Significance
Recent evidence suggests human retinal Müller glia retain a potential for neuronal regeneration. Defining the mechanisms governing retinal repair in robustly regenerative species may provide insights for harnessing this potential therapeutically. Here, we investigated roles of the innate immune system during rod photoreceptor regeneration in zebrafish. Our data establish a role for retinal microglia, the tissue-resident macrophage of the retina, in regulating retinal Müller glia responsiveness to cell death, and thereby controlling photoreceptor regeneration kinetics. Further, we show that immunosuppression can either inhibit or accelerate photoreceptor regeneration kinetics depending on the timing of treatment. We conclude that modulation of immune cell responses to retinal neuron cell death stands as a promising strategy for promoting repair of the human eye.
Keywords: retina, microglia, glucocorticoid, regeneration, macrophage
Abstract
Müller glia (MG) function as inducible retinal stem cells in zebrafish, completely repairing the eye after damage. The innate immune system has recently been shown to promote tissue regeneration in which classic wound-healing responses predominate. However, regulatory roles for leukocytes during cellular regeneration—i.e., selective cell-loss paradigms akin to degenerative disease—are less well defined. To investigate possible roles innate immune cells play during retinal cell regeneration, we used intravital microscopy to visualize neutrophil, macrophage, and retinal microglia responses to induced rod photoreceptor apoptosis. Neutrophils displayed no reactivity to rod cell loss. Peripheral macrophage cells responded to rod cell loss, as evidenced by morphological transitions and increased migration, but did not enter the retina. Retinal microglia displayed multiple hallmarks of immune cell activation: increased migration, translocation to the photoreceptor cell layer, proliferation, and phagocytosis of dying cells. To test function during rod cell regeneration, we coablated microglia and rod cells or applied immune suppression and quantified the kinetics of (i) rod cell clearance, (ii) MG/progenitor cell proliferation, and (iii) rod cell replacement. Coablation and immune suppressants applied before cell loss caused delays in MG/progenitor proliferation rates and slowed the rate of rod cell replacement. Conversely, immune suppressants applied after cell loss had been initiated led to accelerated photoreceptor regeneration kinetics, possibly by promoting rapid resolution of an acute immune response. Our findings suggest that microglia control MG responsiveness to photoreceptor loss and support the development of immune-targeted therapeutic strategies for reversing cell loss associated with degenerative retinal conditions.
Neurodegenerative diseases are caused by the loss of discrete neuronal cell types. The goal of regenerative medicine is to reverse this process. One strategy for doing so involves harnessing the regenerative potential of endogenous neural stem cells. Unfortunately, although adult neural stem cells exist in the mammalian CNS, innate potentials for neuronal repair remain dormant in the absence of exogenous stimulation. Conversely, many other species are endowed with remarkable capacities for neuronal regeneration. For instance, in the zebrafish eye, retinal Müller glia (MG) cells can dedifferentiate to a stem cell-like state and give rise to progenitors that replace lost neurons and restore visual function (1, 2). Intriguingly, human MG cells are able to give rise to new neurons in culture (3) that can restore visual function when transplanted into mammalian disease models (4), suggesting that human MG retain a stem cell-like capacity for mediating retinal repair. Mechanisms controlling the regenerative potential of MG are therefore of great interest. We and others study zebrafish to learn more about how retinal regeneration is controlled, with the hope of revealing therapeutic strategies for stimulating repair of the human eye.
Over the past decade, significant progress has been made in revealing molecular factors that regulate retinal regeneration (5–7). However, less is known about the cellular composition of the retinal stem cell niche. Recent studies have implicated innate immune cells in regulating tissue regeneration in the limb (8, 9), heart (10), and brain (11). In addition, discrete immune cell types react to selective neuronal cell ablation in the peripheral (12) and central nervous systems in regenerative species (13–15). However, whether the immune system regulates regeneration following the selective loss of individual neuronal cell types (i.e., in paradigms akin to neurodegenerative disease) remains unknown. We therefore investigated whether specific innate immune cell types impact the regenerative potential of zebrafish MG following selective ablation of rod photoreceptor cells, a physiological model of retinitis pigmentosa.
Zebrafish MG function as stem cells across a wide range of injury paradigms with most studies involving light damage, toxin injection, or puncture wounding (5–7). The scale of injury likely affects which immune cell types are responsive, potentially altering which roles the immune system plays during regeneration. We adapted an inducible cell-type specific ablation system (16) to zebrafish for the purpose of modeling degenerative diseases in a regenerative species (17). This strategy involves cell-specific transgenic expression of the bacterial prodrug-converting enzyme nitroreductase (NTR). Exposing transgenic fish to prodrug substrates such as metronidazole (Mtz) results in the selective elimination of NTR-expressing cells, thus facilitating cell-specific regenerative biology paradigms (17). Importantly, the NTR/Mtz-based ablation system elicits apoptotic cell death (18), a hallmark of neurodegenerative disease (19). To model retinitis pigmentosa, we created a transgenic line expressing a YFP-nitroreductase fusion protein specifically in rod photoreceptor cells―Tg(rho:YFP-Eco.NfsB)gmc500 (hereafter, rho:YFP-NTR)―enabling Mtz-induced ablation of rod cells (20). To investigate neuroimmune interactions occurring during retinal degeneration, intravital multicolor time-lapse imaging (14, 21, 22) was used to monitor and quantify the behavioral responses of transgenically labeled immune cell types to rod cell death.
Immune cell subtypes play well-defined roles during infection and wound healing (23). Somewhat paradoxically, during neurodegenerative disease, microglia (resident macrophages of the CNS) and/or monocyte-derived macrophages have been reported to play neuroprotective (24) and neurotoxic roles (25). Macrophages can cycle between proreparative (M2) and antireparative (M1) polarization states (26). However, whether this conceptual framework applies to microglia is controversial (27). Simplified, acute immune system reactivity to CNS damage may be beneficial if the inflammatory response is resolved in a timely manner (28). Conversely, chronic immune system activation in the CNS is associated with deleterious inflammatory signaling cascades (25, 28). Comparisons between regenerative and nonregenerative model systems could help clarify which aspects of immune system reactivity are pro- versus antireparative. However, correlations between the polarization states of reactive immune cells during injury and repair in regenerative model systems have begun to be explored only recently (29).
Microglia have been shown to translocate to damaged retinal layers in mammalian (30), chick (31), and fish retinas (32), but functional roles during retinal regeneration remain undefined. In the chicken retina, microglia and/or macrophages are required for exogenous factor stimulation of MG/progenitor cell proliferation following excitotoxic injury (33). Conversely, activated microglia-associated signaling molecules, such as IL-6, CXCL10, and TNF-α, have been shown to inhibit human retinal stem/progenitor proliferation and differentiation in culture (34). However, because chicken and mammalian retinas have a limited capacity to regenerate (35), it can be unclear to what extent retinal stem/progenitor cell proliferation in these systems is indicative of a pro- or anti-reparative response (i.e., proliferative gliosis).
In zebrafish, the innate immune system is active by day 1 of embryogenesis (36). In contrast, the adaptive immune system is not functional until ∼4 wk later (37). This period provides a window to explore the roles played by innate immune cells in isolation from adaptive immune system involvement. We previously developed high-resolution intravital imaging techniques for characterizing interactions between discretely labeled cell types during development (21) and regeneration in zebrafish (14, 22). This approach was used here to determine which innate immune cell subtypes are responsive to rod photoreceptor apoptosis. Neutrophil, microglia, and macrophage migration patterns were quantified following the induction of rod cell loss and were compared with retinal puncture wounds to explore how the scale of injury impacts the responsiveness of different immune cell types. Importantly, the ability to track migratory behaviors of individual cells over time allowed us to delineate retinal microglia responses from those of peripheral macrophages, two innate immune cell types that often are conflated because of a lack of definitive markers (33).
To test functional roles during regeneration, we coablated innate immune cell subtypes and rod cells or applied immune suppression and quantified the kinetics of MG/progenitor cell proliferation and rod cell regeneration. The data showed that MG/progenitor proliferation and rod cell regeneration kinetics were dependent on macrophage/microglia reactivity. Interestingly, by varying the timing of immune suppression, we were able to reveal both pro- and antireparative phases of the immune response, i.e., conditions which either inhibited or accelerated the kinetics of rod cell regeneration depending on when immunomodulation was applied. These data strongly implicate the innate immune system in shaping the responsiveness of MG to selective rod photoreceptor loss and thereby regulating the kinetics of retinal cell regeneration. From these findings, combined with recent related studies in the zebrafish and salamander brain (13, 15, 38), we conclude that microglia function to promote neuronal regeneration following selective neuronal cell loss. Accordingly, a more thorough understanding of the role specific immune cell types play in shaping the regenerative potential of endogenous neural stem cells could help inform the development of therapeutic strategies aimed at reversing damage incurred by neurodegenerative disease.
Results
Intravital Imaging of Innate Immune Cell Responses to Rod Cell Loss in Larval Zebrafish.
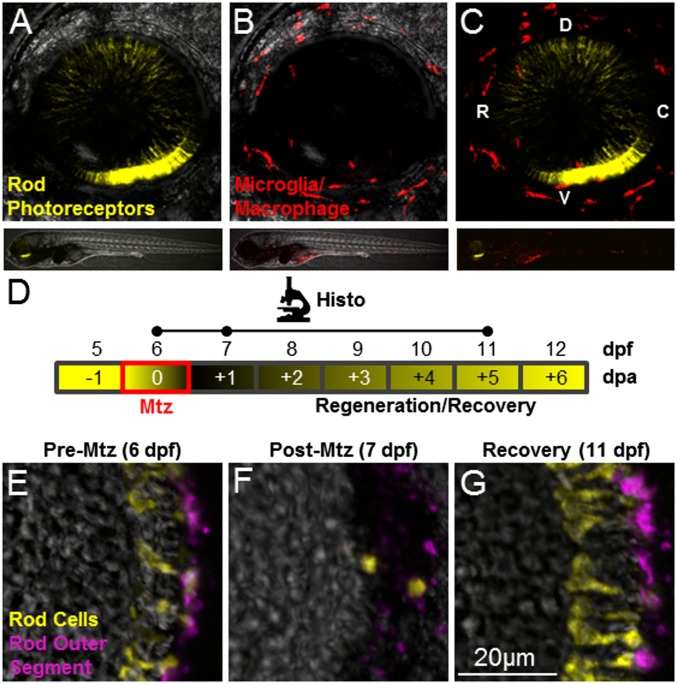
The innate immune system has been implicated in promoting epimorphic and tissue regeneration (8–11); however its roles during cellular regeneration have only begun to be explored (15). We therefore investigated whether the innate immune system is responsive to selective ablation of rod photoreceptors using transgenic larvae in which rod cells and immune cells were labeled with the complementary fluorescent reporters rho:YFP-NTR and mpeg1.1:LOX2272-LOXP-tdTomato-LOX2272-Cerulean-LOXP-EYFP (39) (hereafter, mpeg1:Tomato) (Fig. 1 A–C and Movie S1). To account for dynamic behaviors of activated immune cells, multicolor intravital time-lapse imaging (14, 21, 22) was used to track and quantify immune cell migration patterns. Confocal 3D scans of the entire retina were taken either during or immediately after retinal injury at 10- or 20-min intervals over 20-h time periods. To induce rod cell ablation, larvae were exposed to Mtz for ∼24 h at 6 d postfertilization (dpf), after retinal histogenesis was complete (schematic in Fig. 1D and Fig. 1 E and F). Under these conditions, after 5–6 d of recovery, newly generated rod cells repopulate the outer nuclear layer (ONL) (Fig. 1G).
Fig. 1.
Intravital imaging and prodrug-induced cell ablation. (A–C) Whole-retina z-projection images of double-transgenic (rho:YFP-NTR;mpeg1:Tomato) 6-dpf larvae in which rod cells express YFP-NTR (A and C, yellow), and macrophage/microglia express tdTomato (B and C, red). The upper images show the whole eye (34× magnification); the lower images show the whole fish (4× magnification). (D) Schematic of the assay and analysis timeline for data shown in E–G. Retinal sections were processed for immunohistology (Histo) and imaged at time points indicated by dots on the Histo line. Mtz exposures occurred over a 24-h span at 6 dpf (red box) with loss and subsequent rod cell regeneration (black/yellow gradient) occurring over a weeklong recovery period (−1 to +6 dpa). (E–G) Retinal sections from a rho:YFP-NTR transgenic larva treated with Mtz (10 mM) for 24 h, starting at 6 dpf (E), demonstrating ablation (F), and subsequent regeneration (G) of rod photoreceptors indicated by the loss and recovery of YFP-expressing rod cells (yellow) and a rod outer segment marker (magenta, monoclonal antibody zpr-3, also known as “Fret 11”). C, caudal; D, dorsal; R, rostral; V, ventral.
Neutrophil reactivity.
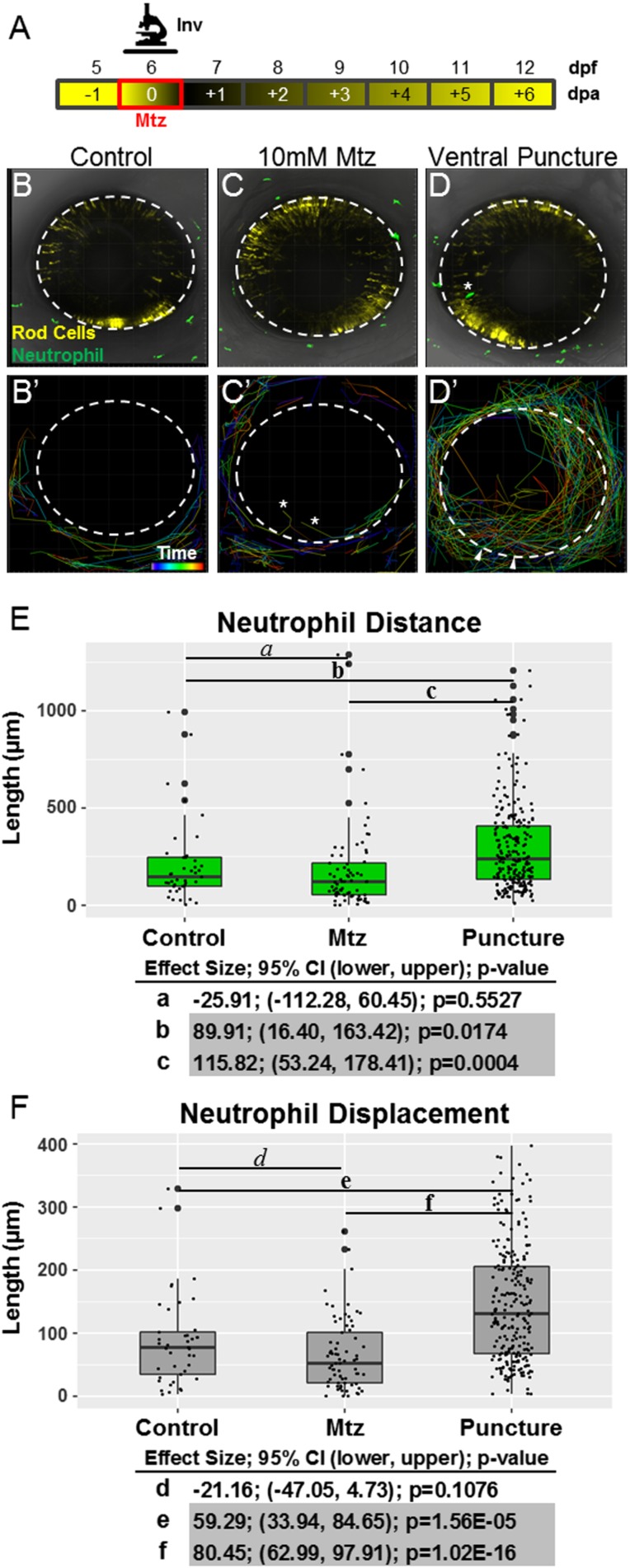
We first asked if neutrophils respond to rod cell loss. A transgenic line labeling neutrophils with GFP, Tg(mpx:GFP)uwm1 (hereafter, mpx:GFP) (40), enabled intravital time-lapse imaging of neutrophil behaviors (Fig. S1A, schematic). Puncture wounding of the eye (6) was used as a control for immune cell activation, and negative-control larvae were exposed only to confocal laser light. No evidence of neutrophil reactivity or infiltration of the eye was seen in negative controls (Fig. S1 B and B′ and Movie S2) or in Mtz-treated retinas (Fig. S1 C and C′and Movie S3). Conversely, upon puncture wounding neutrophils infiltrated the retina (Fig. S1 D and D′and Movie S4) and increased migration distance and displacement (Fig. S1 E and F). These data demonstrate that rod photoreceptor apoptosis is not sufficient to elicit neutrophil reactivity, decreasing the likelihood that neutrophils modulate retinal regeneration following selective retinal cell loss.
Fig. S1.
Neutrophils respond to retinal puncture wounds but not to rod cell ablation. (A) Schematic of timelines for intravital imaging assays performed in B–F. The line denotes the time during which images were collected. (B–D) Whole-retina, 20-h intravital time-lapse sequences (image interval, 20 min) were collected from 6-dpf double-transgenic rho:YFP-NTR;mpx:GFP larvae with YFP-NTR–expressing rod cells (yellow) and GFP-expressing neutrophils (green), and individual neutrophil migration paths were quantified (34× magnification). Representative z-projection confocal images of 6-dpf larvae from untreated control (B and B′), Mtz-treated (C and C′), and puncture wound (D and D′) groups. B′, C′, and D′ show the migration paths of tracked neutrophils; rainbow dragon tails show the position of each neutrophil over the 20-h time-lapse sequence (purple to red line segments). No evidence of neutrophil infiltration into the neural retina was observed in either the untreated control (B′) or rod cell-ablated (C′) condition. The asterisks mark the paths of neutrophils that migrated into extraretinal tissue over the eye. Conversely, puncture wounding (denoted by arrowheads in D′) permitted robust chemotaxis to the injury site and neutrophil infiltration into the neural retina. (E and F) Quantification of neutrophil migration patterns. Box plots show distance (E) and displacement (F) patterns across all three conditions with data from individual neutrophils displayed as a dot blot overlay (larger dots denote outliers). Increased neutrophil migration behaviors are evident only in puncture-wounded retinas. Statistical comparisons (Student’s t test) between paired groups (effect size, 95% confidence intervals, and P value) are provided in the tables below the graphs. (Note: Pairs in which CIs suggest reproducible findings are shaded gray).
Delineating peripheral macrophage and retinal microglia cells.
Because of a lack of definitive markers, it has been difficult to delineate specific roles for peripheral macrophages versus tissue-resident microglia in the CNS, and these cells are often referred to collectively as “macrophage/microglia.” Nevertheless, macrophages and microglia show distinct activity with regard to disease/injury responses (41) and differ in their capacity to assume different pro- and antiinflammatory polarization states (27). Thus, it is important that their relative contributions to different injury paradigms be defined. To circumvent this limitation, we performed 20-h intravital time-lapse imaging assays using 6-dpf transgenic larvae in which macrophages and microglia were labeled with a red fluorescent reporter, mpeg1:Tomato (Fig. 2A). By tracking individual cells, we could determine the location of each cell before injury (Fig. 2 B and B′, C and C′, and D and D′) and their relative reactivity and migration patterns following injury (Fig. 2 B′′–D′′), thus distinguishing peripheral macrophages (Fig. 2 B′–D′, red pseudocolored cells) from retinal microglia (Fig. 2 B′–D′, yellow pseudocolored cells). Previously, we showed that macrophages and/or microglia responded to rod cell loss (12); however, the presence of pigmented cells (i.e., iridophores) limited our ability to track individual cell behaviors accurately. Here we resolved this issue by using a mutant lacking iridophores (roy orbison).
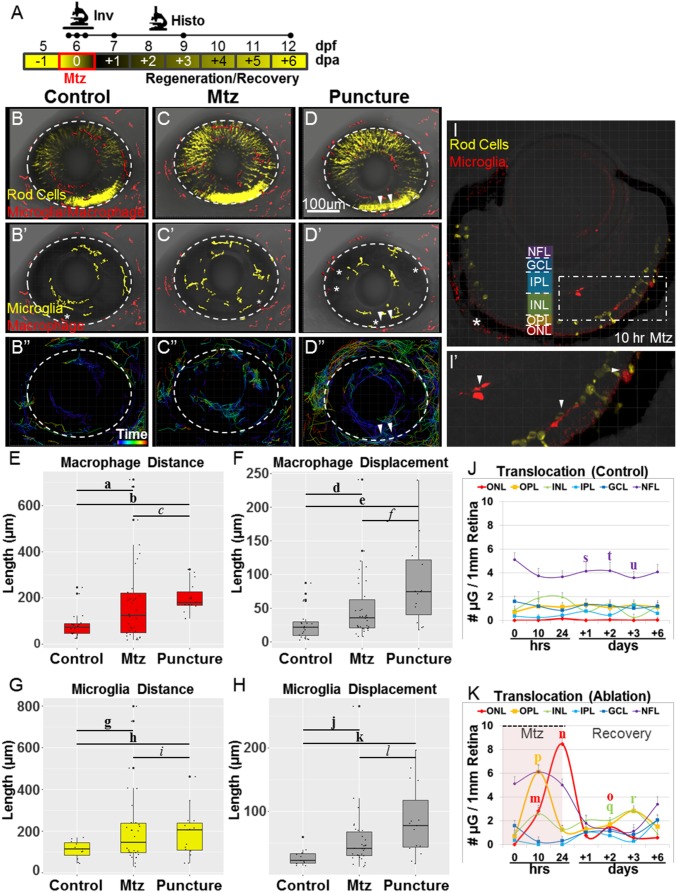
Fig. 2.
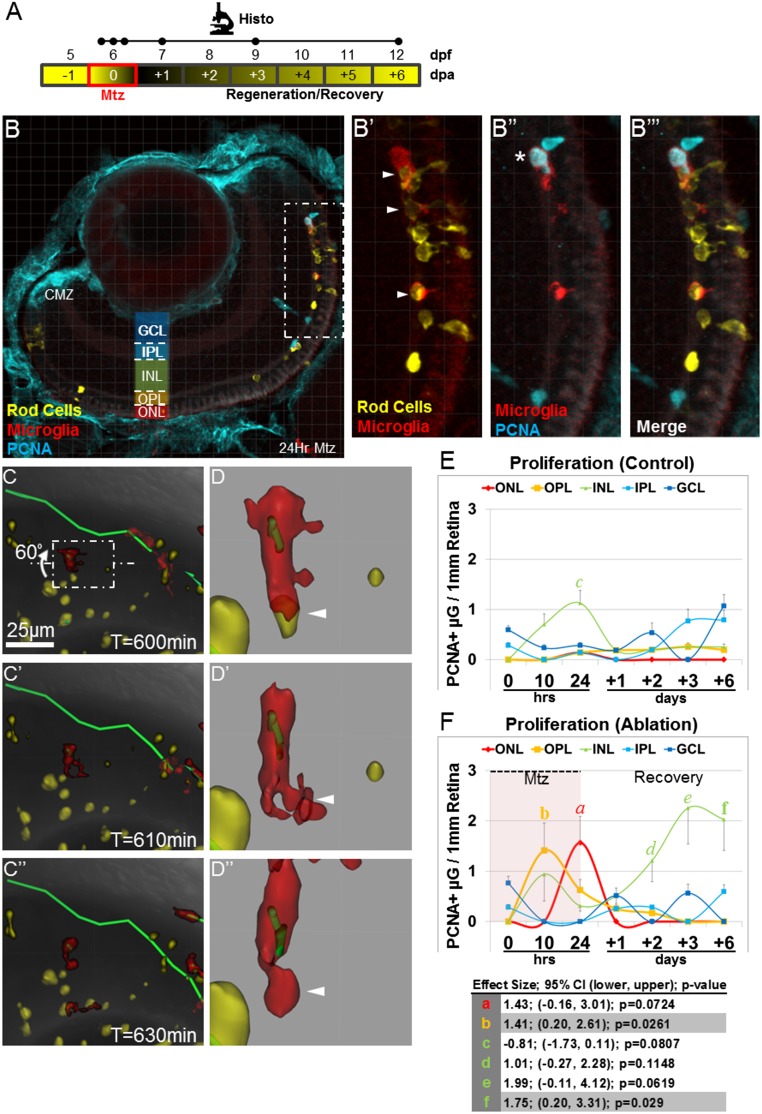
Only microglia act in the retina following rod cell ablation. (A) Schematic of timelines for intravital (Inv; the line denotes a 20-h time-lapse) and histological (Histo; the initial three dots on the Histo line denote t = 0, 10, and 24 h after exposure to Mtz) assays. (B–D) Whole-retina time-lapse sequences (10-min time interval) were collected from 6-dpf larvae with YFP-NTR–expressing rod cells (yellow in B–D) and Tomato-expressing macrophage/microglia (red in B–D). Immune cell migration paths were quantified in noninjured control (B–B′′), Mtz-treated (C–C′′), and puncture-wounded (D–D′′) retinas. B–D show representative z-projection images taken before the induction of rod cell death or puncture wounding (t = 0). B′–D′ are false-color renderings of the mpeg1:Tomato channel showing the initial positions of microglia (yellow) and macrophages (red); asterisks mark cells present in extraretinal tissue. B′′–D′′ show dragon-tail migration paths of tracked microglia and macrophage cells. Macrophages responded to rod cell death by increasing migration (C′′) but entered the retina only after puncture wounding (D′′; puncture sites are denoted by arrowheads). (E–H) Quantification of macrophage (E and F) and microglia (G and H) migration demonstrates reactivity following rod cell ablation and puncture wounding. (I–K) Somal translocation of microglia was evaluated in control (J) and Mtz-treated larvae (I, I′, and K). (I) Seven-day-postfertilization retina following 10 h of Mtz treatment (56× magnification, asterisk indicates autofluorescence). (I′) An enlarged view of the boxed region in I shows microglia in the INL, OPL, and ONL (arrowheads). (J and K) Quantification of microglia somal position showed no evidence of translocation in controls (J) but increased numbers in the OPL and ONL, concomitant to decreases in the NFL, following rod cell ablation (K). Statistics (effect sizes, 95% confidence intervals, and P values for paired comparisons denoted by letters) are provided in Table S1. For J–K, only pairs made between time point-matched control (J) and experimental (K) data showing reproducible differences are labeled with a bolded letter (error bars indicate SEM). For all others, bolded letters denote pairs showing reproducible differences, and italicized letters denote pairs with no discernible differences.
Table S1.
Statistical analysis of data in Fig. 2
| Pair | Effect size | 95% CI | P value |
| a | 102.08 | 32.65, 171.52 | 0.0049 |
| b | 116.63 | 72.29, 160.96 | 9.61E-6 |
| c | 14.54 | −58.33, 87.42 | 0.6987 |
| d | 23.49 | 3.51, 43.46 | 0.0221 |
| e | 58.30 | 22.47, 94.13 | 0.0031 |
| f | 34.82 | −2.78, 72.42 | 0.0679 |
| g | 102.70 | 28.19, 177.21 | 0.0083 |
| h | 76.84 | 9.69, 143.99 | 0.0271 |
| i | −25.86 | −117.00, 65.28 | 0.5698 |
| j | 26.65 | 7.22, 46.08 | 0.0085 |
| k | 57.28 | 25.24, 89.32 | 0.0016 |
| l | 30.63; | −4.37, 65.52 | 0.0835 |
| m | 2.82 | 1.74, 3.90 | 0.0001 |
| n | 8.31 | 6.77, 9.86 | 8.56E-7 |
| o | 1.30 | 0.08, 2.51 | 0.0369 |
| p | 4.93 | 2.71, 7.15 | 0.0002 |
| q | 1.86 | 0.71, 3.02 | 0.0032 |
| r | 3.12 | 1.01, 5.25 | 0.008 |
| s | −2.34 | −3.87, −0.80 | 0.0044 |
| t | −2.10 | −3.91, −0.30 | 0.0239 |
| u | −2.46 | −4.20, −0.71 | 0.0082 |
Bolded letters denote pairs showing reproducible differences; italicized letters denote pairs with no discernible differences.
Macrophage and microglia reactivity.
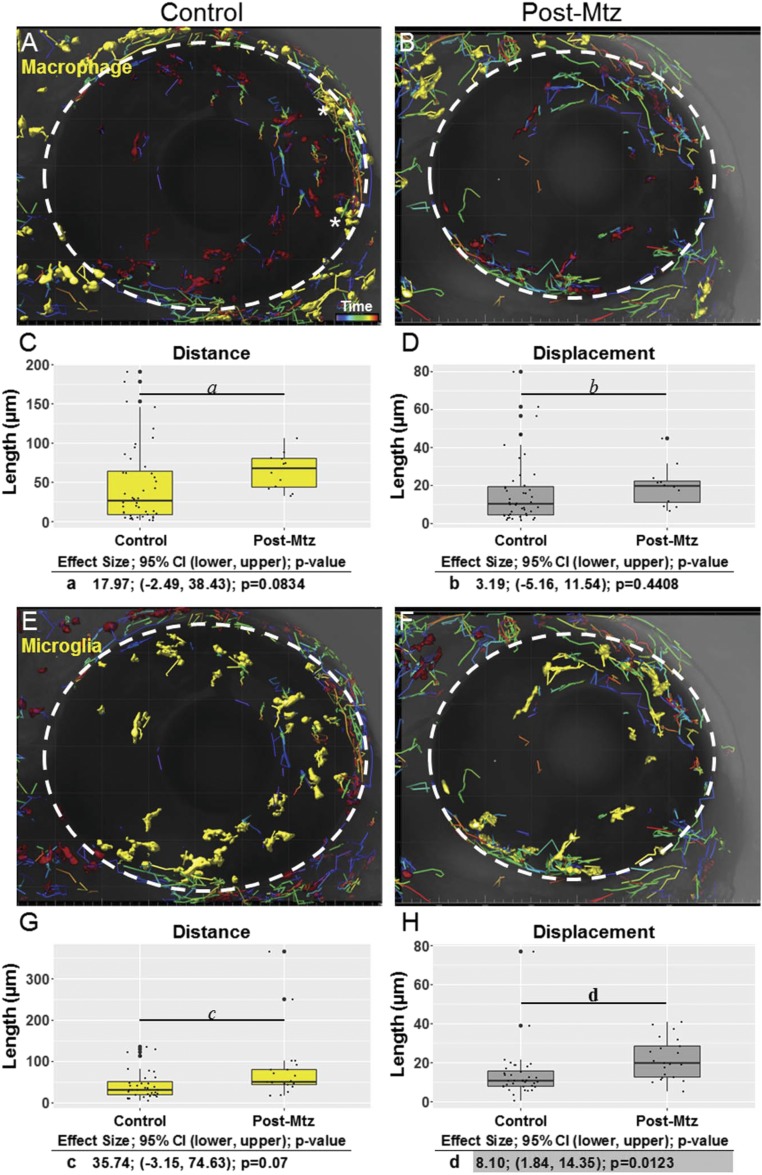
No evidence of macrophage or microglia reactivity was observed in uninjured controls (Fig. 2 B′′, E, and F and Movie S5). Conversely, macrophage and microglia cells transitioned from a ramified to amoeboid morphology and became migratory following rod cell ablation and after puncture wounding (Movies S6 and S7). Quantification of migration patterns confirmed macrophage (Fig. 2 E and F) and microglia (Fig. 2 G and H) reactivity to rod cell ablation and puncture wounds, showing increased migration distance (Fig. 2 E and G) and displacement (Fig. 2 F and H). Interestingly, however, despite showing clear reactivity, peripheral macrophages were never observed to enter the retina following rod cell loss (Movie S6). Conversely, macrophages did infiltrate the retina following puncture wounding (Movie S7). Thus, no evidence was found for a direct role of macrophages within the retina in the absence of a physical disruption of the blood–retina barrier (BRB). Macrophage and microglia migration behaviors were investigated further by collecting time-lapse images during the first day of recovery, i.e., day 7 or +1 d postablation (dpa) (Fig. S2). Macrophages (Fig. S2 A and B) showed an increase in migration distance (Fig. S2C) but no change in migration displacement (Fig. S2D), whereas microglia cells (Fig. S2 E and F) exhibited increased migration distance (Fig. S2G) and displacement (Fig. S2H). These data suggest that any role macrophages play in shaping retinal stem cell reactivity to rod cell death would need to be mediated by secreted factors (e.g., cytokines). Conversely, reactive microglia have direct physical access to dying cells, MG stem cells, and MG-derived progenitors following selective retinal neuron loss. Thus, of the three innate immune cell types evaluated, we concluded that microglia exhibit the greatest potential for influencing the regenerative process, and we concentrated subsequent efforts on further characterization of microglia cells during retinal injury and repair.
Fig. S2.
Macrophage/microglia migration postablation during the recovery phase. Confocal time-lapse microscopy was used to characterize cell migration after Mtz-induced ablation of rod photoreceptor cells in the retina. (A and B) The macrophages selected for tracking are highlighted (yellow) in control (A) and ablated (B) retinas. (C and D) Boxplots representing migration path distance (yellow, C) and displacement (gray, D) of macrophages over 20 h in control (untreated) fish and after Mtz-induced ablation. (E and F) Microglia selected for tracking are highlighted (yellow) in control (E) and ablated (F) retinas. (G and H) Boxplots representing migration path distance (yellow, G) and displacement (gray, H) over 20 h of microglia in both control (untreated) retinas and in retinas with Mtz-induced ablation. The macrophages marked by asterisks in A are subconjunctival, exterior to the RPE. The eye boundary is indicated by the dashed circle in A, B, E, and F (34× magnification). In C, D, G, and H, outliers are represented by large black dots centered on the y axis. For statistical comparisons, see the tables below the respective graphs.
Additional Hallmarks of Microglia Activation Following Rod Cell Ablation.
Microglia translocation to the photoreceptor layer.
We next asked whether microglia translocated to the photoreceptor layer following rod cell death. For this analysis, a time series of fixed retinal sections spanning several days (Fig. 2A) was imaged (Fig. 2 I and I′), and the number of microglia cell bodies per each retinal layer was quantified. Embryonic and larval zebrafish microglia cell bodies have been shown to reside in the nerve fiber layer (NFL, or vitreal surface), ganglion cell layer, and inner nuclear layer (INL) but to be largely excluded from the photoreceptor and plexiform layers (42), in contrast to observations in mammalian systems (43). Data from uninjured controls are in agreement with these findings; the majority of microglia localized to the NFL and showed no evidence of active translocation between different retinal layers in the absence of injury (Fig. 2J). Strikingly, microglia migrated to the somal and synaptic photoreceptor layers (the ONL and the outer plexiform layer, OPL) within 10 h of Mtz exposure (Fig. 2K), coinciding with the rapid induction of rod photoreceptor death observed previously (14). Following Mtz removal, microglia occupation of the outer retinal layers decreased (Fig. 2J), presumably through either reverse migration or apoptosis (13). Interestingly, microglia did not appear to return to the NFL immediately but remained proximal to MG/progenitor cells (Fig. 2K).
Microglia phagocytosis of rod cell debris.
Phagocytosis of dying cells is a hallmark of activated immune cells (44). We therefore asked whether YFP-labeled photoreceptor debris could be found within microglia cell bodies following the induction of rod cell death (Fig. S3A). Phagocytosis of apoptotic photoreceptor fragments by microglia was observed both in retinal sections (Fig. S3 B–B′′′) and through intravital time-lapse microscopy (Fig. S3 C–C′′′) during the Mtz treatment period and after Mtz removal (i.e., over the next 24 h of the recovery phase). For the latter period, we show a microglia cell making initial contact with a large photoreceptor fragment and engulfing it over the next 30 min (arrowheads in Fig. S3 D–D′′ and Movie S8).
Fig. S3.
Microglia exhibit additional hallmarks of innate immune cell activation following rod cell ablation. (A) Schematic of assay timelines for data shown in B–F. (B) Representative retinal section of 7-dpf rho:YFP-NTR;mpeg1:Tomato larvae immunostained for PCNA (blue) after 24-h exposure to Mtz (56× magnification). The boxed region in B (enlarged in B′ and B′′) shows evidence of microglia phagocytosis of rod cell debris and microglia proliferation. Arrowheads in B′ denote yellow rod cell debris within red microglia. The asterisk in B′′ denotes red/blue double-positive microglia. A merged image is shown in B′′′. (C–C′′) Time-lapse sequence showing microglia (red cell) phagocytosis of rod cell debris (yellow). (D–D′′) The boxed region in C was enlarged and rotated 60° to provide additional detail (arrowheads). (E and F) Quantification of microglia proliferation (PCNA immunoreactivity) within defined somal regions in untreated control (E) and Mtz-treated (F) retinas, plotted over time. (E) Control retinas showed little to no proliferation in most somal layers. (F) Mtz-treated retinas showed increased microglia proliferation in photoreceptor regions (OPL, yellow line; ONL, red line) during the time of rod cell death and in the INL (green line) at later time points. Paired statistical comparisons (Student’s t test) made between time point-matched control (E) and experimental (F) data are denoted by a unique letter color coded according to retinal layer. Error bars in E and F indicate SEM. Effect size, 95% confidence intervals, and P values are provided in the table. (Note: Only pairs showing statistically validated differences are listed.) CMZ, ciliary margin zone.
Microglia proliferation in response to rod cell death.
Reactive innate immune cells are known to proliferate at the site of injury (44). To investigate the possibility that microglia cells proliferate in response to rod cell loss, we performed immunohistochemical staining of retinal sections using an antibody against proliferating cell nuclear antigen (PCNA). Concomitant with microglia chemotaxis to the ONL and OPL (Fig. 2), an increase in microglia (PCNA+/Tomato+) proliferation at the site of rod cell ablation was observed (Fig. S3 E and F). Following Mtz removal, microglia underwent a second wave of proliferation within the INL during the recovery phase. Interestingly, this second wave was coincident with the initiation of rod cell regeneration (20).
Microglia/Macrophages Promote Retinal Stem Cell/Progenitor Cell Responsiveness to Rod Cell Ablation.
To define the roles played by microglia/macrophages during retinal regeneration, we investigated the consequences of coablating microglia/macrophages and rod photoreceptors. A transgenic line coexpressing YFP and an improved NTR mutant protein (45) in microglia/macrophages (8), Tg(mpeg1.1:NTR-EYFP)w202 (hereafter, mpeg1:NTR-YFP), was crossed to rho:YFP-NTR;mpeg1:Tomato fish to enable Mtz-induced coablation. In addition, to facilitate visualization of MG, a fourth transgenic line labeling MG with GFP (46), Tg(gfap:GFP)mi2001 (hereafter, gfap:GFP), was introduced to rho:YFP-NTR;mpeg1:Tomato;mpeg1:NTR-YFP fish. To delineate GFP+ MG from YFP+ microglia and rod cells, previously established methods for separating YFP and GFP were applied (22).
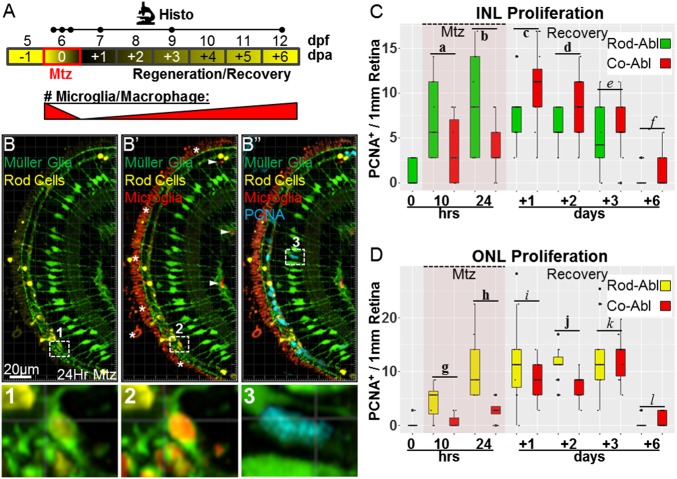
To determine whether ablating microglia/macrophages had any effect on retinal regeneration, we first quantified MG/progenitor cell proliferation rates. Larvae were exposed to Mtz for 24 h, and retinas from coablated and control (triple transgenic, rod cell-ablated only) larvae were fixed at six time points encompassing the loss and regeneration of rod cells (Fig. 3A). Additionally, we observed MG clearing apoptotic cells (Fig. 3B, Inset 1) and saw evidence that MG may clear phagocytic microglia after they have expired (Fig. 3B′, inset 2). As above, antibodies to PCNA were used to mark proliferating cells (Fig. 3B′′, PCNA+/GFP+ MG cell, Inset 3). In coablated retinas the number of proliferating cells in the INL was reduced during the 24-h Mtz treatment (Fig. 3C). Subsequently, INL proliferation increased relative to the control so that the total number of cells proliferating in the INL over the entire ablation/recovery period was indistinguishable in the rod-ablated and coablated conditions [average of 4.3 and 4.7 cells per 1 mm of retina, respectively; 0.42 effect size; 95% CI (−1.74, 1.58); P = 0.475], suggesting that compensation for delayed proliferation is complete by day 3 of recovery (Fig. 3C). Similarly, presumptive rod-committed progenitors and/or MG-derived progenitors present in the ONL (PCNA+ only) showed deficits in proliferation rates in the coablated condition (Fig. 3D), which returned to normal by the day 3 of recovery (Fig. 3D). Unlike in the INL, no evidence of proliferative compensation was observed in the ONL over the time course evaluated. These data suggest that ablation of microglia/macrophages causes a delay in MG/progenitor cell proliferation following rod cell death. However, INL-localized MG/progenitor cells appear to compensate for initial deficits. These results are consistent with microglia/macrophage ablation resulting in a reduction in the kinetics of regeneration following photoreceptor loss.
Fig. 3.
Coablation results in delayed MG and progenitor cell proliferation. (A) Schematic of the assay timeline and the paradigm for rod cell and macrophage/microglia coablation for data shown in B–D (dots on the Histo line indicate time points as in Fig. 2). (B–B′′) Representative retinal section of a 7-dpf rho:YFP-NTR;mpeg1:Tomato;gfap:GFP larva immunostained for PCNA (blue) after 24-h exposure to Mtz. Enlarged views of boxed regions 1 and 2 show evidence of MG (green cells) phagocytosis of microglia (red cells) containing rod cell debris (yellow cells). Boxed region 3 shows an example of a proliferating MG cell following rod cell ablation (blue/green double-positive cell). Note: The prominent red signal outside the ONL in B′ and B′′ (asterisks in B′) is autofluorescence from the retinal pigment epithelium (RPE) layer and blood vessels. (C and D) Quantification of proliferating (PCNA-immunoreactive) MG (GFP+/PCNA+) and presumptive progenitor cells (PCNA+ only) in the INL (C) and ONL (D) following rod photoreceptor ablation (C, green boxes; D, yellow boxes) or rod cell and macrophage/microglia coablation (C and D, red boxes) normalized to the number of cells per 1 mm of retina (measured at the OPL). Note that retinal microglia were eliminated from the analysis of proliferating cells based on expression of the Tomato transgene (arrowheads in B′). (C) An early deficit in INL proliferation rates is evident in the coablated condition during Mtz treatment, but increased proliferation at later time points appears to compensate. (D) Deficits in ONL progenitor proliferation rates in coablated retinas persist through the second day of recovery before matching the levels observed in retinas in which only rod cells were ablated. Statistics (effect sizes, 95% confidence intervals, and P values for paired comparisons) are provided in Table S2; bolded letters denote pairs showing reproducible differences, italicized letters denote pairs with no discernible differences. Co-Abl, coablated retinas; Rod-Abl, rod photoreceptor ablated retinas.
Table S2.
Statistical analysis of data in Fig. 3
| Pair | Effect size | 95% CI | P value |
| a | -3.66 | −6.76, −0.56 | 0.0225 |
| b | -4.85 | −9.14, −0.57 | 0.0299 |
| c | 3.65 | 0.84, 6.47 | 0.0129 |
| d | 2.75 | 0.93, 4.59 | 0.0045 |
| e | 1.37 | −1.77, 4.51 | 0.369 |
| f | 0.53 | −0.49, 1.56 | 0.299 |
| g | −3.71 | −5.87, -1.55 | 0.0025 |
| h | −7.83 | −12.46, −3.20 | 0.0039 |
| i | −3.77 | −9.24, 1.69 | 0.1592 |
| j | −4.44 | −6.12, −2.77 | 9.97E-6 |
| k | 0.20 | −4.96, 5.36 | 0.9343 |
| l | 0.56 | −0.28, 1.40 | 0.1808 |
Bolded letters denote pairs showing reproducible differences; italicized letters denote pairs with no discernible differences.
Microglia/Macrophages Are Necessary for Normal Rod Photoreceptor Regeneration Kinetics.
To assess the possible effects of microglia/macrophage ablation on the kinetics of rod cell regeneration, IMARIS-based 3D rendering was used to quantify YFP-expressing rod cells in intravital images collected at day 1, 3, 4, and 6 of recovery (see schematic, Fig. 4A). Images of uninjured control retinas exhibited a minor loss in observable fluorescence caused by increased pigmentation (Fig. 4 B–B′′′). Retinas in which only rod cells were ablated demonstrated effective loss and regeneration of YFP rod cells (Fig. 4 C–C′′′), consistent with previous results (20). Coablated retinas exhibited effective loss of YFP cells and a reduction in rod cell regeneration kinetics through day 4 of recovery (Fig. 4 D–D′′′). To quantify rod cell-associated YFP signal volumes in coablated larvae accurately, YFP-NTR–expressing microglia had to be subtracted out of the analysis. To do so, the transgenic line labeling microglia/macrophages with a red fluorescent reporter (mpeg1:Tomato) was used to colabel all YFP-NTR–targeted microglia/macrophage (Fig. 4 E–E′′). All colabeled cells then were subtracted from the YFP channel in 3D-rendered images. Isolated photoreceptor volumes (in cubic micrometers) were normalized to the control average for each time point (to account for effects of pigmentation). YFP volume quantification confirmed that ablation of rod cells alone led to full YFP volume recovery by the fourth day of recovery (Fig. 4F, light red bars). Strikingly, in coablated retinas there was a substantial delay in YFP volume recovery, with no regeneration evident through day 3 of recovery and ∼55% rod cells regenerated by day 4 of recovery (Fig. 4F). However, by day 6 of recovery ∼85% of rod cells had been replaced (Fig. 4F; no statistical difference with controls). Collectively, these data suggest that microglia/macrophages regulate stem/progenitor cell responsiveness to rod cell death, thereby impacting the regeneration kinetics of lost retinal cells. Compensatory proliferation observed in the INL at days +1 and +2 dpa (Fig. 3) appears to be sufficient to restore rod cells to normal levels, albeit at a delayed rate of recovery.
Fig. 4.
Coablation results in delayed PR regeneration. (A) Schematic of assay timeline and paradigm for rod cell and macrophage/microglia coablation for data shown in B–F. Whole-retina intravital images across the indicated time series (dots in the Inv line) track changes in YFP expression in individual fish over time. (B–D) Representative z-projection confocal images of rho:YFP-NTR;mpeg1:Tomato;mpeg1:NTR-YFP larvae from untreated control (B–B′′′), rod-ablated (C–C′′′), and coablated (D–D′′′) groups at days 1, 3, 4, and 6 of recovery. (E–E′′) Images showing complete overlap between mpeg1:NTR-YFP (E) and mpeg1:Tomato (E′) reporter expression (40× magnification), which facilitated subtraction of NTR-YFP–expressing microglia cells from all retinal YFP volume calculations. A merged image is shown in E′′. (F) Quantification of YFP expression intensity—as an indication of rod cell number—for all conditions at each time point. For both ablation conditions, rod cell loss is evident at +1 d of recovery (Upper Left). In retinas in which only rod cells were ablated, YFP intensity begins to approach control levels at +3 d of recovery (Upper Right). In contrast, coablation causes a delay in rod cell regeneration such that evidence of new rod cells cannot be detected until day 4 of recovery (Lower Left) before finally approaching control levels at +6 d of recovery (Lower Right). Statistics: The Wilcoxon rank-sum test was used for paired comparisons; corresponding P values are provided in Table S3; bolded letters denote pairs showing reproducible differences; italicized letters denote pairs with no discernible differences.
Table S3.
Statistical analysis of data in Fig. 4
| Pair | P value |
| a | 0.0047 |
| b | 0.0190 |
| c | 0.7879 |
| d | 0.3357 |
| e | 0.0061 |
| f | 0.0040 |
| g | 0.6282 |
| h | 0.0667 |
| i | 0.0242 |
| j | 0.6990 |
| k | 0.0930 |
| l | 0.1790 |
Bolded letters denote pairs showing reproducible differences; italicized letters denote pairs with no discernible differences.
Pharmacological Immune Suppression Can Delay or Accelerate Rod Cell Regeneration Kinetics Depending on the Timing of the Treatment.
Preablation.
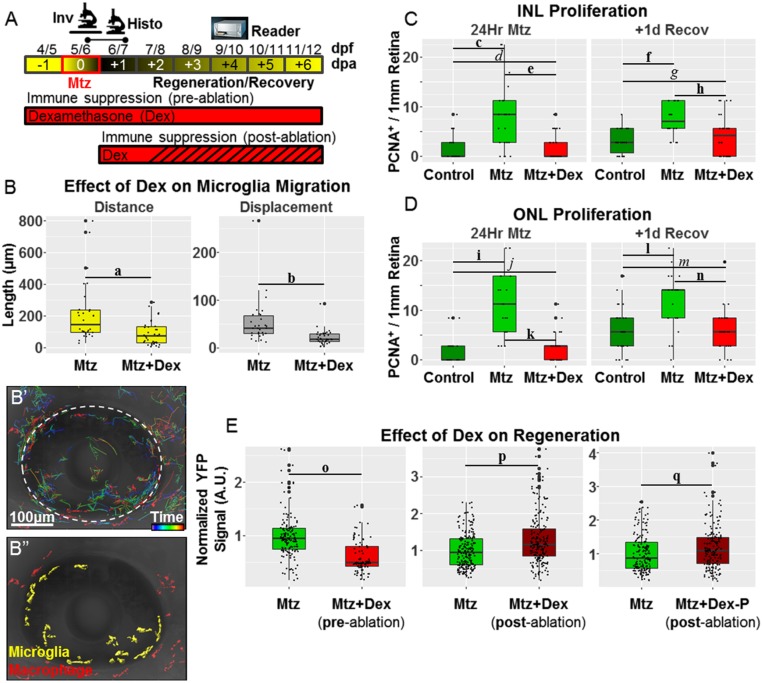
To investigate further roles of the innate immune system in retinal regeneration, we asked if chemical suppression of immune cell reactivity impacts the kinetics of rod cell replacement. Dexamethasone (Dex), a glucocorticoid receptor agonist, has been used to identify inflammatory signaling as a factor contributing to CNS regeneration in adult zebrafish following traumatic brain (11) and spinal cord (15) injuries. However, a role for inflammation in regulating regeneration following selective neuronal cell ablation has yet to be investigated. Activation of glucocorticoid signaling has been shown to reduce microglial reactivity in the mammalian brain (47, 48) and chick retina (49). Therefore, using the intravital imaging techniques detailed above, we first investigated whether exposing larvae to Dex before Mtz-induced rod cell ablation would result in a reduction in microglia migration. Initial titration assays determined that 5-µM Dex treatments produced no toxicity in 5- to 9-dpf larvae. One day before Mtz exposure, and daily thereafter, larvae were immersed in 5 µM Dex (Fig. 5A). Quantification of microglia migration patterns showed reductions in distance and displacement in the presence of Dex (Fig. 5B), consistent with the inhibition of microglia reactivity to rod cell death upon activation of glucocorticoid signaling.
Fig. 5.
Immunomodulatory control of rod cell regeneration kinetics. (A) Schematic of assay timelines and Dex treatment paradigms for data shown in B–F. (B–B′′) Whole-retina intravital time-lapse sequences from Mtz-treated 6-dpf rho:YFP-NTR;mpeg1:Tomato larvae and microglia migration patterns quantified ± preablation Dex exposure. (B) Exposing larvae to Dex before Mtz-induced rod cell death suppressed microglia responsiveness. (B′) Representative dragon-tail microglia migration data from the preablation Dex-treated retina group. (B′′) False-colored image showing microglia (yellow) and macrophages (red) at t = 0. (C and D) Retinal sections were processed for PCNA immunoreactivity and imaged at the indicated time points (dots in the Histo line in A). (C and D) Quantification of proliferative MG/progenitor cells in the INL (C) and ONL (D) from uninjured control retinas (dark green boxes), Mtz-treated retinas (light green boxes), and Mtz+Dex (preablation)-treated retinas (red boxes) normalized to the number of cells per 1 mm of retina (measured at the OPL). Dex pretreatment causes a decrease in proliferation in the INL and ONL after 24 h Mtz and at +1 d recovery. (E) Plate reader-based (i.e., ARQiv) quantification of rod cell regeneration kinetics (i.e., YFP intensity) in individual rho:YFP-NTR larvae at +4 d of recovery following Mtz-induced rod cell death and ± Dex exposure (pre- or postablation). Comparisons between larvae treated with Mtz alone (green boxes) or with Mtz +Dex (red boxes) preablation (Left) or postablation (Center), or with Mtz + Dex-P postablation (Right) showed that pretreatment with Dex inhibited rod cell replacement, whereas posttreatment with Dex (or Dex-P) accelerated rod cell regeneration kinetics. (Note that for logistical reasons associated with ARQiv assays, Mtz was applied at 5 dpf for this experiment.) Statistics (effect sizes, 95% confidence intervals, and P values for paired comparisons) are provided in Table S4; bolded letters denote pairs showing reproducible differences; italicized letters denote pairs with no discernible differences.
Table S4.
Statistical analysis of data in Fig. 5
| Pair | Effect size | 95% CI | P value |
| a | −122.17 | −196.39, −47.95 | 0.0019 |
| b | −31.93 | −50.39, −13.48 | 0.0013 |
| c | 5.63 | 2.57, 8.69 | 7.72E-4 |
| d | −0.22 | −1.75, 1.31 | 0.7719 |
| e | −5.85 | −8.81, −2.89 | 0.0004 |
| f | 4.47 | 2.72, 6.21 | 7.97E-6 |
| g | 1.13 | −0.73, 2.99 | 0.2279 |
| h | −3.34 | −5.47, −1.21 | 0.0029 |
| i | 9.31; | 6.07, 12.55 | 1.95E-6 |
| j | −0.01 | −1.74, 1.73 | 0.9958 |
| k | -9.34; | −12.41, −6.22 | 1.52E-6 |
| l | 6.42 | 3.39, 9.45 | 0.0001 |
| m | 0.32 | −1.98, 2.62 | 0.7797 |
| n | −6.10; | −9.10, −3.10 | 0.0002 |
| o | −0.38 | −0.47, −0.29 | 1.16E-16 |
| p | 0.29 | 0.13, 0.41 | 1.26E-6 |
| q | 0.17 | 0.05, 0.31 | 0.007 |
Bolded letters denote pairs showing reproducible differences; italicized letters denote pairs with no discernible differences.
We next asked whether pretreatment with Dex resulted in a reduction in MG/progenitor cell proliferation rates akin to findings in the chick retina (50). Immunostaining for PCNA was used to quantify the number of proliferative cells in the INL and ONL immediately after Mtz exposure and after 1 d of recovery (Fig. 3) in the presence or absence of Dex. Preexposure to Dex resulted in an inhibition of MG/progenitor cell proliferation rates to levels equivalent to uninjured controls at both time points and in both the INL (Fig. 5C) and ONL (Fig. 5D). This result suggests that microglia reactivity is a key regulator of MG responsiveness to rod cell death, in keeping with findings from our coablation studies. Further, the data implicate inflammatory signaling in promoting MG dedifferentiation to a proliferative stem-like state.
Given the inhibitory effects on MG/progenitor cell proliferation, we were interested in determining if Dex pretreatment resulted in a reduction in the rate of rod cell replacement. The kinetics of rod cell regeneration was assessed using fluorescent plate reader-based quantification (i.e., automated reporter quantification in vivo, ARQiv) of YFP signal levels in individual larvae (20, 51, 52). Using this method, we had previously shown full regeneration of rod cells as early as day 5–6 of recovery (20). For comparison with Dex-treated larvae, the level of YFP signal observed in Mtz-treated control larvae at day 4 of recovery was normalized to 100% regeneration. Preexposure to Dex resulted in reduced rod cell replacement rates with ∼60% YFP levels relative to Mtz-alone controls (Fig. 5E, Left, −0.38 effect size).
Postablation.
In addition to inhibiting immune cell activation, glucocorticoid signaling also promotes M2 polarization, an “alternatively activated” macrophage state (53). The M2 polarization state is correlated with the resolution of inflammatory signaling events and thus is considered proreparative. Accordingly, we were interested in testing whether stimulating glucocorticoid signaling after the initial response to rod cell death had occurred could accelerate retinal regeneration kinetics by hastening the resolution of associated inflammatory signals. We therefore evaluated the effects of treatment with Dex at day 1 of the recovery phase after the induction of rod cell ablation (see schematic, Fig. 5A).
Intriguingly, Dex posttreatment regimens resulted in accelerated rod cell regeneration kinetics: ∼130% regeneration relative to the control at 4 d of recovery (Fig. 5E, Center, 0.29 effect size). The insoluble nature of Dex suggested that methods for improving bioavailability in water could enhance efficacy in our assays. We therefore asked whether a water-soluble prodrug version (e.g., Dex sodium phosphate, Dex-P) would show greater efficacy in enhancing rod cell regeneration kinetics. However, the data suggest no improvement relative to Dex-treated controls (Fig. 5E, compare center and right graphs). The cellular mechanisms by which Dex posttreatment accelerates rod cell regeneration kinetics await further characterization. Intriguingly, however, this result suggests that accelerating the rate at which inflammatory signaling events attending neuronal cell apoptosis are resolved can function to promote neuronal regeneration.
Discussion
The CNS was once thought to have little functional interaction with the immune system in the absence of trauma or disease. However, in the past decade neuroimmune crosstalk—direct interplay between neuronal and immune cells—has been shown to be critical for neurodevelopment (54), synaptic homeostasis (55), and adult-stage neurogenesis (56) and may play beneficial roles during neurodegenerative disease (24, 28). For instance, acute activation of microglia may function to protect neurons from accumulations of cytotoxic derivatives of dying cells and stress-related signaling pathways during the early stages of neurodegeneration. In keeping with this notion, efficient phagocytosis of apoptotic cells is not generally associated with an inflammatory response (57). Conversely, chronic inflammation and related immune cell infiltration of the CNS are believed to exacerbate neurodegenerative pathologies (43). Interestingly, zebrafish regenerate neural tissue despite mounting an inflammatory response to injury (58). Indeed, inflammatory signaling may even stimulate neural stem cell proliferation (11, 33, 38). Therefore, whether immune cells act in a beneficial or deleterious manner in the nervous system is likely to be context dependent. Because zebrafish display robust reparative capacities across multiples levels of CNS injury, it is an excellent model system for exploring the roles played by the immune system during neuronal regeneration.
The NTR ablation system creates inducible physiological models of degenerative diseases by enabling the induction of apoptosis in genetically targeted cell types (17). By using this approach, macrophages were recently implicated as being critical to appendage regeneration in the salamander and zebrafish (8, 9). Reactive macrophage/microglia also have been observed in the fish brain after widespread NTR/Mtz-induced neuronal death (13) and in the retinal photoreceptor layer following light damage (32). Intriguingly, inflammatory signaling regulates neural stem cell and progenitor proliferation rates following traumatic brain injury in zebrafish (11). In addition, macrophage/microglia cells have been shown to be necessary for exogenous factor stimulation of MG-derived progenitor cell proliferation following excitotoxin injections in the chicken retina (33). Here we investigated innate immune cell reactivity and function following the selective loss of rod photoreceptor cells in the zebrafish retina.
Because of the highly dynamic nature of reactive immune cells and difficulties in imaging the brain, detailed analyses of neuroimmune interactions have been elusive. Over the past decade, however, intravital imaging has provided key insights into immune cell behavior and function (59). The zebrafish system enables intravital imaging of fluorescently labeled cell types at high spatial and temporal resolution. We therefore applied multicolor in vivo time-lapse imaging (21, 22) to characterize innate immune cell behaviors during retinal regeneration. More specifically, we investigated neutrophil, macrophage, and microglia reactivity to selective rod photoreceptor loss and retinal puncture wounds in larval zebrafish. Of note, the adaptive immune system does not develop in zebrafish until the juvenile stage (60). By performing our studies in larval zebrafish, we were able to assess innate immune system function independent of adaptive immune system interactions.
Neutrophils Are Unresponsive to Rod Cell Death.
Neutrophils are known to be first responders to infection and nonsterile injuries (40) and to play key roles during wound healing and infection, but their ability to influence regenerative processes has begun to be explored only recently (61). The blood–brain barrier (BBB) and BRB normally prevent monocyte infiltration; however, upon disruption of the barrier, neutrophils can invade. The potential for BRB disruption during retinal neurodegenerative conditions suggests possible involvement of neutrophils. The superficial system (precursor to the choroid) and hyaloid vasculature of the larval zebrafish retina are conceivable points of neutrophil infiltration following the induction of rod cell death. Although neutrophils are not normally associated with phagocytosis of apoptotic cells (57), they can exhibit chemotaxis toward apoptotic cells (62). Moreover, neutrophils respond to lysed apoptotic bodies (i.e., secondary necrosis) upon release of ATP metabolites. Therefore, a full characterization of innate immune system responses to NTR-mediated neuronal cell loss required that we account for possible neutrophil involvement.
We found that neutrophils were unresponsive to rod cell death. This finding is consistent with studies of widespread NTR-mediated ablation of neurons in the zebrafish forebrain (13) and suggests that any role for neutrophils in modulating retinal cell regeneration would need to be mediated by secreted factors. In contrast, retinal puncture wounds resulted in increased migration rates and infiltration of neutrophils into the neural retina. This finding is not surprising, because an ocular puncture wound is expected to disrupt the BRB, elicit an osmotic shock, and produce classic signaling cascades known to recruit immune cells to sites of traumatic injury. Importantly, this result highlights the potential for neutrophil involvement in retinal injury models that disrupt the BRB, e.g., light damage (7, 63), puncture wounds (6), or toxin injection (31). Moreover, these data suggest that caution should be exercised when relating findings across regenerative paradigms involving different scales of injury, particularly with respect to immune system-related signaling cascades.
Peripheral Macrophages Are Responsive to Rod Cell Death but Do Not Enter the Retina.
Macrophages are efficient phagocytes that contribute to the clearance of dead cells (64). Moreover, macrophages have been implicated as beneficial to healing/regenerative processes in a diverse set of injury paradigms including muscle (65), limb (8, 9), lateral line (12, 66), heart (10), brain (11, 38), spinal cord (67, 68), and retina (33, 69). However, there is controversy over the extent to which monocyte-derived macrophages are involved in CNS degenerative diseases. The controversy is largely the result of a lack of cell-specific markers for delineating microglia from macrophages, although headway has been made in this regard recently (70). Of note, time-lapse imaging overcomes this limitation by enabling tracking of individual cell behaviors following induction of injury. Using this approach, we observed an increase in macrophage migration following rod cell loss (Fig. 2). However, although macrophages entered the retina rapidly following puncture wounding, they remained restricted to the periphery after rod cell death. This observation suggests that macrophages are responsive to rod cell death but do not infiltrate the retina, likely because of the maintenance of the BRB. This behavior contrasts with intravital imaging observations following widespread Mtz-induced neuronal cell loss in the larval zebrafish brain in which presumptive macrophages were the first to respond, and microglia arrived later, remained longer, and appeared to clear apoptotic macrophage debris (13). The source of this discrepancy is unclear; however, differences in the extent of neuronal damage could impact the composition of the immune response and/or the relative integrity of the BBB and BRB. A recent study has shown that neuronal apoptosis drives infiltration of anterior lateral plate mesoderm-derived macrophages into the brain during development (71). Widespread Mtz-induced apoptosis of hindbrain neurons at 3 dpf resulted in supernumerary infiltration of macrophages; however, because macrophages normally enter the developing brain until 4 dpf (71), there is no discrepancy between these findings and our observations. One caveat of our study is that the transgenic lines we used label mature immune cells but not circulating monocytes. Thus, it remains possible that monocytes extravasate into the retina following the induction of rod cell death and differentiate into phagocytes, as has now been shown for retinas exposed to intense light and irradiated retinas in mice (70). Investigation of this possibility will require new transgenic toolsets for monitoring monocytes, a cell type that has only recently begun to be characterized in zebrafish. For now, the data suggest that within the context of selective rod cell death, any modulation of retinal stem/progenitor cell responsiveness or rod cell regeneration kinetics by peripheral macrophages would need to be mediated by secreted factors.
Microglia Exhibit Several Hallmarks of Phagocyte Activation in Response to Rod Cell Death.
Microglia are gaining recognition as key regulators of development, homeostasis, proliferation, and disease. Microglia may be useful sentinels of neurodegeneration at early stages and have long been known to be responsive to neuronal cell apoptosis (30), including in a number of human retinal diseases (72). Thus, we expected to see activation of these cells upon rod cell ablation. However, the extent acute microglia responses serve beneficial (73, 74) versus deleterious (75) roles during neurodegenerative processes remained unclear. Similarly, although microglia have been implicated as being necessary for exogenous factor stimulation of MG/progenitor proliferation following excitotoxic injury in the chicken retina (33), the avian retina does not display robust regenerative capacities (35). Thus, roles for microglia during retinal regeneration remain largely undefined. Accordingly, we explored potential roles for microglia during the loss and regeneration or rod photoreceptors in zebrafish.
Migratory monocytes colonize the embryonic zebrafish retina by 30 h postfertilization (hpf). By 48 hpf there are ∼30–35 microglia residing in the eye (42). In the uninjured retina, microglia were found to reside within inner retinal layers, consistent with observations in adult zebrafish (32), and exhibited exuberant ramified process behaviors consistent with immune surveillance (Movie S5). Following rod cell ablation, microglia adopted amoeboid morphologies and exhibited marked increases in migration (Movie S6). Time-series quantification of microglia migration over several days confirmed the behavioral trends observed during confocal time-lapse imaging: Within 10 h of Mtz treatment microglia translocated to layers where photoreceptors reside (i.e., the OPL and ONL). Over the course of the recovery period, the microglia presence diminished in the outer retina but remained elevated in the INL. The significance of sustained microglia presence in the INL during the recovery phase is unknown; however, it is tempting to postulate that it serves to promote interaction with MG/progenitor cell bodies.
Using both time-lapse imaging and immunolabeling, we observed microglia phagocytosis of dying rod photoreceptors. Time-lapse imaging revealed that from the initial moment of contact to complete engulfment, loss of rod cell-associated YFP signals took ∼30 min. In mammals, phagocytosis of apoptotic cells can induce activated macrophages to adopt an antiinflammatory (or regulatory) phenotype (76), typified by secretion of antiinflammatory cytokines, e.g., TGF-β (77). Microglia of the human CNS also exhibit this phenomenon, although possibly exhibiting fewer differences between polarization states (27). Importantly, acutely activated microglia are known to express neuroprotective and regenerative factors, e.g., TGFβ, IGF1, IL-10, LIF, and MANF (74, 78), some of which have been shown to stimulate MG proliferation (5, 6). Combined, these observations suggest that after an initial reactive response to neuronal cell death microglia may adopt an antiinflammatory phenotype to stimulate stem/progenitor cell proliferation.
Finally, microglia exhibited two waves of proliferation in response to rod cell loss. The first wave occurred at the OPL/ONL boundary proximal to photoreceptor injury, consistent with observations in the chick retina following excitotoxic injury (33). A second wave of microglial proliferation occurred within the INL at days 2–6 of recovery. These results suggest that expansion of the resident microglia population was necessary to mount an adequate response to the extent of rod cell death and/or that microglia numbers needed to be replenished during the regenerative process. There is considerable controversy over whether resident microglia maintain their own numbers or if macrophages need to enter the CNS to repopulate stressed microglia populations. Our results suggest that, within the context of limited neuronal cell death, microglia proliferate locally to maintain their numbers. In summary, using intravital imaging and immunohistology, we showed that retinal microglia exhibit multiple hallmarks of immune cell activation following rod photoreceptor ablation in zebrafish larvae: (i) increased migration, (ii) translocation, (iii) phagocytosis, and (iv) proliferation. We next turned to investigating potential roles for microglia during retinal regeneration.
Disrupting Microglia Reactivity to Cell Death Inhibits MG and Progenitor Cell Proliferation Rates and Delays Rod Photoreceptor Regeneration.
Various methods for altering immune cell numbers or reactivity are useful for testing function (61). For instance, liposome-encapsulated clodronate was developed to ablate phagocytic cells and can be used to ablate macrophages and microglia (79). This strategy was used recently to demonstrate that macrophages are necessary for tissue/appendage regeneration (8–10), hair cell replacement (66), and retinal neuroprotection (49, 80). Similarly, we used the NTR system to coablate microglia/macrophage and rod cells and then quantified effects on MG/progenitor cell proliferation rates and rod cell regeneration kinetics. We found that coablation reduced stem/progenitor cell proliferation rates in the INL over the first few days of the regenerative process. Similar examples of MG and microglia cross-talk mediating injury responses in the retina have been observed in other species as well (81). Interestingly, the effects on proliferation were temporary, with INL MG/progenitor cells seeming to compensate for early deficits at days 1–2 of recovery. Nevertheless, initial deficits in proliferation rates were correlated with delays in rod cell regeneration kinetics. Similarly, we found that pretreating larvae with the immune suppressant Dex, a glucocorticoid agonist, eliminated microglia reactivity to rod cell death and inhibited proliferation in the INL and ONL. Combined with other recent studies in regenerative model species, the data suggest that macrophage/microglia play critical roles in promoting proper tissue and cellular regeneration.
Elegant studies from the Fischer laboratory have shown that in the chick retina reactive microglia/macrophages are required to promote the generation of MG-derived progenitor cells (MGPCs) (33) and that Dex injections can inhibit microglia reactivity and decrease proliferation of MGPCs after excitotoxic retinal injury (50). Further, their data suggest that inhibition of microglia activation is an indirect effect of activated glucocorticoid signaling in MG, the primary site of glucocorticoid receptor expression in chick and several other vertebrate species investigated (49). In contrast to our findings, Dex functioned as a neuroprotectant in the injured chick retina. Interestingly, the neuroprotective effects of Dex were only partially dependent on the presence of microglia/macrophages, suggesting that MG were the primary target of glucocorticoid agonist action; this possibility requires further exploration in the fish retina.
Immunomodulator-Induced Resolution of Activated Microglia Accelerates Rod Cell Regeneration Kinetics.
Macrophages can transition between phenotypic polarization states that have been characterized as anti- (M1) and proreparative (M2). The M1 phase is associated with initial immune cell reactivity, whereas the M2 phase is linked to resolution. Our functional assays indicated that an initial, presumably M1-type, microglia response was required for proper stem and progenitor cell responses to rod cell loss and, thereby, for proper regeneration. We were motivated, however, to test what effect a delay in immune suppression would have. We posited that hastening the resolution of microglia/macrophages following an acute activation might enhance rod cell regeneration. Immune suppression was therefore delayed until the day after rod cell death was initiated, i.e., after 24-h Mtz treatments. Intriguingly, this treatment regimen led to accelerated rod cell regeneration kinetics. This result suggests that accelerating the resolution of an immune response to neuronal cell death can serve to enhance the regenerative process and is consistent with recent findings regarding the regeneration of dopaminergic neurons in the salamander brain (38). Although multiple reports have described factors and conditions that stimulate increased MG and progenitor cell proliferation following retinal injury (5–7), whether such manipulations equate to improvements in retinal cell regeneration kinetics remains largely unexplored. Here, we report chemically enhanced rod photoreceptor regeneration kinetics in zebrafish. Somewhat confoundingly, the glucocorticoid antagonist RU486 has been shown to promote the regeneration of amacrine cells in the chick retina (49). However, this promotion appears to be an effect of MG-derived progenitors on differentiation, because amacrine cells are restored at the expense of MG. We currently are exploring the cellular and molecular mechanisms underlying Dex-enhanced regeneration in the fish retina.
Collectively, these results and other recent findings support the notion that manipulation of neuroimmune interactions could be a useful means of stimulating reparative processes in patients with degenerative the CNS disorders (43). One promising immunomodulatory candidate is the mesencephalic astrocyte-derived neurotrophic factor (MANF). MANF was recently shown to be required for retinal repair in Drosophila and could act as a neuroprotectant, as well as promoting engraftment of transplanted progenitors/photoreceptors and improving visual recovery, in the mouse (78). Determining the role of MANF and other immune-related factors in regulating the activation, proliferation, and differentiation of MG and their progenitors stands as a promising path to stimulating endogenous regenerative mechanisms in the human retina.
Summary
We have defined a role for the innate immune system in regulating neuronal regeneration following selective loss of rod photoreceptor cells in the zebrafish retina. Intravital imaging was used to track and quantify the responses of specific immune cell subtypes to rod cell death. Microglia, resident macrophages of the retina, exhibited multiple hallmarks of immune cell activation. Using targeted cell ablation and immunomodulation, microglia/macrophages were shown to be critical for proper rod cell regeneration kinetics, i.e., for stimulating the activation of retinal MG to a stem cell-like state. Our results support the theory that microglia play key roles in shaping systemic responses to neuronal cell death and are consistent with the possibility that microglia regulate fibrotic versus reparative programs of endogenous neural stem cells, thereby controlling regenerative potential in the CNS. Supported by studies across multiple model systems, immune modulation now stands as a promising approach for stimulating endogenous retinal repair mechanisms and/or promoting engraftment of exogenously delivered stem cells, therapeutic strategies aimed at restoring visual function to patients.
Materials and Methods
The majority of the procedures applied and all transgenic lines used, Tg(rho:YFP-Eco.NfsB)gmc500, Tg(mpx:GFP)uwm1, Tg(mpeg1.1:LOX2272-LOXP-dTomato-LOX2272-Cerulean-LOXP-EYFP)w201, Tg(mpeg1.1:NTR-EYFP)w202, and Tg(gfap:GFP)m2001, have been previously published (8, 20, 22, 39, 41, 47). Brief descriptions of the experimental design are outlined here; additional details can be found in SI Materials and Methods and references provided.
NTR/Mtz-Mediated Rod Photoreceptor Ablation and Puncture Wounding.
NTR-expressing larvae were separated into equal-sized groups at 5 dpf: (i) nontreated controls and (ii) larvae treated with 10 mM Mtz (Acros) for 24 h starting at 5 or 6 dpf. After Mtz treatment, fish were rinsed and kept in 0.3× Danieau’s solution until imaged or killed. Retinal punctures were performed using a stereotactic-directed 10-µm-diameter pulled-glass capillary inserted through the ventral conjunctiva and into the retina.
Intravital Imaging.
All intravital imaging applied previously detailed protocols (14, 21, 22). Confocal z-stacks encompassing the entire orbit of the eye (step size, 5 microns) were collected at 10- or 20-min intervals over a total of 20 h. Image analysis was performed using FiJi (i.e., ImageJ v1.49b; NIH) or Imaris (v7.6.5; Bitplane) to quantify migration distance (total length traveled) and displacement (length of vector between initial and final position) for selected cells. Additional details can be found in SI Materials and Methods.
4D Quantification of Innate Immune Cell Activity.
Confocal retinal z-stacks were transferred to ImageJ, and the Correct 3D Drift plugin was applied. Imaris then was used for 3D surface rendering and subsequent cell tracking. Specific sets of migration paths were selected for measurement. The migration distance (total length traveled) and migration displacement (the length of the vector between the initial and final position of each cell) was quantified.
IMARIS Volumetric Rendering and Quantification.
Larval whole-retina images were collected as described above using identical acquisition and IMARIS processing parameters across all conditions. Photoreceptor volume was calculated using IMARIS local background-based volumetric rendering of YFP signals after microglia coexpressing YFP/tdTomato were subtracted from the total YFP volume.
Tissue Preparation, Immunohistochemistry, Cell Counting, and Analysis.
Zebrafish larval retina tissue preparation and immunohistochemistry were performed using established protocols. Full details are provided in SI Materials and Methods.
Dexamethasone Treatment and ARQiv Scans.
Transgenic rho:YFP-NTR larvae were treated with Mtz ± Dex, and regeneration kinetics were analyzed using the ARQiv system, as previously described (20, 52). In the preablation Dex regimen, 5-dpf larvae were exposed to Dex (2.5 μM in 0.1% DMSO) for 24 h before Mtz-induced ablation at 6 dpf. Fresh Dex was replaced daily until the ARQiv scan on day 9. In the postablation regimen, 5-dpf larvae were exposed to 10 mM Mtz for 20–22 h and then were treated with a single dose of 2.5 µM Dex. The ARQiv scan was performed on day 10.
Statistical Analysis.
Data were processed with a custom R-based package (ggplot2) (82) to generate box plots showing the first quartile (lower box), median (bold line), third quartile (upper box), upper and lower adjacent (whiskers), and raw data (dot plot; large dots denote outlier observations) for each experimental condition. Statistical analyses were carried out with R 3.3.1 and RStudio 0.99.893. Student’s t test was used to calculate effect size between paired groups with effect size, 95% confidence intervals, and P values provided. For groups with fewer than 10 observations, a Wilcoxon rank-sum test was used for comparison with the P values provided.
SI Materials and Methods
Intravital Imaging and Quantification of Migration Patterns.
To facilitate in vivo confocal imaging in larvae (e.g., 5–11 dpf), transgenic fish were propagated in the roy orbison (royA9) pigmentation mutant background which lacks iridophores (21). In addition, 200 µM PTU (1-phenyl-2-thiolurea; Acros Organics) was added to aquaculture medium daily to inhibit melanogenesis and melanin-dependent pigmentation. Larvae at ages 6–11 dpf were anesthetized, mounted in 0.25% low-melt agarose, and imaged using an upright FV1000 laser-scanning confocal microscope (Olympus). Confocal z-stacks encompassing the entire orbit of the eye (step size, 5 microns) were collected every 10–20 min (e.g., 5 min collection, 5 min rest) over a total of 20 h.
Tissue Preparation, Immunohistochemistry, Cell Counting, and Analysis.
For immunohistochemistry, larval zebrafish were killed in 20× tricaine (15 mM) and then were fixed in 4% paraformaldehyde (PFA) (Acros Organics) for 4 h at 22 °C and were washed three times in 1× PBS (EMD Millipore) for 60 min before storing at 4 °C in PBS containing 30% sucrose for cryoprotection. Samples were mounted in embedding medium (Cryo-Gel; InstruMedics) within the next 2 d and were frozen in liquid nitrogen. All blocks were kept at −80 °C until sectioned in the sagittal plane at 20-µm thickness with a cryostat (Leica). Sliced sections were transferred serially onto microscope slides (Fisher Scientific), briefly dried, and stored at 4 °C.
For immunohistochemistry, slides were warmed to room temperature for 30 min protected from light. Samples were rinsed three times in 1× PBS for 5 min each (all subsequent rinses are 5 min) to remove embedding medium, were blocked with a PAP pen (Sigma), and then were fixed to the slides with 4% PFA (in 1× PBS) for 15 min. Three rinses with PBS plus 0.1% Tween20 (PBST) (Fisher Scientific) followed to remove trace amounts of PFA. Antigen retrieval was achieved via 5-min incubation in 1% SDS (Fisher Scientific) in 1× PBS. Section blocking was performed with 3% normal goat serum (Sigma) in 1× PBS, 1% BSA, 1% DMSO, 0.1% Triton X-100 (PBDT) (Fisher Scientific) for 60 min. Afterward, the primary antibody was applied in 1% goat serum in PBDT overnight at 4 °C. The following morning, the primary antibody was washed from the slides with five consecutive rinses in PBST, and then the slides were incubated with the appropriate secondary antibody in PBDT for 2 h protected from light. Antibody incubations were maintained in a humidified chamber to prevent evaporation, and 22 × 50 mm coverslips (Fisher Scientific) were used to distribute antibody solution uniformly. Four additional washes with PBST were performed to remove trace secondary antibody, followed by a final rinse in 1× PBS to remove trace detergent. The samples then were protected with VECTASHIELD (Vector Labs) covered with a 24 × 50 mm coverslip (Fisher Scientific) and sealed carefully with clear nail polish.
Primary antibodies were applied at the following concentrations: mouse monoclonal anti-PCNA (a standard marker for proliferation; clone PC10; Sigma-Aldrich) 1:1,000; mouse monoclonal anti-ZPR3 (Zebrafish International Resource Center) 1:500. Secondary antibodies were conjugated either to Alexa Fluor 405, or Alexa Fluor 635 (Molecular Probes) diluted at 1:500. Imaging was performed using an upright confocal laser-scanning microscope (FV1000; Olympus) fitted with an oil immersion objective (40×, 1.3NA) and six laser lines (405, 440, 488, 515, 559, and 635 nm). Analysis was performed on z-stacks, with each stack containing multiple channels and z-sections. Image analysis was processed as described below using ImageJ (version 1.49b; NIH) and Imaris (version 7.6.5; Bitplane).
Confocal z-stacks of immunolabeled retinal sections (20-μm thickness) were obtained via 40× oil immersion objective (2.5-μm step size, 130-μm confocal aperture). A total of ∼20 retinal sections (centered around the optic nerve) from 10 or more retinas (distributed over a minimum of six fish) were imaged and saved as a .OIB file (Olympus image format). The data were transferred to ImageJ. Stacks were transformed via z-projection (SD method) for representative images and were compared with uncompressed stacks to ensure that labeled cells were not missed or double counted. All manual cell counts were normalized as the number of cells per 1 mm of retina (measured at the OPL).
Supplementary Material
Acknowledgments
We thank members of the J.S.M. laboratory for helpful discussions and Drs. Chao-Tsung Yang, Lalita Ramakrishnan, Anna Huttenlocher, and Pamela Raymond for providing transgenic lines. This research was funded by National Eye Institute Grant R01EY022810 and National Center for Advancing Translational Sciences Grant R41TR000945.
Footnotes
Conflict of interest statement: J.S.M. holds patents regarding nitroreductase-expressing zebrafish and their use for discovering genes and chemical compounds that regulate regeneration.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1617721114/-/DCSupplemental.
References
- 1.Yurco P, Cameron DA. Responses of Müller glia to retinal injury in adult zebrafish. Vision Res. 2005;45:991–1002. doi: 10.1016/j.visres.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 2.Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Müller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26:6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giannelli SG, Demontis GC, Pertile G, Rama P, Broccoli V. Adult human Müller glia cells are a highly efficient source of rod photoreceptors. Stem Cells. 2011;29:344–356. doi: 10.1002/stem.579. [DOI] [PubMed] [Google Scholar]
- 4.Jayaram H, et al. Transplantation of photoreceptors derived from human Muller glia restore rod function in the P23H rat. Stem Cells Transl Med. 2014;3:323–333. doi: 10.5966/sctm.2013-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenkowski JR, Raymond PA. Müller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retin Eye Res. 2014;40:94–123. doi: 10.1016/j.preteyeres.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman D. Müller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014;15:431–442. doi: 10.1038/nrn3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorsuch RA, Hyde DR. Regulation of Müller glial dependent neuronal regeneration in the damaged adult zebrafish retina. Exp Eye Res. 2014;123:131–140. doi: 10.1016/j.exer.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrie TA, Strand NS, Yang CT, Rabinowitz JS, Moon RT. Macrophages modulate adult zebrafish tail fin regeneration. Development. 2014;141:2581–2591. doi: 10.1242/dev.098459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci USA. 2013;110:9415–9420. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aurora AB, et al. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382–1392. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyritsis N, et al. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science. 2012;338:1353–1356. doi: 10.1126/science.1228773. [DOI] [PubMed] [Google Scholar]
- 12.Villegas R, et al. Dynamics of degeneration and regeneration in developing zebrafish peripheral axons reveals a requirement for extrinsic cell types. Neural Dev. 2012;7:19. doi: 10.1186/1749-8104-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Ham TJ, et al. Intravital correlated microscopy reveals differential macrophage and microglial dynamics during resolution of neuroinflammation. Dis Model Mech. 2014;7:857–869. doi: 10.1242/dmm.014886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White DT, Mumm JS. The nitroreductase system of inducible targeted ablation facilitates cell-specific regenerative studies in zebrafish. Methods. 2013;62:232–240. doi: 10.1016/j.ymeth.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohnmacht J, et al. Spinal motor neurons are regenerated after mechanical lesion and genetic ablation in larval zebrafish. Development. 2016;143:1464–1474. doi: 10.1242/dev.129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark AJ, et al. Selective cell ablation in transgenic mice expression E. coli nitroreductase. Gene Ther. 1997;4:101–110. doi: 10.1038/sj.gt.3300367. [DOI] [PubMed] [Google Scholar]
- 17.Curado S, et al. Conditional targeted cell ablation in zebrafish: A new tool for regeneration studies. Dev Dyn. 2007;236:1025–1035. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- 18.Cui W, Gusterson B, Clark AJ. Nitroreductase-mediated cell ablation is very rapid and mediated by a p53-independent apoptotic pathway. Gene Ther. 1999;6:764–770. doi: 10.1038/sj.gt.3300873. [DOI] [PubMed] [Google Scholar]
- 19.Adler R. Mechanisms of photoreceptor death in retinal degenerations. From the cell biology of the 1990s to the ophthalmology of the 21st century? Arch Ophthalmol. 1996;114:79–83. doi: 10.1001/archopht.1996.01100130075012. [DOI] [PubMed] [Google Scholar]
- 20.Walker SL, et al. Automated reporter quantification in vivo: High-throughput screening method for reporter-based assays in zebrafish. PLoS One. 2012;7:e29916. doi: 10.1371/journal.pone.0029916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mumm JS, et al. In vivo imaging reveals dendritic targeting of laminated afferents by zebrafish retinal ganglion cells. Neuron. 2006;52:609–621. doi: 10.1016/j.neuron.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ariga J, Walker SL, Mumm JS. Multicolor time-lapse imaging of transgenic zebrafish: Visualizing retinal stem cells activated by targeted neuronal cell ablation. J Vis Exp. 2010;(43):1–6. doi: 10.3791/2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin P, Leibovich SJ. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 25.Karlstetter M, Ebert S, Langmann T. Microglia in the healthy and degenerating retina: Insights from novel mouse models. Immunobiology. 2010;215:685–691. doi: 10.1016/j.imbio.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ransohoff RM. A polarizing question: Do M1 and M2 microglia exist? Nat Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- 28.Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen-Chi M, et al. Identification of polarized macrophage subsets in zebrafish. eLife. 2015;4:e07288. doi: 10.7554/eLife.07288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thanos S. Sick photoreceptors attract activated microglia from the ganglion cell layer: A model to study the inflammatory cascades in rats with inherited retinal dystrophy. Brain Res. 1992;588:21–28. doi: 10.1016/0006-8993(92)91340-k. [DOI] [PubMed] [Google Scholar]
- 31.Fischer AJ, Seltner RL, Poon J, Stell WK. Immunocytochemical characterization of quisqualic acid- and N-methyl-D-aspartate-induced excitotoxicity in the retina of chicks. J Comp Neurol. 1998;393:1–15. [PubMed] [Google Scholar]
- 32.Craig SEL, Calinescu A-A, Hitchcock PF. Identification of the molecular signatures integral to regenerating photoreceptors in the retina of the zebra fish. J Ocul Biol Dis Infor. 2008;1:73–84. doi: 10.1007/s12177-008-9011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer AJ, Zelinka C, Gallina D, Scott MA, Todd L. Reactive microglia and macrophage facilitate the formation of Müller glia-derived retinal progenitors. Glia. 2014;62:1608–1628. doi: 10.1002/glia.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dick AD. Influence of microglia on retinal progenitor cell turnover and cell replacement. Eye (Lond) 2009;23:1939–1945. doi: 10.1038/eye.2008.380. [DOI] [PubMed] [Google Scholar]
- 35.Wilken MS, Reh TA. Retinal regeneration in birds and mice. Curr Opin Genet Dev. 2016;40:57–64. doi: 10.1016/j.gde.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 36.Herbomel P, Thisse B, Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development. 1999;126:3735–3745. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- 37.Davidson AJ, Zon LI. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene. 2004;23:7233–7246. doi: 10.1038/sj.onc.1207943. [DOI] [PubMed] [Google Scholar]
- 38.Kirkham M, Berg DA, Simon A. Microglia activation during neuroregeneration in the adult vertebrate brain. Neurosci Lett. 2011;497:11–16. doi: 10.1016/j.neulet.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Pagán AJ, et al. Myeloid growth factors promote resistance to mycobacterial infection by curtailing granuloma necrosis through macrophage replenishment. Cell Host Microbe. 2015;18:15–26. doi: 10.1016/j.chom.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathias JR, et al. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol. 2006;80:1281–1288. doi: 10.1189/jlb.0506346. [DOI] [PubMed] [Google Scholar]
- 41.Yamasaki R, et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med. 2014;211:1533–1549. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herbomel P, Thisse B, Thisse C. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through a M-CSF receptor-dependent invasive process. Dev Biol. 2001;238:274–288. doi: 10.1006/dbio.2001.0393. [DOI] [PubMed] [Google Scholar]
- 43.Karlstetter M, et al. Retinal microglia: Just bystander or target for therapy? Prog Retin Eye Res. 2015;45:30–57. doi: 10.1016/j.preteyeres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol. 2007;81:1345–1351. doi: 10.1189/jlb.0207114. [DOI] [PubMed] [Google Scholar]
- 45.Mathias JR, Zhang Z, Saxena MT, Mumm JS. Enhanced cell-specific ablation in zebrafish using a triple mutant of Escherichia coli nitroreductase. Zebrafish. 2014;11:85–97. doi: 10.1089/zeb.2013.0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernardos RL, Raymond PA. GFAP transgenic zebrafish. Gene Expr Patterns. 2006;6:1007–1013. doi: 10.1016/j.modgep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 47.Nadeau S, Rivest S. Glucocorticoids play a fundamental role in protecting the brain during innate immune response. J Neurosci. 2003;23:5536–5544. doi: 10.1523/JNEUROSCI.23-13-05536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carrillo-de Sauvage MÁ, et al. Potent and multiple regulatory actions of microglial glucocorticoid receptors during CNS inflammation. Cell Death Differ. 2013;20:1546–1557. doi: 10.1038/cdd.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gallina D, Zelinka CP, Cebulla CM, Fischer AJ. Activation of glucocorticoid receptors in Müller glia is protective to retinal neurons and suppresses microglial reactivity. Exp Neurol. 2015;273:114–125. doi: 10.1016/j.expneurol.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallina D, Zelinka C, Fischer AJ. Glucocorticoid receptors in the retina, Müller glia and the formation of Müller glia-derived progenitors. Development. 2014;141:3340–3351. doi: 10.1242/dev.109835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang G, et al. First quantitative high-throughput screen in zebrafish identifies novel pathways for increasing pancreatic β-cell mass. eLife. 2015;4:e08261. doi: 10.7554/eLife.08261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White DT, et al. ARQiv-HTS, a versatile whole-organism screening platform enabling in vivo drug discovery at high-throughput rates. Nat Protoc. 2016;11:2432–2453. doi: 10.1038/nprot.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mantovani A, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 54.Bessis A, Béchade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55:233–238. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- 55.Schafer DPP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walton NM, et al. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- 57.Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: Apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013;5:a008748. doi: 10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas JL, Ranski AH, Morgan GW, Thummel R. Reactive gliosis in the adult zebrafish retina. Exp Eye Res. 2016;143:98–109. doi: 10.1016/j.exer.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 59.Oosterhof N, Boddeke E, van Ham TJ. Immune cell dynamics in the CNS: Learning from the zebrafish. Glia. 2015;63:719–735. doi: 10.1002/glia.22780. [DOI] [PubMed] [Google Scholar]
- 60.Lam SH, Chua HL, Gong Z, Lam TJ, Sin YM. Development and maturation of the immune system in zebrafish, Danio rerio: A gene expression profiling, in situ hybridization and immunological study. Dev Comp Immunol. 2004;28:9–28. doi: 10.1016/s0145-305x(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 61.Keightley MC, Wang CH, Pazhakh V, Lieschke GJ. Delineating the roles of neutrophils and macrophages in zebrafish regeneration models. Int J Biochem Cell Biol. 2014;56:92–106. doi: 10.1016/j.biocel.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 62.Chen Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 63.Zweypfenning R, Putting B, Vrensen G, Oosterhuis J, van Best J. Dysfunction of the blood-retina barrier following white light exposure. A tracer study with horseradish peroxidase and ferrous gluconate. Graefes Arch Clin Exp Ophthalmol. 1992;230:561–568. doi: 10.1007/BF00181779. [DOI] [PubMed] [Google Scholar]
- 64.Stefater JA, 3rd, Ren S, Lang RA, Duffield JS. Metchnikoff’s policemen: Macrophages in development, homeostasis and regeneration. Trends Mol Med. 2011;17:743–752. doi: 10.1016/j.molmed.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun D, et al. Bone marrow-derived cell regulation of skeletal muscle regeneration. FASEB J. 2009;23:382–395. doi: 10.1096/fj.07-095901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carrillo SA, et al. Macrophage recruitment contributes to regeneration of mechanosensory hair cells in the zebrafish lateral line. J Cell Biochem. 2016;117:1880–1889. doi: 10.1002/jcb.25487. [DOI] [PubMed] [Google Scholar]
- 67.Becker T, Becker CG. Regenerating descending axons preferentially reroute to the gray matter in the presence of a general macrophage/microglial reaction caudal to a spinal transection in adult zebrafish. J Comp Neurol. 2001;433:131–147. doi: 10.1002/cne.1131. [DOI] [PubMed] [Google Scholar]
- 68.Lotan M, Schwartz M. Cross talk between the immune system and the nervous system in response to injury: Implications for regeneration. FASEB J. 1994;8:1026–1033. doi: 10.1096/fasebj.8.13.7926367. [DOI] [PubMed] [Google Scholar]
- 69.London A, et al. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J Exp Med. 2011;208:23–39. doi: 10.1084/jem.20101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Koren EG, Mathew R, Saban DR. Fate mapping reveals that microglia and recruited monocyte-derived macrophages are definitively distinguishable by phenotype in the retina. Sci Rep. 2016;6:20636. doi: 10.1038/srep20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casano AM, Albert M, Peri F. Developmental Apoptosis Mediates Entry and Positioning of Microglia in the Zebrafish Brain. Cell Reports. 2016;16:897–906. doi: 10.1016/j.celrep.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 72.Ma W, Wong WT. Aging Changes in Retinal Microglia and their Relevance to Age-related Retinal Disease. Adv Exp Med Biol. 2016;854:73–78. doi: 10.1007/978-3-319-17121-0_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harada T, et al. Microglia-Müller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J Neurosci. 2002;22:9228–9236. doi: 10.1523/JNEUROSCI.22-21-09228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang M, Ma W, Zhao L, Fariss RN, Wong WT. Adaptive Müller cell responses to microglial activation mediate neuroprotection and coordinate inflammation in the retina. J Neuroinflammation. 2011;8:173. doi: 10.1186/1742-2094-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao L, et al. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol Med. 2015;7:1179–1197. doi: 10.15252/emmm.201505298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fadok VA, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neves J, et al. Immune modulation by MANF promotes tissue repair and regenerative success in the retina. Science. 2016;353:aaf3646. doi: 10.1126/science.aaf3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delemarre FG, Kors N, Kraal G, van Rooijen N. Repopulation of macrophages in popliteal lymph nodes of mice after liposome-mediated depletion. J Leukoc Biol. 1990;47:251–257. doi: 10.1002/jlb.47.3.251. [DOI] [PubMed] [Google Scholar]
- 80.Arroba AI, Alvarez-Lindo N, van Rooijen N, de la Rosa EJ. Microglia-Müller glia crosstalk in the rd10 mouse model of retinitis pigmentosa. Adv Exp Med Biol. 2014;801:373–379. doi: 10.1007/978-1-4614-3209-8_47. [DOI] [PubMed] [Google Scholar]
- 81.Wang M, Wong WT. Microglia-Müller cell interactions in the retina. Adv Exp Med Biol. 2014;801:333–338. doi: 10.1007/978-1-4614-3209-8_42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wickham H. ggplot2 - Elegant Graphics for Data Analysis. 2nd Ed Springer International Publishing, Cham; Switzerland: 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.