Significance
Fungus-cultivating termites are icons of ecologically successful herbivores in (sub)tropical ecosystems, cultivating Termitomyces fungi for overcoming the rigid lignin barrier of wood resources. To date, research on these ectosymbiotic fungi has only identified laccases, rather than the typical ligninolytic peroxidases. Using 2D gel-state NMR, we chemically tracked the fate of lignin from the original poplar wood throughout the complex food-processing system in a farming termite. We found young worker termites rapidly depolymerize and degrade even the most recalcitrant wood lignin structures, facilitating polysaccharide cleavage by symbiotic fungi. These results suggest that the natural systems for lignin degradation/pretreatment are far beyond the systems currently recognized and are potential sources of novel ligninolytic agents, enabling more efficient plant cell wall utilization.
Keywords: lignin, carbohydrate, NMR, symbiosis, age polyethism
Abstract
Depolymerizing lignin, the complex phenolic polymer fortifying plant cell walls, is an essential but challenging starting point for the lignocellulosics industries. The variety of ether– and carbon–carbon interunit linkages produced via radical coupling during lignification limit chemical and biological depolymerization efficiency. In an ancient fungus-cultivating termite system, we reveal unprecedentedly rapid lignin depolymerization and degradation by combining laboratory feeding experiments, lignocellulosic compositional measurements, electron microscopy, 2D-NMR, and thermochemolysis. In a gut transit time of under 3.5 h, in young worker termites, poplar lignin sidechains are extensively cleaved and the polymer is significantly depleted, leaving a residue almost completely devoid of various condensed units that are traditionally recognized to be the most recalcitrant. Subsequently, the fungus-comb microbiome preferentially uses xylose and cleaves polysaccharides, thus facilitating final utilization of easily digestible oligosaccharides by old worker termites. This complementary symbiotic pretreatment process in the fungus-growing termite symbiosis reveals a previously unappreciated natural system for efficient lignocellulose degradation.
Lignin is a heterogeneous plant cell wall polymer derived primarily from hydroxycinnamyl alcohols via combinatorial radical coupling reactions (1). Its depolymerization is challenging, making lignin a major barrier to gaining access to stored energy within lignocellulosic materials (2). Woody biomass, such as that contained in pine and poplar trees, represents the most abundant renewable energy resource in our terrestrial ecosystems. Because of a high content of lignin, a complex polymer that contains ether– and carbon–carbon linkages between monomer-derived units (1, 3), biotechnological efforts to use this material for bioenergy require expensive chemical, physical, or biological pretreatment processes to partially delignify lignocellulose to allow access to plant polysaccharides (2).
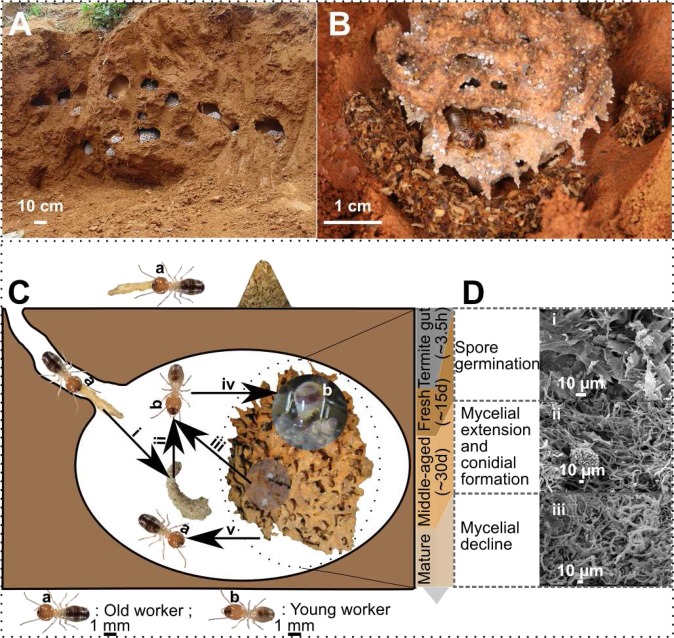
Termites are remarkably efficient at degrading woody biomass (4). Within hours, they digest 74–99% of cellulose and 65–87% of hemicellulose, leaving the lignin-rich residues as feces; that is, they process far more efficiently than other herbivores that can digest the less-lignified forages, such as grasses, leaves, and forest litter (5). Among termites, the subfamily Macrotermitinae form a monophyletic group of diverse species that originated in Africa and have cultivated specialized fungi (Termitomyces spp.) in their nests using woody or litter-based biomass for about 31 million years, and are able to almost completely decompose lignocellulose (6, 7). These termites enable the consumption of more than 90% of dead plant tissues in some arid tropical areas (7, 8), and thus play central ecological roles in Old World (sub)tropical carbon cycling (Fig. 1 A and B) (8). Throughout their evolutionary history, fungus-cultivating termites have evolved in mutualistic association with multiple microbial symbionts, not only with the lignocellulose-digesting basidiomycete fungi Termitomyces spp., but also with gut microbiota and the bacterial community associated with their fungus comb (8, 9). The Macrotermitinae termite–fungus–bacterial symbiosis is a system that enables the extensive conversion of plant materials (9, 10). In addition, the plant substrate processing in fungus-cultivating termites is completed by the termite worker castes with complex age-related labor division (genera Odontotermes and Macrotermes) (11, 12) (Fig. 1C). Older workers forage for plant material and transport it to the nest, but gain their nutrition by consuming the mature, protein-rich fungus comb. In contrast, young workers ingest the undegraded plant material provided by their older nestmates together with fungal nodules (containing asexual spores), and use their feces, which are rich in fungal spores and lignocellulosic substrates, to build new fungus combs (Movie S1). The fungus comb has a turnover time of ∼45 d (13) (Fig. 1D), making fungus-cultivating termites an efficient system for bio-degradation of lignocellulose (7). Moreover, the evolution of elaborate symbiotic associations, together with age polyethism in plant substrate processing, likely contributes to the ecological success of the Macrotermitinae, in particular the Odontotermes genus that represents more than half of the described Macrotermitinae species (14), and are dominant wood and grass degraders in southeast Asia (14).
Fig. 1.
The plant material food-processing pathway in fungus-cultivating termites. As dominant decomposers, an O. formosanus colony forms in subtropical China with massive fungus combs underground (A), which are made of various plant materials foraged by worker termites (B). Age-related labor division in food processing of fungus-cultivating termites (C). Old workers forage outside and transport plant materials back to nest and form a food store (i); young workers ingest the food store (ii), imbue them with the fungal nodules (that are rich in asexual Termitomyces spores) (iii), and use their feces to build fresh fungus combs (iv); old workers gain their nutrition by consuming the mature fungus comb (v). (D) The development of Termitomyces fungi in fungal comb: the poplar fungal comb has a cycle time of ∼45 d. The Termitomyces spores and plant materials rapidly pass through the gut of young worker (∼3.5 h) and form as fresh comb with the fast spores germination (i); after 15 d of growth, the middle-aged comb with dense fungal mycelia and fungal nodules (ii); and after 30 d more growth, the comb materials complete maturation and containing little recognizable mycelia (iii).
Lignin degradation mechanisms in fungus-cultivating termite systems have remained contentious since it was revealed that the ectosymbiotic fungi Termitomyces spp. from several Macrotermitinae termite hosts apparently lack typical ligninolytic agents, such as lignin peroxidase (LiP), manganese peroxidase (MnP), and versatile peroxidase (VP), that are found in, for example, white-rot fungi (8, 15, 16). Research on these enzymes shows that lignin depolymerization may occur via many mechanisms: (i) LiPs and VPs are known to efficiently oxidize nonphenolic β-ether units in lignin within the wood cell wall—beyond where enzymes are permitted to travel—via single electron transfer, creating aryl cation radical intermediates and cleaving the β-ether unit between its Cα and Cβ carbons (17, 18); (ii) MnPs preferentially oxidize Mn2+ to the reactive Mn3+ which, in its chelated form, can act as a diffusible redox mediator and attack phenolic structures in lignin (19); (iii) unsaturated fatty acids undergo peroxidation by MnP to form organoperoxyl radicals that have also been shown to be potential ligninolytic agents if able to diffuse into the wood cell wall (20). In contrast to characteristic ligninolytic peroxidases, laccase is the only enzyme with the potential to act on lignin that has been found in Termitomyces spp. (15, 16, 21), but its functional role in ligninolysis remains unclear and controversial (22). In addition to genetic and biochemical research approaches, several solid-state NMR studies have shown different peak intensities assigned to lignin structures between spectra of the fresh comb and mature comb (23, 24). Although these findings suggest that lignin degradation occurs within the fungus comb, these earlier experiments characterized comb samples from field colonies, with a potentially wide variety of plant debris collected by the termites, making it difficult to match the chemical compositional and structural changes occurring along with the plant substrate processing within these systems (25, 26). The way Macrotermitinae termites and symbiotic microbiota circumvent the lignin barrier and cleave the polysaccharide into easily digestible simple sugars has therefore remained a mystery (8, 27).
Results and Discussion
General Characteristics of Wood Particles Throughout Substrate Processing.
To investigate lignocellulose degradation, we first physically and chemically characterized the fungus comb and the original poplar wood samples by both scanning electron microscopy and chemical compositional analyses. Comparing the morphology of intact poplar wood to the wood particles obtained by dissection of the young workers' gut showed slight physical disruption (SI Appendix, Fig. S1B). Wood particle sizes were mostly between 20 and 80 μm (SI Appendix, Figs. S2 and S3), and there was significant reduction in length of the fibrils when going from the original wood to mature comb (SI Appendix, SI Text), possibly because of the capacity of microbiome for lignocellulose degradation. Consistent with the physical observation, chemical compositional analysis of the hydrolyzed materials showed that the fungus comb samples had lost 13%, 45% and 60% of their lignin (Klason lignin plus acid-soluble lignin) in the fresh, middle-aged, and mature comb, respectively, and there was significant reduction in their xylan, by 19%, 24%, and 61% (calculation based on percentage of the totals in SI Appendix, Table S1). In contrast, the concentration of glucose in the comb increased from 14% (fresh), to 28% (middle-aged), and 42% (mature) glucose across the three fungus comb strata (SI Appendix, Table S1). These results show that young workers and the fungus-comb microbiome enable the degradation of lignocellulose as the poplar wood passes through this fungus-cultivating termite system. Because other plant material inputs were prevented, these results suggest that young worker termites are also engaged in degrading lignocellulose.
To determine the transit time of wood particles through the guts of young workers to passage through the fungus comb in Odontotermes formosanus, we conducted food-stain marker feeding experiments (details provided in SI Appendix, SI Materials and Methods and Fig. S4A). The passage of stained poplar wood through the young worker gut was ∼3.46 ± 0.04 h (SI Appendix, Fig. S4C). In contrast, the total duration of the passage in a wood-feeding lower termite (nonfungus-cultivating species) Copototermes formosanus was ∼22.51 ± 0.09 h (SI Appendix, Fig. S4 B and C). The transit time in young worker guts of O. formosanus here is consistent with results from a previous study of the rapid passage of food through the intestinal tract of young workers in another fungus-cultivating termite, Macrotermes subhyalinus (28).
Step-Wise Lignocellulose Delignification as Shown by NMR and Methoxyl Analysis.
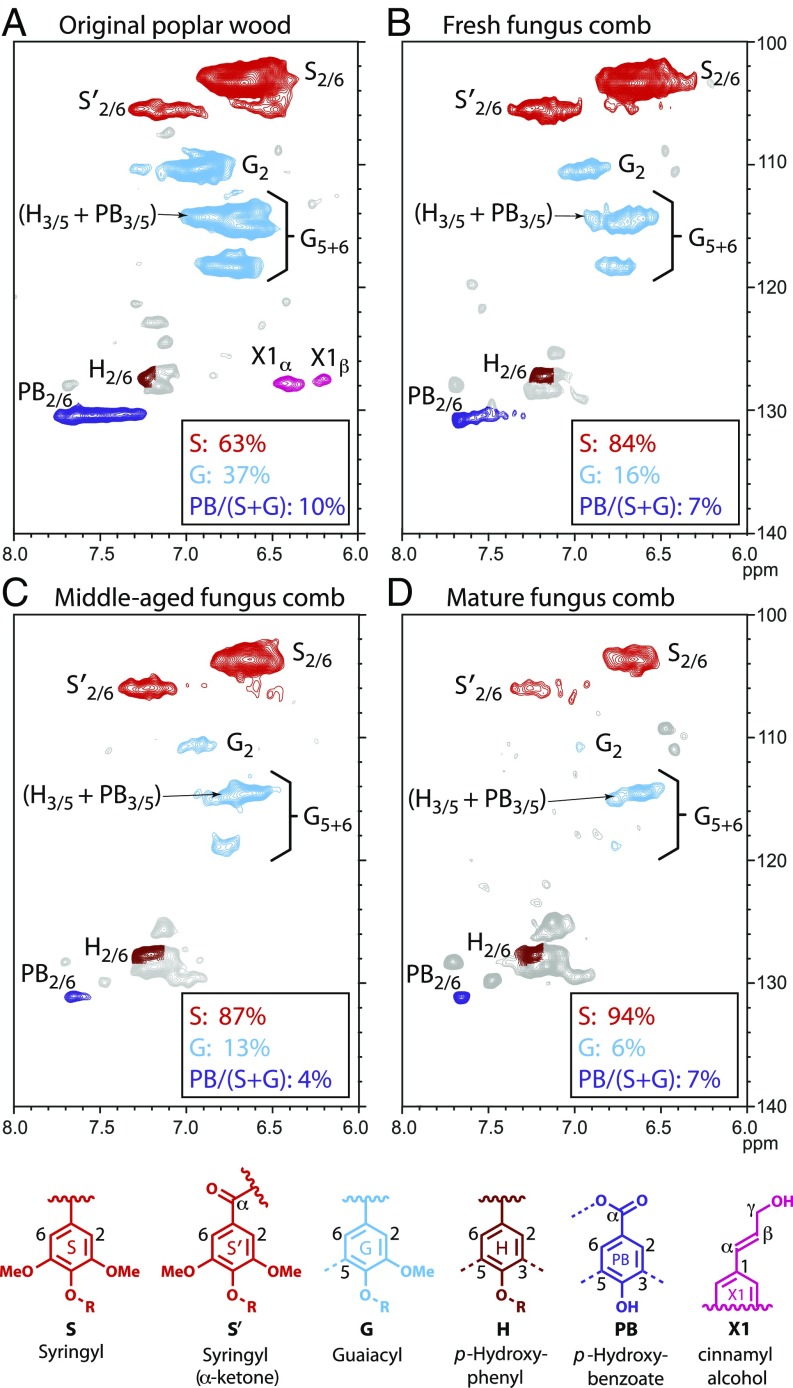
Gel-state 2D-NMR spectroscopy on the whole cell-wall plant material is a well-developed analytical approach that allows us to analyze the complex lignocellulosic macromolecular matrix without requiring fractionation, separation, or depolymerization other than that caused by ball-milling (29, 30), and therefore has been widely used to provide evidence for biological/chemical processes of lignin cleavage (31–34). By using 2D-NMR in conjunction with methoxyl group analysis, we are able to quantitatively determine where and to what degree bond cleavage is occurring in the wood cell wall, and understand the functional roles of the interactions between the multiple partners in the lignocellulosic pretreatment by fungus-cultivating termites. In fact, 2D-NMR is the most diagnostic approach to ascertain the presence and relative levels of the various units within native lignin (35). We elucidated the relative distributions of the various lignin subunits, characterized by their specific interunit linkages, as well as the polysaccharide profile (Figs. 2 and 3). The three treated samples (fresh, middle-aged, and mature fungus comb) were analyzed and compared with the original poplar wood. The spectral assignments of 1H–13C correlations for lignin and polysaccharides are listed in SI Appendix, Tables S2–S4. For fungus combs, the lignin aromatic spectral regions were entirely different from those from the original poplar wood, irrespective of their ages (Fig. 2). Syringyl-to-guaiacyl (S/G) ratios, determined from the aromatic region of fresh, middle-aged, and mature fungus comb were 5.23, 6.61, and 16.39, respectively, compared with 1.68 in the original poplar wood sample (Fig. 2 and Table 1). Therefore, the G units were preferentially degraded through young worker termite gut and fungus combs relative to the S units, to the extent that G units were almost absent in the mature comb. Specific cleavage and removal of G units has been documented in the lower wood-feeding termites (36, 37). Taken together with our results here, this finding suggests that, unlike other wood-feeding insects that preferentially degrade syringyl lignin (37, 38), termites and their gut microbiota appear to favor guaiacyl lignin degradation. Moreover, although the G and S units are depleted, the visual appearance of H units and associated components that remain unidentified remain strong as the comb matures. The p-hydroxybenzoates (PB) were already depleted (from the level in the original poplar wood) by the time the material got to the fresh comb, but did not display a significant decrease in frequency from the fresh to the mature fungus comb (Fig. 2 and Table 1), as indicated by the PB/(S+G) ratios. Our results showed that a large aromatic (lignin) component has been removed throughout the fungus-comb maturation, which implies that the fungus-comb microbiome—including its dense Termitomyces mycelial mass, replete with laccases, and harboring unique bacterial lineages—has the ability to extensively mineralize aromatics (39, 40).
Fig. 2.
1H–13C HSQC NMR spectra of cell wall gels from samples. Lignin aromatic regions are shown for the original poplar wood (A), fresh fungus comb (B), middle-aged fungus comb (C), and mature fungus comb (D). Correlations from the major aromatic ring unit types are well dispersed and color coded by their indicated structures. See SI Appendix, Table S2 for signal assignments.
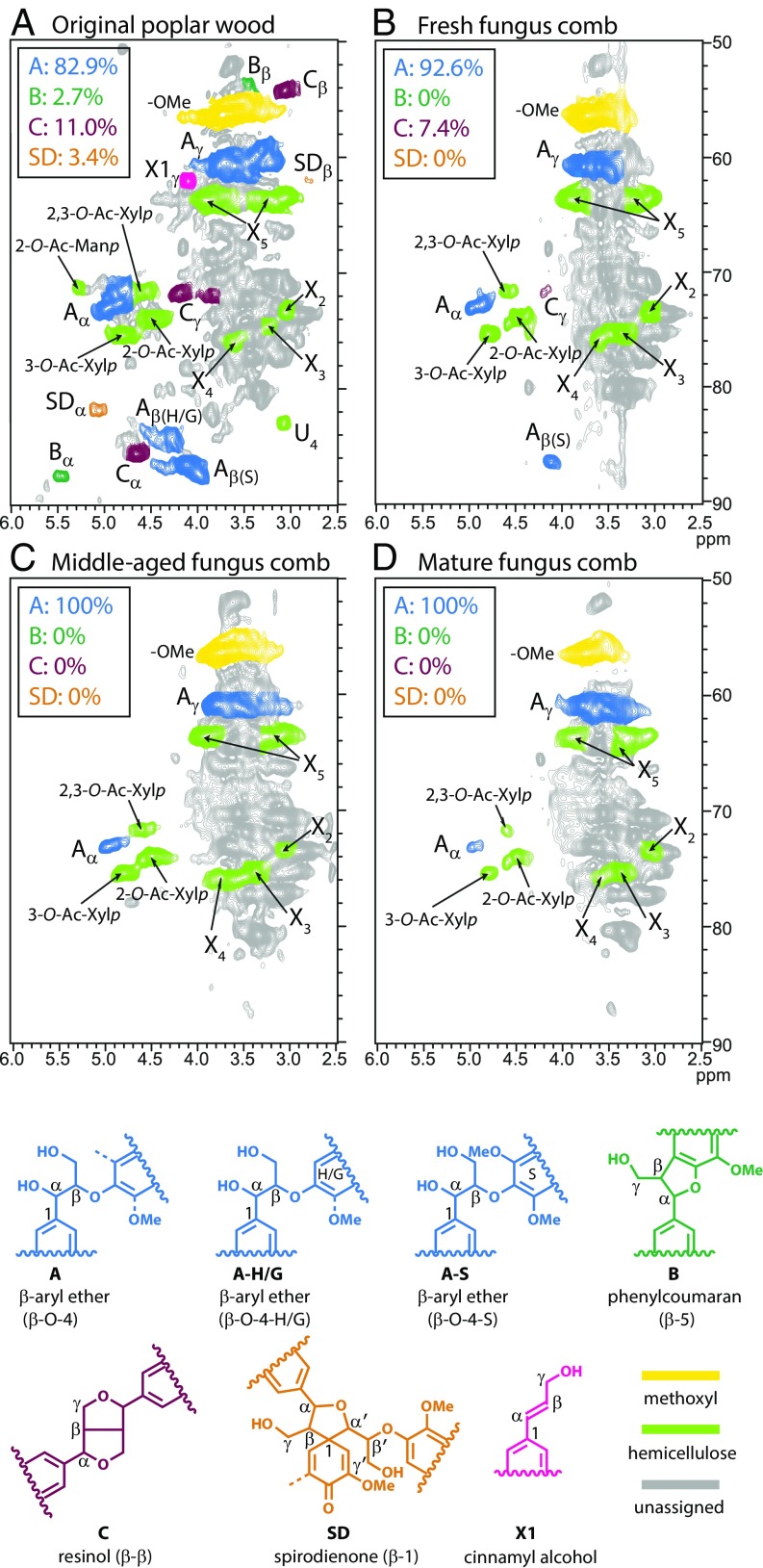
Fig. 3.
1H–13C HSQC NMR spectra of cell wall gels from samples. Lignin aliphatic regions are shown for: (A) original poplar wood, (B) fresh fungus comb, (C) middle-aged fungus comb, and (D) mature fungus comb. Correlation from the major structural units are well dispersed and color coded by their indicated structures with characteristic interunit bonding. See SI Appendix, Table S2 for signal assignments.
Table 1.
Two-dimensional NMR Integral data for interunit linkages relative to the lignin methoxyl, methoxyl analyses results, and aromatic unit ratios
| Unit | Poplar wood | Fresh comb | Middle-aged comb | Mature comb |
| β-Ether A | 0.121 | 0.087 | 0.037 | 0.037 |
| Phenylcoumaran B | 0.004 | — | — | — |
| Resinol C | 0.016 | 0.007 | — | — |
| Spirodienone SD | 0.005 | — | — | — |
| OMe content/sample (%)* | 4.5 | 2.9 | 1.9 | 1.4 |
| OMe content/lignin (%)† | 20.4 | 15.2 | 15.7 | 15.7 |
| OMe decrease (%)† | — | 25 | 23 | 23 |
| S/G ratio | 1.68 | 5.23 | 6.61 | 16.39 |
| PB/(S+G) ratio | 0.10 | 0.07 | 0.04 | 0.07 |
All integral data for lignin methoxyl corrected for actual OMe/lignin obtained from OMe analysis (SI Appendix, SI Materials and Methods). —, not detected.
Based on the dry weight of material analyzed.
Based on the Klason lignin + acid-soluble lignin.
The aliphatic regions of the heteronuclear single-quantum coherence (HSQC) spectra (Fig. 3) provide details regarding the distribution of interunit linkages in the lignin structure. The lignin compositions in the fungus comb were strikingly different from those in the original poplar, even in the fresh comb (Table 1). The β-ether units A decreased by 28% going from the wood to the fresh fungus comb, followed by an additional 57% from fresh to the middle-aged comb, but no further depletion in the mature fungus comb. The resinol units C also showed a substantial decrease with 56% depletion in the fresh fungus comb and complete removal by the middle-aged comb. Phenylcoumarans B, spirodienones SD, and cinnamyl alcohol end-units X1 had also essentially disappeared before the material made it to the fresh fungus comb (Fig. 3B). Methoxyl analysis revealed that the fresh fungus-comb sample had lost ∼25% of its original methoxyls; no further depletion was seen in the progression through the middle-aged and mature fungus combs. The HSQC spectra of the digested wood from the young worker termites shows divergent behavior compared with wood after white- and brown-rot decay also analyzed by 2D-NMR spectroscopy (32, 33). For example, white-rotted wood typically shows simultaneous disappearance of lignin and polysaccharide moieties, whereas brown-rotted wood retains much of the lignin aromatics with some depletion of aliphatic side-chains. Here, our findings reveal that, even during the rapid transit time for the material in the termite gut (∼3.5 h), the lignin is significantly depolymerized. Indeed, ring methoxyl groups are appreciably removed, and lignin sidechains are extensively cleaved, leaving a residue (fresh fungus comb) almost completely devoid of various condensed units that are identifiable by their characteristic carbon–carbon linkages. Apparently, the enzymes in young worker termite guts work in conjunction with intestinal microbiota to depolymerize lignin. Moreover, the comparative studies of fresh and middle-aged fungus comb showed that continuous cleavage of lignin sidechains is occurring throughout this process, but with no further depletion in the mature fungus comb, implying the bacterial lineages in the fresh comb derived from the feces of young workers may have similar lignin-degrading abilities to those in the gut, and thus subsequently contribute to the complete lignin depolymerization (39, 40).
Confirmation of Lignin Depolymerization and Degradation by Pyrolysis-GC-MS and Tetramethylammonium Hydroxide-Pyrolysis-GC-MS.
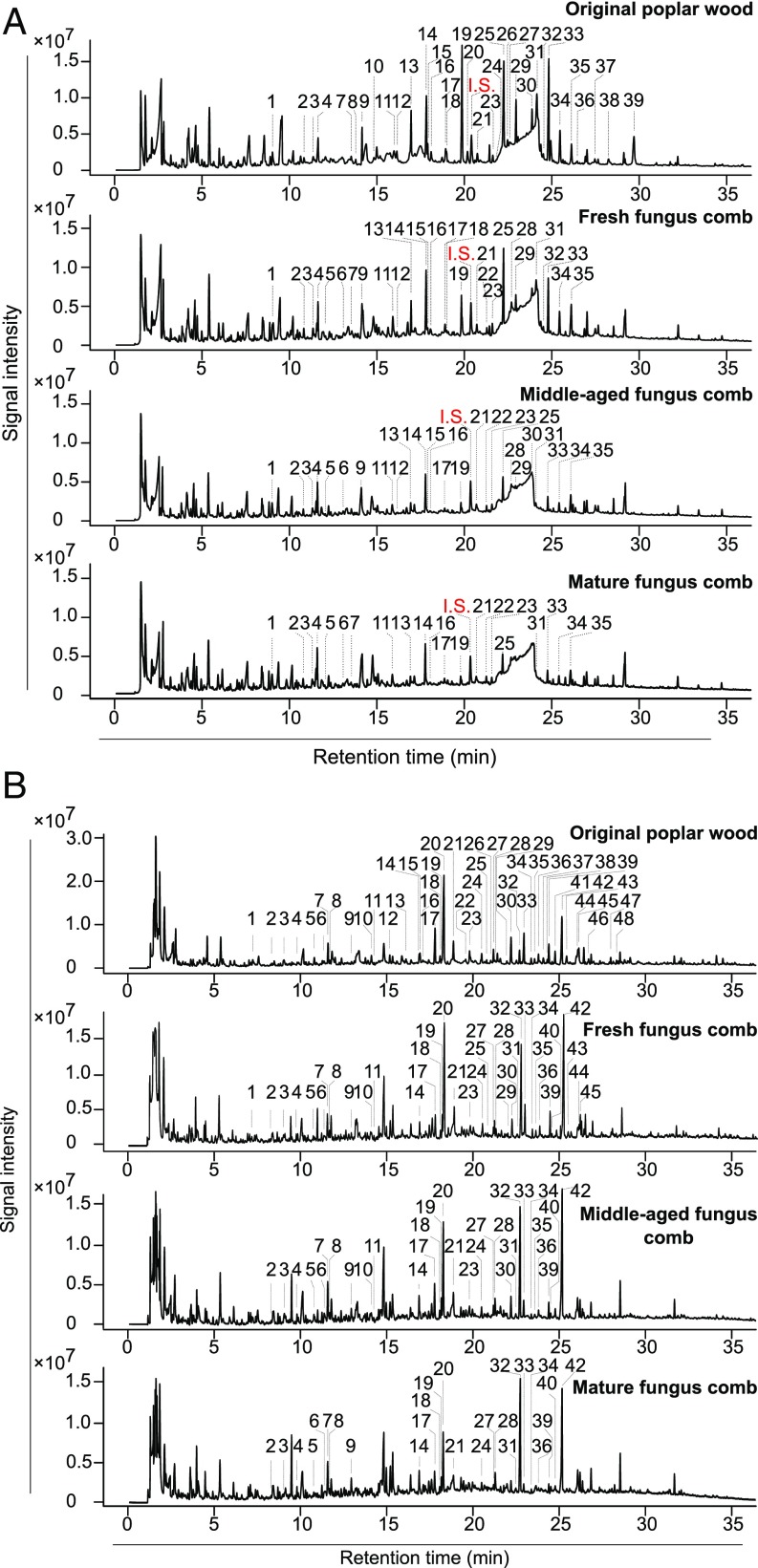
To establish whether a significant decrease in aromatic units had occurred during the fungus-cultivating termite pretreatment, the original poplar wood and fungus comb samples were subjected to pyrolysis (Py)-GC-MS, the pyrograms of which, with their numbered peaks identifying lignin-derived phenols, are shown in Fig. 4A. Differences in the peak profiles of phenolic compounds derived from the lignin moieties in the pyrograms from fungus-comb samples versus those from the original poplar wood reveal details consistent with the NMR structural analysis. For example, the amounts of phenolics released from the fresh, middle-aged, and mature fungus-comb lignin (1 mg total per sample) were 0.13 mg, 0.07 mg, and 0.05 mg, compared with 0.23 mg in the original poplar sample. Moreover, the H/G/S/PB ratios were 5:31:64:0, 8:40:50:0, and 15:28:57:0 compared with 2:23:76:2 in the poplar wood sample (SI Appendix, Table S5). Interestingly, as a common intermediate in β-ether degradation pathways (22), the vanillic acid (peak 28) was highly abundant in pyrograms from the fresh fungus comb (8.3%), represented only minor amounts in the middle-aged comb (2%), and was completely absent from the mature comb, suggesting that the major β-ether cleavage takes place in the young workers' gut, but is followed by a further cleavage by the fresh comb microbiome, again suggesting that activity is retained in the feces.
Fig. 4.
Thermochemolysis profiles of cell walls from samples. (A) Py-GC-MS profiles. Poplar wood, fresh fungus comb, middle-aged fungus comb, and mature fungus comb with added internal standard (I.S.). The peaks labeled are identified phenolics-derived compounds. See SI Appendix, Table S5 for peak identification and relative molar areas. The structures of the labeled peaks are shown in SI Appendix, Fig. S7. (B) TMAH-Py-GC-MS profiles. Poplar wood, fresh fungus comb, middle-aged fungus comb, and mature fungus comb. The peaks labeled are identified phenolics-derived compounds. See SI Appendix, Table S6 for peak identification and relative molar areas. The structures of the labeled peaks are shown in SI Appendix, Fig. S8.
Additional support for our findings of the cleavage of lignin ethers during the rapid passage through the young worker termite gut is provided by investigating pyrolysis of the original poplar wood and fungus-comb samples in the presence of tetramethylammonium hydroxide (TMAH) (Fig. 4B). TMAH present during pyrolysis esterifies acids (and methylates phenols) with remarkable efficiency (41), and has been used to analyze insect-digested and fungal-rotted wood (37, 42). Ether cleavage resulted in the enhanced production of 3,4-dimethoxybenzoic acid methyl ester (peak 32) and 3,4,5-trimethoxybenzoic acid methyl ester (peak 42); an increase in the G-derived ester peak 32 to aldehyde peak 20 and the S ester peak 42 to aldehyde peak 33 ratios is indicative of lignin depolymerization (37, 42). These G-monomer ratios from the fresh, middle-aged, and mature fungus comb were 0.64, 1.08, and 1.88 compared with 0.12 from the poplar wood (SI Appendix, Table S6). Similarly, S-monomer ratios were 4.53, 6.87, and 10.50 compared with 1.62 from the original poplar wood (SI Appendix, Table S6). TMAH-Py-GC-MS results therefore demonstrate the remarkable degree of ether cleavage that has occurred during the gut passage in young worker termites, and are in good agreement with NMR and Py-GC-MS data.
Preferential Carbohydrate Utilization and Polysaccharide Cleavage.
Insight into the polysaccharide conversion in this termite system was gained from HSQC NMR spectra together with high-performance anion-exchange chromatography (HPAEC) analysis. The polysaccharide anomeric regions of the spectra from fungus combs showed distinct differences from those within the original poplar wood (SI Appendix, Fig. S5). For the fresh comb sample, the absence of 4-O-MeGlcA (labeled U1) and α-l-Araf sidechain units of hemicelluloses and a slight decrease in the acetylated xylan hemicellulose, as exemplified by reduction in the level of the 2,3-di-O-Ac-β-d-Xylp (2) contours (Fig. 3 and SI Appendix, Fig. S5), shows that the young workers mainly make use of the wood cell wall’s branched sugars substituted along hemicellulosic backbones, suggesting that the young workers preferentially release the more easily accessible sugars from the sidechains during the rapid transit time. Dominant members of the bacterial family Lachnospiraceae are present in the young workers of O. formosanus (39); such microbiota are ascribed essential and specific roles in both deconstruction and fermentation of noncellulosic poly- and oligosaccharides that have been interpreted as evolutionary adaptations for the rapid digestive niche (43). The enrichment in cellulose (β-d-Glcp), as a result of a decrease in xylan (β-d-Xylp) in the middle-aged and mature combs, was evident from the HSQC spectra. Consistent with this interpretation, the HPAEC analysis revealed that the glucose/xylose ratios in the middle-aged and mature combs increased to 3.80 and 8.20, compared with 2.27 in the original poplar wood (SI Appendix, Table S1). These data suggest that the fungus-comb microbiome preferentially uses xylose and other hemicelluloses, leaving most of the cellulosic substrates.
For the middle-aged and mature fungus comb, strong increases in the intensities of the reducing endgroups both in xylan (α-d-Xylp-R) and mannan (α-d-Manp-R) were also observed. In addition to these NMR spectra, comparisons of the total amount of carbohydrates in hydrolyzed comb samples (SI Appendix, Table S1) indicated that significant insoluble material weight losses were observed as the comb matured, as revealed by the HPAEC analysis of hydrolyzed extractive-free fraction from the comb (SI Appendix, Table S7). In going from the middle-aged comb to the mature comb, 69% of the insoluble carbohydrates were lost (see extract-free fraction in SI Appendix, Table S7). Together with the limited amount of soluble monosaccharides detected in the nonhydrolyzed extractive fractions, large amounts of soluble oligosaccharides were expected in the extractives. Although HPAEC analysis using an oligosaccharides column showed none of the typical 1,4-β-d-glucooligosaccharide and 1,4-β-d-xylooligosaccharide compounds, two unknown compounds had accumulated in the extractives phase of the mature comb sample (SI Appendix, Fig. S6). Attempts to identify these compounds were not successful (details provided in SI Appendix, SI Materials and Methods). Consistent with previous reports that Termitomyces fungi are genetically enriched in polysaccharide backbone-degrading enzymes and depleted in oligosaccharide-splitting enzymes (9, 16), our results demonstrate that the fungus-comb microbiome contributes to significant polysaccharide cleavage/depolymerization, leaving the mature comb enriched in oligosaccharides, particularly cellulosic oligomers/polymers.
The ecological success of fungus-cultivating termites derives from their striking ability to effect the almost complete decomposition of a broad range of plant-derived biomass, including litter, grasses, and lignin-rich woods. In this study, we show highly efficient deconstruction of wood in a fungus-cultivating termite O. formosanus, which sheds considerable new light on the delignification in the fungus-cultivating termite symbiosis. Indeed, both our NMR and thermochemolysis data compellingly reveal that the rapid first gut passage through young workers considerably depolymerizes and degrades lignin. Far beyond the young termite’s traditionally held role to simply mix asexual spores and lignocellulosic substrates (11, 28), these observations align with previous studies, providing evidence that lower termites (nonfungus-cultivating species) enable lignin sidechain oxidation (37, 44). After the first gut-passage step, the lignin degradation products in the woody residue are continuously cleaved/removed by the fungus-comb microbiome. Finally, our combined polysaccharide analyses demonstrate a complementary cooperation in polysaccharide conversion among symbionts in O. formosanus, consistent with recent genetic evidence from another fungus-cultivating species Macrotermes natalensis (9). In total, these findings establish the role of the fungus comb as the external digestive system for the fungus-cultivating termite superorganism.
Conclusion
Woody biomass has substantial potential for diverse lignocellulose industries, but its lignin makes it particularly recalcitrant, requiring the use of expensive pretreatments to depolymerize lignin. Although the white-rot/brown-rot fungi have been viewed as the paradigm for wood decay, our findings add to the growing body of contention that ectosymbiotic fungi of fungus-cultivating insects (ants and termites) are likely to lose the key delignification potential throughout evolutionary modifications (45). In the fungus-cultivating termite symbiotic system, lignin depolymerization takes place during the rapid passage through the pH-neutral gut of young worker termites (46) in a process that is striking in its speed and efficiency at destroying the traditionally considered most-recalcitrant C–C-bonded lignin structural units, thereby facilitating efficient degradation of the substrate by processes subsequently occurring via the fungus-comb microbiome. This efficient woody biomass bio-pretreatment comprised of step-wise delignification and cleavage of polysaccharides under mild conditions, therefore has considerable biotechnological potential.
Experimental Procedures
We qualitatively and quantitatively examined the mechanisms of biological pretreatment of poplar wood’s lignin and carbohydrates through each stage of the plant substrate flow using our well-developed laboratory rearing colonies of O.formosanus (12). Specifically, we: (i) combined feeding experiments through laboratory rearing colony and follow the passage of material throughout substrate processing; (ii) applied scanning electron microscopy to determine changes in plant morphology; and (iii) used chemical measurements of lignocellulosic composition, as well as 2D-NMR, Py-GC-MS, and TMAH-Py-GC-MS analyses of lignocellulosic structure to track the fate of poplar wood’s lignin and carbohydrates. All detailed experimental procedures are provided in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank D. K. Aanen, I. T. Baldwin, A. Brune, H. Horn, M. Poulsen, and R. Pi for valuable comments and discussion on this paper; R. Sun and J. Wen from Beijing Forestry University for providing the fresh poplar wood; and K. Hirth from US Forest Products Laboratory for methoxyl determination. This study was funded by the National Natural Science Foundation of China Project 31170611 (to J.M.); Zhejiang Provincial Natural Science Foundation Project Z3100211 (to J.M.); National Natural Science Foundation of China Grant Project 31500528 (to H.L.); and the Department of Energy Great Lakes Bioenergy Research Center Office of Science Grant DE-FC02-07ER64494 (to C.R.C. and J.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1618360114/-/DCSupplemental.
References
- 1.Ralph J, et al. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem Rev. 2004;3:29–60. [Google Scholar]
- 2.Chen F, Dixon RA. Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol. 2007;25:759–761. doi: 10.1038/nbt1316. [DOI] [PubMed] [Google Scholar]
- 3.Wilkerson CG, et al. Monolignol ferulate transferase introduces chemically labile linkages into the lignin backbone. Science. 2014;344:90–93. doi: 10.1126/science.1250161. [DOI] [PubMed] [Google Scholar]
- 4.Warnecke F, et al. Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature. 2007;450:560–565. doi: 10.1038/nature06269. [DOI] [PubMed] [Google Scholar]
- 5.Brune A. Symbiotic digestion of lignocellulose in termite guts. Nat Rev Microbiol. 2014;12:168–180. doi: 10.1038/nrmicro3182. [DOI] [PubMed] [Google Scholar]
- 6.Aanen DK, et al. The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc Natl Acad Sci USA. 2002;99:14887–14892. doi: 10.1073/pnas.222313099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohkuma M. Termite symbiotic systems: Efficient bio-recycling of lignocellulose. Appl Microbiol Biotechnol. 2003;61:1–9. doi: 10.1007/s00253-002-1189-z. [DOI] [PubMed] [Google Scholar]
- 8.Bignell DE. The role of symbionts in the evolution of termites and their rise to ecological dominance in the tropics. In: Hurst CJ, editor. The Mechanistic Benefits of Microbial Symbionts. Springer; Dordrecht, The Netherlands: 2016. pp. 121–172. [Google Scholar]
- 9.Poulsen M, et al. Complementary symbiont contributions to plant decomposition in a fungus-farming termite. Proc Natl Acad Sci USA. 2014;111:14500–14505. doi: 10.1073/pnas.1319718111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aylward FO, et al. Convergent bacterial microbiotas in the fungal agricultural systems of insects. MBio. 2014;5:e02077. doi: 10.1128/mBio.02077-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aanen DK. As you reap, so shall you sow: Coupling of harvesting and inoculating stabilizes the mutualism between termites and fungi. Biol Lett. 2006;2:209–212. doi: 10.1098/rsbl.2005.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, et al. Investigation of age polyethism in food processing of the fungus-growing termite Odontotermes formosanus (Blattodea: Termitidae) using a laboratory artificial rearing system. J Econ Entomol. 2015;108:266–273. doi: 10.1093/jee/tou005. [DOI] [PubMed] [Google Scholar]
- 13.Traniello JF, Leuthold RH. Behavior and ecology of foraging in termites. In: Abe Y, Bignell DE, Higashi T, editors. Termites: Evolution, Sociality, Symbioses, Ecology. Springer; Dordrecht, The Netherlands: 2000. pp. 141–168. [Google Scholar]
- 14.Krishna K, Grimaldi DA, Krishna V, Engel MS. Introduction. In: Krishna K, Grimaldi DA, Krishna V, Engel MS, editors. Treatise on the Isoptera of the World. Vol 1. American Museum of Natural History; New York: 2013. pp. 1–200. [Google Scholar]
- 15.Taprab Y, et al. Symbiotic fungi produce laccases potentially involved in phenol degradation in fungus combs of fungus-growing termites in Thailand. Appl Environ Microbiol. 2005;71:7696–7704. doi: 10.1128/AEM.71.12.7696-7704.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johjima T, Taprab Y, Noparatnaraporn N, Kudo T, Ohkuma M. Large-scale identification of transcripts expressed in a symbiotic fungus (Termitomyces) during plant biomass degradation. Appl Microbiol Biotechnol. 2006;73:195–203. doi: 10.1007/s00253-006-0570-8. [DOI] [PubMed] [Google Scholar]
- 17.Martínez AT. Molecular biology and structure-function of lignin-degrading heme peroxidases. Enzyme Microb Technol. 2002;30:425–444. [Google Scholar]
- 18.Harvey PJ, Schoemaker HE, Palmer JM. Veratryl alcohol as a mediator and the role of radical cations in lignin biodegradation by Phanerochaete chrysosporium. FEBS Lett. 1986;195:242–246. [Google Scholar]
- 19.Wariishi H, Valli K, Gold MH. In vitro depolymerization of lignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1991;176:269–275. doi: 10.1016/0006-291x(91)90919-x. [DOI] [PubMed] [Google Scholar]
- 20.Kapich AN, Jensen KA, Hammel KE. Peroxyl radicals are potential agents of lignin biodegradation. FEBS Lett. 1999;461:115–119. doi: 10.1016/s0014-5793(99)01432-5. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Deng T, Pan C, Chen C, Mo J. Purification of a laccase from fungus combs in the nest of Odontotermes formosanus. Process Biochem. 2010;45:1052–1056. [Google Scholar]
- 22.Bugg TD, Ahmad M, Hardiman EM, Rahmanpour R. Pathways for degradation of lignin in bacteria and fungi. Nat Prod Rep. 2011;28:1883–1896. doi: 10.1039/c1np00042j. [DOI] [PubMed] [Google Scholar]
- 23.Hyodo F, Inoue T, Azuma JI, Tayasu I, Abe T. Role of the mutualistic fungus in lignin degradation in the fungus-growing termite Macrotermes gilvus (Isoptera; Macrotermitinae) Soil Biol Biochem. 2000;32:653–658. [Google Scholar]
- 24.Mathew GM, et al. Synergistic collaboration of gut symbionts in Odontotermes formosanus for lignocellulosic degradation and bio-hydrogen production. Bioresour Technol. 2013;145:337–344. doi: 10.1016/j.biortech.2012.12.055. [DOI] [PubMed] [Google Scholar]
- 25.Hyodo F, et al. Differential role of symbiotic fungi in lignin degradation and food provision for fungus-growing termites (Macrotermitinae: Isoptera) Funct Ecol. 2003;17:186–193. [Google Scholar]
- 26.Nobre T, Rouland-Lefèvre C, Aanen DK. Comparative biology of fungus cultivation in termites and ants. In: Bignell DE, Roisin Y, Lo N, editors. Biology of Termites: A Modern Synthesis. Springer; Dordrecht, The Netherlands: 2010. pp. 193–210. [Google Scholar]
- 27.Ono K, Hata T, Yoshimura T, Kinjo K. Wood decaying properties of the termite mushroom Termitomyces eurrhizus. J Wood Sci. 2017;63:83–94. [Google Scholar]
- 28.Leuthold R, Badertscher S, Imboden H. The inoculation of newly formed fungus comb with Termitomyces in Macrotermes colonies (Isoptera, Macrotermitinae) Insectes Soc. 1989;36:328–338. [Google Scholar]
- 29.Mansfield SD, Kim H, Lu F, Ralph J. Whole plant cell wall characterization using solution-state 2D NMR. Nat Protoc. 2012;7:1579–1589. doi: 10.1038/nprot.2012.064. [DOI] [PubMed] [Google Scholar]
- 30.Kim H, Ralph J. Solution-state 2D NMR of ball-milled plant cell wall gels in DMSO-d6/pyridine-d5. Org Biomol Chem. 2010;8:576–591. doi: 10.1039/b916070a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yelle DJ, Ralph J, Lu F, Hammel KE. Evidence for cleavage of lignin by a brown rot basidiomycete. Environ Microbiol. 2008;10:1844–1849. doi: 10.1111/j.1462-2920.2008.01605.x. [DOI] [PubMed] [Google Scholar]
- 32.Martínez AT, et al. Selective lignin and polysaccharide removal in natural fungal decay of wood as evidenced by in situ structural analyses. Environ Microbiol. 2011;13:96–107. doi: 10.1111/j.1462-2920.2010.02312.x. [DOI] [PubMed] [Google Scholar]
- 33.Yelle DJ, Wei D, Ralph J, Hammel KE. Multidimensional NMR analysis reveals truncated lignin structures in wood decayed by the brown rot basidiomycete Postia placenta. Environ Microbiol. 2011;13:1091–1100. doi: 10.1111/j.1462-2920.2010.02417.x. [DOI] [PubMed] [Google Scholar]
- 34.Tran F, Lancefield CS, Kamer PCJ, Lebl T, Westwood NJ. Selective modification of the β-β linkage in DDQ-treated Kraft lignin analysed by 2D NMR spectroscopy. Green Chem. 2015;17:244–249. [Google Scholar]
- 35.Shuai L, et al. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science. 2016;354:329–333. doi: 10.1126/science.aaf7810. [DOI] [PubMed] [Google Scholar]
- 36.Katsumata KS, Jin Z, Hori K, Liyama K. Structural changes in lignin of tropical woods during digestion by termite, Cryptotermes brevis. J Wood Sci. 2007;53:419–426. [Google Scholar]
- 37.Geib SM, et al. Lignin degradation in wood-feeding insects. Proc Natl Acad Sci USA. 2008;105:12932–12937. doi: 10.1073/pnas.0805257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ke J, Laskar DD, Chen S. Biodegradation of hardwood lignocellulosics by the western poplar clearwing borer, Paranthrene robiniae (Hy. Edwards) Biomacromolecules. 2011;12:1610–1620. doi: 10.1021/bm2000132. [DOI] [PubMed] [Google Scholar]
- 39.Li H, et al. Age polyethism drives community structure of the bacterial gut microbiota in the fungus-cultivating termite Odontotermes formosanus. Environ Microbiol. 2016;18:1440–1451. doi: 10.1111/1462-2920.13046. [DOI] [PubMed] [Google Scholar]
- 40.Mathew GM, Ju YM, Lai CY, Mathew DC, Huang CC. Microbial community analysis in the termite gut and fungus comb of Odontotermes formosanus: The implication of Bacillus as mutualists. FEMS Microbiol Ecol. 2012;79:504–517. doi: 10.1111/j.1574-6941.2011.01232.x. [DOI] [PubMed] [Google Scholar]
- 41.del Río JC, Gutiérrez A, Rodríguez IM, Ibarra D, Martínez AT. Composition of non-woody plant lignin and cinamic acid by Py-GC-MS, Py/TMAH and FT-IR. J Agric Food Chem. 2007;79:39–46. [Google Scholar]
- 42.Filley T, Hatcher P, Shortle W, Praseuth R. The application of 13C-labeled tetramethylammonium hydroxide (13C-TMAH) thermochemolysis to the study of fungal degradation of wood. Org Geochem. 2000;31:181–198. [Google Scholar]
- 43.Pope PB, et al. Adaptation to herbivory by the Tammar wallaby includes bacterial and glycoside hydrolase profiles different from other herbivores. Proc Natl Acad Sci USA. 2010;107:14793–14798. doi: 10.1073/pnas.1005297107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ke J, Laskar DD, Singh D, Chen S. In situ lignocellulosic unlocking mechanism for carbohydrate hydrolysis in termites: Crucial lignin modification. Biotechnol Biofuels. 2011;4:17. doi: 10.1186/1754-6834-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nygaard S, et al. Reciprocal genomic evolution in the ant-fungus agricultural symbiosis. Nat Commun. 2016;7:12233. doi: 10.1038/ncomms12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H, et al. Physicochemical conditions and metal ion profiles in the gut of the fungus-growing termite Odontotermes formosanus. J Insect Physiol. 2012;58:1368–1375. doi: 10.1016/j.jinsphys.2012.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.