Epstein–Barr virus (EBV) is a ubiquitous herpesvirus infecting more than 95% of the population by adulthood. EBV is the first human virus known to associate with human cancers and the latent infection by EBV poses a public health burden worldwide (1). In the past five decades, scientific discoveries linked the risk of EBV-associated malignancies with the impaired function of the immune system, particularly by HIV and the immunosuppressive therapies in patients undergoing organ transplantation. EBV is strictly a human virus and scientists have been trying to delineate the mechanistic and causal roles of EBV in cancers in vivo using small animal models to recapitulate the dynamic events in the human body. In PNAS, Minamitani et al. (2) offer new insights, describing how two EBV-encoded latent membrane proteins (LMPs), LMP1 and LMP2A, synergistically drive malignant transformation of germinal center (GC) B cells.
EBV takes advantage of a key pathway in B-cell development: the GC reaction. The GC is a structure within a B-cell follicle in lymph nodes that is important for B-cell differentiation and maturation into antibody-secreting plasma cells or memory B cells; the latter is where EBV establishes life-long persistence (3). EBV LMP1 and LMP2A mimic signals from the host CD40 receptor and the B-cell receptor (BCR), respectively (4). In a normal T-dependent immune response resulting in B-cell activation, the BCR on B cells recognizes cognate antigens, becomes activated, and provides the important “first signal” for B cells. CD40 activation provides the “second signal” or costimulatory signal for B-cell activation via binding to its ligand on T-helper cells. These two signals are imperative for B-cell proliferation, as well as the differentiation from naïve to memory B cells, a maturation path where traversing through the GC is critical.
Both LMP1 and LMP2A are found in a number of GC-derived lymphomas, including Hodgkin, Burkitt, posttransplant, and AIDS-associated lymphoma (4). LMP2A expression in different stages of mouse B-cell development promotes survival of B lymphocytes, can replace or rescue destructive mutations in Ig genes affecting the expression of BCR (5, 6), and can promote the cell cycle (7). The replacement of the BCR signal with an LMP2A signal may be especially important in the case of classic Hodgkin lymphoma (cHL), where it can complement the absence of BCR expression on the Hodgin and Reed-Sternberg (HRS) cell, a malignant multinucleated cell originating from GC B cells. LMP2A alone is not sufficient to drive tumor development but can accelerate tumor onset when expressed in combination with the dysregulated expression of the c-MYC oncogene (8). In contrast to LMP2A, transgenic mouse models of LMP1 generated by different groups exhibit different outcomes. Earlier studies used a mouse model in which LMP1 is expressed at an early stage of B-cell development (under the IgH promoter). These mice develop lymphoma in an immunocompetent situation (9). However, expression of LMP1 in normal mice at a slightly later stage of B-cell differentiation (CD19-driven expression) results in the elimination of the LMP1-expressing cells by the immune system. The depletion of T cells thus allows these LMP1+ cells to progress into lymphoma cells (10, 11).
Recent work has attempted to delineate the roles of both LMPs in GC-specific manners. Wirtz et al. (10) used Cγ1 transcription to drive LMP2A and LMP1 expression in GC B cells. Upon immunization of these mice, expression of the LMPs resulted in an immune response causing the elimination of GC B cells expressing the LMPs. By depleting T cells after the immunization, the combination of LMP1 and LMP2A expression drove fatal lymphoma in all mice by 60 d (10). In PNAS, Minamitani et al. (2) controlled the expression of both LMPs under the AICDA locus encoding the GC-specific enzyme called activation-induced cytidine deaminase. T-cell depletion alone in this model was not sufficient to allow the outgrowth of LMP1/LMP2A double-expressing GC B cells, but the codepletion of both T and NK cells resulted in lymphoma in all mice by 12 d. Furthermore, Minamitani et al. found that LMP1 and LMP2A coexpression in GC B cells induces transcriptional changes that overlap with cHL signature genes. Notably, the up-regulated genes included those encoding CD30, a marker and therapeutic target in cHL, as well as non–B-lineage perforin and granzyme, which are uniquely found in HRS cells. This finding further emphasizes the oncogenic roles of EBV in HL pathogenesis.
Although all studies are in agreement that LMP2A alone is not sufficient to drive lymphoproliferative disease, the findings from Wirtz et al. (10) and Minamitani et al. (2) are in contrast to an earlier work using double-transgenic mice expressing both LMPs from early B-cell development stages (IgHLMP1 and EμLMP2A), whereby coexpression of LMP1 and LMP2A did not result in lymphoma development (12). The results from both Wirtz et al. (10) and Minamitani et al. (2) show the synergistic effect of LMPs in lymphoma development; however, both differ in terms of requirement of T or NK cells in controlling LMP1/LMP2A GC B-cell outgrowth. One explanation of the differences in these findings may be because of the differences in the levels or the timing of LMP1 expression. The latest study from Minamitani et al. (2) reveals the up-regulation of CXCL9, CXCL10, and CXCL11, all of which induce the migration of T cells. One histopathological hallmark of cHL is the presence of HRS cells surrounded by various inflammatory immune cells. Therefore, the attraction of inflammatory cells plays an important contribution in the development of cHL. The levels of LMP1 in the earlier study may not be sufficient to induce such immunological response, and thus lack the key critical step in the malignant formation.
The unique HRS cells undergo various transcriptional reprogramming to down-regulate B-cell markers and genes, as well as the destructive mutation in Ig genes affecting the expression of BCR on the cell surface (13). About 40% of cHL cases are associated with EBV, suggesting that LMP2A can replace necessary signals from BCR, and LMP1 provides growth-promoting yet immunogenic signals during the malignant transformation of HRS cells. It is noteworthy that HRS cells account for only 1–2% of the tumor tissue, suggesting that the malignant process is complex, involving several steps and factors. The Minamitani et al. (2)
Minamitani et al. offer new insights, describing how two EBV-encoded latent membrane proteins (LMPs), LMP1 and LMP2A, synergistically drive malignant transformation of germinal center (GC) B cells.
study highlights a paradoxical role of EBV. On one hand the virus promotes the outgrowth of infected B cells when the immune surveillance is impaired. On the other hand, it also attracts inflammatory responses, which are critical in the malignant transformation, particularly in the case of cHL (Fig. 1).
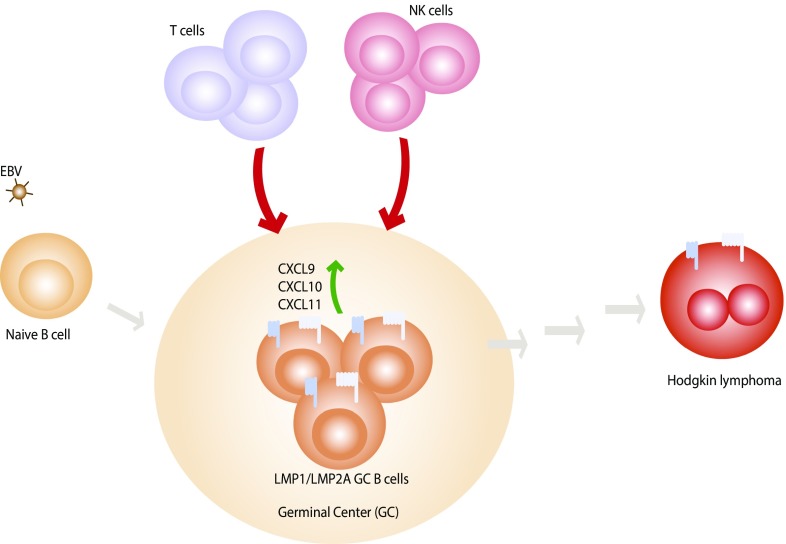
Fig. 1.
LMP1 and LMP2A synergistically drive the malignant process in GC B cells. After the initial infection of EBV, B cells enter the GC, where LMP1 and LMP2A mimic the signals from the host CD40 and BCR, respectively. LMP1/LMP2A-expressing GC B cells are eliminated by T and NK immunosurveillance and the depletion of T and NK cells allows the outgrowth of fatal lymphoma. Minamitani et al. (2) show that LMP1/LMP2A-expressing GC B cells undergo transcriptional changes, some of which recapitulate the gene signatures of cHL and genes encoding proinflammatory chemokines CXCL9, CXCL10, and CXCL11. Both LMP1 and LMP2A can be found in HRS cells in cHL although the malignant transformation requires multiple steps, including the effects the infiltrating immune cells and additional genetic mutations.
Although the new mouse model provides new details of oncogenic mechanisms of both LMPs and offers a potential murine model for EBV-associated cHL, there are some limitations in the use of transgenic models. The current study (2) shows that LMP1 and LMP2A expression suppress genes required for GC maintenance and induce key factors required for plasma cell differentiation, a feature that is not normally found in EBV latent infection, because the virus latently persists in the memory B-cell compartment. This observation suggests that the virus has a check-balance system, where the synergistic effects of both LMPs are dynamic and transient. In this model, EBV LMPs are not expressed under their native viral promoters, which can also be influenced by other microenvironment factors, such as cytokines.
From the virus perspective, EBV is a successful pathogen infecting almost everyone, as the virus prefers to “fly under the radar” until it finds an opportunity to transmit to the next host. In a perfect storm, when immune surveillance is impaired and the right viral products are expressed at the right place and the right time, fatal malignant events could occur. The current study (2) showcases the specific oncogenic roles of LMP1 and LMP2A in GC B cells. Several studies demonstrated the pathogenic roles of the host BCR and CD40 in lymphoma development (14, 15). An interesting question is: why does EBV evolve to express the viral version of these two cellular signals? Studying the comprehensive signals from LMP1 and LMP2A helps understand the unique features and regulation of the viral proteins compared with cellular counterparts. Although the discovery of EBV is more than 50 y old, we have just started to understand the molecular enigma of the role of EBV in human cancers.
Footnotes
The authors declare no conflict of interest.
See companion article on page 4751.
References
- 1.Longnecker R, Kieff E, Cohen JI. Epstein-Barr virus. In: Knipe DM, Howley PM, editors. Fields Virology. 6th Ed. Vol 2. Lippincott Williams & Wilkins; Philadelphia, PA: 2013. pp. 1898–1959. [Google Scholar]
- 2.Minamitani T, et al. Mouse model of Epstein–Barr virus LMP1- and LMP2A-driven germinal center B-cell lymphoproliferative disease. Proc Natl Acad Sci USA. 2017;114:4751–4756. doi: 10.1073/pnas.1701836114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity. 1998;9:395–404. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- 4.Young LS, Yap LF, Murray PG. Epstein-Barr virus: More than 50 years old and still providing surprises. Nat Rev Cancer. 2016;16:789–802. doi: 10.1038/nrc.2016.92. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell RG, Wilson JB, Anderson SJ, Longnecker R. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity. 1998;9:405–411. doi: 10.1016/s1074-7613(00)80623-8. [DOI] [PubMed] [Google Scholar]
- 6.Mancao C, Altmann M, Jungnickel B, Hammerschmidt W. Rescue of “crippled” germinal center B cells from apoptosis by Epstein-Barr virus. Blood. 2005;106:4339–4344. doi: 10.1182/blood-2005-06-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fish K, Chen J, Longnecker R. Epstein-Barr virus latent membrane protein 2A enhances MYC-driven cell cycle progression in a mouse model of B lymphoma. Blood. 2014;123:530–540. doi: 10.1182/blood-2013-07-517649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bieging KT, Amick AC, Longnecker R. Epstein-Barr virus LMP2A bypasses p53 inactivation in a MYC model of lymphomagenesis. Proc Natl Acad Sci USA. 2009;106:17945–17950. doi: 10.1073/pnas.0907994106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulwichit W, et al. Expression of the Epstein-Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc Natl Acad Sci USA. 1998;95:11963–11968. doi: 10.1073/pnas.95.20.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wirtz T, et al. Mouse model for acute Epstein-Barr virus infection. Proc Natl Acad Sci USA. 2016;113:13821–13826. doi: 10.1073/pnas.1616574113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang B, et al. Immune surveillance and therapy of lymphomas driven by Epstein-Barr virus protein LMP1 in a mouse model. Cell. 2012;148:739–751. doi: 10.1016/j.cell.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vrazo AC, Chauchard M, Raab-Traub N, Longnecker R. Epstein-Barr virus LMP2A reduces hyperactivation induced by LMP1 to restore normal B cell phenotype in transgenic mice. PLoS Pathog. 2012;8:e1002662. doi: 10.1371/journal.ppat.1002662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Küppers R. The biology of Hodgkin’s lymphoma. Nat Rev Cancer. 2009;9:15–27. doi: 10.1038/nrc2542. [DOI] [PubMed] [Google Scholar]
- 14.Weniger MA, Küppers R. NF-κB deregulation in Hodgkin lymphoma. Semin Cancer Biol. 2016;39:32–39. doi: 10.1016/j.semcancer.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat Rev Drug Discov. 2013;12:229–243. doi: 10.1038/nrd3937. [DOI] [PMC free article] [PubMed] [Google Scholar]