Significance
Severe human fibrotic diseases are devastating and without effective treatments. We found that c-JUN expression is increased in many human fibrotic diseases and that systemic induction of c-Jun in mice resulted in development of fibrosis of multiple organs. These results suggest that many fibrotic diseases share a common pathomechanism that converges on c-Jun induction. Thus, common treatment strategies could potentially be developed for these seemingly different fibrotic disease entities. Moreover, the in vivo c-Jun induction represents a mouse model for these devastating diseases that could be used for preclinical evaluation of candidate antifibrotic treatments. Indeed, we show that blockade of the antiphagocytotic signal CD47 and the AKT and VEGF receptor pathways reverses tissue fibrosis in mice.
Keywords: c-JUN, anti-CD47 antibody therapy, signaling pathways, fibrotic disease, scleroderma
Abstract
Fibrotic diseases are not well-understood. They represent a number of different diseases that are characterized by the development of severe organ fibrosis without any obvious cause, such as the devastating diseases idiopathic pulmonary fibrosis (IPF) and scleroderma. These diseases have a poor prognosis comparable with endstage cancer and are uncurable. Given the phenotypic differences, it was assumed that the different fibrotic diseases also have different pathomechanisms. Here, we demonstrate that many endstage fibrotic diseases, including IPF; scleroderma; myelofibrosis; kidney-, pancreas-, and heart-fibrosis; and nonalcoholic steatohepatosis converge in the activation of the AP1 transcription factor c-JUN in the pathologic fibroblasts. Expression of the related AP1 transcription factor FRA2 was restricted to pulmonary artery hypertension. Induction of c-Jun in mice was sufficient to induce severe fibrosis in multiple organs and steatohepatosis, which was dependent on sustained c-Jun expression. Single cell mass cytometry revealed that c-Jun activates multiple signaling pathways in mice, including pAkt and CD47, which were also induced in human disease. αCD47 antibody treatment and VEGF or PI3K inhibition reversed various organ c-Jun–mediated fibroses in vivo. These data suggest that c-JUN is a central molecular mediator of most fibrotic conditions.
The fibrotic response is an important component of normal repair processes that, if uncontrolled, can lead to various life-threatening conditions, like idiopathic pulmonary fibrosis (IPF), primary myelofibrosis, and scleroderma (1–5). It is not known whether similar molecular mechanisms are responsible for the fibrotic response in different diseases, and some studies came to conflicting conclusions (6). The molecular processes driving fibrogenesis are not well-understood, involving but not limited to transforming growth factor B (TGFB), platelet-derived growth factor (PDGF), connective-tissue growth factor (CTGF), vasoactive peptide, integrin signaling, and increased tissue stiffness (7, 8). At the cellular level, efforts have been made to characterize fibroblasts by molecular markers (9), and fibrosis is thought to involve the cross-talk of hematopoietic and mesenchymal stroma cells (4, 6).
Although the few currently available animal models are useful, all of them have certain limitations: e.g., bleomycin-induced lung or skin fibrosis develops acutely in response to chemical injury and is self-resolving; however, human diseases such as idiopathic pulmonary fibrosis or scleroderma are not (10). A more recent genetic mouse model showed some fibrosis features but predominantly exhibited vasoocclusive alterations more reminiscent of pulmonary artery hypertension (PAH) (11).
On the other hand, the genetic basis of fibrotic diseases is just emerging but already promises to gain fundamental insights into pathomechanisms: e.g., FAN1 mutations have been associated with kidney fibrosis, PNLAP3 with liver fibrosis, mutations in JAK2, MPL, or calreticulin with bone marrow fibrosis in myeloproliferative neoplasm (12), and telomerase reverse transcriptase (TERT) and mucin 5B (MUC5B) with lung fibrosis, as well as alterations in DNA methylation, and some microRNAs (5) have been shown to play a role in lung fibrosis. Some of these molecular findings have guided current standard of care treatments in, e.g., lung fibrosis and primary myelofibrosis.
We previously developed mouse models of primary myelofibrosis associated with myeloproliferative disease (13, 14) and wished to investigate their molecular downstream effectors. Gene expression analysis suggested the dysregulation of cJUN, an AP-1 transcription factor that is a well-established regulator of critical cell biological processes and involved in cancer and other human diseases (15, 16). We subsequently investigated the expression of AP-1 transcription factors in most human fibrotic diseases and found increased c-JUN expression in SMA+ fibroblasts. In addition, c-JUN was responsible for the pathologically increased proliferation of fibroblasts of patients with idiopathic pulmonary fibrosis. Based on our observations in patients with various clinical pictures of fibrosis, we generated a mouse model with inducible c-Jun. In these mice, we were able to induce fibrosis via the c-JUN pathway, which closely resembled the various organ manifestations in patients. Remarkably, we found that fibroblasts selectively responded to c-Jun despite ubiquitous c-Jun expression. Single cell mass cytometry analysis in mouse fibrosis revealed that transcriptional effects of c-Jun lead to a profound rewiring of active signaling pathways, which we exploited for effective therapeutic intervention in mice.
Results
c-JUN Is Expressed in All Major Human Fibrotic Conditions.
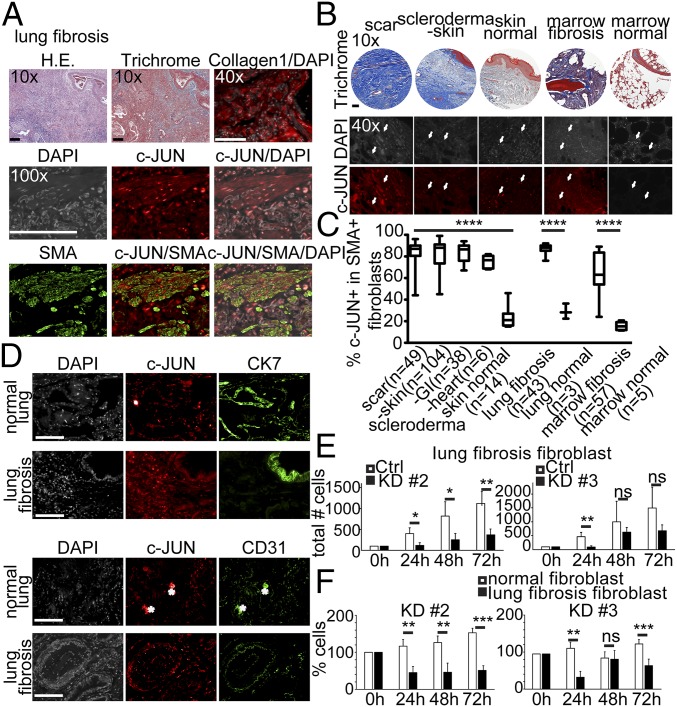
Previously, we had observed the up-regulation of AP-1 transcription factors in a mouse model of polycythemia vera that also recapitulated features of bone marrow fibrosis (14). We therefore sought to analyze the expression of c-JUN in human fibrotic conditions. Given the cellular heterogeneity in fibrotic lesions, we performed a thorough immunohistochemical analysis. A total of 454 biopsies from patients with different fibrotic conditions were stained with H&E, with trichrome, and with antibodies against Collagen1, c-JUN, and SMA (Fig. 1). Then, 148 biopsies were analyzed from patients with scleroderma [skin lesions (n = 104), gastrointestinal (n = 38), and heart (n = 6)], 36 biopsies from patients with idiopathic lung fibrosis, 57 biopsies from patients with primary myelofibrosis, 164 biopsies from patients with liver fibrosis [related to nonalcoholic steatohepatitis (NASH)/hemochromatosis, ethanol (ETOH)/hepatitis C (HCV), alpha-1-anti-trypsin (A1A) deficiency, and chronic rejection], and biopsies from kidneys [systemic lupus erythematosus (SLE)-related and reflux-related) and the bladder, the pancreas, and the heart, but also biopsies involving intraabdominal and pleural adhesions; biopsies were matched with normal tissues if available (Fig. 1 A and B and Fig. S1 A and C). Quantification of the immunofluorescence analysis demonstrated that a much higher fraction of SMA+ fibroblasts expressed nuclear c-JUN in all these fibrotic diseases compared with three control conditions (P < 0.0001) (Fig. 1C and Fig. S1A). Costaining of c-JUN with SMA (smooth muscle, fibroblasts), CK7 (bronchoepithelium), and CD31 (blood vessel endothelium) demonstrated that c-JUN is predominantly expressed in fibroblasts and a subset of bronchoepithelial cells and macrophages but only rarely detected in other cell types in lung biopsies of lung fibrosis patients (Fig. 1 A and D).
Fig. 1.
Elevated c-JUN expression in various human fibrotic diseases. (A) SMA+ fibroblasts in lung fibrosis strongly expressed nuclear c-JUN (red) in fibrotic plaques as revealed by trichrome, Collagen1, and SMA staining. Shown are representative patient biopsies of lung fibrosis (n = 43) stained for indicated markers. (Scale bar: 100 µm.) (B) Example micrographs showing high c-JUN expression in trichrome+, fibrotic plaques of various indicated fibrotic conditions. Arrows indicate examples of nuclear c-JUN expression. (C) Quantification of c-JUN expression in SMA+ fibroblasts in various fibrotic conditions, including scar tissue (49 patient biopsies), scleroderma (104 patient biopsies of scleroderma, diffuse proliferative type), and lung fibrosis (43 patient lung biopsies) and in marrow fibrosis (57 patient marrow biopsies) but not in normal skin, lung, and marrow. ****P < 0.0001; ANOVA test. We quantified coexpression of c-JUN and SMA in the entire tissue of each sample. (D) Nuclear c-JUN is strongly expressed in mesenchymal cells in fibrotic plaques in lung fibrosis but only weakly in a small subset of bronchoepithelia (CK7 in green) and endothelia (CD31 in green). DAPI, nuclear counterstain in gray. Asterisks indicate auto fluorescence; one out of seven lung fibrosis and one out of six normal lung patient biopsies are shown. Three biological replicates for each stain were performed. (Scale bar: 100 μm.) (E) Lung fibroblasts from lung fibrosis patients require c-JUN to proliferate. Filled bars show cell numbers at indicated time points after c-JUN hairpin induction with doxycycline, open bars after doxycycline of control infections. (F) Normal lung fibroblasts do not require c-JUN to proliferate, unlike lung fibrosis fibroblasts. Shown are relative cell numbers before and after doxycycline-induced c-JUN knockdown normalized to the condition before knockdown. Patient lung fibroblast cultures were infected with one of two lentiviral shRNA hairpins also expressing RFP or a control vector expressing GFP. Infected cells were sorted by FACS and plated at a density of 6,000 cells per well, and the number of red and green fluorescent cells was counted 24, 48, and 72 h after plating. Data (mean ± SEM) represent four replicates (Student’s t test). *P < 0.05, **P < 0.01, ***P < 0.001.
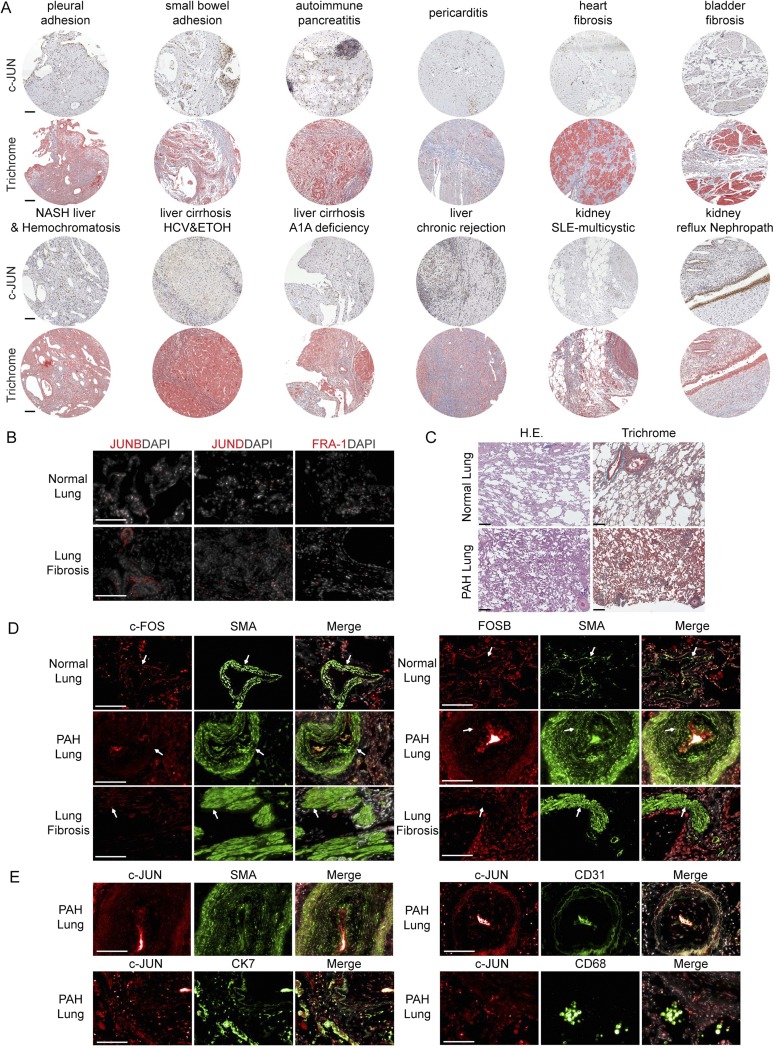
Fig. S1.
c-JUN is highly expressed in most human fibrotic conditions and coexpressed with FOS in lung fibrosis. (A) Representative histology of patient biopsies of indicated fibrotic conditions out of 454 biopsies total were stained with c-JUN (brown nuclear staining) and trichrome (blue staining) and show increased collagen-producing fibroblasts with high level expression of c-JUN in densely fibrotic areas. (B) Immunohistochemical stains of JUNB, JUND, and FRA-1 (red) were negative in normal lung and lung fibrosis tissues. (C) Representative histology of normal lung and pulmonary arterial hypertension (PAH) lung tissue stained with H&E and trichrome with minimal perivascular fibrosis (blue on trichrome stain) and a pathological thickened vessel (PAH-lung, Lower Right). (D) c-FOS and FOSB (red), SMA (green), and DAPI (gray/merge) staining of lung tissue from normal, PAH, and lung fibrosis showed a subset of fibroblasts in lung fibrosis expressing nuclear c-FOS and FOSB. The arrows highlight representative nuclear staining. (E) c-JUN (red) costaining with cell type–specific markers SMA, CK7, CD31, and CD68 (green) showed coexpression of c-JUN in a subset of bronchoepithelial cells (CK7), but not in the majority of smooth muscle cells (SMA), endothelial cells (CD31), or macrophages (CD68). For B–E, one of seven idiopathic pulmonary fibrosis patient biopsies, one of five PAH patient biopsies, and one of six normal lung biopsies are shown. Three biological replicates for each stain were performed. (Scale bars: 100 μm.)
Nuclear c-JUN Is the Predominant AP-1 Transcription Factor Identified in Pathogenic Fibroblasts in Fibrotic Plaques of Human Lung Fibrosis and Other Fibrotic Diseases.
Because the c-JUN–related factor FRA-2 has been previously described in a murine model of pulmonary vasoocclusion and subsequent fibrosis (11), we tested whether FRA-2 would be coexpressed with c-JUN in lung fibrosis and other fibrotic conditions. Indeed, resembling the described mouse model remarkably closely, nuclear FRA2 was readily detectable in biopsies of pulmonary artery hypertension (PAH), in particular in pathologically thickened PAH vessels (Fig. S2A, Upper). However, we failed to detect nuclear FRA-2 staining in the majority of fibroblasts in other fibrotic diseases, including the most common interstitial pneumonia-type of idiopathic pulmonary fibrosis (IPF) (Fig. S2A, Lower).
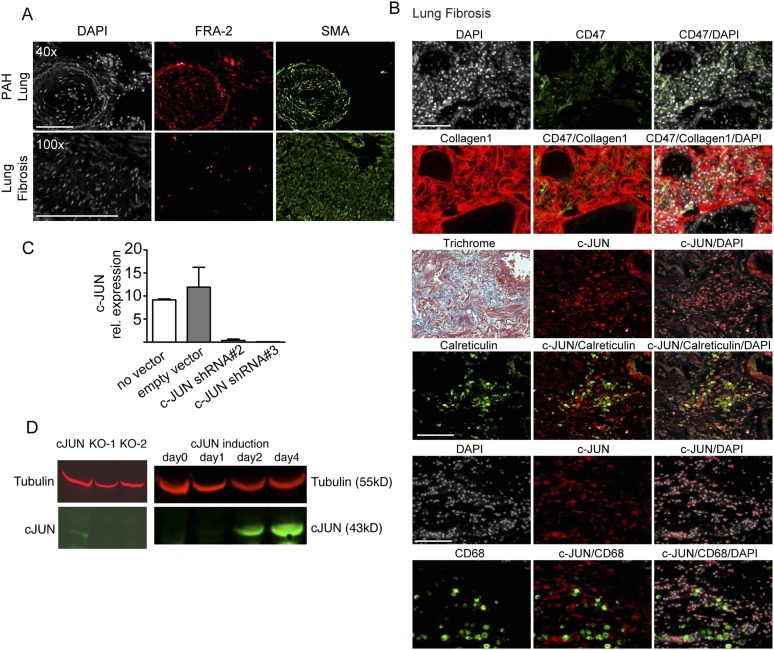
Fig. S2.
Don't-eat-me signal CD47 and calreticulin (eat-me signal) are expressed in lung fibrosis. (A) Representative lung sections from pulmonary arterial hypertension (PAH) and lung fibrosis lungs showed pathologically thickened vessels comprising FRA-2–positive smooth muscle cells. One of seven idiopathic pulmonary fibrosis and one of five PAH patient biopsies are shown. (Scale bars: 100 μm.) (B) Continuous sections of biopsies of lung fibrosis patients were costained as indicated and demonstrated expression of don’t-eat-me signal CD47 (green) in c-JUN+ (red) fibroblasts also expressing Collagen1 (green). Trichrome costains highlighted a fibrotic plaque with a small bronchus (Top Right) and otherwise highly abnormal lung histology. Costaining of c-JUN (red) and calreticulin (green) suggested expression of the eat-me signal in bronchoepithelium and macrophages (CD68, green). (Scale bars: 100 μm.) (C) We demonstrated efficient knockdown of c-JUN with hairpin no. 2 and no. 3 with quantitative RT-PCR in primary lung fibroblasts derived from lung fibrosis patient. (D) Western blot of c-JUN antibody revealed the loss of the specific 43-kDa band upon c-JUN knockout with CRISPER and a c-JUN–specific 43-kDa band upon doxycycline–mediated c-JUN induction in primary lung fibroblasts, supporting the specificity of the c-JUN antibody used for immunostaining.
This finding prompted us to systematically investigate the expression of additional AP-1 transcription factors. Immunohistochemical stains of lung, liver, kidney, bladder, pancreas, and heart fibrosis and visceral adhesions confirmed strong nuclear c-JUN expression. c-JUN was coexpressed with FOS and FOS-B occasionally in IPF, but not nuclear FRA-2 (Fig. S1 D and E). None of the remaining AP-1 transcription factors JUNB, JUND, and FRA-1 were detectable (Fig. S1B).
Pathogenic but Not Normal Fibroblasts Require c-JUN for Rapid Proliferation.
To further investigate a functional role of c-JUN in pathogenic fibrosis, we knocked down c-JUN in primary lung-derived fibroblasts isolated from patients with idiopathic pulmonary fibrosis and normal lung tissue with two different hairpins. First, we confirmed that both hairpins achieved substantial decrease of c-JUN mRNA (Fig. S2C). Both hairpins substantially reduced the proliferation of IPF fibroblasts compared with the control hairpin at various time points after plating (Fig. 1E, filled bars). Surprisingly, we found that normal healthy lung-derived fibroblasts do not require c-JUN; they continue to grow upon c-Jun knockdown (Fig. 1F, open bars).
c-JUN–Positive Fibroblasts Are Surrounded by Macrophages and Express the CD47 “Don’t-Eat-Me” Signal in Idiopathic Pulmonary Fibrosis Lungs.
A complex interplay between the immune system and mesenchymal cells is considered important for the pathogenesis of fibrotic conditions (5, 6). We therefore analyzed lung samples of patients with idiopathic lung fibrosis for the presence of immune cells and observed large numbers of CD68+ macrophages interspersed with c-JUN–expressing fibroblasts (Fig. S2B, Bottom) (Fig. S2D demonstrates specificity of the human c-JUN antibody). This finding raised the question why fibroblasts are not phagocytized by macrophages. We therefore investigated the expression levels of antiphagocytic don’t-eat-me signals and found that CD47 was up-regulated on fibroblasts. In contrast, calreticulin, considered an “eat-me” signal, was expressed in macrophages and a subset of bronchoepithelial cells (Fig. S2B, Top, Middle, and Bottom).
c-Jun Induces Widespread Fibrosis in Many Organs.
In addition to bone marrow fibrosis (Figs. S3 and S4), detailed histopathologic analysis of systemically c-Jun–induced mice revealed severe fibrosis of the skin, which is illustrated by the blue trichrome staining that reveals that over 80% of the dermis is replaced with extracellular collagen deposition (Fig. 2D, Upper). Moreover, several gastroenteric organs displayed similarly severe fibrosis in the distal esophagus, stomach, and small intestine (Fig. 2D, Lower). Other organs were much less affected, such as the lung that showed only a mild interstitial fibrosis. Fibrotic alterations of the skin, gastroesophageal junction, and lung are characteristic diagnostic criteria for the human condition of scleroderma.
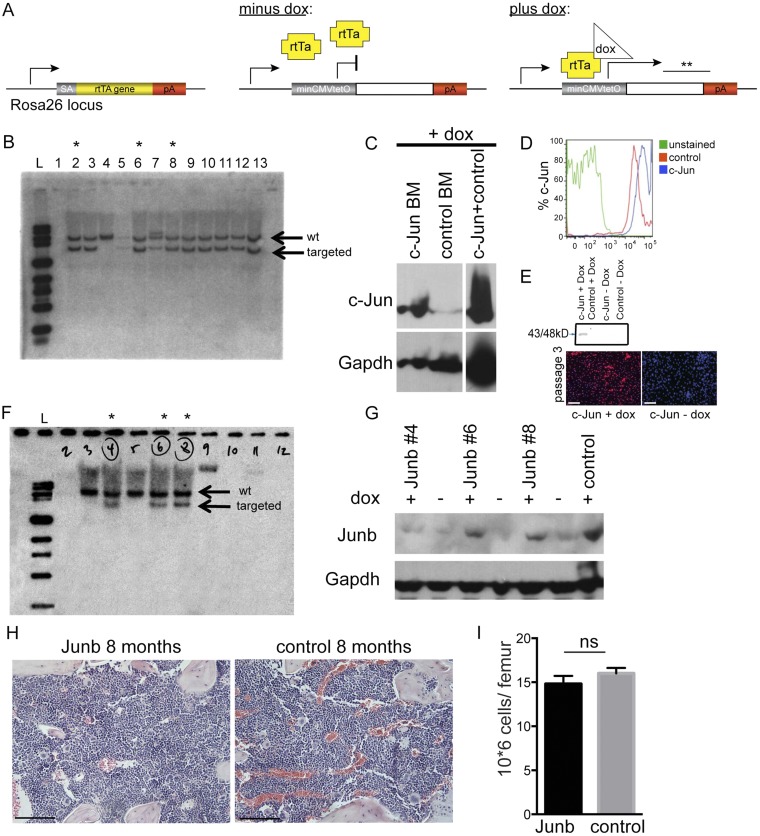
Fig. S3.
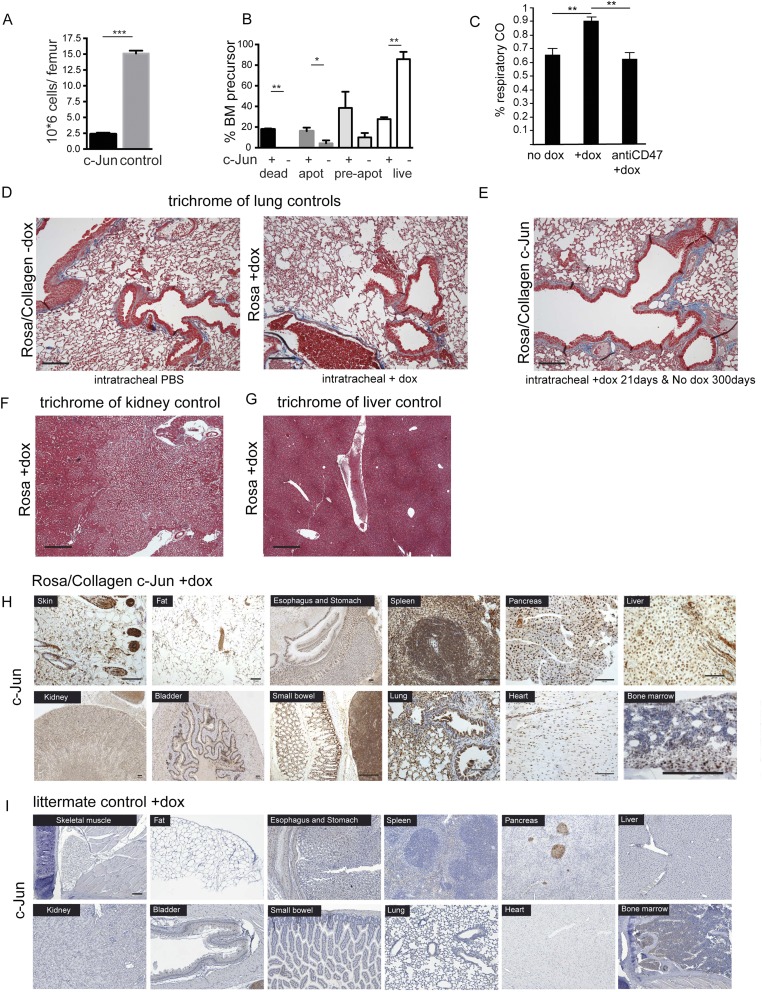
c-Jun but not Junb causes skin, visceral, and marrow fibrosis in adult mice. (A) Targeting construct of c-Jun doxycycline-inducible mice. (B) Southern blot showing positively targeted ES cells with c-Jun. (C and D) Protein expression of c-Jun in mouse marrow by Western plot after 48 h of induction with doxycycline and after 7 d by intracytoplasmic flow cytometric analysis. (E) c-Jun expression and antibody specificity were confirmed by Western blot and immune staining in primary marrow-derived fibroblasts from c-Jun rtTA mouse after three passages in culture on day 2 of doxycycline induction and control (without doxycycline). (F) Southern blot showing positively targeted ES cells with Junb. (G) Protein expression of Junb by Western plot after 48 h of induction with doxycycline. (H) Representative histology of the marrow of Junb-expressing mice (n = 5). (I) Single femur counts revealed normocellular marrows for age. Data (mean ± SEM) are from five independent replicates. P = not significant (ns); paired Student’s t test was used to determine significant changes. (Scale bars: 100 μm.)
Fig. S4.
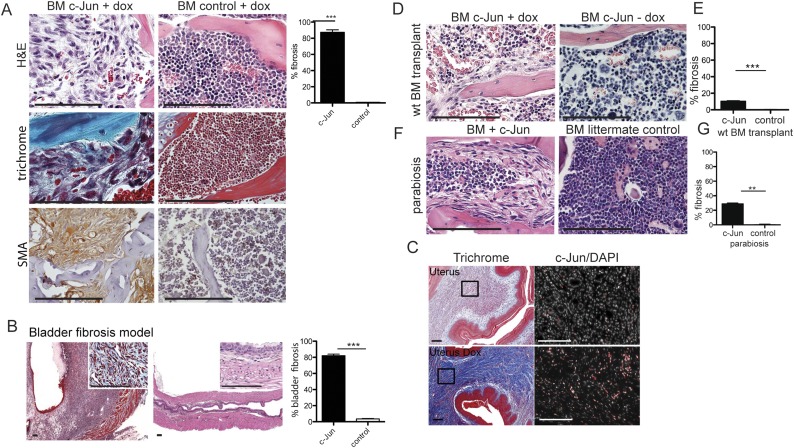
c-Jun expression in stroma cells caused severe marrow and visceral fibrosis that was mildly suppressed by hematopoietic cells. (A) Histological analysis revealed c-Jun–mediated dense fibrosis in the marrow in c-Jun–expressing mice (Left) quantified at 80% (graph, Far Right). H&E stains (Top) demonstrated that hematopoietic cells are significantly decreased and replaced by fibroblasts. Trichrome stains (Middle) highlighted the densely fibrotic areas with pathologic deposition of extracellular collagen (representative images, n = 17 mice analyzed) and fibroblasts coexpressed smooth muscle actin (SMA, brown cytoplasmic staining, Bottom). Trichrome stains label cross-linked collagen and are standard of care in clinical practice to grade organ involvement by fibrosis. n = 17 mice. (B) Histological analysis revealed fibrosis of the bladder wall 38 d after dox-mediated c-Jun induction on H&E and trichrome (Inset) stain and quantified at 82%. (C) Histological analysis revealed dense fibrosis of the uterus (82%) on H&E and trichrome 8 d after dox-mediated c-Jun induction. The black frames indicate the areas shown on higher magnification to the right. C-Jun staining demonstrated increased expression of c-Jun 8 d after c-Jun induction. (D and F) c-Jun–induced fibrosis was partially rescued by WT hematopoietic cells after bone marrow transplantation and also in c-Jun–induced mice parabiosed to WT syngeneic mice (n = 10 transplanted mice, n = 2 parabiosed mouse pairs). (E and G) Extent of fibrosis was quantified at 10.3% in sections of c-Jun–induced animals transplanted with WT bone marrow and at 29% in sections of parabiosed c-Jun–induced animals. To assess the percent involvement by fibrosis, we quantified the trichrome+ areas of 10 high power fields (40x) in all cases. **P < 0.01, ***P < 0.001, Student’s t test. (Scale bars: 100 μm.)
Fig. 2.
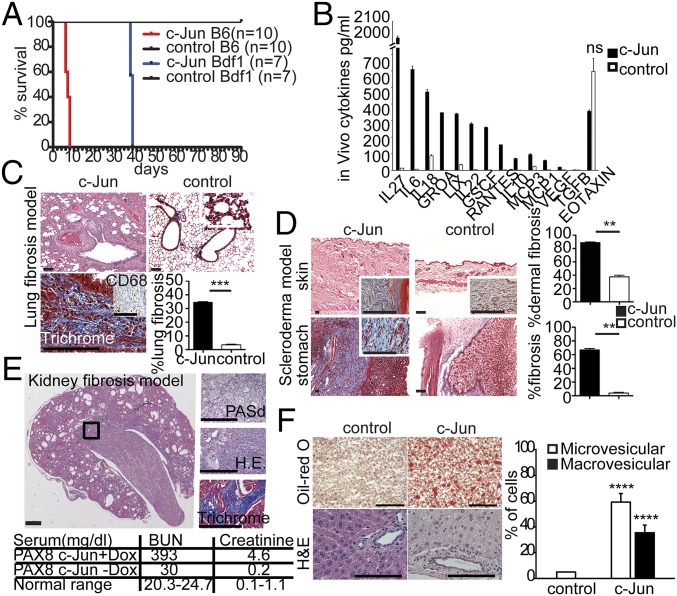
Development of multiple organ fibrosis in mice after induction of c-Jun in vivo. (A) c-Jun–induced lethality in mice in a strain-dependent manner. B6, F1 129/C57BL/6; Bdf1, F1 129/BDF1. The red curve represents c-Jun B6 mice (n = 10, P < 0.0001), and the blue curve c-Jun Bdf1 mice (n = 7 mice, P < 0.0001 by Kaplan–Meyer survival analysis and two independent experiments). (B) Mice (B6) released many pro- and antiinflammatory cytokines in the blood 48 h after dox-mediated ubiquitous c-Jun induction. We quantified 38 mouse cytokines/chemokines by multiplex assay and found 13 to be increased significantly (**P < 0.01; ns, not significant) as indicated (n = 3 animals per group, repeated once, P values have been calculated by Student's t test). (C) Dox-mediated c-Jun expression in the lung led to “honeycomb” fibrosis reminiscent of human idiopathic lung fibrosis. Fibrotic plaques with extensive interstitial collagen (blue stained areas on trichrome) and intermixed with interstitial macrophages (CD68+, Inset) were identified peribronchial and subpleural and represented 34% of the surface area. We quantified the trichrome+ areas of 10 high power fields (40x), n = 3 animals per group (***P < 0.001). (D) Dox-mediated ubiquitous c-Jun expression led to thickening and fibrosis of the dermal skin and the gastroesophageal junction, a pattern of fibrosis reminiscent of human scleroderma. We quantified fibrosis in the trichrome+ areas of 10 high power fields (40x) of trichrome-stained sections (Insets) and detected 89% in the dermal skin and 67% in the stomach wall, n = 5 animals. (E) Kidney-restricted c-Jun expression using a Pax8-rtTA strain resulted in interstitial fibrosis and tubular atrophy (quantified at 30 to 40%) with elevated kidney enzymes as indicated in the table reminiscent of a primary tubulointerstitial nephropathy in patients. (Insets) High power views (40x) of the abnormal areas. A PASd stain (Top) labels intact basement membranes of glomeruli and tubuli, the H&E stain demonstrates increased interstitial fibrosis (Middle), and a trichrome stain (blue stain) shows abundant abnormal extracellular collagen matrix deposition (Bottom). n = 4 animals. (F) Systemic c-Jun expression caused fatty liver changes of the micro- and macrovesicular type in mice as shown by the small and large intrahepatic orange deposits on Oil-red O and the intracellular vesicles on H&E stains. We quantified 400 hepatocytes for intracellular lipid droplets in representative areas of Oil-Red O-stained liver sections as indicated. ****P < 0.0001; paired Student's t test. All data (Fig. 2 C–F) (mean ± SEM) represent two replicates of two independent experiments. Representative histology at 20x magnification, n = 4 animals. (Scale bars: 100 μm.)
Given the short time window of analysis due to rapid death with systemic induction, we next asked whether tissue-restricted induction of c-Jun may cause fibrosis also in additional organs.
We subsequently established fibrosis restricted to the lung by c-Jun induction via dox aerosol administration. Indeed, this treatment resulted in striking fibrosis, with over 30% of the lung parenchymal tissue replaced with extracellular collagen as shown by trichrome stain (Fig. 2C). The collagen was distributed in a patchy interstitial pattern peripleurally and surrounding the major airways. This process was reversible when dox administration was discontinued after 21 d, and animals completely resolved the fibrosis and demonstrated normal histopathology at 300 d at study end point (Fig. S5E). In addition, we did not notice any fibrotic changes after PBS aerosol administration in c-Jun–inducible mice or with dox aerosol in littermate controls lacking c-Jun (Fig. S5D). In mice with lung fibrosis, inflammatory cells were admixed and comprised of abundant CD68+ macrophages with fewer lymphocytes, plasma cells, and neutrophils (Fig. 2C, Inset, Lower Left). Because conventional volumetric assessment of lung function proved challenging in these sick mice, we measured CO gas diffusion, which showed a functional impairment in gas exchange (Fig. S5C). Thus, the characteristic histopathological abnormalities and clinical presentation of mice after lung-restricted c-Jun induction closely recapitulated the human condition of idiopathic pulmonary fibrosis, in particular the most common usual interstitial pneumonia-type.
Fig. S5.
c-Jun–mediated fibrosis is specific to c-Jun induction in vivo. (A) Single femur counts revealed severe cytopenia in the bone marrow. Data (mean ± SEM) are from four independent replicates. ***P < 0.001 (paired Student’s t test). (B) 7AAD/annexin V staining demonstrated increased apoptosis in c-Jun–expressing hematopoietic precursors. Data (mean ± SEM) represent three replicates and two independent experiments. *P < 0.05, **P < 0.01; paired Student’s t test. (C) Lung-specific c-Jun expression resulted in interstitial fibrosis in the lung, and, as a consequence, CO accumulated in the respiratory air. CD47 antibody resolved interstitial fibrosis and thus normalized CO diffusion in the lung. Data (mean ± SEM) represent four replicates and two independent experiments; **P < 0.01; Student's paired t test. (D) Long-term intratracheal aerosol treatment of control mice with doxycycline and control or c-Jun mice with PBS did not result in any fibrosis in the lung. (E) Established c-Jun–mediated lung fibrosis (after 21 d of intratracheal dox treatment) normalized 300 d after c-Jun was switched off, and we show normal lung histomorphology with only minimal fibrosis on trichrome stain. (F and G) Representative kidney and liver sections of control mice stained with trichrome to highlight fibrosis. (H and I) c-Jun stains highlight increased c-Jun expression in all cell types and most organs 48 h after dox-mediated c-Jun induction in c-Jun mice (+dox) but not controls. Similarly, c-Jun is expressed in many different hematopoietic cell types in the bone marrow. (Scale bars: 100 μm.)
Next we crossed the tetO-c-Jun allele to Pax8-rtTA mice to accomplish kidney-specific c-Jun induction. After 8 wk on dox water, animals appeared moribund. Histopathologic analysis demonstrated 30 to 40% interstitial fibrosis of the kidney, with abundant abnormal extracellular collagen matrix deposition labeled in blue with trichrome stain and tubular atrophy consistent with a primary tubulointerstitial nephropathy. In addition, serologic markers for kidney function, such as blood urinary nitrate (BUN) and creatinine, were significantly increased (Fig. 2E and Fig. S5F).
cJUN expression caused drastic thickening and fibrosis of the bladder wall (80% fibrosis after 38 d of dox in the drinking water in c-Jun BDF1 mice) (Fig. S4B) and uterus fibrosis (Fig. S4C).
Development of Steatohepatosis-Like Alterations After c-Jun Induction.
Steatohepatosis is associated with increased fat accumulation in liver cells and considered a precursor of liver cirrhosis/fibrosis. Indeed, 7 to 10 d after systemic c-Jun induction, we noticed small and large vacuoles in the majority of hepatocytes (Fig. 2F and Fig. S5G) (which were positive by oil-red O, a stain that highlights lipids) but no fibrosis. We qualitatively assessed liver fat content after cJUN expression in mice and detected 60% micro- and 40% macrovesicular changes in contrast to less than < 5% in noninduced controls. A recent report showed similar liver changes in mice mediated by distinct AP1 dimers; however, unlike our findings, the steatohepatic abnormalities required liver injury (17).
In summary, these findings demonstrate that c-Jun expression results in fibrosis in virtually all organs.
c-Jun Induces Feedback Loops to Rewire Signaling Pathways in a Cell Context-Specific Manner.
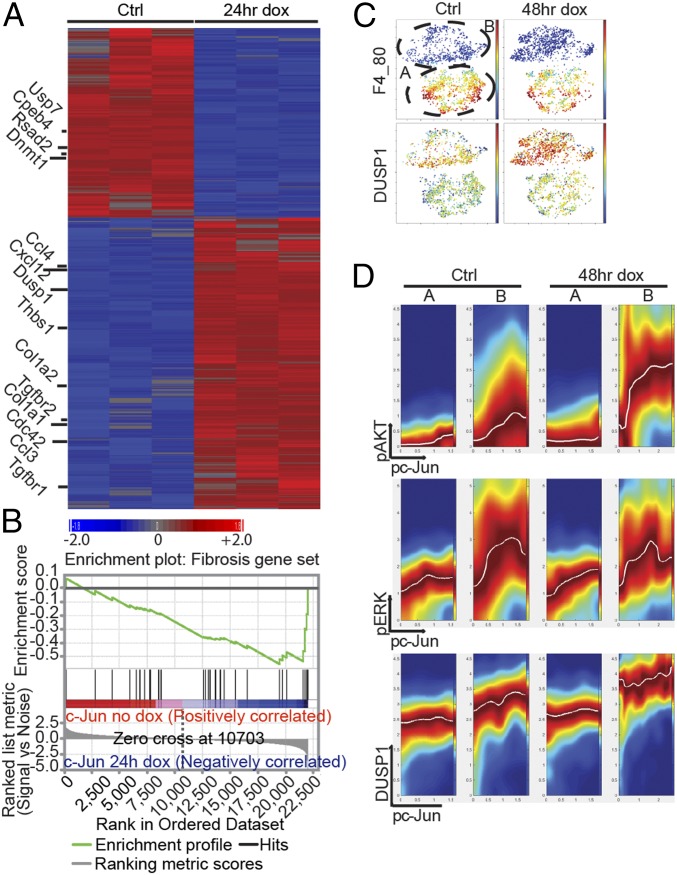
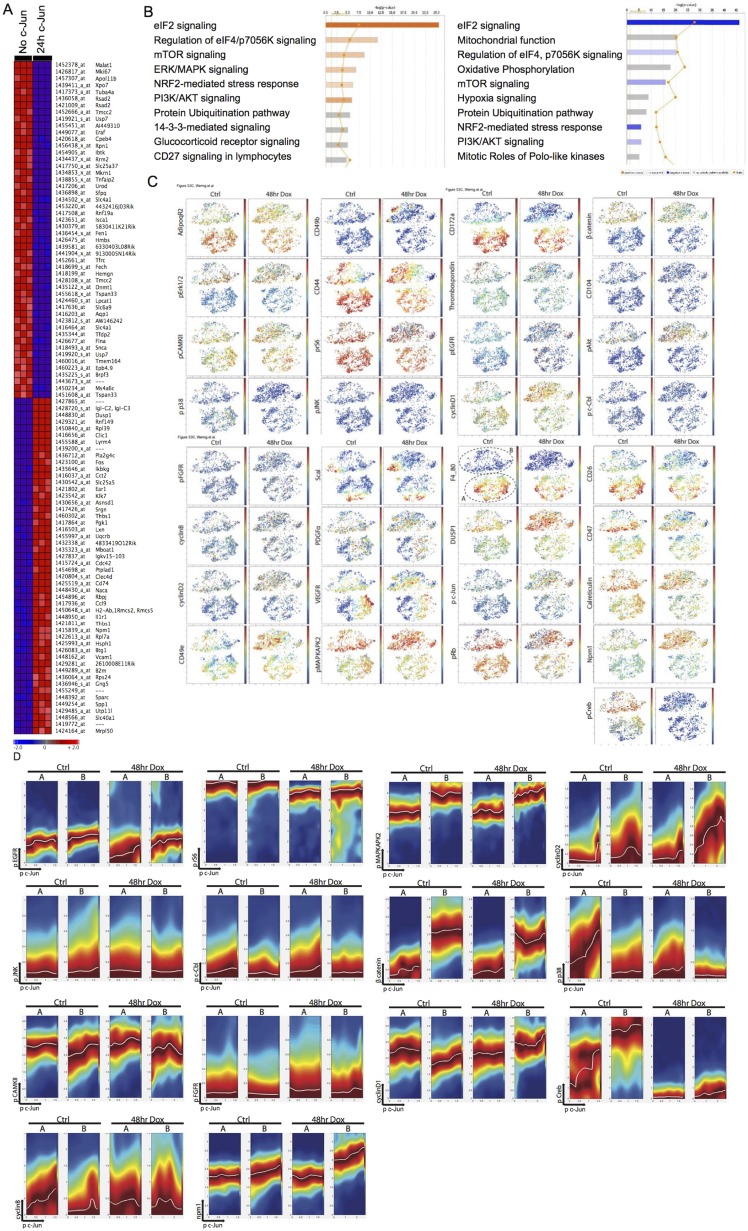
To further explore the molecular pathways involved in c-Jun–induced fibrosis, we performed genome-wide transcriptional profiling. We profiled whole bone marrow 24 h after dox treatment of c-Jun–inducible and control mice in vivo. Strikingly, already at this early time point, many fibrogenesis-associated genes were induced, such as Cdc42, Cxcl12 (also known as Sdf1), Tgfbr1 and -2, Ccl3, Ccl4, Collagen1a1, Collagen1a2, Collagen5a2, and Adiponectin, some of which are involved in migration (Fig. 3A and Fig. S6A). Among the top 100 up-regulated genes were other representatives of the AP1 family, most notably c-Fos (Fig. S6A). To confirm our RNA expression data, we tested 43 cytokines and surface epitopes by direct immunofluorescence or flow cytometry and found Cxcl12 (Sdf1), Cxcr4, Thrombospondin-1, CD51, Vegfr, and CD47 to be increased in c-Jun–expressing cells (SI Results, SI Materials and Methods, and Dataset S1). Specific interrogation of a fibrosis signature using gene set enrichment analysis (GSEA) revealed a significant enrichment in the c-Jun–induced cells (Fig. 3B). We next asked whether c-Jun, a target of the MAPK/JNK pathway itself, may in turn control the transcription of genes involved in upstream signaling pathways. To that end, we performed ingenuity pathway analysis and found transcriptional up-regulation of the MAPK pathway 24 h post–c-Jun induction (Fig. S6B).
Fig. 3.
c-Jun rewires transcriptional and signaling pathways in fibroblasts in vivo. (A) Genome-wide transcriptional profiling in mouse marrow after 24 h of dox-mediated c-Jun expression in vivo demonstrated up-regulation of c-Jun and Fos and indicated genes, many of which were previously associated with fibrotic conditions. We selected 1,144 probes out of 45,101 based on greater than ±2-fold change. Gene expression studies were performed in triplicate per each time point, and bone marrow of three mice was pooled per array. (B) Fibrosis signature genes are significantly enriched by gene set enrichment analysis (GSEA) in the up-regulated genes 24 h after dox-mediated c-Jun expression. (C) ViSNE maps of primary, marrow-derived fibroblast cultures before (Ctrl, 0 h dox) and after c-Jun induction (48 h dox) were generated by considering all 12 surface markers and represent its degree of F4_80 (Upper) or Dusp1 (Lower) expression; blue colors represent low expression and yellow to red colors high expression. (D) Conditional density visualization (DREVI plots) of the relationship between Phospho-c-Jun (pc-Jun) and Phospho-Akt (pAkt) (Top), pc-Jun and pErk (Middle), and pc-Jun and Dusp1 (Bottom) in F4_80-negative (subset B) versus F4_80-positive (subset A) marrow-derived adherent cells 48 h after c-Jun induction. The visualization method described how the y axis molecule changes as a function of the x axis molecules. Dark red (maximal color) represents the most likely y axis molecule value in the corresponding x axis molecular value. A response function (white curve) is fit to the region of highest conditional density. Representative data of two independent series are shown.
Fig. S6.
c-Jun modifies wiring of signaling and transcriptional response in fibrosis. (A) We performed gene expression studies and selected the top 100 differentially expressed genes of 45,101 based on greater than ±2-fold change 24 h after c-Jun induction in vivo. Gene expression studies were performed in triplicate per each time point, and bone marrow of three mice was pooled per array. (B) Ingenuity analysis revealed activation of MAPK pathway 24 h after c-Jun induction. (C) viSNE map of bone marrow-derived primary fibroblast cocultures before (Ctrl, 0 h dox) and after (“48hr Dox”) c-Jun induction showed distinct subsets based on macrophage lineage marker F4_80 and CD172a expression (A-F4_80-positive and B-F4_80-negative subsets). Each point in the viSNE map represents an individual cell, and color represents the expression level of indicated markers from high (red) to low (blue). (D) DREVI plots in all panels represent the relationship between pc-Jun on the x axis and the indicated genes on the y axis. (C and D) Representative data of one out of four experiments.
These transcriptional changes indicated that c-Jun once stimulated by upstream mechanisms may induce feedback loops to rewire the intracellular signaling networks that in turn may lead to fibrogenesis. To trace the origin and consequences of c-Jun–mediated signaling in primary bone marrow stroma, we performed CyTOF (mass cytometry) analysis. Primary bone marrow stroma cells with or without 48 h of c-Jun induction were fixed and stained with 34 metal-conjugated antibodies against fibrogenesis-associated proteins (such as CD104, CD26, Sca1, Pdgfra, Fgfr, Vegfr, AdipoqR2, CD49b, and CD49e), genes up-regulated from transcriptional analysis (such as CD47, Npm1, Dusp1, and thrombospondin), and major signaling pathways related to c-Jun [such as the ERK1/2 MAPK pathway (pERK1/2 and pS6), the p38 pathway (pMAPKAPKII), pJNK, the AKT-mTOR pathway (pAKT and pS6), and Dusp1] (Dataset S1, SI Results, and SI Materials and Methods). The visualizing data using t-Distributed Stochastic Neighbor Embedding (t-SNE) algorithm (viSNE) maps generated based on all 11 surface markers to the dataset showed two clearly distinct subpopulations. One was characterized by high expression of macrophage lineage markers, including CD172a and F4/80. The other subpopulation, CD172a and F4/80-negative cells, exhibited a much higher induction of phospho-c-Jun and c-Jun–induced molecules, such as Dusp1 (Fig. 3C and Fig. S6C). To better understand the effect of c-Jun induction on signaling networks in both subpopulations, we took advantage of the inherent stochasticity at the phosphor-c-Jun level between individual cells within each subpopulation and asked whether the state of a dependent signaling node could be correlated with the abundance of phospho-c-Jun. A conditional density-based algorithm, DREVI, was recently developed for this purpose to provide a way to visualize relationships between signaling nodes, which is well-suited to characterize the rewired signaling network after c-Jun induction (18, 19). As we show in Fig. 3D and Fig. S6D, this method computed the conditional density of the dependent signaling nodes on the y axis for the corresponding pospho-c-Jun values on the x axis. Among the measured signaling molecules, the DREVI plot revealed a digital type of response in the relationship between phospho-c-Jun and phospho-Akt only 48 h after c-Jun induction in CD172a and F4/80-negative cells, where a sharp transition between low and high phospho-Akt was observed (Fig. 3D). In contrast, components of MAPK pathways were the major up-regulated genes in the transcriptomic analysis at 24 h. We did not detect any increase in the phospho-Erk phospho-c-Jun relationship before and after c-Jun induction, and our data suggest that c-Jun increased Dusp1 levels, which negatively regulate the MAPK pathway. Remarkably, the F4-80+ cells showed only little response to c-Jun induction. These results demonstrate that the induction of transcriptional regulator c-Jun can rewire intracellular signaling networks by robustly activating the PI3K-Akt pathway while maintaining homeostasis in the MAPK pathway.
Functional Evaluation of Signaling Pathways Mediating the Fibrotic Response.
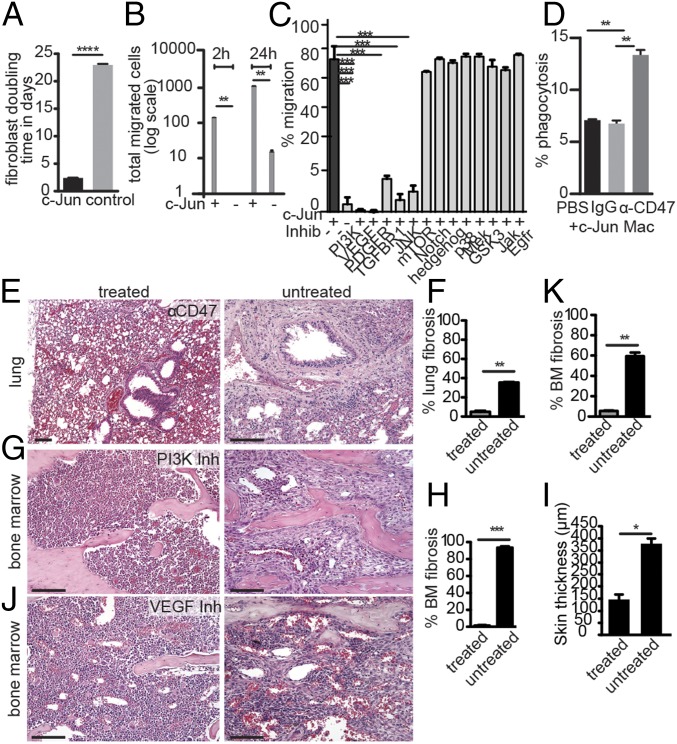
We next wished to assess the functional relevance of the signaling pathway rewiring induced by transcriptional activation of c-Jun. To enable systematic pharmacological interrogation, we sought to develop a c-Jun–dependent in vitro assay. We assessed two cell biological parameters in cultured bone marrow-derived stromal cells from c-Jun–inducible mice. First, we assessed cell growth and observed a shortening of the cell doubling time from about 22 d in control to about 2 d in c-Jun–induced fibroblasts (Fig. 4A). Second, we measured cell migration of marrow-derived stromal cells in a transwell migration assay as a functional readout of c-Jun induction and observed a 100-fold increase in migration at 2 h and 24 h in dox-mediated c-Jun–expressing cells compared with noninduced cells (Fig. 4B).
Fig. 4.
Anti-CD47, PI3K and VEGF inhibitors block c-Jun–mediated fibrosis. (A) Assessment of the doubling time (in days) of primary bone marrow fibroblasts with or without c-Jun induction revealed an about 10-fold increased proliferation rate upon c-Jun induction. Data (mean ± SEM) represent three replicates and two independent experiments; ****P < 0.0001; paired Student’s t test. (B) Transwell migration of primary marrow fibroblasts from c-Jun–inducible mice increased about 100-fold upon dox treatment at both 2 and 24 h after seeding. Data (mean ± SEM) represent three replicates and two independent experiments; **P < 0.01; paired Student’s t test. (C) c-Jun–mediated transwell migration of primary marrow fibroblasts in the presence of indicated small molecule inhibitors. Migration was impaired by PI3K, Vegf, Pdgfr, and Tgfb pathway inhibitors. Data (mean ± SEM) represent two replicates and two independent experiments; ***P < 0.001; paired Student’s t test. (D) In vitro phagocytosis assay of primary c-Jun–expressing fibroblasts and primary macrophages in the presence or absence of anti-CD47 antibodies. Anti-CD47 increased the phagocytosis rate. Data (mean ± SEM) represent two replicates and two independent experiments; **P < 0.01; paired Student’s t test. (E) H&E stain of lung sections of anti-CD47–treated (Left) and untreated (Right) mice after airway-restricted doxycycline delivery, which resulted in c-Jun–induced honeycomb-type lung fibrosis. The fibrosis was histomorphologically reversed with anti-CD47 antibody (MIAP 410, also known as clone 3). (F) Quantification of the extent of fibrosis in lung sections revealed that the residual fibrosis in the treatment group was 5%. n = 3 mice per experiment, two independent experiments; **P < 0.01, paired Student’s t test. (G and J) H&E histological analysis revealed that c-Jun–mediated bone marrow fibrosis was eliminated and replaced with normal hematopoietic cells after systemic treatment of PI3K and VEGF pathway antagonists. (H) Quantification of marrow fibrosis of PI3K inhibitor-treated and control mice revealed almost complete absence of fibrosis in treated animals. N = 3 animals, two independent experiments; ***P < 0.001. BM, bone marrow. (K) Quantification of marrow fibrosis of VEGF inhibitor-treated and control mice. n = 3 animals, two independent experiments; **P < 0.01. (I) c-Jun–mediated scleroderma-type dermal skin thickening was substantially reduced with PI3K inhibitor treatment. Data (mean ± SEM) represent n = 3 replicates, two independent experiments; *P < 0.05; paired Student’s t test. (Scale bars: 100 μm.)
Given its much greater dynamic range, we then tested a series of small molecule inhibitors in the transwell migration assay. Consistent with our mass cytometry findings, the c-Jun–induced migration was reduced to almost baseline levels in the presence of PI3K pathway inhibitors but not blockers of MAPK (such as MEK, p38), Jak, mTOR, Notch, hedgehog, GSK3, and EGFR (Fig. 4C).
Blockade of the VEGF and PI3K Pathways Reverses Marrow and Skin Fibrosis in Vivo.
Encouraged by these findings, we sought to evaluate the efficacy of blocking the PI3K-AKT pathway in c-Jun–mediated fibrosis in vivo. Wortmannin, a potent PI3K inhibitor, was systemically administered to mice (for 14 d) that were induced to express c-Jun 2 d before drug. Remarkably, this treatment resulted in complete suppression of bone marrow and skin fibrosis (Fig. 4 G, H, and I). In addition to the PI3K-AKT pathway, we also tested the effect of blocking the VEGF pathway on c-Jun–induced phenotypes both in vitro and in vivo because we found that VEGF was highly expressed in the primary bone marrow-derived adherent cells (Fig. S6C). As shown in Fig. 4 C, J, and K, the specific VEGFR inhibitor PD173074 was able to abolish c-Jun–mediated migration in vitro and significantly decreased bone marrow fibrosis, but not skin fibrosis, in vivo (SI Results and SI Materials and Methods).
Prophagocytic Anti-CD47 Treatment Reduces Fibrosis in Vivo.
We had noticed the prominent appearance of infiltrating macrophages in lung fibrosis, both in patient biopsies and in c-Jun–induced mice (Fig. S2B and Fig. 2C), which enclosed pathogenic fibroblasts expressing the antiphagocytotic CD47 signal (Fig. S2B). This pattern was reminiscent of macrophages surrounding CD47+ cancer cells or atherosclerotic plaques and raised the question of whether blocking the CD47 signal may stimulate phagocytosis of pathogenic fibroblasts by these infiltrating macrophages (20). Anti-CD47 antibodies have been shown to eliminate various human cancer cells and atherosclerosis in different preclinical models (21), but elimination of nontransformed normal cells has not been observed yet. To evaluate whether macrophages could be stimulated to phagocytose fibroblasts, we first tested the effect of blocking anti-CD47 antibodies in vitro. We cocultured primary mouse macrophages with c-Jun–induced primary fibroblasts and observed that, in the presence of anti-CD47 antibodies, the percentage of phagocytosed fibroblasts was indeed significantly increased (Fig. 4D). These compelling results prompted us to assess the effects of CD47 antibody treatment in our lung-restricted c-Jun fibrosis model. Indeed, daily i.p. injections for 21 d with an anti-CD47 antibody substantially reduced fibrosis (Fig. 4 E and F). Most importantly, animals administered anti-CD47 antibody treatment survived longer than control group, and their lung function was significantly improved as indicated by normalized carbon monoxide diffusion (Fig. S5C). These data suggest that macrophages can eliminate even nontransformed cells that contribute to disease formation when antiphagocytotic stimuli are blocked.
SI Results
c-Jun, but Not JunB, Induces Severe Marrow Fibrosis in Mice.
To dissect the functional relevance of AP-1 transcription factor up-regulation that we observed in patients, we generated c-Jun and JunB doxycycline (dox)-inducible mice by gene targeting in mouse embryonic stem cells, followed by blastocyst injection of the transduced cells (Fig. S3 A, B, and F). In this expression system, the reverse tetracycline transactivator (rtTA) is ubiquitously expressed from the Rosa26 locus, and the inducible cassette is targeted downstream of the Col1a1 locus, leading to robust and reliable drug-dependent transgene induction in most tissues (25). In both mouse strains, c-Jun and JunB were expressed in all cell types and organs (Fig. S5 H and I), and proteins were readily induced after 2 d of dox administration in the drinking water (Fig. S3 C, D, and G). Surprisingly, only 4 d after induction of c-Jun, hybrid F1 129/C57BL/6 mice became moribund and died between 6 and 8 d due to systemic cytokine release (Fig. 2B). In the BDF1 genetic background, the mice also died almost synchronously, but survival was prolonged to about 38 d (Fig. 2A). Before death, the mice exhibited scruffy fur and stiff skin, difficulties breathing, and prolonged bleeding. Initially, we focused our histopathologic analysis on the marrow, which revealed the lack of hematopoietic cells and striking fibrosis with collagen deposition replacing 87% of the marrow space, fulfilling all clinico-pathological criteria of grade 3 marrow fibrosis (Fig. S4A). Initially, we focused our histopathologic analysis on the bone marrow, which architecture was completely effaced by dense fibrosis with collagen deposition by trichrome stain. There were only a few pockets with residual trilineage hematopoiesis and mild megakaryocytic dysplasia. These findings are compatible with the clinico-pathological criteria of grade 3 marrow fibrosis, replacing 87% of the marrow space. (Fig. S4A). Time course analysis of the bone marrow demonstrated that pancytopenia and fibrosis in c-Jun–induced animals were progressive between days 2 through 6. The fibrotic infiltrate comprised spindle shaped cells that stained positive with trichrome and smooth muscle actin (Fig. S4A, Middle and Bottom). Gross morphologic paleness and single femur cell counts revealed severe cytopenia in the marrow, with an over ninefold reduction in the total number of bone marrow cells (2.5 × 106 versus 15 × 106, P < 0.01) in c-Jun–induced mice (Fig. S5A). Annexin V/7AAD stains indicated distinct c-Jun–mediated cell type–specific response 4 d after induction in vivo, which was a significant increase in the apoptosis in c-Jun-expressing hematopoietic precursors, suggesting cell death rather than migration as the primary mechanism accounting for the rapid disappearance of hematopoietic cells from the fibrotic bone marrow (Fig. S5B).
In contrast, none of the JunB-inducible mice showed any signs of disease after up to 8 mo of doxycycline treatment. Histopathological analysis revealed bone marrow cellularity and composition indistinguishable from control mice of the same age (Fig. S3 H and I). Thus, fibrogenesis is specifically induced by c-Jun and not by the related AP-1 transcription factor JunB.
c-Jun–Induced Marrow Fibrosis Is Primarily Mediated by Stroma Cells, with Some Contribution from Hematopoietic Lineage Cells.
We next sought to address whether c-Jun induction in the hematopoietic or mesenchymal cell population is responsible for fibrogenesis and whether the effects are mediated in a cell-autonomous manner as could be expected from a transcription factor. First, we transplanted whole bone marrow of c-Jun–inducible mice into WT recipients. Despite nearly 100% blood chimerism, no fibrosis was detected after dox treatment, suggesting that the hematopoietic compartment alone is insufficient to induce fibrogenesis. Next, we injected 1 × 106 CD45.1 WT whole bone marrow into c-Jun–inducible mice expressing CD45.2. After 25 d, the bone marrow was nearly completely reconstituted with WT donor cells. Upon dox treatment, fibrosis formation occurred but was substantially suppressed compared with nontransplanted c-Jun–inducible mice (Fig. S4 F and G, compare with B). To independently confirm these results, we performed a parabiosis experiment allowing blood exchange after anastomosis of the circulation of c-Jun–inducible and littermate control mice for 3 wk, a time point at which peripheral blood chimerism is known to be established. One week after dox treatment, the bone marrow fibrosis was present but markedly suppressed in c-Jun mice parabiosed to WT mice (Fig. S4 F and G, compare with A). To understand which blood cell subsets are contributing to fibrosis, we investigated c-Jun–mediated fibrogenesis in NOD-SCID IL2R-gamma and RAG2-gamma knockout mice lacking B, T, and NK cells. Fibrosis developed in all conditions, demonstrating that other blood cell types, such as macrophages, modulate the fibrotic process. To understand whether cytokine release contributed to c-Jun–mediated fibrosis and rapid mortality of B6 mice, we quantified cytokines in mouse serum 48 h after dox-mediated c-Jun expression by multiplex assay. We found that cJun mice released many pro- and antiinflammatory cytokines at high levels, leading to death between 6 and 8 d (Fig. 2B).
In summary, these observations establish that fibrogenesis can result from the direct induction of c-Jun in mesenchymal stroma cells, which leads to a systemic release of potent proinflammatory chemo- and cytokines, and that induction in the hematopoietic compartment alone is insufficient. However, WT transplanted or circulating hematopoietic cells, likely macrophages, can partially suppress fibrosis in c-Jun marrows. Thus, the fibrosis is driven in a cell-autonomous manner by tissue fibroblasts but is modulated in a non cell-autonomous manner by hematopoietic cells compatible with the well-characterized interaction of inflammatory and fibrotic mechanisms in sclerotic disease.
Additional Results: Blockade of the VEGF and PI3K Pathways Reverses Marrow and Skin Fibrosis in Vivo.
Any effects on gastroesophageal or bladder were not assessed because the fibrosis in these organs takes longer to develop. Both Rosa26-rtTA control mice were treated with dox, and c-Jun–inducible mice were treated with PBS. No fibrosis developed in either control condition in lung or other organs over the same time span (Fig. S5D). Thus, the PI3K and VEGFR pathways are critical mediators of c-Jun–induced fibrosis in mice and may also be involved in the development of human fibrotic conditions. We note that a recent study showed initial clinical benefit to idiopathic pulmonary fibrosis patients of VEGFR/FGFR/PDGFR pathway inhibition (1).
SI Materials and Methods
General Statement Regarding Human Samples and Animal Studies.
Deidentified patient specimens in paraffin and discarded fresh patient tissues were used for our studies as approved in IRB11177. Experiments were conducted on c-Jun tetracycline-inducible transgenic mice in accordance with guidelines established by the Stanford University Administrative Panel on Laboratory Animal Care (SU-APLAC 30911, 30912, 31026).
Generation of Tetracycline-Inducible Transgenic Mice.
The tetracycline-inducible system was used to generate transgenic mice overexpressing c-Jun and JunB in vivo as described exactly in ref. 25. Briefly, it consists of two components, one encoding the tetracycline-controllable transactivator (rtTA) and the other consisting of the tetracycline operator minimal promoter (tetOP) driving the gene of interest. We targeted the ColA1 locus that encodes the type I collagen protein, and transgenic tetracycline-inducible mice were generated. ES cells carrying both the R26-M2rtTA allele and the flp-in tetO-c-Jun or flp-in tetO-JunB alleles were screened by Southern blot. Subsequently, targeted ES cells were injected into blastocysts and viable chimeras were generated; alternatively, mice were derived by tetraploid embryo complementation. Mice were genotyped by PCR for the presence of both alleles, and 6- to 8-wk-old mice were treated with doxycycline administered in the drinking water (1 mg/mL) for 7 d up to 36 d, and tissues were harvested and analyzed by immunocytochemistry. Transgenic mice that were not exposed to doxycycline as well as mice harboring the rtTA only showed no detectable c-Jun or JunB expression, and those were used as controls as indicated.
Bone Marrow Transplantation and Parabiosis.
Transplant studies and parabiosis surgery were performed exactly as previously described (14) and in accordance with the guidelines established by the Stanford University for the humane care and use of animals (APLAC).
Tissue Processing, Immunostaining, and Apoptosis Assessment.
Animals were killed at times indicated based on an APLAC-approved protocol that includes assessment of morbidity by >10% loss of weight, scruffy appearance, and lethargy. For in vivo studies, tetracycline-inducible transgenic mice were put on doxycycline, and, consecutively, tissues from all major organ systems were collected, fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin or, to assess for fibrosis, stained with reticulin, trichrome, anti-SMA, and c-Jun. Images of histological slides were obtained on a Nikon Eclipse E400 microscope (Nikon) equipped with a SPOT RT color digital camera (model 2.1.1; Diagnostic Instruments). Images were analyzed in Adobe Photoshop (Adobe Systems). For flow cytometry, cells were washed in PBS, washed in 2% FBS, blocked with Fc-Block (BD PharMingen) for 10 min on ice, and stained with primary antibodies in PBS and 2% FCS for 30 min on ice. A comprehensive list of antibodies used, including brand names and dilutions, is included in Dataset S1. For assessment of apoptosis, marrow-derived cells were flushed from the leg bones of c-Jun mice. The cells were then washed, and the red cells were lysed on ice with RBC lysis buffer (Gentra). Pooled progenitor populations were sorted and analyzed as previously reported, and apoptosis analysis was performed using the Annexin V Apoptosis Detection Kit (BD Pharmingen). Flow cytometry was performed on a FACS Aria cytometer (BD Biosciences), at least 10,000 events were acquired, and data were analyzed using FlowJo software. The results are presented as graphs and representative dot plots of viable cells selected on the basis of scatter and 7-AAD staining.
Tissue sections (4 μm thickness) were cut from tissue blocks of archival deidentified human biopsies using a microtome for immunofluorescence staining. The sections were baked at 65 °C for 20 min, deparaffinized in xylene, and rehydrated via a graded ethanol series. The sections were then immersed in epitope retrieval buffer (10 mM sodium citrate, pH 6) and placed in a pressure cooker for 45 min. The sections were subsequently rinsed twice with dH2O and once with wash buffer (TBS, 0.1% Tween, pH 7.2). Residual buffer was removed by gently touching the surface with a lint-free tissue before incubating with blocking buffer for 30 min. Blocking buffer was subsequently removed, and the sections were stained overnight at 4 °C in a humidified chamber. The following morning, the sections were rinsed twice in wash buffer, and secondary antibody (Invitrogen) was used for visualization of signal. Images of histological slides were obtained on a Leica Eclipse E400 microscope (Leica) equipped with a SPOT RT color digital camera (model 2.1.1; Diagnostic Instruments). We validated all of the antibodies we used for immunostaining first by staining positive and negative controls of healthy human and mouse tissues, but also lung cancer, breast cancer, adrenal, placenta, tonsils, peripheral blood mononuclear cells, and spleen. To find the optimal antibody concentration and decrease nonspecific staining, we subsequently titrated the respective antibodies by serial dilution from 1:50, 1:100, and 1:200 to 1:500. We included positive and negative control tissues and sections stained with isotype control with each subsequent immunostain. In addition, to proof specificity of the c-JUN antibody, we engineered mouse and human c-JUN–inducible cell lines and generated a c-JUN knockout cell line by crisper/cas9, as shown in Figs. S2D and S3 C and E.
Expression Arrays and Computational Methods.
Time course experiments of c-Jun protein expression in vivo were performed by harvesting marrow from mice exposed to doxycycline on day 0 and day 1 and applied to standard affymetrix mouse arrays. We processed the raw gene expression values with the robust multiarray analysis (RMA) algorithm using BioConductor software. We then filtered out unchanging genes that had an absolute change less than 70 and minimum fold change less than 3 across any two samples. We set thresholds of a minimum of 20 and a maximum of 20,000. Using these preprocessed data, we then identified differentially expressed genes by performing permutation testing with the Comparative Marker Selection algorithm using GenePattern software. We used the signal-to-noise ratio to rank-order the genes that distinguish two classes of gene expression samples. In addition to finding differentially expressed genes, we also ran gene set enrichment analysis to measure enrichment of fibrosis gene sets in our data.
Single Cell Mass Cytometry.
Samples were processed as described. Briefly, primary bone marrow-derived adherent cells from c-Jun mice were induced with doxycycline for 48 h, labeled with IdU to assess cell proliferation as previously described, washed once with PBS, treated with 25 μM cisplatin for 1 min for live–dead cell discrimination, washed once with RPMI medium containing 10% FBS, treated with 1× TrypLE (Invitrogen) for 5 min at 37 °C, dissociated into single-cell suspension by trituration, and washed with PBS containing 0.5% BSA. The cell samples were then fixed with 2% paraformaldehyde at room temperature for 20 min, followed by two washes with PBS containing 0.5% BSA. Formaldehyde-fixed cell samples were incubated with metal-conjugated antibodies against surface markers for 1 h, washed once with PBS containing 0.5% BSA, permeabilized with methanol on ice for 15 min, washed twice with PBS containing 0.5% BSA, and then incubated with metal-conjugated antibodies against intracellular molecules for 1 h. Cells were washed once with PBS containing 0.5% BSA and then incubated at room temperature for 20 min with an iridium-containing DNA intercalator (Fluidigm) in PBS containing 2% paraformaldehyde. After intercalation/fixation, the cell samples were washed once with PBS containing 0.5% BSA and twice with water before measurement on a CyTOF mass cytometer (Fluidigm). Normalization for detector sensitivity was performed as previously described. After measurement and normalization, the individual FCS files were analyzed by first gating out doublets, debris, and dead cells based on cell length, DNA content, and cisplatin staining. viSNE maps were generated with software tools available at https://www.cytobank.org/ by considering all surface markers. The F4/80 and CD172a positive or negative subpopulations were gated on a viSNE map, and the events in each gated population were exported for a DREVI plot using a software package available at www.c2b2.columbia.edu/danapeerlab/html/dremi.html.
Isolation of Marrow-Derived Fibroblasts.
Primary mouse bone marrow-derived fibroblasts were generated as such. The femoral and tibial bones of the donor mice were collected, and the adherent soft tissue was removed. Both ends of the bones were cut away from the diaphysis with bone scissors, the bone marrow plugs were then hydrostatically expelled from the bones, and the dispersed cells were plated into 10-cm polystyrene tissue culture dishes (Corning, Inc.) and cultured in alpha DMEM (alpha DMEM; Gibco Laboratories) containing selected lots of 10% FCS (FCS; JR Scientific Inc.) and antibiotics (penicillin G, 100 U/mL; streptomycin, 100 μg/mL; amphotericin B, 0.25 μg/mL; Gibco Laboratories) at 37 °C in a humidified atmosphere of 5% CO2. Three days later, the medium was changed, and nonadherent cells were discarded and cultured until confluence was reached.
Transwell Migration and Proliferation Assays.
For transwell migration assays, Costar transwell chambers were used according to the manufacturer's directions, and transmigrated adherent cells were fixed and stained and counted by microscopy after periods of 2 h and 24 h. Alternatively, a cytoselect 24-well cell migration assay (12-μm fluorometric format) was purchased from Cell Biolabs, Inc. and used according to the manufacturer's instructions. Briefly, 1 × 104 cells per milliliter for mouse fibroblasts and 2 × 104 cells per milliliter for human idiopathic lung fibrosis and WT fibroblasts were prepared in 300 μL of serum-free medium containing DMEM with 0.5% BSA, 2 mM CaCl2, and 2 mM MgCl2 and added to the inside of each insert. Then, 500 μL of DMEM containing 20% FBS or respective inhibitor were added to the lower well of the migration plate and incubated for 4 and 72 h at 37 °C. Subsequently, the migrated cells were treated with cell detachment solution and lysis buffer and stained, and the fluorescence was assessed with a fluorescence plate reader at 480 nm/520 nm according to the manufacturer's instructions or manually counted under the microscope. Briefly, cell proliferation was assessed in cell lines derived from lung biopsies of human lung fibrosis patients or normal control lungs. We purchased two hairpins directed against human c-JUN and control hairpins in a pTRIPZ vector-based system engineered to be Tet-On from Thermo Scientific. Primary fibroblastic cell lines were infected with hairpin no. 2 or no. 3 directed against human c-JUN or hairpin controls, and 6 × 103 cells per well were plated in duplicate. We quantified cell proliferation every 24 h up to 72 h by manually counting the total cell numbers and performed Edu staining to assess cell division as outlined in the click-iT EdU Alexa Fluor 488 Imaging Kit. Subsequently, we acquired images of proliferating and migrating cells at the Stanford Microscopy Core Facility.
In Vitro Inhibitory Screen.
Inhibitors such as EGFR/ErbB-2 inhibitor (324673) (10 μM), PDGFR inhibitor (10 μM and 4 μM), wortmannin/PI3K (1 μM), GSK/lithium (1 μM), rapamycin (1 μM), Dapt/Notch (1 μM), Cyclopamin (1 μM), gamma-secretase inhibitor (1 μM), U0126 Mek inhibitor (10 μM), SB203580 inhibitor (1 μM), SU5402/FGF1/VEGF (10 μM), PI3 kinase inhibitor Ly294002 (10 μM), and JAK2 Inhibitor II (420132) (10 μM) were purchased from Calbiochem/Selleckchem and dosed as indicated, and anti-mouse CD47 clone 3 was used as described previously (21, 24).
Anesthesia and CO Diffusion Testing.
Mice were anesthetized with isoflurane gas or avertin (0.025 mL/g mouse of a 20 mg/mL filter sterilized solution) in saline via i.p. injection. For the measurement of pulmonary diffusing capacity for carbon monoxide (DFCO), a gas mixture of CH4/CO/air was used that is similar to the one used in humans for CO diffusion capacity measurements. Anesthetized mice were intubated with a 22-gauge stub needle cannula for the administration of intratracheal doxycycline or DFCO measurements. In a 3-mL syringe, 0.8 mL from the commercially available gas mixture was withdrawn, connected with a syringe to the tracheal cannula, and inflated in the lung. After 9 s, 0.8 mL of gas mixture was withdrawn and further analyzed with mass spectrometry.
Cytokine/Chemokine Multiplex Assay.
The following 38 mouse cytokines/chemokines have been quantified by cytokine/chemokine multiplex assay by the Stanford core facility: G-SCF/CSF-3, IL10, IL-3 LIF IL-1B, IL-2, M-CSF, IP-10, VEGF-A, IL4, IL-5, IL-6, TGFB, IFN-a, IL-22, IL-9, IL-13, IL-27, IL-23, IFN-g, IL-12P70, GM-CSF, GRO-a, RANTES, TNF-a, MIP-1a, MCP-3, MCP-1, IL-17A, IL-15/IL-15R, MIP-2, IL-1a, LIX, EOTAXIN, IL-28, IL-18, MIP-1b, IL-31; the mean fluorescent intensity has been measured and the concentrations of each cytokine/chemokine has been quantified by the standard curve method in pg/mL; the experimental details can be found at iti.stanford.edu/himc.html, the Stanford Human Immune Monitoring Center.
In Vivo Drug Application.
Six- to 12-wk-old c-Jun transgenic and control mice were either maintained on doxycycline containing water or induced intratracheally and concomitantly systemically treated with anti-CD47 antibody (100 μL i.p.), VEGF inhibitor PD173074 (2 mg/kg once per day i.p.), and a PI3K inhibitor wortmannin (2 mg/kg 3x/wk i.p.). Intratracheal intubation was performed under isoflurane anesthesia using a 22-gauge catheter, a light source, and an intubation platform with the administered volume not exceeding >125 μL per mouse.
Protein Lysates and Western Blot Analysis.
For protein assays, protein extracts were prepared by cell lysis in buffer containing protease inhibitors, subjected to SDS/PAGE, and analyzed by Western blot using primary antibodies directed against c-Jun and JunB as indicated throughout.
Statistics.
The results are expressed as the mean ± SEM for n given samples. Data were analyzed using the two-tailed Student’s t test or ANOVA with any P value less than or equal to 0.05 being considered significant. Survival was monitored and analyzed by Kaplan–Meyer analysis. Numbers of recipient mice are indicated, and the P value was derived by log-rank test.
Discussion
Here, we report that c-JUN, a well-characterized AP1 transcription factor, is expressed in many different fibrotic diseases. We found decreased proliferation of patient fibroblasts from fibrotic lungs after knockdown of c-JUN. We detected activated c-Jun and Akt as well as up-regulation of CD47 expression in vivo in endstage fibrosis lungs. We further showed that c-Jun can induce rapid and widespread fibrosis in all organs in mice and is also expressed in fibrotic areas of abdominal adhesions in WT mice. c-JUN is widely expressed in skin epithelium and many other epithelial cells, but not highly in stromal cells. c-JUN is also part of the acute phase response cascade, has a role in bone formation, and has a reputation as an oncogene, and its up-regulation has been shown in various cancers (16). Although c-JUN’s role in cell cycle promotion has been well established primarily in vitro (16, 22), we observed a striking cell context-dependent fibrotic response in vivo. Despite ubiquitous c-Jun induction, we observed primarily fibrotic changes, indicating that the proproliferative and promigratory effects of c-Jun require the specific cellular context of tissue fibroblasts. Systemic induction of c-Jun in hematopoietic precursors caused rapid apoptosis; induction in the liver caused a pronounced hepatosteatosis. This unique c-Jun responsiveness seems to be shared among fibroblasts of many different tissues although fibroblasts are considered highly heterogeneous and tissue-specific (23). The fibrogenic response in multiple tissues and organs also contrasts with previously developed fibrosis models, suggesting that induction of c-Jun could be a common molecular mechanism across different human fibrotic conditions.
We further provide evidence that the transcription factor c-Jun, which is a downstream target of MAPK-signaling cascades, can itself rewire and stabilize a specific pattern of multiple signaling pathways. We assume that the remodeling of signaling pathways will be different in different cell types, leading to the opposing cell biological effects of c-Jun in different cell types.
Importantly, our mouse model also confirmed the functional relevance of several signaling pathways, some of which were previously associated with fibrosis and were targeted in past clinical trials (23), and idiopathic pulmonary fibrosis is currently treated with a combination of small molecule inhibitors targeting four different pathways: VEGF/FGFR/PDGFR and TGFBR (1). This finding suggests that c-Jun may be a central node controlling these essential pathways. Although combination therapy is in principle an attractive strategy, in practice, it is difficult to identify the right combination of pathways to target. In particular, for clinical trials, it is not feasible to evaluate combination therapy in a systematic manner. Our discovery that c-Jun coordinates several signaling pathways leading to fibrosis in vivo provides a unique opportunity to identify all relevant signaling pathways and predict the most effective therapeutic drug combinations. Moreover, it may be possible to develop therapeutic strategies interfering with the activity of c-Jun directly, which would eliminate the need to search for the most effective combination by eliminating the key disease-driving element.
Several other mouse models have been established previously and have served to gain important insight into some specific aspects of disease (4). The most widely used model is a bleomycin-induced lung fibrosis isolated or in combination with the genetic model of Marfan syndrome. This model suggested the involvement of the TGFBR pathway in fibrosis, which we could confirm to also play a role in our c-Jun–induced model. In particular, the genetic model of Marfan syndrome was very instructive regarding the dissection of the contribution of dendritic helper cells toward the pathomechanism of skin and pulmonary fibrosis (4). Another model system uses carbon tetrachloride (CCl4) or bile duct ligation to study fibrosis in the liver, which has been shown to be linked to loss or constitutive activation of PDGFR-β in stellate cells.
Unlike these mouse models, the c-Jun–induced model is a purely genetically driven model. Importantly, it recapitulates many aspects of the respective human disease conditions and is not limited to one organ system (such as only lung or skin) akin to the multiorgan disease of scleroderma. Furthermore, c-Jun is highly expressed in all human fibrotic conditions analyzed, and thus in vivo c-Jun induction is likely more physiologically relevant than chemical or infectious conditions that are not involved in the pathogenesis of human disease. We would argue for these reasons that our mouse model will be an important tool to further dissect the pathomechanisms leading to human disease.
One such application was our surprising discovery that endogenous macrophages can be exploited to eliminate pathologic fibroblasts. We showed that fibrogenic cells expressed high levels of the self-protective don’t-eat-me epitope CD47. It had been shown in various solid cancers and hematopoietic malignancies, and most recently in atherosclerosis, that blockade of CD47 by antibodies or artificial, high-affinity Sirpα analogs prevents this repressive signal in macrophages, leading to their activation and active phagocytosis (24). The remarkable low toxicity of anti-CD47 treatments, however, suggested that additional alterations in cancer cells are required to induce phagocytosis (21, 25). Here, we show that this property is not limited to cancer cells because fibrosis was effectively reversed with anti-CD47 treatment by elimination of fibroblasts by macrophages. Studies are needed to identify the common mechanisms between fibrotic cells and cancer cells that allow effectiveness of the anti-CD47 treatment and to identify which other noncancerous diseases may benefit from such a therapy.
In conclusion, our study revealed the unexpected role of c-Jun as a key and selective driver of organ fibrosis in most human fibrotic diseases. Our findings suggest that diverse fibrotic syndromes may have different etiologies but share common pathomechanisms centered around activation of c-Jun. The c-Jun mouse model may well be suitable to further dissect the pathogenesis of all types of pathologic fibrotic conditions and to develop new and effective therapies.
Materials and Methods
Animal studies were approved by Stanford University Administrative Panels for Lab Animal Care (SU-APLAC 30911, 30912, 31026) and human research under IRB11177. We generated the flp-in tetO-c-JUN or flp-in tetO-JUNB transgenic mice as previously described (25) and induced the mice with tetracycline. CyTOF studies were performed in vivo exactly as described (18, 19). Details can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Erika Dobos for pathology expertise, Daniel Haag for ingenuity analysis and GSEA analysis, Samuele Marro for help with heat maps, Norm Cyr for help with digital images, Farnaz Khameneh for help with genotyping PCRs, Patrick Sweeney and Shirley Kwok for help with tissue array assembly, and the Stanford Immuno core facility for help with cytokine/chemokine quantification. For these studies, I.L.W. was funded by the Virginia and D. K. Ludwig Fund for Cancer Research; the National Heart, Lung, and Blood Institute; and the National Cancer Institute of the National Institutes of Health under Grants U01HL099999 and R01CA86017, respectively. G.W. was funded by the Stanford Cancer Institute Fellowship Award; the Stanford Physician Scholar Society Award; and Institute for Immunity, Transplantation and Infection Young Investigator Award grants. The CyTOF studies were supported by NIH Grants U19AI057229, U19AI100627, R33CA183654, R33 CA0183692, R01GM10983601, R01-CA184968, R01CA19665701, R21-CA183660, R01-NS08953301, 5UH2AR067676, and R01HL120724; Northrop-Grumman Corporation; Novartis Grant CMEK162AUS06T; Pfizer Grant 123214; Juno Therapeutics Grant 122401; Department of Defense Grants OC110674 and W81XWH-14-1-0180; Gates Foundation Grant OPP1113682; and Food and Drug Administration Grant BAA-15-00121.
Footnotes
Conflict of interest statement: I.L.W. and G.W. have filed a patent, Docket No. S14-256: “(CD47) CD47 as potential single or combinatorial treatment in idiopathic lung fibrosis and systemic sclerosis.” I.L.W. cofounded 47Inc, a company developing anti-CD47 antibody treatment as anticancer therapy.
Data deposition: The microarray data displayed in Fig. 3 in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE84838).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1621375114/-/DCSupplemental.
References
- 1.Hunninghake GM. A new hope for idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2142–2143. doi: 10.1056/NEJMe1403448. [DOI] [PubMed] [Google Scholar]
- 2.Maier C, Distler JH, Beyer C. Deciphering the pro-fibrotic phenotype of fibroblasts in systemic sclerosis. Exp Dermatol. 2014;23:99–100. doi: 10.1111/exd.12237. [DOI] [PubMed] [Google Scholar]
- 3.Kuter DJ, Bain B, Mufti G, Bagg A, Hasserjian RP. Bone marrow fibrosis: Pathophysiology and clinical significance of increased bone marrow stromal fibres. Br J Haematol. 2007;139:351–362. doi: 10.1111/j.1365-2141.2007.06807.x. [DOI] [PubMed] [Google Scholar]
- 4.Gerber EE, et al. Integrin-modulating therapy prevents fibrosis and autoimmunity in mouse models of scleroderma. Nature. 2013;503:126–130. doi: 10.1038/nature12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rockey DC, Bell PD, Hill JA. Fibrosis: A common pathway to organ injury and failure. N Engl J Med. 2015;373:96. doi: 10.1056/NEJMc1504848. [DOI] [PubMed] [Google Scholar]
- 6.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tager AM, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 8.Liu F, et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2015;308:L344–L357. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driskell RR, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto T. The bleomycin-induced scleroderma model: What have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006;297:333–344. doi: 10.1007/s00403-005-0635-z. [DOI] [PubMed] [Google Scholar]
- 11.Eferl R, et al. Development of pulmonary fibrosis through a pathway involving the transcription factor Fra-2/AP-1. Proc Natl Acad Sci USA. 2008;105:10525–10530. doi: 10.1073/pnas.0801414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vainchenker W, Constantinescu SN, Plo I. Recent advances in understanding myelofibrosis and essential thrombocythemia. F1000 Res. 2016;5:5. doi: 10.12688/f1000research.8081.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wernig G, et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell. 2008;13:311–320. doi: 10.1016/j.ccr.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Wernig G, et al. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood. 2006;107:4274–4281. doi: 10.1182/blood-2005-12-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jochum W, Passegué E, Wagner EF. AP-1 in mouse development and tumorigenesis. Oncogene. 2001;20:2401–2412. doi: 10.1038/sj.onc.1204389. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Bergami P, Lau E, Ronai Z. Emerging roles of ATF2 and the dynamic AP1 network in cancer. Nat Rev Cancer. 2010;10:65–76. doi: 10.1038/nrc2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasenfuss SC, Bakiri L, Thomsen MK, Hamacher R, Wagner EF. Activator Protein 1 transcription factor Fos-related antigen 1 (Fra-1) is dispensable for murine liver fibrosis, but modulates xenobiotic metabolism. Hepatology. 2014;59:261–273. doi: 10.1002/hep.26518. [DOI] [PubMed] [Google Scholar]
- 18.Krishnaswamy S, et al. Systems biology: Conditional density-based analysis of T cell signaling in single-cell data. Science. 2014;346:1250689. doi: 10.1126/science.1250689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mingueneau M, et al. Single-cell mass cytometry of TCR signaling: Amplification of small initial differences results in low ERK activation in NOD mice. Proc Natl Acad Sci USA. 2014;111:16466–16471. doi: 10.1073/pnas.1419337111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaiswal S, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao MP, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber M, et al. Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev. 1999;13:607–619. doi: 10.1101/gad.13.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinkevich Y, et al. Skin fibrosis: Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science. 2015;348:aaa2151. doi: 10.1126/science.aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kojima Y, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beard C, Hochedlinger K, Plath K, Wutz A, Jaenisch R. Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells. Genesis. 2006;44:23–28. doi: 10.1002/gene.20180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.