug transporters and cytidine deaminase (CDA) (a key enzyme that metabolizes gemcitabine following its uptake). Overall, these findings highlight a cell-physiologic mechanism of drug resistance. “Switching off” the Hippo signaling pathway and thus activating YAP could present a strategy to overcome drug resistance in pancreatic cancer and other cancers.
Results
In trying to profile pathways for drug resistance, we unexpectedly stumbled over a large inconsistency in the published studies of the cellular response to gemcitabine (SI Appendix, Table S1). The same pancreatic cancer cell line had been reported as sensitive or resistant in different publications; this was true to differing degrees for 15 cell lines with varying genetic backgrounds. Furthermore, there was little consensus among published large scale Cancer Genome Project studies that measured the effects of gemcitabine on a large panel of genomically annotated cancer cell lines (6, 7). Because varying assay conditions such as the duration, the method of detection, and the density of seeding were used in these previous studies, we opted to repeat these studies using a real-time (kinetic) cell growth assay.
Cell–Cell Contact-Dependent Response to Gemcitabine in Pancreatic Cancer.
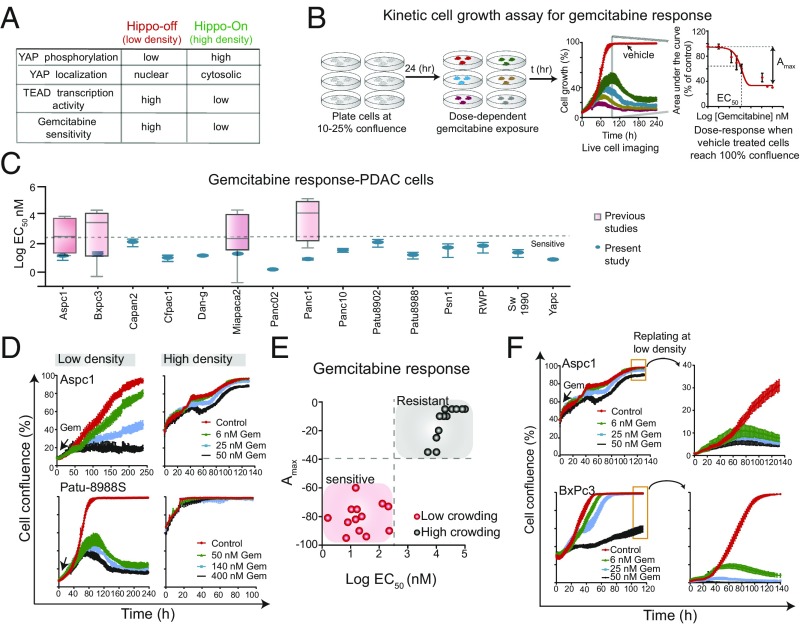
We used a cell growth assay (Fig. 1B) to determine the effects of gemcitabine on a panel of pancreatic cancer cell lines. Cells were plated at low densities (10–25% confluence) and 24 h later exposed to gemcitabine in a dose-dependent manner. They were imaged every 1–3 h until control (vehicle)-treated cells reached 100% confluence. This assay is not confounded by the fact that the time required for each cell line to reach 100% confluence may be very different (as the cell lines have different doubling times). The dose–response effect of gemcitabine on cell growth for 15 pancreatic cancer cell lines is shown in Fig. 1C and SI Appendix, Fig. S1 and Table S2, where the range of previous studies is also shown. In our experiments, all cell lines tested under these conditions were sensitive to gemcitabine (EC50 < 200 nM) (Fig. 1C and SI Appendix, Fig. S1). We found similar responses to gemcitabine in liver cancer cell lines (Huh7 and FOCUS) and untransformed (HEK293) cell lines (SI Appendix, Fig. S1).
Fig. 1.
Cell density-dependent response to gemcitabine in pancreatic cancer. (A) A table illustrating YAP nuclear localization and TEAD transcriptional activity in low- and high-cell density conditions. (B) A schematic showing live-cell kinetic cell growth assay used to characterize the phenotypic effect of gemcitabine in a panel of pancreatic cancer cell lines. Gemcitabine-mediated GC50 (50% inhibition in growth compared with control) for each cell line was calculated. (C) A plot showing the effect on gemcitabine on growth of 15 pancreatic cancer cell lines. Literature-curated values of cell line-specific GC50 are also indicated. See SI Appendix, Table S1 for details. (D) Cell density affects gemcitabine response. Plots show cell growth curves of Aspc1 (Top) and Patu-8988S (Bottom) cells grown in low- or high-density conditions. (E) All cell lines tested were sensitive or resistant to gemcitabine in low- or high-density conditions, respectively. (F) Replating cells at low density restored sensitive to gemcitabine.
In the course of these experiments, we inadvertently found that cells grown in more crowded/dense conditions (40–60% confluence) were much less sensitive to gemcitabine, relative to cells grown in less crowded/dense conditions (10–25% confluence) (Fig. 1D). Every PDAC cell line showed this effect. This was reflected in the EC50 as well as the Amax, as shown in Fig. 1E, which demonstrates the striking disparity of sensitivities at high and low densities. Furthermore, replating cells at low density restored sensitivity to gemcitabine (Fig. 1F).
The in vitro crowding conditions had no obvious relevance to the growth conditions in human tumors. Nevertheless, we were curious how extrinsic factors, such as cell density, could so dramatically affect drug sensitivity. One possible explanation was depletion of the culture medium. Changing culture medium or addition of insulin or fresh serum has been shown to stimulate macromolecular synthesis and cell division in postconfluent cultures (8–10). Replenishing fresh medium, containing serum or supplemented with 15 different growth factors, including EGF, FGF, IGF, HGF, PDGF, Wnt3a, Wnt5a, TGFβ, and IL 6, did not increase the sensitivity of insensitive cells at high-density conditions to gemcitabine (SI Appendix, Fig. S1). However, these growth factors had activated their cognate downstream signaling proteins even in the high crowding conditions (SI Appendix, Fig. S1). For example, stimulation by IL-6 led to phosphorylation of Stat3, whereas stimulation with HGF and EGF caused increased phosphorylation of ERK, MEK, and S6 proteins (SI Appendix, Fig. S1). Increased Mg2+ concentrations, which have also been shown to play a role in modulating protein and DNA synthesis and cell proliferation in cultured cells (11), also did not increase susceptibility to gemcitabine. Although supplemental Mg2+ can cause a marginal increase in the growth, it had no effect on gemcitabine sensitivity in Bxpc3, Aspc1, and Panc10.05 cells (SI Appendix, Fig. S2). Conditioned medium from dermal fibroblasts has recently been shown to cause gemcitabine resistance in colorectal and pancreatic cancer cells, implying that changes in the tumor microenvironment could alter drug resistance (12). However, exposure of pancreatic cancer cells to the conditioned media of human dermal fibroblast, vascular endothelial cells, or other mesenchymal cancer cells (Panc1) had no effect on gemcitabine response in Bxpc3 and Panc02.13 cells (SI Appendix, Fig. S2). Finally, coculture of sparse GFP-labeled Panc02.13 cells with fibroblast or other cancer cells as a way of achieving high overall cell density produced the same resistance to gemcitabine found in dense tumor cell culture (SI Appendix, Fig. S2). These data suggest that a wide variety of extrinsic cell growth conditions do not affect the sensitivity of pancreatic cancer cells to gemcitabine in crowded conditions.
It was suggested to us that pancreatic cancer cells might have become temporarily resistant to apoptosis in high-density growth conditions. We find that there is no change in the protein levels of 29 apoptotic signaling proteins, including Bad, Bax, and Bcl2, in response to crowding conditions (SI Appendix, Fig. S2). Furthermore, Panc02.13 cells exposed to UV radiation in crowded conditions underwent apoptosis as assessed by cleaved caspase 3 and 7, and PARP levels (SI Appendix, Fig. S2), suggesting that crowded cells are not intrinsically resistant to apoptosis. Finally, replating Aspc1 and Bxpc3 cells at low density (using the original growth medium containing gemcitabine) immediately reestablished their sensitivity (Fig. 1E), further suggesting that the gemcitabine response in pancreatic cancer cells is a function of cell density and not dependent on extrinsic cell culture conditions.
To establish whether the effect of density is related to some very special characteristic of gemcitabine’s mechanism of action, we examined the effect of cell crowding on a set of seven diverse cytotoxic drugs. We initially tested the sensitivity of seven PDAC cell lines grown at varying density conditions to these seven cytotoxic drugs, commonly used in chemotherapy. The cellular response to both gemcitabine and doxorubicin (a topoisomerase II inhibitor) was dependent on cell density (using a >100-fold difference in EC50 as the threshold), whereas the response to camptothecin, paclitaxel, docetaxel (taxane), and oxaliplatin (platinum) showed weak or no correlation with cell density (SI Appendix, Fig. S3). That several cytotoxic inhibitors such as taxanes were equally sensitive in low- or high-crowding conditions further corroborates our conclusion that cells in high-crowding conditions are susceptible to apoptosis (SI Appendix, Fig. S3). Overall, these data suggest that the cellular response of pancreatic cancer cells to cytotoxic drugs, such as gemcitabine, is greatly influenced by cell–cell interactions and that this property is shared by some but certainly not by all cytotoxic drugs. It further shows that the affect is drug specific, because responses to most antiproliferative drugs are unaffected by density.
The Hippo–YAP Pathway Controls Sensitivity to Gemcitabine.
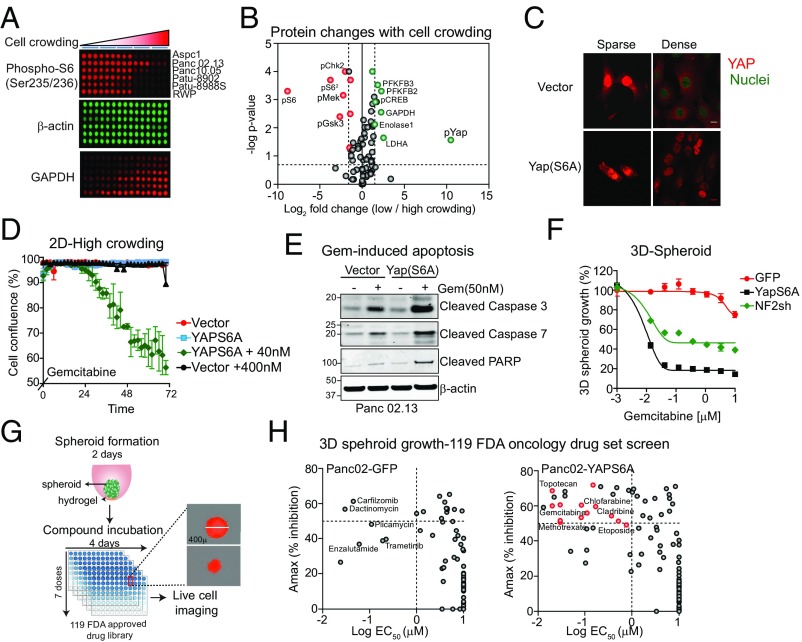
To identify signaling pathways that might mediate the density-dependent responses to gemcitabine, we used reverse-phase protein arrays to measure 75 signaling proteins in a panel of six pancreatic cancer cell lines grown in various crowding conditions (Fig. 2A). As expected, when cell growth is slowed down at high-cell density, the activities of many growth factor signaling proteins such as Erk, Akt, and S6 ribosomal proteins are down-regulated (Fig. 2 A and B and SI Appendix, Fig. S3). More interestingly, we observed (>10-fold) an increase in phosphorylation of YAP at elevated cell density (Fig. 2B), which was confirmed by Western blotting in several PDAC cell lines (SI Appendix, Fig. S3). We also saw smaller but highly significant increases in the levels of glycolytic enzymes, a significant response that remains unexplained. YAP is a potent transcriptional coactivator that functions via binding to the TEAD transcription factor in the Hippo pathway (Fig. 1A); it plays a critical role in the control of organ size and in tumorigenesis (13, 14). Pathway activation inactivates the YAP protein through its phosphorylation by upstream kinases, such as the LATS kinases. Phosphorylation causes YAP to be excluded from the nucleus and be retained or degraded in the cytoplasm, where it can no longer activate transcription (15). YAP phosphorylation and localization was already known to be controlled by cell density (16). In agreement with these observations, we observed crowding-dependent nuclear localization of YAP in pancreatic cancer cells, that is, nuclear localization was only found in cells at low confluence (Fig. 2C).
Fig. 2.
YAP activation sensitizes pancreatic cancer cells to cytotoxic drugs. (A) Proteomic changes in six pancreatic cancer cell lines grown in five different crowding conditions was performed using reverse-phase protein arrays. Representative images show levels of phosho-S6, β-actin, and GAPDH. (B) A plot showing changes in protein levels or phosphorylation that occur in Aspc1 cells grown under low- or high-crowding conditions. Many growth factor signaling proteins such as Erk, Akt, and S6 ribosomal proteins are down-regulated when cells are grown in dense cultures (shown in red). Increase in phosphorylation of YAP in density-dependent manner is also observed (shown in green). (C) Confocal images showing expression of YAP in Panc02.13 cells expressing vector only or YAPS6A expression constructs under sparse or dense cultures. (D) Suppressing Hippo pathway by expression of nonphospho, active form of YAP (YAPS6A) sensitizes pancreatic cancer cells to gemcitabine. A plot showing the effect of gemcitabine on the growth of Panc02.13 cells expressing vector only or YAPS6A construct grown at high cell density. (E) Western blots showing expression of YAPS6A sensitizes cells to gemcitabine and activates apoptosis. Apoptosis was measured by immunoblotting with cleaved caspases 3/7 or PARP. Blots were also stained with anti–β-actin for loading control. (F) Suppressing Hippo pathway by expression of YAPS6A or knockdown of NF2 sensitizes pancreatic cancer cells to gemcitabine 3D spheroid culture. A dose–response curve of gemcitabine treated Panc02.13 cells expressing GFP vector, YAPS6A plasmid, or NF2shRNA grown as 3D spheroid. (G) A schematic showing 3D spheroid assay used for chemical screening. Cells were grown in round-bottom plates for 2 d to form spheroid of ∼400 μm, followed by dose-dependent drug treatment and live-cell imaging for 4 d. A dose–response curve is then used to determine the effect of each drug on spheroid growth. (H) A total of 119 FDA-approved oncology drugs was tested in pancreatic cancer cells using 3D spheroid growth assays. (Left) A plot showing most of the drugs are ineffective in Panc02.13 GFP-expressing cells with EC50 > 1 μM. Some of the drugs that blocked spheroid growth in parental Panc02.13 cells are indicated. (Right) YapS6A expressing Panc02.13 are sensitive to 15 additional drugs, which include antimetabolites, anthracyclines, topoisomerase inhibitors, and kinase inhibitors (indicated in red). Inhibitors that affected both GFP- and YAPS6A-expressing cells are indicated in gray.
Although there is increasing evidence for a role of the Hippo pathway in cell proliferation, the observed effects here, particularly at high density, when cells are resistant to gemcitabine, is a previously uncharacterized feature of this pathway. Although knockdown of YAP in three different pancreatic cancer cell lines mildly depressed proliferation (SI Appendix, Fig. S3), it had no effect on the gemcitabine response. It was also known that Hippo pathway inactivation, which leaves YAP unphosphorylated and in the nucleus, can trigger tumorigenesis in mice and that altered expression of a subset of Hippo pathway genes can be found in several human cancers (17). When the Hippo pathway is inactivated, YAP is localized in the nucleus in 60% of hepatocellular carcinomas, 15% of ovarian cancers, and 65% of non–small-cell lung cancers (17). However, only a small fraction of human pancreatic tumors exhibited intense nuclear staining for YAP in late-stage tumors (18). We surmise that the human tumors show the “crowded, gemcitabine-resistant phenotype.” Verteporfin (a YAP-TEAD small-molecule inhibitor) (19) had a potent effect on pancreatic cancer cell growth in low-density growth conditions (Hippo-OFF, EC50, <0.5 μM), but had little effect on pancreatic cancer cell growth in 3D spheroid assays (Hippo-ON, EC50, >5 μM) (SI Appendix, Fig. S4).
In cells growing at low density, YAP is localized to the nucleus, cells are sensitive to gemcitabine, and presumably YAP-dependent transcription is turned on. At high cell density, YAP is in the cytoplasm, YAP-dependent transcription is impaired, and resistance to gemcitabine is high. Given these correlations, we asked whether inactivation of Hippo pathway could restore gemcitabine sensitivity under crowded growth conditions. Expression of a nonphosphorylatable form of YAP (YAPS6A) in Panc02.13 pancreatic cancer cells causes constitutive nuclear localization of exogenous YAP even at high cell density (Fig. 2C). Expression of YAPS6A in crowded cells led to an increase in the expression of YAP-TEAD target genes including AMOTL2 (>10-fold), CTGF (>3-fold), AXL (>3-fold), and BIRC5 (>2-fold) (SI Appendix, Fig. S4). Although cells expressing the YAPS6A mutant or knockdown of NF2 (an upstream stimulator of YAP phosphorylation) (20) showed altered morphology and a mildly increased rate of cell growth (SI Appendix, Fig. S4), the increased sensitivity to gemcitabine (and 5-flurouracil) as measured by growth retardation or increased apoptosis was much more striking (Fig. 2 D and E and SI Appendix, Fig. S5). NF2 depletion in Panc02.13 cells also restored sensitivity to verteporfin in a high-density spheroid assay (SI Appendix, Fig. S4). Together, these data suggest YAP phosphorylation (and its export from the nucleus) is the critical determinant of resistance to gemcitabine and perhaps other drugs.
To determine whether the Hippo–YAP pathway regulates the sensitivity of pancreatic cancer cells to a broader set of oncology drugs, we screened 119 FDA-approved oncology drugs using the 3D spheroid (high-crowding condition) assay. In this assay, cells are plated in a round-bottom, hydrogel-coated wells for 2 d to form compact 3D spheroids (Fig. 2G). Cells are then treated with small-molecule inhibitors at varying concentrations (10−9 to 10−5 M) and imaged over 4 d (Fig. 2G). A dose–response curve for each inhibitor is calculated based on control (no inhibitor/DMSO)-treated wells. Most of the oncology drugs tested were ineffective in blocking the growth of Panc02.13 cells (EC50, >1,000 nM; Amax, <50%). This is yet more evidence that cell density does not generally affect resistance to antiproliferative drugs; such resistance is true for only a minority of drugs. Only carfizomob and dactinomycin showed significant inhibition in these high-density growth conditions (Fig. 2H). To test the role of the Hippo pathway in regulating sensitivity, we then exposed Panc02.13 cells expressing the YAPS6A mutant to the same drugs. We found that 15 drugs showed significantly enhanced sensitivity (EC50, <1,000 nM; Amax, >50%) (Fig. 2H and SI Appendix, Fig. S5). These drugs include antimetabolites, anthracyclines, topoisomerase inhibitors, and kinase inhibitors, suggesting that the role of the Hippo pathway in altering the efficacy is not in any simple way related to the drug’s mechanism of action.
The Hippo–YAP Pathway Modulates Gemcitabine Metabolism and Export.
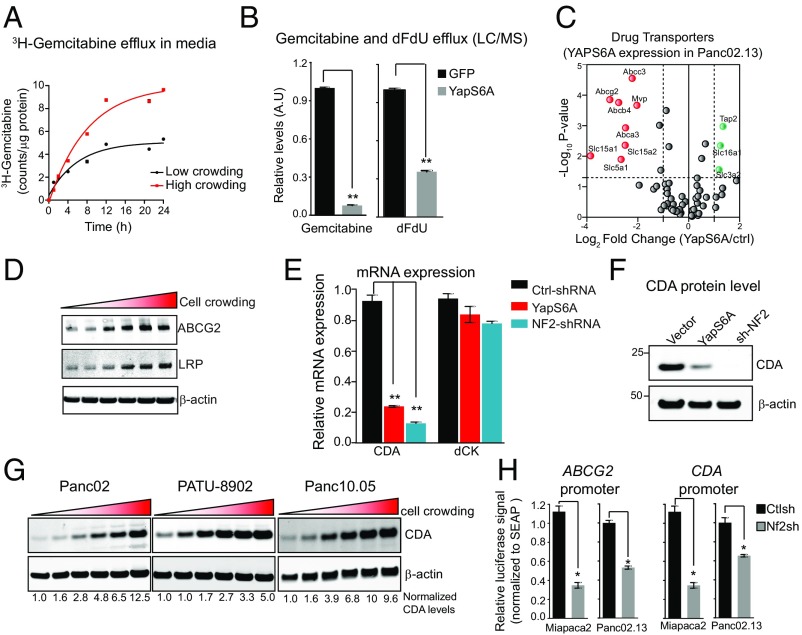
The diverse chemotypes affected by the Hippo pathway suggested that a general process of drug availability rather than regulation of a specific cellular pathway is responsible for the effects. Drug availability mediated by transport or binding or export from the cell is known to be a major determinant of the sensitivity to chemotherapy (21). We first checked that gemcitabine was not lost from the medium due to lability or enzymatic degradation and found that gemcitabine is not labile in culture media (SI Appendix, Fig. S5). Furthermore, conditioned media collected from Panc02.13 cells exposed to gemcitabine after 5 d retained 96.7%. We next considered whether the Hippo pathway might affect the efflux of gemcitabine and/or its metabolites. To assess directly gemcitabine efflux in conditioned media of pancreatic cancer cells, we used both radiolabeled gemcitabine and liquid chromatography tandem-mass spectrometry (LC-MS/MS)-based methods. Panc02.13 cells grown in highly crowded conditions (Hippo-ON) pumped out twofold to threefold more radiolabeled gemcitabine (counts per microgram of protein) in contrast to cells grown in less crowded conditions (Hippo-OFF) (Fig. 3A). Another pathway of inactivation and export is the enzymatic conversion of gemcitabine to a uracil derivative [2′,2′-difluorodeoxyuridine (dFdU)] by deamination catalyzed by CDA (22). We measured the efflux of gemcitabine and its deaminated metabolite, dFdU, by LC-MS/MS (23) in Panc02.13 cells expressing YAPS6A or vector control after gemcitabine treatment (Fig. 3B). Panc02.13 cells expressing YAPS6A (Hippo-OFF) effluxed significantly less gemcitabine (10-fold, P < 0.05) compared with GFP-expressing cells, in agreement with the radiolabel measurements (Fig. 3B). YAPS6A-expressing Panc02.13 cells also effluxed significantly less dFdU (fivefold, P < 0.05) compared with GFP-expressing cells. Together, these data suggest that activation of the Hippo–YAP pathway in high-density cultures increases efflux of gemcitabine and its metabolic conversion to dFdU, resulting in a lower intracellular gemcitabine concentration (Fig. 3B).
Fig. 3.
Hippo–YAP pathway affects gemcitabine availability by modulating its efflux and metabolism. (A) Plot showing increased gemcitabine efflux (release in the medium) in Panc02.13 cells either grown at low/high-crowding conditions. (B) Plots showing gemcitabine and dFdU efflux in Panc02.13 cells expressing either vector control or YAPS6A measured using LC/MS. **P < 0.01. (C) Activation of YAP decreases expression of several multidrug transporters. mRNA expression profiles comparing 84 drug transporters in Panc02.13 cells expressing vector control or YAPS6A. Expression of drug transporters that are significantly down-regulated (P < 0.05) are indicated in red, whereas significantly up-regulated transporters are indicated in green. (D) Western blots showing increase in protein expression of drug transporters ABCG2 and LRP with cell crowding. (E) Activation of YAP decreases expression of CDA. mRNA expression of CDA is significantly decreased in Panc02.13 cells expressing YAPS6A or NF2shRNA compared with vector-only control. The mRNA expression of dCK does not change with overexpression of YAPS6A or knockdown of NF2 (NF2shRNA). **P < 0.01. (F) Western blots showing protein expression of CDA in Panc02.13 cells expressing vector control, YAPS6A, or NF2shRNA. (G) Protein levels of CDA change with cell crowding. Western blots showing protein levels of CDA in three different pancreatic cancer cell lines. (H) Hippo–YAP pathway negatively regulates ABCG2 and CDA expression. ABCG2 and CDA expression levels were measured using promoter reporter construct in Panc02.13 cells expressing NF2shRNA or control siRNA. Data were normalized to internal control (SEAP) activity. *P < 0.05.
Drug efflux transporters can reduce the concentration of cytotoxic drugs in the cell, allowing cancer cells to survive (24). To investigate which transporters might be regulated by the Hippo pathway, we profiled the expression of 84 drug efflux transporters in Panc02.13 cells expressing YAPS6A or a control vector by quantitative PCR. Those include the ATP-binding cassette (ABC) transporters, solute-carrier (SLC) transporters, and other transporters, such as voltage-dependent anion channels, aquaporins, and copper pumps. We found that the mRNA expression levels of eight transporters, mostly from the ABC transporter family, significantly decreased (4- to 16-fold, P < 0.05) in Panc02.13 cells expressing the YAPS6A mutant vector compared with GFP-expressing cells (Fig. 3C). Quantitative Western blotting also confirmed these findings and revealed that the protein levels of these receptors were reduced when the Hippo pathway is inhibited (SI Appendix, Fig. S6). Similar results were seen in Panc1, Patu8988S, and Patu8902 cells (SI Appendix, Fig. S6). Many of these transporters including ABCG2, ABCC3, and lung cancer resistance protein (LRP) have previously been implicated in gemcitabine resistance and/or are highly expressed in pancreatic tumors (23, 25, 26). Expression levels of the monocarboxylate transporter (SLC3A2), the antigen peptide transporter (TAP2), and an amino acid transporter (SLC16A1) were mildly increased (twofold to fourfold, P < 0.05) in Panc02.13 expressing the YAPS6A construct (Fig. 3C). Because cell crowding inhibits the phosphorylation and activity of YAP, which then is retained in the nucleus (Fig. 2 B and C) (16), it would be expected that the expression of these drug transporters (ABCG2, LRP, and ABCC3) would be significantly increased (Fig. 3D and SI Appendix, Fig. S6). On the other hand, the mRNA levels of uptake transporters for gemcitabine (SLC29A1, SLC29A2) were not affected by cell crowding or YAP activity (SI Appendix, Fig. S6). These data show that the activation of the Hippo pathway at high cell density decreases the expression of drug efflux transporters, thereby increasing the effective intracellular concentration of gemcitabine.
The activity of the Hippo pathway not only increased the efflux of gemcitabine but also the production of its major metabolite, dFdU (Fig. 3B). Switching off the Hippo pathway (by depletion of NF2 or expression of YAPS6A) significantly decreased both the mRNA (5- to 8-fold, P < 0.05) and protein levels (5- to 10-fold, P < 0.05) of CDA; these changes should also increase gemcitabine levels (Fig. 3 E and F). Similar results were seen in four other pancreatic cancer cell lines (Panc1, Patu8988S, YAPC, and Patu8902) (SI Appendix, Fig. S6). By contrast, the level of deoxycytidine kinase (dCK) (the enzyme involved in the first phosphorylation and activation of gemcitabine) was not affected by the Hippo pathway (Fig. 3E and SI Appendix, Fig. S6). Consistently, we found that cell crowding increased the levels of CDA (5- to 10-fold, P < 0.05) in several other pancreatic cancer cell lines (Fig. 3G), which should contribute to the drop in gemcitabine levels and increased drug resistance. Finally, verteporfin treatment of Panc02.13 cells, which decreases YAP activity, should be a phenocopy of growth of cells at high density, where YAP is inactivated and degraded. As expected, verteporfin caused a significant increase in CDA levels (threefold, P < 0.05) (SI Appendix, Fig. S6), suggesting expression of CDA is negatively regulated by the Hippo pathway and that this does not require direct interaction with a nucleoside analog.
To further delineate the molecular mechanism of how the Hippo pathway regulates the levels of gemcitabine efflux pumps and CDA activity, we assessed TEAD binding sites in the promoter region of ABCG2 and CDA. Transcription factor ChIP-seq data from the Encyclopedia of DNA Elements (27) revealed multiple TEAD4 consensus binding sites in the promoter region of ABCG2, ABCC3, LRP, and CDA. To validate these findings, we designed synthetic promoter activity constructs comprising the promoter region of either ABCG2 or CDA followed by a luciferase gene. Promoter activity of both ABCG2 and CDA was significantly decreased in cells expressing YAPS6A mutant in both Panc02.13 (twofold, P < 0.05) and Miapaca2 (threefold, P < 0.05) cells compared with GFP vector-expressing cells (Fig. 3H). We conclude that the Hippo–YAP pathway affects gemcitabine action in at least two ways: by negatively regulating mRNA expression of drug resistance proteins and by negative regulating the mRNA for CDA, thereby modulating export and metabolism of gemcitabine.
Inhibition of Hippo–YAP Pathway Activity Increases Sensitivity to Gemcitabine in Tumors.
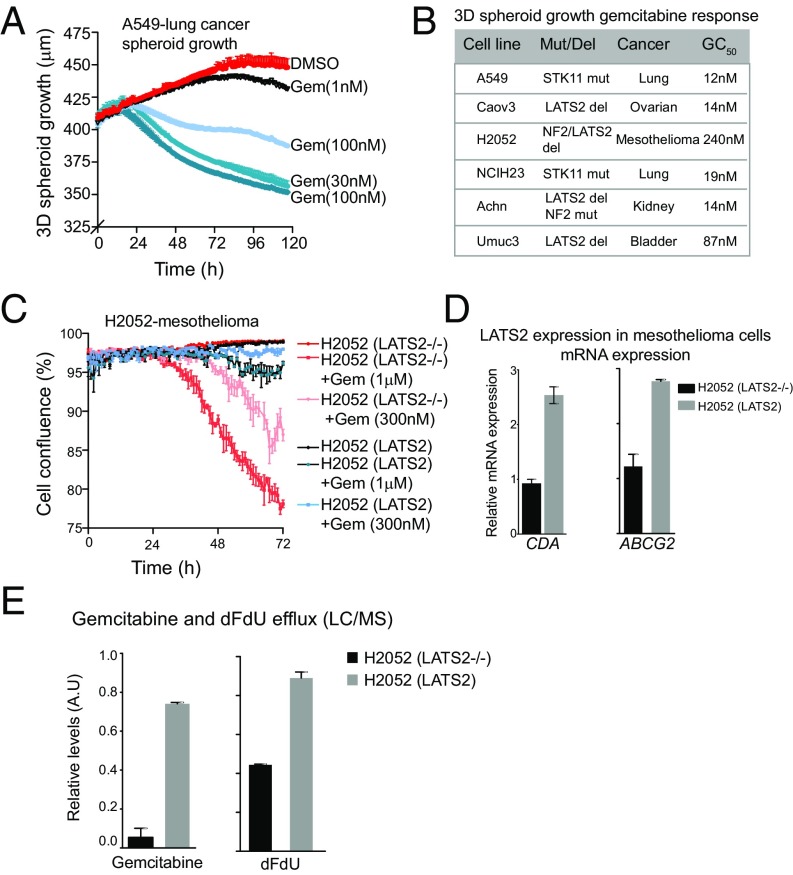
Genetic defects that inhibit the Hippo pathway and increase YAP levels can induce tumors in model organisms. Such mutations occur in a broad range of human carcinomas, including lung, mesothelioma, colorectal, ovarian, and liver cancers (17) (SI Appendix, Table S3). Mutations in NF2 and LATS2 are found in ∼30% of mesotheliomas and mutations in STK11 are found in 18% of lung cancers (SI Appendix, Table S3). Previous studies have shown that aberrations in LATS2 and NF2 inactivate the Hippo pathway and overcome crowding-mediated YAP inhibition (28). Despite the oncogenic effect of Hippo pathway mutations, the above studies would predict that the same inactivating mutations in the Hippo pathway genes (NF2, LATS2, and STK11) could have an important positive effect, which can be exploited for chemotherapy: they might be hypersensitive to gemcitabine even in highly crowded conditions and increase the effectiveness of treatment. Indeed, we find that gemcitabine treatment of a broad panel of cancer cell lines harboring Hippo pathway genetic alterations from five diverse cancer types significantly reduced 3D spheroid growth (EC50, <1,000 nM) (Fig. 4 A and B). Interestingly, each of these cell lines had been previously found to be extremely sensitive to gemcitabine in in vitro and some even in mouse xenograft models; however, the mechanism of sensitivity was unclear (29–35). Furthermore, previous studies have shown that mutations in STK11 (LKB1) in lung cancer cell lines confer sensitivity to gemcitabine, whereas ectopic expression of STK11 causes resistance (36, 37). STK11 has been identified as an upstream kinase that negatively regulates YAP activity (38). Increases in the phosphorylation of YAP (3- to 4-fold) and in the levels of CDA (12-fold) due to cell crowding were observed in lung cancer cells expressing wild-type STK11, whereas relatively subtle changes (pYAP, 1.5-fold; CDA, 2-fold) were observed in STK11 mutant lung cancer cells (SI Appendix, Fig. S6). We posit that genetic aberrations in the Hippo pathway might be predictive biomarkers for response to gemcitabine.
Fig. 4.
Hippo pathway genetic aberrations confer sensitivity to gemcitabine in several cancer types. (A) A plot showing dose-dependent effect of gemcitabine on growth of A549 cells (carrying STK11 mutation) in 3D spheroid. (B) A table summarizing the effect of gemcitabine on growth of six different cancer cell lines carrying Hippo pathway mutations. The relative GC50 and mutated or deleted Hippo pathway gene for each cell line is also listed. (C) Mesothelioma cells harboring LATS2 deletion are sensitive to gemcitabine, and restoring LATS2 expression confers drug resistance. A plot showing the effect of gemcitabine on growth of H2052-mesothelioma cells in the presence or absence of LATS2 expression. (D) Ectopic expression of LATS2 increases the expression of ABCG2 and CDA in H2052 cells. (E) Plots showing relative levels of gemcitabine and dFdU effluxed from H2052 parental or H2052 expressing LATS2 cells.
Are defects in the Hippo pathway the major cause of gemcitabine sensitivity? We find that restoration of LATS2 expression in H2052 mesothelioma cells (lacking NF2 and LATS2 expression) causes resistance to gemcitabine in high-density growth (Fig. 4C). In crowded conditions, exposure of a low dose (<300 nM) of gemcitabine to parental H2052 cells (LATS2−/−) significantly decreases their viability in response to gemcitabine, compared with the same cells complemented with wild-type LATS2 (Fig. 4C). Restoring the levels of LATS2 in H2052 cells caused an increase in the mRNA and proteins levels of ABCG2 and CDA (Fig. 4D and SI Appendix, Fig. S6). LC-MS/MS–based measurement also showed significantly higher amounts of effluxed gemcitabine (∼10-fold) and dFdU (2- to 3-fold) in the media of H2052 (LATS2) compared with parental H2052 (LATS2−/−) cells (Fig. 4E).
Hippo Pathway Inactivation Sensitizes a Diverse Panel of Human Tumors to Gemcitabine in Mouse Xenografts, and Patient-Derived Xenograft Models.
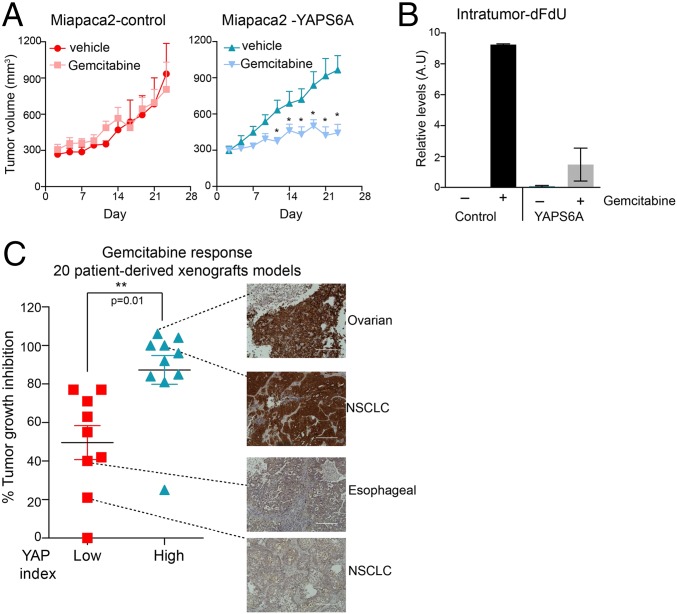
To assess the gemcitabine response to Hippo pathway inactivation in tumors, we used a mouse xenograft model of pancreatic carcinoma cells and patient-derived xenograft (PDX) models from a variety of solid tumors including human cancers from non–small-cell lung, esophagus, breast, mesothelium, ovary, colon, head and neck, sarcoma, and cholangiocarcinoma (SI Appendix, Table S4). In mouse xenograft studies, two human pancreatic cancer cell lines (Miapac2 and Panc02.13) expressing GFP or YAPS6A were injected into athymic mice. Both parental or GFP-expressing cells grew rapidly, producing palpable tumors in 5–10 d. When the tumors were ∼200 mm3 (as measured using a caliper), mice were randomized into treatment and control groups. The former received i.p. saline injections on alternate days for 2 wk, and the latter received gemcitabine (20 mg/kg in Miapaca2-YAPS6A and 50 mg/kg in Panc02.13-YAPS6A cohorts). We observed that gemcitabine treatment had no effect on the growth of Miapaca2-GFP xenografts as previously observed (39), whereas the growth of Miapaca2-YAPS6A was significantly slowed (Fig. 5A). Similar results were seen in Panc02.13 xenografts where gemcitabine treatment had no effect on the growth of Panc02.13-parental xenografts, whereas gemcitabine treatment of Panc02.13-YAPS6A (50 mg/kg) led to significant regression in the tumor volume (SI Appendix, Fig. S7). Intratumor measurements of the levels of dFdU showed significant reduction (greater than fourfold, P < 0.01) in accumulation of dFdU in Miapca-YAPS6A xenografts compared with parental controls xenografts (Fig. 5B). Consistently, we observed greater than twofold induction in apoptosis (measured by levels of cleaved caspase 7 and phosphor-H2aX) in Miapca-YAPS6A xenografts compared with parental controls (SI Appendix, Fig. S7). These data imply that switching off the Hippo–YAP pathway overcomes intrinsic drug resistance in these models of pancreatic ductal carcinoma.
Fig. 5.
YAP activation sensitizes pancreatic tumors to gemcitabine in mouse xenograft models. (A) Gemcitabine treatment of YAPS6A expressing Miapaca2 xenografts showed significantly reduced tumor growth in nude mice. (B) Bar graph showing relative levels of intratumor dFdU in Miapaca2 xenografts measured using LC/MS. (C) High YAP-expressing tumors show significantly heightened sensitivity to gemcitabine (P = 0.01, Mann–Whitney test). A plot showing tumor growth inhibition in response to gemcitabine in PDX models. Representative images of YAP staining among high and low YAP group are also shown. NSCLC, non–small-cell lung carcinoma. (Scale bar, 200 μm.)
It would be natural to try to test gemcitabine response in a mouse model of pancreatic cancer, particularly one that shows a stromal response of connective tissue growth, known as desmoplasia. Unfortunately, the best established mouse models (such as KPC, KrasLSL.G12D/+; p53R172H/+; PdxCretg/+) unlike the human tumors show no activation of YAP (the nonphosphorylated YAP remains in the nucleus). These tumors would not be expected to be sensitive to gemcitabine. In fact, this mouse model and others are already known to be completely resistant to gemcitabine [the median survival upon gemcitabine treatment is ∼15 d compared with 10.5 d in vehicle control (40)]. There may be many interesting features in these mouse PDA models, but, unfortunately, they are not appropriate for studying the Hippo pathway and gemcitabine responsiveness.
An alternative to an endogenous mouse models for capturing effects of the tumor environment are PDX models. PDX models have been shown to retain the architecture and stromal components of the original tumor and therefore are thought to more accurately represent the complex biochemical and physical interactions between the cancer cells and their microenvironment (41, 42). At the cellular level, PDX models also preserve the intratumoral heterogeneity, as well as the molecular characteristics of the original cancer, including copy number variants, single-nucleotide polymorphisms, and gene expression profiles (43, 44). Moreover, studies have found that clinical response of PDXs to therapeutics is correlated with response in patients (45). When we used PDX models to assess whether YAP activation sensitizes solid tumors to gemcitabine, we found significant effects. Tumors with high YAP activity (YAP staining index) showed significantly better response to gemcitabine (approximately twofold difference in percentage tumor growth index, P = 0.01) (Fig. 5C). Notably, there was no correlation between gemcitabine response and tumor doubling time (r = −0.07) (SI Appendix, Fig. S7). In addition, percentage tumor growth index in response to other cytotoxic drugs including carboplatin and cisplatin was not affected by YAP staining index (SI Appendix, Fig. S7). These in vivo data further demonstrate that inactivation of the Hippo–YAP pathway conferred sensitivity to gemcitabine in a diverse panel of cancers.
Gemcitabine is a first-line treatment for locally advanced and metastatic pancreatic cancer. Therefore, in looking retrospectively at clinical response, it is reasonable to assume that the vast majority of patients were treated with gemcitabine. If Hippo pathway aberrations affect the response of pancreatic cancer to gemcitabine during clinical treatment, this might be revealed by comparing the survival of patients with tumors bearing mutations in the Hippo pathway to those with tumors in which the Hippo pathway was intact. In two independent studies using exome sequencing of DNA from pancreatic cancers, we found that high levels of YAP-dependent genes (AMOTL2, CTGF, AXL, ABCG2, ABCC3, MVP, and CRB3) were associated with longer patient survival (SI Appendix, Fig. S7). Specifically, patients with high expression of YAP-TEAD downstream target genes had median survival of 870 d compared with patients with low expression of YAP-TEAD downstream target genes (median survival of 360 d) (SI Appendix, Fig. S7). In lung cancers (∼20% carry STK11 mutations), high expression of CTGF (a YAP-TEAD gene target) correlated with better overall survival (SI Appendix, Fig. S8), although in this case, the data provide no clue to treatment history. Similarly, patients with intrahepatic cholangiocarcinomas that expressed high levels of CTGF have less chance of tumor recurrence and fare better overall survival than those with tumors that lack CTGF expression (46). Gastric cancer patients who received 5-FU–based adjuvant therapy showed better overall survival when the Hippo pathway was inactivated (low NF2 or high CTGF) (SI Appendix, Fig. S8); unfortunately, it is not known whether the more positive responders were treated with one of the 15 drugs that respond to the inactivation of the Hippo pathway and this omission strongly limits our interpretation. Finally, a recent study has also shown that high YAP downstream gene signature correlates with better prognosis in breast cancers (47). These findings collectively raise the possibility that Hippo pathway inactivation might play a role in overall survival in certain chemotherapy regimens, although most of the existing data are inadequate for reaching definitive conclusions.
Discussion
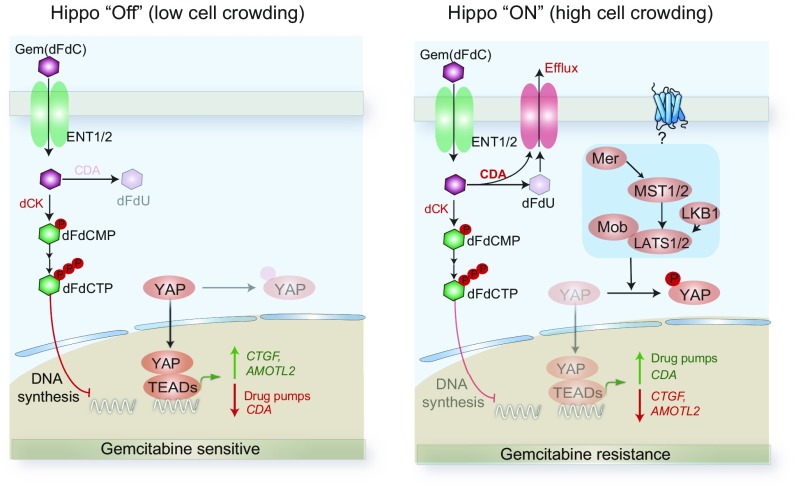
Pancreatic cancer responds poorly to chemotherapy (48); most pancreatic cancer trials have failed, and the current standard-of-care therapy, gemcitabine, has a median overall survival of only 6 mo (49, 50). Gemcitabine is also used to treat advanced-stage lung and breast cancers; however, the determinants of sensitivity and/or resistance to this agent are not fully understood. Comparatively little effort has been directed recently by large drug companies to improving well-established cytotoxic therapies or even to trying to understand why they succeed or fail. Quite understandably, it seemed much more productive to look for new therapies. However, an alternative would be to search for general cellular mechanisms that would differentially affect the activity of these well-established drugs in different people. On that assumption, we searched for pathways that could tune the resistance to gemcitabine in pancreatic cancer. This led us to a previously unknown role of the Hippo–YAP pathway in mediating sensitivity to several chemotherapeutic drugs including gemcitabine (Fig. 6).
Fig. 6.
Hippo–YAP pathway mediates physiological resistance to gemcitabine. In low-crowding conditions or in the case of Hippo pathway genetic aberrations, Hippo pathway is inactive, leading to lower levels of CDA and efflux pumps. This increases intracellular concentration of gemcitabine causing enhanced killing. In high-crowding conditions, Hippo pathway is active, leading to higher levels of CDA and efflux pumps. This reduces intracellular concentration of gemcitabine, leading to drug resistance.
At the onset of our experiments with gemcitabine in pancreatic cancer cells, we were surprised to find that there was a large inconsistency in the published results (Fig. 1B and SI Appendix, Table S1). The same cell lines in different studies were reported as sensitive or resistant, and this was true in all 15 cell lines tested. We found that differences in sensitivity depended very strongly on the cell density (Fig. 1D), and this stands as the most likely explanation for these findings and perhaps for others in drug-profiling efforts (6). Discrepancy in experimental findings is often blamed on sloppiness and has been excoriated as part of an epidemic of irreproducible scientific experiments (51). A fairer lesson is that inconsistency may reflect the extreme sensitivity of some biological phenomena to experimental conditions. History has shown that trying to resolve these can inspire discovery, as we believe has happened here. The resolution to the discrepant findings with gemcitabine is in large part due to the action of the Hippo–YAP pathway, which was activated when cells were grown at high density (Fig. 2B). Conversely, inactivation of Hippo–YAP pathway, which naturally occurs under sparse growth conditions, confers sensitivity to gemcitabine and some other cytotoxic drugs. Experimentally inactivating this pathway by expressing nonphosphorylatable YAP confers sensitivity to crowded cells in 2D and in 3D spheroid culture and also in mouse xenografts (Figs. 2 and 5). Most of the interest in the Hippo pathway in cancer is in its role as a tumor suppressor. Paradoxically, our data, along with a disparate list of published studies, suggest that up-regulating some oncogenes (such as YAPS6A) and down-regulating tumor suppressors (such as RB, p53, NF2, or LATS2) can promote the action of certain drugs (52–55). This appears to be true for gemcitabine and pancreatic cancer, as we have found that cancer patients carrying a deletion of or inactivating mutation in certain tumor suppressor genes in the Hippo pathway appear to live longer on gemcitabine therapy (Fig. 5 and SI Appendix, Fig. S7).
The data on gemcitabine in pancreatic cancer can be linked to clinical outcomes because it has been the first-line therapy. In other tumors, the link is much less clear because the treatment history is not generally known. There are hints, however, that mutations in the Hippo pathway can lead to better-than-expected treatment outcomes. In lung cancers (∼20% carry STK11 mutations), high expression of CTGF (a YAP-TEAD gene target) correlated with better overall survival (SI Appendix, Fig. S8), although in this case the data provide no clue to treatment history. Similarly, patients with intrahepatic cholangiocarcinomas that expressed high levels of CTGF have less chance of tumor recurrence and fare better overall survival than those with tumors that lack CTGF expression.
There is some recognition of the positive value of down-regulating tumor suppressors under other circumstances. A few tumor suppressors have also been used as predictive biomarkers for therapy. Loss of BRCA1 enhances sensitivity to apoptosis induced by antimicrotubule agents such as paclitaxel and vinorelbine, but inhibits apoptosis induced by DNA-damaging agents such as cisplatin and etoposide (56). Rb status has also been shown to be predictive of response to certain drugs such as cisplatin and etoposide in both xenograft mouse models and clinical studies (53–55). Similarly, p53 disruption rendered colorectal cancer cells resistant to the antimetabolite 5-FU but sensitized these cells to the DNA-damaging drug doxorubicin (52). These scattered findings are intriguing and suggest that oncogenes and tumor suppressors may have different effects in drug responses than they do in tumor initiation.
Drug efflux and metabolism can be major determinants of drug efficacy. Several drug transporters are known to regulate gemcitabine efflux and associated resistance (22, 23, 25, 57, 58). However, drug efflux as a target has generally been unsuccessful for pharmacology (59). Due to redundancy in substrate specificity, inhibiting a single ABC transporter has had limited success in blocking gemcitabine efflux (23). Our genetic perturbation experiments revealed YAP-TEAD down-regulates expression of a suite of multidrug transporters (ABCG2, MVP, ABCC3, ABCC5) as well as CDA, resulting in an effective increase in the intracellular availability of gemcitabine (Fig. 4). The expression of many of these transporters including ABCG2, ABCC3, and ABCC5 and CDA has been shown to be up-regulated in pancreatic carcinoma compared with normal pancreatic tissue (SI Appendix, Fig. S8) (60, 61). In particular, a recent study has shown that ABCG2 expression regulates gemcitabine response in pancreatic cancer (62). There is some specificity because we found no correlation between overall survival and the levels of Hippo-independent drug transporters in pancreatic cancers (SI Appendix, Fig. S8). Finally, an increased level of CDA (twofold to threefold, P < 0.05) was also detected in gastric cancer cells that had acquired resistance to gemcitabine (SI Appendix, Fig. S6). A recent study has shown that LKB1 (STK11), another activator of the Hippo pathway, enhances chemoresistance to gemcitabine by up-regulating CDA in a basal triple-negative breast cancer line (36). STK11 deletion in mouse Schwann cells led to sixfold increase in CDA expression levels (SI Appendix, Fig. S6) (63). Furthermore, previous studies have shown that poor vascularization of pancreatic tumors limits the intratumor availability of gemcitabine (64). We propose that inefficient availability of gemcitabine is an intrinsic property of pancreatic cancer cells and is a major contributor to its drug resistance. Thus, inhibiting Hippo–YAP pathway, which coordinately affects many relevant targets, could modulate the drug efflux pumps that mediate gemcitabine resistance.
In addition to gemcitabine, several other cytotoxic agents such as antimetabolites and topoisomerase inhibitors are also affected by Hippo–YAP pathway. Therefore, physiological cell crowding seems to mediate the response of several drugs, but it is certainly not a completely general condition for all cytotoxic drugs. It is plausible but unproven that the Hippo–YAP sensitization to drugs other than gemcitabine is through modulating intracellular drug levels or drug metabolism. ABCG2 and ABCC3 are known to be broad-spectrum drug efflux pumps; substrates of ABCG2 include many drugs that were identified in our screen such as gemcitabine, cladribine, epirubicin, etoposide, imatinib, methotrexate, mitoxantrone, topotecan, and teniposide (65) (Fig. 2 and SI Appendix, Fig. S5). Alternatively, the intracellular distribution of the drug may be altered by the Hippo pathway, thereby reducing the drug concentration at the site of action. For example, Lung Resistance Protein expression is associated with a redistribution of doxorubicin from the nucleus to the cytoplasm without changes in total drug intracellular concentration (66). The underlying mechanisms of how cell-to-cell contact affects sensitivity to other drugs and how the Hippo–YAP pathway is involved in regulating response to other drugs warrants further study.
The FDA has approved over 100 drugs for use in oncology, and there is still a great need to discover more drugs. Although drug discovery holds great potential, we can also make important gains through better understanding of how existing drugs work and, perhaps, even more importantly, how they fail (67). We have begun to appreciate how the Hippo pathway plays a role in gemcitabine response and how the status of this pathway might be used as a prognostic marker. Recently attention has turned to mutational status rather than tissue of origin to assess new drugs in so-called “basket trials” (68). The view is that it may be harder to connect a drug to a tissue than it would be to connect it to a mutated or overexpressed gene. Furthermore, drugs that are shown to be effective by that route could have much wider utility than they would be if they were only certified for a specific subset of patients with an organ-specific tumor. The situation may be very similar with drugs affected by the Hippo pathway. Although mutations in the Hippo pathway are relatively uncommon in any given tumor, when specified by organ of origin, in the aggregate they represent a significant frequency of tumor occurrence. Therefore, it could be worth taking into consideration the Hippo pathway status, when considering first-line therapy for tumors that harbor Hippo pathway defects. After further study, the utility of other drugs that appear to be regulated by the Hippo–YAP pathway could be considered. With a better understanding of the physiologically adaptive responses of cancer cells to cytotoxic drugs, and the use of molecular markers to identify patients who might therefore qualify as exceptional responders, it may be possible to extend personalized treatment to the category of cytotoxic drugs.
Materials and Methods
Cell Lines and Reagents.
Pancreatic cancer cell lines Panc1, Panc02.13, BcPC3, Miapaca2, Panc10.05, Capan2, YAPC, CFPAC1, PATU-8902, PATU-8988S, DANG, and ASPC1 cells and mesothelioma cell line H2052 were obtained from American Type Culture Collection. Panc1, Miapaca2, PATU-8902, and PATU-8988S were maintained in DMEM supplemented with 10% (vol/vol) FBS (FBS), 2 mM glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin. Panc02.13, BxPC3, Panc10.05, Capan2, YAPC, CFPAC1, DANG, ASPC, and H2052 cells were maintained in RPMI supplemented with 10% (vol/vol) FBS, 2 mM glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin.
Small Molecules.
Gemcitabine hydrochloride (catalog #G-4177) was purchased from LC Labs. Radiolabeled gemcitabine was purchased from American Radiolabeled Chemicals. Irrinotecan (catalog #S1198), paclitaxel (catalog #S1150), docetaxel (catalog #S1148), oxaliplatin (catalog #S1224), etoposide (catalog #S1225), and camptothecin (catalog #S1288) were purchased from Selleckchem. A set of FDA-approved anticancer drug library consisting of 119 agents was obtained from the Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health (NIH).
Expression Constructs and RNAi.
YAP expression construct with serine-to-alanine mutations at S61A, S109A, S127A, S128A, S131A, S163A, S164A, and S381A was purchased from Addgene (plasmid ID: 42562). GIPZ Lentiviral shRNAmir clones for human YAP1 or NF2 were purchased from Dharmacon.
Kinetic Cell Growth Assay.
The effect of gemcitabine on pancreatic cancer cell growth was studied using a kinetic cell growth assay. Pancreatic cancer cells were plated on 96-well plates (Essen ImageLock; Essen Instruments) at varying densities (2–4 × 103 for low-density or 15–20 × 103 for high-density experiments). Small-molecule inhibitors at different doses were added 24 h after plating, and cell confluence was monitored with Incucyte Live-Cell Imaging System and software (Essen Instruments). Confluence was observed every hour for 48–144 h or until the control (DMSO-only) samples reached 100% confluence.
Supplementary Material
Acknowledgments
We thank the staff of the Nikon Imaging Center and Systems Biology Flow Cytometry Facility at Harvard Medical School for help and support. We thank Drs. Harold Varmus, Jeff Settleman, Victor Luria, and Christine Hagen for critical review of our data and providing helpful feedback. This study was supported by awards from the NIH (R01 HD073104 and R01 GM103785), Novartis Institutes for BioMedical Research–Harvard collaboration, and Ellison Foundation. T.S.G. is a Human Frontier Science Program Fellow.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703096114/-/DCSupplemental.
References
- 1.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: An evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 2.Chang DK, Grimmond SM, Evans TR, Biankin AV. Mining the genomes of exceptional responders. Nat Rev Cancer. 2014;14:291–292. doi: 10.1038/nrc3723. [DOI] [PubMed] [Google Scholar]
- 3.Weigelt B, Reis-Filho JS, Swanton C. Genomic analyses to select patients for adjuvant chemotherapy: Trials and tribulations. Ann Oncol. 2012;23:x211–x218. doi: 10.1093/annonc/mds323. [DOI] [PubMed] [Google Scholar]
- 4.Burris HA, 3rd, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haibe-Kains B, et al. Inconsistency in large pharmacogenomic studies. Nature. 2013;504:389–393. doi: 10.1038/nature12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garnett MJ, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483:570–575. doi: 10.1038/nature11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffiths JB. Role of serum, insulin and amino acid concentration in contact inhibition of growth of human cells in culture. Exp Cell Res. 1972;75:47–56. doi: 10.1016/0014-4827(72)90518-6. [DOI] [PubMed] [Google Scholar]
- 9.Sanford KK, Barker BE, Woods MW, Parshad R, Law LW. Search for “indicators” of neoplastic conversion in vitro. J Natl Cancer Inst. 1967;39:705–733. [PubMed] [Google Scholar]
- 10.Leontieva OV, Demidenko ZN, Blagosklonny MV. Contact inhibition and high cell density deactivate the mammalian target of rapamycin pathway, thus suppressing the senescence program. Proc Natl Acad Sci USA. 2014;111:8832–8837. doi: 10.1073/pnas.1405723111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin H. Magnesium: The missing element in molecular views of cell proliferation control. BioEssays. 2005;27:311–320. doi: 10.1002/bies.20183. [DOI] [PubMed] [Google Scholar]
- 12.Straussman R, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camargo FD, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 14.Zhao B, Li L, Lei Q, Guan K-L. The Hippo-YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev. 2010;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- 16.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal. 2014;7:ra42. doi: 10.1126/scisignal.2005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu-Chittenden Y, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang N, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connor R. The pharmacology of cancer resistance. Anticancer Res. 2007;27:1267–1272. [PubMed] [Google Scholar]
- 22.Veltkamp SA, et al. New insights into the pharmacology and cytotoxicity of gemcitabine and 2′,2′-difluorodeoxyuridine. Mol Cancer Ther. 2008;7:2415–2425. doi: 10.1158/1535-7163.MCT-08-0137. [DOI] [PubMed] [Google Scholar]
- 23.Rudin D, et al. Gemcitabine cytotoxicity: Interaction of efflux and deamination. J Drug Metab Toxicol. 2011;2:1–10. doi: 10.4172/2157-7609.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polli JW, et al. The role of efflux and uptake transporters in [N-3-chloro-4-[(3-fluorobenzyl)oxy]phenyl-6-[5-([2-(methylsulfonyl)ethyl]aminomethyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008;36:695–701. doi: 10.1124/dmd.107.018374. [DOI] [PubMed] [Google Scholar]
- 25.Hagmann W, Jesnowski R, Löhr JM. Interdependence of gemcitabine treatment, transporter expression, and resistance in human pancreatic carcinoma cells. Neoplasia. 2010;12:740–747. doi: 10.1593/neo.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, et al. ABCC3 as a marker for multidrug resistance in non-small cell lung cancer. Sci Rep. 2013;3:3120. doi: 10.1038/srep03120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ENCODE Project Consortium The ENCODE (ENCyclopedia of DNA elements) project. Science. 2004;306:636–640. doi: 10.1126/science.1105136. [DOI] [PubMed] [Google Scholar]
- 28.Murakami H, et al. LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res. 2011;71:873–883. doi: 10.1158/0008-5472.CAN-10-2164. [DOI] [PubMed] [Google Scholar]
- 29.Achiwa H, et al. Determinants of sensitivity and resistance to gemcitabine: The roles of human equilibrative nucleoside transporter 1 and deoxycytidine kinase in non-small cell lung cancer. Cancer Sci. 2004;95:753–757. doi: 10.1111/j.1349-7006.2004.tb03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohde D, Hayn HK, Blatter J, Jakse G. The efficacy of 2′,2′-difluorodeoxycytidine (gemcitabine) combined with interferon in human renal cell carcinoma cell lines. Int J Oncol. 1998;12:1361–1366. doi: 10.3892/ijo.12.6.1361. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda R, et al. Isolation and characterization of gemcitabine-resistant human non-small cell lung cancer A549 cells. Int J Oncol. 2011;38:513–519. doi: 10.3892/ijo.2010.866. [DOI] [PubMed] [Google Scholar]
- 32.Ratner ES, et al. A KRAS variant is a biomarker of poor outcome, platinum chemotherapy resistance and a potential target for therapy in ovarian cancer. Oncogene. 2012;31:4559–4566. doi: 10.1038/onc.2011.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boven E, Schipper H, Erkelens CA, Hatty SA, Pinedo HM. The influence of the schedule and the dose of gemcitabine on the anti-tumour efficacy in experimental human cancer. Br J Cancer. 1993;68:52–56. doi: 10.1038/bjc.1993.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damaraju VL, et al. Characterization of nucleoside and nucleobase transporters in a human mesothelial cell line: Evaluation of nucleoside and nucleobase antimetabolites for application in malignant mesothelioma. Cancer Res. 2006;66:141. [Google Scholar]
- 35.Damaraju D, et al. Cytotoxic activities of nucleoside and nucleobase analog drugs in malignant mesothelioma: Characterization of a novel nucleobase transport activity. Biochem Pharmacol. 2008;75:1901–1911. doi: 10.1016/j.bcp.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Xia C, et al. Liver kinase B1 enhances chemoresistance to gemcitabine in breast cancer MDA-MB-231 cells. Oncol Lett. 2014;8:2086–2092. doi: 10.3892/ol.2014.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang C. 2014. LKB1 deficient non-small cell lung cancer cells are vulnerable to energy stress induced by ATP depletion. MS thesis (University of Texas, Houston)
- 38.Mohseni M, et al. A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nat Cell Biol. 2014;16:108–117. doi: 10.1038/ncb2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen D, et al. Inhibition of AKT2 enhances sensitivity to gemcitabine via regulating PUMA and NF-κB signaling pathway in human pancreatic ductal adenocarcinoma. Int J Mol Sci. 2012;13:1186–1208. doi: 10.3390/ijms13011186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobetz MA, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garber K. From human to mouse and back: “Tumorgraft” models surge in popularity. J Natl Cancer Inst. 2009;101:6–8. doi: 10.1093/jnci/djn481. [DOI] [PubMed] [Google Scholar]
- 42.Tentler JJ, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9:338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeRose YS, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17:1514–1520. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi SY, et al. Lessons from patient-derived xenografts for better in vitro modeling of human cancer. Adv Drug Deliv Rev. 2014;79-80:222–237. doi: 10.1016/j.addr.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Hidalgo M, et al. Patient-derived xenograft models: An emerging platform for translational cancer research. Cancer Discov. 2014;4:998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gardini A, et al. Expression of connective tissue growth factor is a prognostic marker for patients with intrahepatic cholangiocarcinoma. Dig Liver Dis. 2005;37:269–274. doi: 10.1016/j.dld.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 47.von Eyss B, et al. A MYC-driven change in mitochondrial dynamics limits YAP/TAZ function in mammary epithelial cells and breast cancer. Cancer Cell. 2015;28:743–757. doi: 10.1016/j.ccell.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 48.Oberstein PE, Olive KP. Pancreatic cancer: Why is it so hard to treat? Therap Adv Gastroenterol. 2013;6:321–337. doi: 10.1177/1756283X13478680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 50.Conroy T, et al. Groupe Tumeurs Digestives of Unicancer PRODIGE Intergroup FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 51.Freedman LP, Cockburn IM, Simcoe TS. The economics of reproducibility in preclinical research. PLoS Biol. 2015;13:e1002165. doi: 10.1371/journal.pbio.1002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bunz F, et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zagorski WA, Knudsen ES, Reed MF. Retinoblastoma deficiency increases chemosensitivity in lung cancer. Cancer Res. 2007;67:8264–8273. doi: 10.1158/0008-5472.CAN-06-4753. [DOI] [PubMed] [Google Scholar]
- 54.Treré D, et al. High prevalence of retinoblastoma protein loss in triple-negative breast cancers and its association with a good prognosis in patients treated with adjuvant chemotherapy. Ann Oncol. 2009;20:1818–1823. doi: 10.1093/annonc/mdp209. [DOI] [PubMed] [Google Scholar]
- 55.Herschkowitz JI, He X, Fan C, Perou CM. The functional loss of the retinoblastoma tumour suppressor is a common event in basal-like and luminal B breast carcinomas. Breast Cancer Res. 2008;10:R75. doi: 10.1186/bcr2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quinn JE, et al. BRCA1 functions as a differential modulator of chemotherapy-induced apoptosis. Cancer Res. 2003;63:6221–6228. [PubMed] [Google Scholar]
- 57.Zhou J, et al. Persistence of side population cells with high drug efflux capacity in pancreatic cancer. World J Gastroenterol. 2008;14:925–930. doi: 10.3748/wjg.14.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hauswald S, et al. Histone deacetylase inhibitors induce a very broad, pleiotropic anticancer drug resistance phenotype in acute myeloid leukemia cells by modulation of multiple ABC transporter genes. Clin Cancer Res. 2009;15:3705–3715. doi: 10.1158/1078-0432.CCR-08-2048. [DOI] [PubMed] [Google Scholar]
- 59.Pérez-Tomás R. Multidrug resistance: Retrospect and prospects in anti-cancer drug treatment. Curr Med Chem. 2006;13:1859–1876. doi: 10.2174/092986706777585077. [DOI] [PubMed] [Google Scholar]
- 60.König J, et al. Expression and localization of human multidrug resistance protein (ABCC) family members in pancreatic carcinoma. Int J Cancer. 2005;115:359–367. doi: 10.1002/ijc.20831. [DOI] [PubMed] [Google Scholar]
- 61.Wang F, et al. hsa-miR-520h downregulates ABCG2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br J Cancer. 2010;103:567–574. doi: 10.1038/sj.bjc.6605724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He X, et al. Hypoxia regulates ABCG activity through the activivation of ERK1/2/HIF-1alpha and contributes to chemoresistance in pancreatic cancer cells. Cancer Biol Ther. 2016;17:188–198. doi: 10.1080/15384047.2016.1139228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beirowski B, et al. Metabolic regulator LKB1 is crucial for Schwann cell-mediated axon maintenance. Nat Neurosci. 2014;17:1351–1361. doi: 10.1038/nn.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olive KP, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cusatis G, Sparreboom A. Pharmacogenomic importance of ABCG2. Pharmacogenomics. 2008;9:1005–1009. doi: 10.2217/14622416.9.8.1005. [DOI] [PubMed] [Google Scholar]
- 66.Dalton WS, Scheper RJ. Lung resistance-related protein: Determining its role in multidrug resistance. J Natl Cancer Inst. 1999;91:1604–1605. doi: 10.1093/jnci/91.19.1604. [DOI] [PubMed] [Google Scholar]
- 67.Kirschner M. Marc Kirschner. Nat Rev Drug Discov. 2011;10:894. doi: 10.1038/nrd3613. [DOI] [PubMed] [Google Scholar]
- 68.Hollingsworth SJ. Precision medicine in oncology drug development: A pharma perspective. Drug Discov Today. 2015;20:1455–1463. doi: 10.1016/j.drudis.2015.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.