Abstract
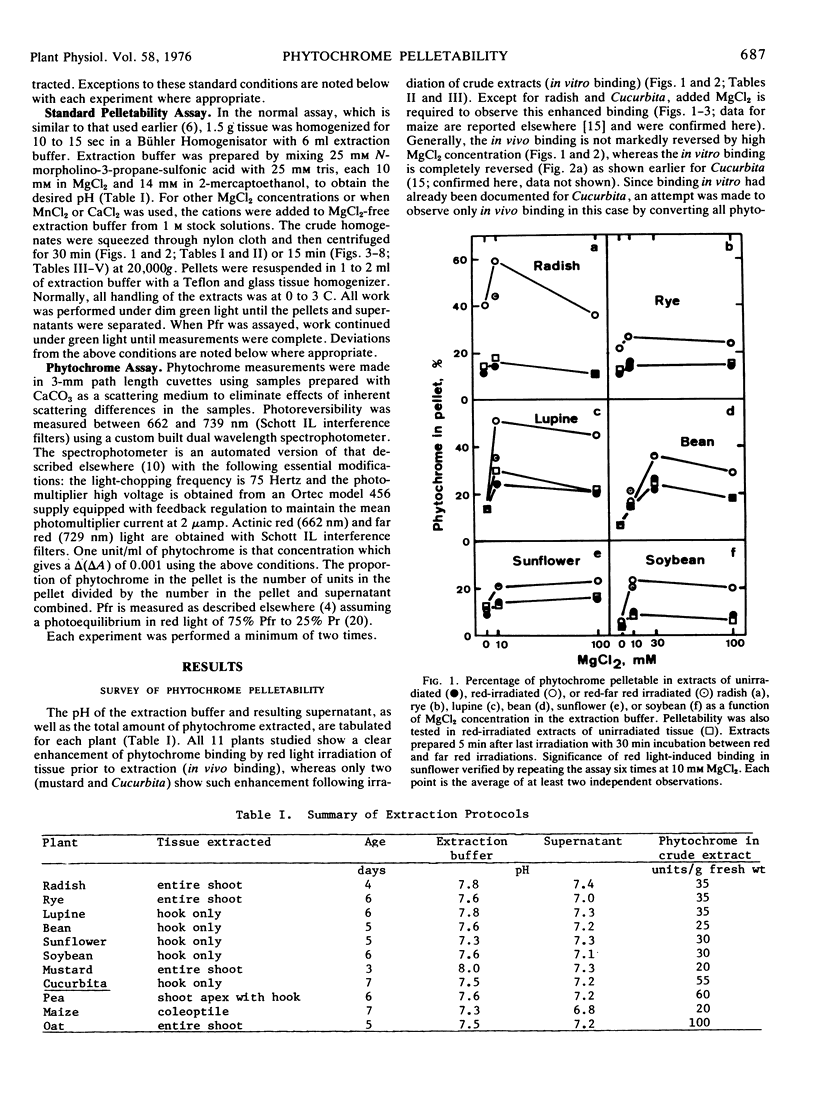
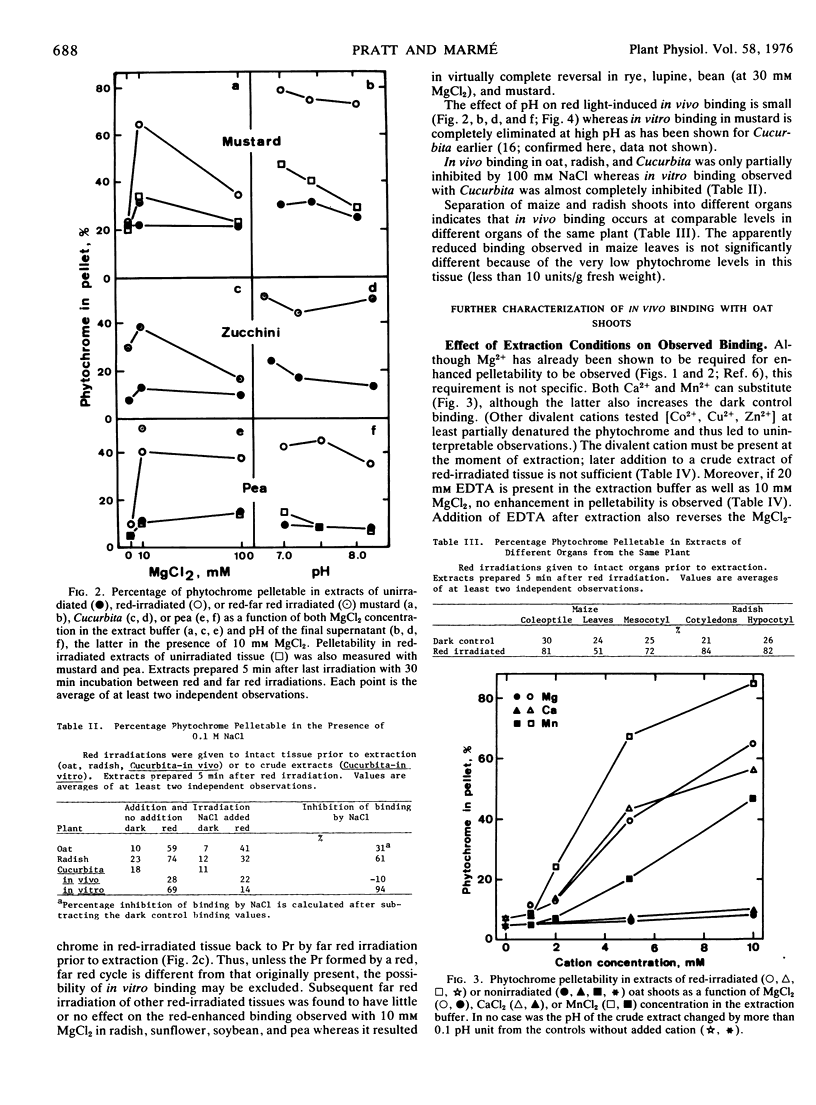
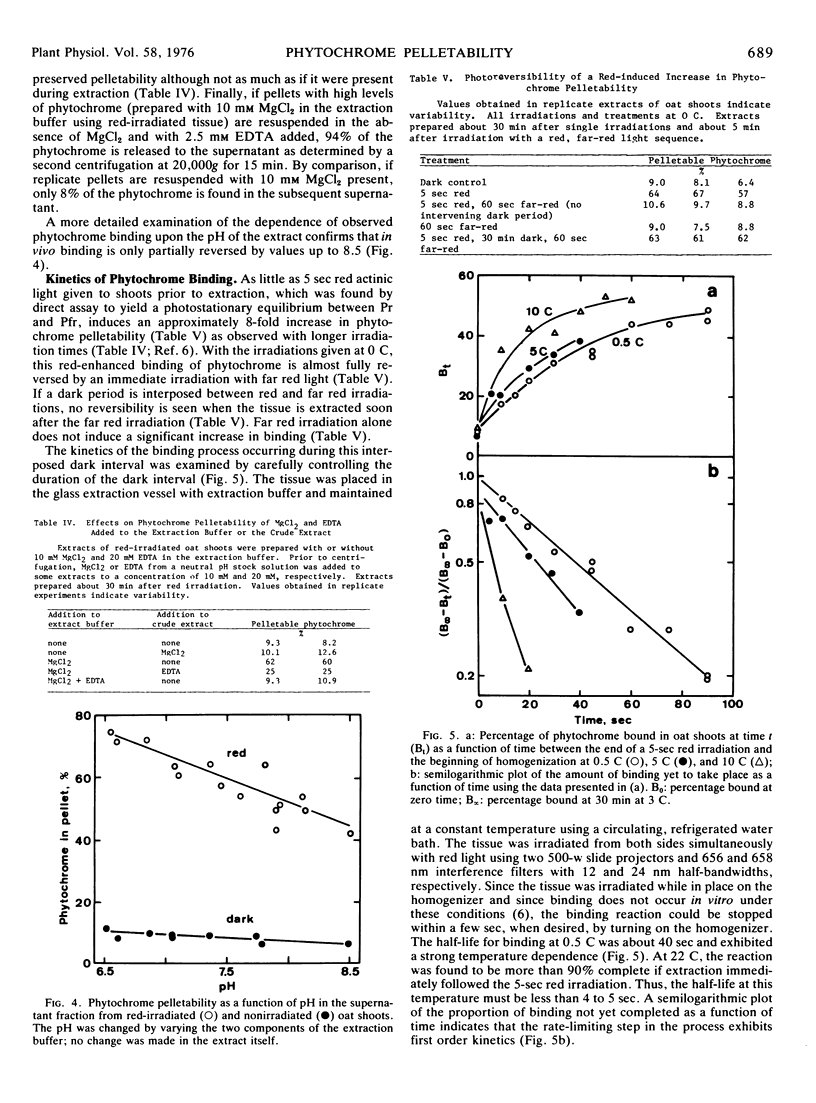
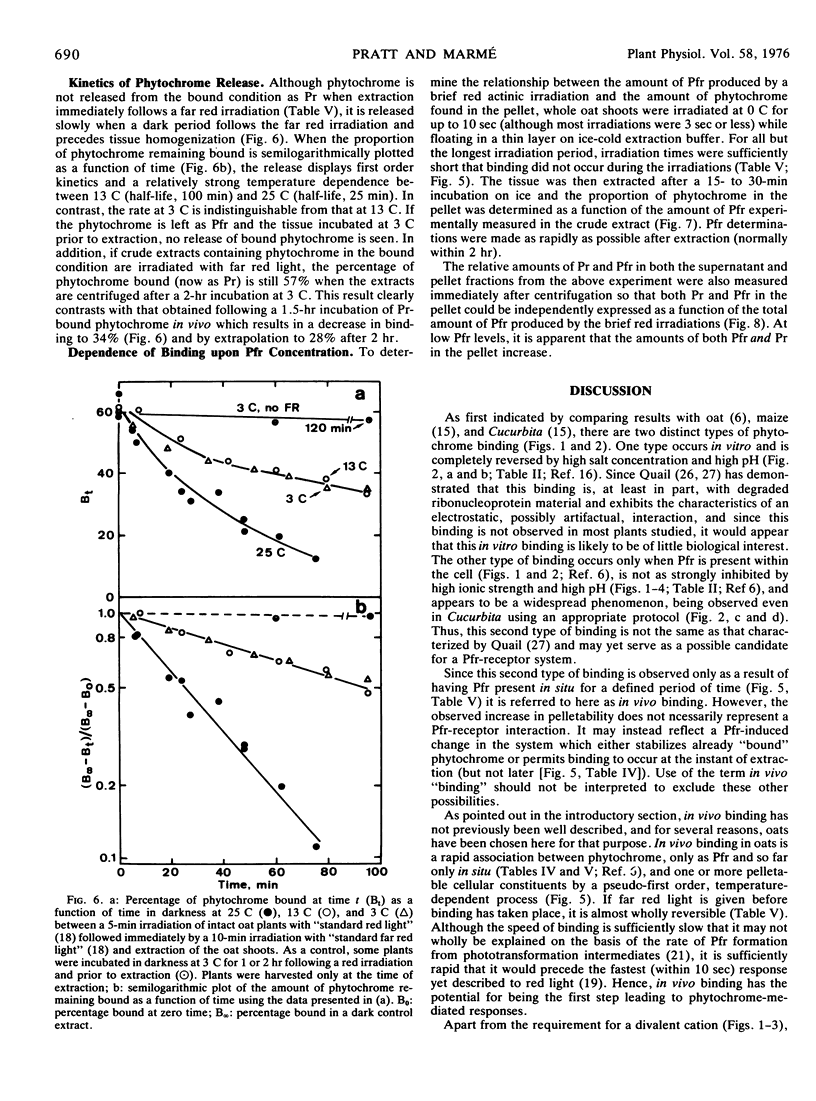
Red light-enhanced pelletability of phytochrome was observed in extracts of all 11 plants tested: Avena sativa L., Secale cereale L., Zea mays L., Cucurbita pepo L., Sinapis alba L., Pisum sativum L., Helianthus anuus L., Raphanus sativus L., Glycine max (L.) Merr., Phaseolus vulgaris L., and Lupinus albus L. This enhanced pelletability was observed in all 11 plants following in situ irradiation (in vivo binding) but only in Sinapis and Cucurbita after irradiation of crude extracts (in vitro binding). In vivo binding was not strongly dependent upon pH and, with few exceptions, was not markedly sensitive to high salt concentration, whereas in vitro binding was completely reversed by both high pH and high salt concentration. However, both binding phenomena were observed only with a divalent cation in the extract buffer. In vivo binding was further characterized using Avena which showed an increase in pelletability from less than 10% in dark control extracts to more than 60% in extracts of red light-irradiated shoots. The half-life for binding was 40 seconds at 0.5 C and was strongly temperature-dependent, binding being complete within 5 to 10 sec at 22 C. If pelletable phytochrome in the far red-absorbing form was photoconverted back to the red-absorbing form in situ, phytochrome was released from the pelletable condition with a half-life of 25 minutes at 25 C and 100 minutes at both 13 C and 3 C. No cooperativity in red light-enhanced pelletability with respect to phytochrome-far red-absorbing form was observed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boisard J., Marmé D., Briggs W. R. In Vivo Properties of Membrane-bound Phytochrome. Plant Physiol. 1974 Sep;54(3):272–276. doi: 10.1104/pp.54.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick H. A., Hendricks S. B., Schneider M. J., Taylorson R. B., Toole V. K. The high-energy light action controlling plant responses and development. Proc Natl Acad Sci U S A. 1969 Oct;64(2):479–486. doi: 10.1073/pnas.64.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Lane H. C. Dark Transformations of Phytochrome in vivo. II. Plant Physiol. 1965 Jan;40(1):13–17. doi: 10.1104/pp.40.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd G. H., Pratt L. H. Phytochrome destruction: an apparent requirement for protein synthesis in the induction of the destruction mechanism. Plant Physiol. 1973 Oct;52(4):309–311. doi: 10.1104/pp.52.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie J. M., Jr, Coleman R. A., Briggs W. R., Pratt L. H. Reversible redistribution of phytochrome within the cell upon conversion to its physiologically active form. Proc Natl Acad Sci U S A. 1975 Mar;72(3):799–803. doi: 10.1073/pnas.72.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli A. L., Rabino I. Photocontrol of Anthocyanin Synthesis: IV. Dose Dependence and Reciprocity Relationships in Anthocyanin Synthesis. Plant Physiol. 1975 Sep;56(3):351–355. doi: 10.1104/pp.56.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmé D. Binding properties of the plant photoreceptor phytochrome to membranes. J Supramol Struct. 1974;2(5-6):751–768. doi: 10.1002/jss.400020518. [DOI] [PubMed] [Google Scholar]
- Marmé D., Boisard J., Briggs W. R. Binding properties in vitro of phytochrome to a membrane fraction. Proc Natl Acad Sci U S A. 1973 Dec;70(12 Pt 1-2):3861–3865. doi: 10.1073/pnas.70.12.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmé D., Mackenzie J. M., Boisard J., Briggs W. R. The isolation and partial characterization of a membrane fraction containing phytochrome. Plant Physiol. 1974 Sep;54(3):263–271. doi: 10.1104/pp.54.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H., Butler W. L. The temperature dependence of phytochrome transformations. Photochem Photobiol. 1970 May;11(5):361–369. doi: 10.1111/j.1751-1097.1970.tb06008.x. [DOI] [PubMed] [Google Scholar]
- Quail P. H., Marmé D., Schäfer E. Particle-bound phytochrome from maize and pumpkin. Nat New Biol. 1973 Oct 10;245(145):189–191. doi: 10.1038/newbio245189a0. [DOI] [PubMed] [Google Scholar]
- Quail P. H., Schäfer E. Particle-bound phytochrome: a function of light dose and steady-state level of the far-red-absorbing form. J Membr Biol. 1974;15(4):393–404. doi: 10.1007/BF01870097. [DOI] [PubMed] [Google Scholar]
- Quail P. Interaction of phytochrome with other cellular components. Photochem Photobiol. 1975 Dec;22(6):299–301. doi: 10.1111/j.1751-1097.1975.tb06755.x. [DOI] [PubMed] [Google Scholar]
- Razin S. Reconstruction of biological membranes. Biochim Biophys Acta. 1972 Apr 18;265(2):241–296. [PubMed] [Google Scholar]
- Schäfer E. Analysis of the binding of phytochrome to particulate fractions. Photochem Photobiol. 1975 Mar;21(3):189–191. doi: 10.1111/j.1751-1097.1975.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Van Tol A. On the occurrence of a temperature coefficient (Q10) of 18 and a discontinuous Arrhenius plot for homogeneous rabbit muscle fructosediphosphatase. Biochem Biophys Res Commun. 1975 Feb 3;62(3):750–756. doi: 10.1016/0006-291x(75)90463-5. [DOI] [PubMed] [Google Scholar]


