Summary
We describe an observational survey of diagnostic pathways in 104 patients attending four specialist allergy clinics in the United Kingdom following perioperative hypersensitivity reactions to chlorhexidine reactions. The majority were life‐threatening. Men undergoing urological or cardiothoracic surgery predominated. Skin prick testing and specific immunoglobulin (sIg)E testing were the most common tests used for diagnosis. Fifty‐three per cent of diagnoses were made on the basis of a single positive test. Where multiple tests were performed the sensitivity of intradermal, basophil activation and skin prick testing was 68% (50–86%), 50% (10–90%) and 35% (17–55%), respectively. Seven per cent were negative on screening tests initially, and 12 cases were only positive for a single test despite multiple testing. Intradermal tests appeared most sensitive in this context. Additional sensitization to other substances used perioperatively, particularly neuromuscular blocking agents (NMBA), was found in 28 patients, emphasizing the need to test for possible allergy to all drugs to which the patient was exposed even where chlorhexidine is positive.
Keywords: anaesthesia, anaphylaxis, chlorhexidine allergy, skin tests, specific IgE
Introduction
Chlorhexidine is recognized increasingly as a significant allergen in the perioperative setting 1. We aimed to describe and compare a larger series of cases from multi‐centre British specialist allergy clinics 1, 2. This increase is thought to be driven by increased use of chlorhexidine and increased awareness of allergy, even though there remains some evidence of under‐diagnosis 2, 3, 4. Unlike most perioperative reactions 5, the majority of reported patients have been men, frequently undergoing urological or cardiothoracic surgery in non‐UK and single‐centre studies.
The performance of tests for chlorhexidine allergy has been estimated in single centres and there is published guidance on how to conduct tests for chlorhexidine allergy 6, 7, but it is not clear if these observations can be generalized to other clinic cohorts or countries 7. We also set out to determine whether we could estimate sensitivity for the different tests available for diagnosing chlorhexidine allergy in a routine clinical setting and identify the most effective diagnostic strategy for determining sensitization. Finally, multiple reactivity has been reported in some individuals with well‐documented chlorhexidine allergy 3. We evaluate how frequently potentially misleading multiple sensitization is noted in our clinics.
Materials and methods
Data collection
Data on all patients diagnosed with chlorhexidine allergy was collected retrospectively from records of four regional UK Allergy Centres (Sheffield Teaching Hospitals, Central Manchester University Hospitals NHS Foundation Trust, Southampton University Hospitals and Leeds Teaching Hospitals) between 2009 and 2015. The patients were seen following routine referral into anaesthetic drug reaction clinics. The investigations carried out were not harmonized across clinics. Many of our series had only one test [most commonly skin prick test (SPT) or specific immunoglobulin (sIg)E)] and the first positive test prevented further testing. The sequence of further testing differed between centres [only one offered a basophil activation test (BAT)], between patients and across time [increasing use of the intradermal skin test (IDT) in patients who were negative in screening tests in some centres]. As the data were collected as part of routine clinical audit, ethics committee approval was not required. Gender, chlorhexidine preparation used, the clinical setting, details of the reaction, investigations performed (SPT and/or IDT, sIgE and basophil activation test) and final clinical diagnosis were obtained.
SPT was carried out using undiluted clear or pink Hydrex® [chlorhexidine gluconate solution 20% BP (Ph Eur) 2·5% v/v, denatured ethanol B 96%, purified water BP, carmosine (E122)] with positive (histamine 10 mg/ml) and negative (normal saline) controls: SPT was positive if a wheal ≥ 3 mm than negative control was present at 15–20 min, as reported previously and as per 2011 guidance 6, 7. All other drug SPT were carried out in accordance with 2011 guidance. Both pink and clear Hydrex was used in some centres to exclude any possible reactions due to reactors to the colourant.
IDT was performed using 20 μl injections of chlorhexidine gluconate (clear or pink or both, as appropriate to the clinic) 1 : 1000 dilution and normal saline, administered on the volar aspect of the forearm. The results were interpreted as described previously 3, 7. A positive IDT was defined as the mean of orthogonal weal diameters of at least 3 mm greater than the negative control in the presence of a flare 3, 7.
Chlorhexidine sIgE was measured by immunoassay (ImmunoCAP) on the Phadia ImmunoCap 1000 Analyser (Thermo Scientific, Loughborough, UK). A sIgE level > 0·35 kUA/l was deemed positive in Sheffield, Leeds and Southampton and ≥ 0·4 kUA/l in Manchester (functionally equivalent to > 0·35, as this laboratory reported measurement to a single decimal place only). All laboratories performed daily internal quality control and participated in the UK National External Quality Assurance Scheme for allergen‐specific IgE with satisfactory performance.
At Sheffield and Southampton, BAT (Buhlmann FlowCast, Switzerland) were analysed on a Beckman Coulter EPICS XL flow cytometer. The chlorhexidine used to stimulate the basophils was from the same source as the SPT. Chlorhexidine was used at concentrations of 0·05%, 0·005%, 0·0005% and 0·00005% for Hydrex® ‘clear’ and 0·02%, 0·002% and 0·0002% for Hydrex® ‘pink’. A wide range of concentrations were used to assess the strength of sensitization and exclude potential irritant or toxic concentrations in patients and controls in view of the lack of experience, harmonization and validation of this test.
Positive controls [high‐affinity IgE receptor (FcεRI) and N‐formylmethionyl‐leucyl‐phenylalanine (fMLP)], negative control (background) and a normal volunteer control were performed for each run. Fluorescently labelled antibody to CCR3 was used to identify basophils. Activated basophils were differentiated from resting basophils using a fluorescently labelled antibody to CD63, which becomes expressed on the cell surface only when basophils are activated 8. A positive response was present if two or more concentrations gave > 5% basophil activation and a stimulation index > 2. The stimulation index was calculated by dividing the percentage of activated basophils at each concentration by the percentage of activated basophils in the background tube.
Clinical reaction grading was in accordance with international guidance on reactions taking place in the perioperative setting (grade 1: cutaneous signs; grade 2: measurable but not life‐threatening physiological abnormalities; grade 3: life‐threatening physiological abnormalities; grade 4: cardiac and/or respiratory arrest) 6.
Patient inclusion criteria
The clinical history of type I hypersensitivity required the involvement of two or more systems with defined symptoms 9. This diagnosis was made by the submitting clinician. The perioperative period was defined as admission for an invasive procedure, to their discharge or death. In the absence of an agreed diagnostic gold standard for establishing chlorhexidine allergy, or a recognized and harmonized provocation test, we accepted a diagnosis of chlorhexidine allergy when there was a consistent clinical history for type I hypersensitivity along with one or more positive tests demonstrating sensitization; i.e. the potential for an IgE‐mediated mechanism had been demonstrated 7.
Because each patient had different combinations of tests and test specificity was unknown, we adopted a pragmatic strategy to assess test performance and compare with previous work. In the absence of a gold standard test, such as provocation, assessment of individual tests to estimate sensitivity is challenging. Many of our series had only one test and the first positive test prevented further testing. For patients with multiple tests we required an extremely rigorous demonstration of sensitization for each test, with at least two additional positive allergy tests, as has been reported previously for chlorhexidine and rocuronium 7, 10. For the purposes of estimating individual test sensitivity, the result of the test being assessed for performance was omitted from diagnostic decision‐making and results of the remaining tests were used to determine sensitization status for chlorhexidine. For example, when the sensitivity of SPT was being calculated, results of sIgE, IDT and BAT were used to determine allergy to chlorhexidine (where two confirmatory positive tests were present). We were unable to estimate specificity using these data, because we did not analyse a series of patients without allergy to whom the same tests were applied.
Results
Clinical features
One hundred and thirty‐four patients were identified with a clinical diagnosis of chlorhexidine reaction; 18 patients had no positive tests (of whom 11 had received only one test), one patient had not been tested and 12 patients had no evidence of perioperative reactions (referred because of occupational exposure or unexplained symptoms). These 30 patients were excluded from the analysis.
One hundred and four patients met our inclusion criterion of having had a probable perioperative anaphylactic reaction to chlorhexidine; 66 patients were men. Details of surgical interventions were available for 70 patients, of whom 16 had cardiac procedures and 13 had urological procedures. Other specialities appear to be under‐represented; for example, obstetrics and gynaecology procedures had been carried out in only two patients.
The route of chlorhexidine exposure was reported in 53 patients, of whom 26 had been exposed only to chlorhexidine skin preparations. Three patients had been exposed to chlorhexidine‐coated central venous catheters (CVC), three to sterile lubricating gel (InstillagelTM) and three to chlorhexidine mouthwash. One patient had been exposed to chlorhexidine mouth spray only, and the rest to a combination of these products. There were no clear relationships between the type of surgery and the chlorhexidine products used. For example, cardiac patients were exposed to combinations of chlorhexidine skin preparation, lubricating gel and coated CVCs (data not shown).
The grade of reaction was available in 101 patients 6. Most were severe grades 3 or 4, including grade 1 (nine patients), grade 2 (12 patients), grade 3 (72 patients) and grade 4 arrests (eight patients). Grade 4 reactions were not associated with any particular type of surgery. One of four patients in our series, who were exposed only to chlorhexidine mouthwash/spray, experienced grade 4 anaphylaxis.
Hypotension was the most common individual symptom and was described in 75 patients. Generalized urticaria was seen in 64, bronchospasm in 33 and angioedema in 21 patients. Localized urticaria was present in three and generalized flushing in 11. There was no relationship between the presence of individual symptoms and different types of operation.
Details of the timing of reactions were available in only 19 patients: 15 were described as ‘immediate perioperative’ (i.e. within 15 min) and only four were delayed at 30 min (grade 1 reaction) and 30, 45 and 90 min (all grade 3 reactions). Sequential mast cell tryptase results were available in 11 of these cases and showed a rise above baseline in 10 (not shown).
Test results for entire series
Because there was no harmonized testing pathway, different numbers and combinations of tests were used. SPT were most common, performed in 93 of 104 patients and positive in 72 (77%). sIgE was assayed in 78 of 104 patients and positive in 62 (80%). IDT was performed in 23 of 104 and positive in 21 (91%). BAT was performed in six patients and positive in three (50%). The distribution of positive tests is shown in Fig. 1. The mean sIgE levels and positive allergy tests for other substances for all 104 patients are shown in Table 1.
Figure 1.
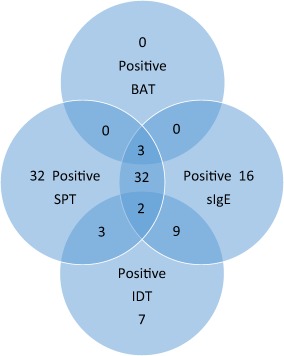
The distribution of positive tests for all 104 patients. [Colour figure can be viewed at wileyonlinelibrary.com.]
Table 1.
Combinations of test results in 104 cases of perioperative chlorhexidine allergy
| Positive tests | Number of patients | Mean Chlorhexidine sIgE (kUA/l) (95% CI) | Patients with other positive allergy tests* | Patients with positive NMBA allergy tests | Patients with other positive allergy tests |
|---|---|---|---|---|---|
| Single pos IDT | 7 | 0·34 (0–0·35) | 3 |
1 × atracurium SPT 1 × vecuronium & atracurium IDT |
1 × gelatin sIgE |
| Single pos IgE | 16 | 4·34 (0–16·52) | 6 |
1 × rocuronium SPT 1 × cisatracurium IDT 2 × NMBA IDT & sIgE |
1 × QAM** sIgE 1 × morphine sIgE 1 × teicoplanin SPT |
| Single pos SPT | 32 | 0·34 (0–0·35) | 4 | 1 × atracurium SPT |
1 × carmosine SPT 1 × penicillin SPT 1 × latex SPT |
| Double pos IDT, sIgE | 9 | 8·50 (0–21·82) | 7 |
1 × vecuronium IDT & sIgE 1 × all NMBAs IDT & sIgE 1 × atracurium IDT 1 x atracurium sIgE |
1 × teicoplanin IDT & SPT 1 × amoxicillin sIgE 1 × gentamycin IDT 1 × morphine IDT |
| Double pos SPT, IDT | 3 | 0·34 (0–0·34) | 1 | 0 | 1 × gelatin IDT |
| Double pos SPT, sIgE | 32 | 8·96 (0–27·92) | 6 |
2 × all NMBA IDT 1 × rocuronium SPT & suxamethonium sIgE 1 × atracurium SPT 1 × suxamethonium IDT & sIgE |
1 x QAM sIgE 1 × morphine, amoxicillin sIgE |
| Triple pos SPT, IDT, sIgE | 2 | 1·08 (0·17–1·99) | 1 | 1 × suxamethonium sIgE | 1 × morphine SPT |
| Triple pos SPT, sIgE, BAT | 3 | 6·18 (0–13·56) | 0 | 0 | 0 |
There was no correlation between the number and type of positive tests or specific immunoglobulin (sIg)E level and reaction grade for the cohort of 104 cases (not shown). *Tests potentially relevant to the differential diagnosis of the reaction. **QAM = Quaternary Ammonium Moiety (e.g. Thiocholine or Suxamethonium sIgE). SPT = skin prick test; BAT = basophil activation test; IDT = intradermal skin test; NMBA = neuromuscular blocking agent; CI = confidence interval.
Results for patients who had three or more tests
Figure 2 shows the distribution of positive tests among the 25 patients who had three tests. Table 2 shows the mean sIgE levels and positive allergy tests for other substances for these patients.
Figure 2.
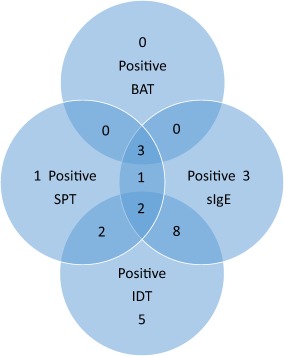
Twenty‐five patients had three tests. All had skin prick test (SPT) and specific immunoglobulin (sIg)E. Six patients also had a basophil activation test (BAT) and 19 also had an intradermal skin test (IDT). [Colour figure can be viewed at wileyonlinelibrary.com.]
Table 2.
The mean specific immunoglobulin (sIg)E levels and distribution of positive allergy tests for other substances for 25 patients who tested positive for two or more chlorhexidine tests
| Patients who had three tests performed | Number of patients | Mean chlorhexidine SIgE (KUA/l) (95% CI) | Patients with other positive allergy tests | Patients with positive NMBA allergy tests | Patients with other positive allergy tests |
|---|---|---|---|---|---|
| Single pos IDT | 5 | 0·34 (0–0·34) | 2 | 1 × vecuronium & atracurium IDT | 1 × gelatin sIgE |
| Single pos IgE | 3 | 1·34 (0·59–2·09) | 1 | 1 × NMBA IDT & sIgE1 | 0 |
| Single pos SPT | 1 | 0·34 | 1 | 0 | 1 × carmosine SPT |
| Double pos IDT, sIgE | 8 | 8·88 (0–23·07) | 6 |
1 × vecuronium IDT & sIgE 1 × all NMBA IDT & sIgE 1 × atracurium IDT 1 × atracurium sIgE |
1 × teicoplanin IDT & SPT 1 × amoxicillin sIgE 1 × gentamycin IDT 1 × morphine IDT |
| Double pos SPT, IDT | 2 | 0·34 (0–0·35) | 1 | 0 | 1 × gelatin IDT |
| Double pos SPT, sIgE | 1 | 9·74 | 0 | 0 | 0 |
| Triple pos SPT, sIgE, BAT | 3 | 6·18 (0–20·88) | 0 | 0 | 0 |
| Triple pos SPT, IDT, sIgE | 2 | 1·08 (0·17–1·99) | 1 | 1 × suxamethonium sIgE | 1 × morphine SPT |
In the head‐to‐head comparison of the skin prick test (SPT), specific immunoglobulin (sIg)E and intradermal skin test (IDT), IDT was positive in 17 of 19 cases where three tests were performed, more frequently than any other test. BAT = basophil activation test; NMBA = neuromuscular blocking agent; CI = confidence interval.
For analysis of the 25 patients who had three tests, we used two positive tests as a gold standard for making the diagnosis of chlorhexidine allergy in the presence of definitive sensitization. Using this approach, we were able to estimate the sensitivity and demonstrate that all three test modalities should be used when the screening test is negative, as shown in Table 3.
Table 3.
Sensitivity of each test modality in the 25 patients with at least two positive chlorhexidine tests
| SPT | sIgE | IDT | BAT | |
|---|---|---|---|---|
| True positives | 9 | 17 | 17 | 3 |
| False negatives | 16 | 8 | 2 | 3 |
|
Sensitivity in cases with at least three tests (95% CI) |
36%*
(17–55%) |
68% (50–86%) |
89% (75–100%) |
50% (10–90%) |
|
Published sensitivity [7] |
95% | 100% | 68% | Not published |
*The majority of cases were diagnosed on the basis of skin prick test (SPT) or specific immunoglobulin (sIg)E and this subgroup represents cases where multiple tests were used, predominantly because the initial screening test was negative. IDT = intradermal skin test; BAT = basophil activation test; CI = confidence interval.
Sensitization to other potential triggers
Twenty‐eight of 104 chlorhexidine allergic patients had evidence of reactivity to other potentially relevant allergens, including neuromuscular blocking agents (NMBA; 17 patients), morphine (four patients) and a small number of other agents (see Table 1).
Some patients had extreme multi‐reactivity; for example, one patient who had two grade 3 anaphylactic reactions during orthopaedic procedures had positive SPT and sIgE to chlorhexidine (10·4 kUA/l) and also had positive rocuronium IDT and positive sIgE to suxamethonium, morphine and amoxicillin.
NMBA‐positive patients appeared more likely to be chlorhexidine sIgE‐positive than NMBA‐negative patients (13 of 15 versus 49 of 63). This was not true for chlorhexidine IDT (six of seven versus 15 of 16) or SPT (six of 13 versus 66 of 80); i.e. NMBA positivity correlated more closely with sIgE than IDT or SPT testing.
Multiple allergen reactivity was confirmed by the results of the patients who had three or more tests. Twelve of the 25 patients who underwent three different chlorhexidine allergy tests also showed evidence of allergy to other substances (Table 2), including seven patients with evidence of NMBA allergy. Of these seven patients with evidence of NMBA allergy, six had positive chlorhexidine sIgE or IDT, while only one had positive chlorhexidine SPT.
Discussion
Our report describes the largest single series of patients with perioperative chlorhexidine allergy published so far from routine clinical assessment. It confirms and extends previous reports. Our observations are based on data from routine clinical practice; therefore, not all patients underwent the same tests. Conversely, the data reflect existing clinical practice in the United Kingdom and should be less prone to bias than smaller reports of very specific types of reaction; for example, those triggered by chlorhexidine‐coated CVCs.
It is notable that our clinical data are consistent with previous descriptions of perioperative chlorhexidine allergy outside the United Kingdom. For example, the majority of our patients were men, and most reactions took place in urology or cardiothoracic surgery, as described previously 3, 4.
Severe reactions are common but may be subject to selection bias, as these cases have been selected to be referred for specialist assessment, as is true for most previous series. The explanation for the apparent under‐representation of obstetric and gynaecological procedures is not clear. There is no clear reason why referral patterns for patients undergoing these procedures should differ from other surgical interventions, as the majority of clinic referrals are made by anaesthetists.
The majority of the reactions experienced by our patients were severe grade 3 reactions, most commonly including hypotension, with cardiac arrest occurring in a significant minority, as described previously 1, 3, 4. Hypotension is not a unique characteristic of reactions to chlorhexidine, and has been shown to be a dominant feature of most perioperative allergic reactions 2, 11. In contrast, allergic reactions to penicillins or wasp venom appear to cause hypotension less frequently 1, 3, 4, 12, 13.
It is possible that perioperative allergic reactions, including those to chlorhexidine, tend to be more severe because the patient is unconscious and cannot respond to early symptoms. In addition, many patients undergoing surgery have cardiorespiratory co‐morbidity.
Diverse sources of chlorhexidine were triggers, and it is noteworthy that chlorhexidine mouthwash caused cardiac arrest in one patient. Fatal reactions to topical chlorhexidine have been reported 14.
Hidden chlorhexidine exposure is a known problem; in a recent systematic review of published cases of perioperative chlorhexidine allergy, coated CVCs accounted for a third of cases but infrequently caused cardiac arrest 4. In our group of patients, CVC exposure to chlorhexidine was not particularly common, but frequently caused hypotension and cardiac arrest. It is not clear why our data on reactions triggered by chlorhexidine coated CVCs are different, but reporting bias may be relevant in small case series. In addition, protocols for using chlorhexidine‐coated CVCs may differ between centres. It is also possible that reactions to chlorhexidine‐coated CVCs are under‐referred to our services. Anaphylaxis to chlorhexidine‐coated CVCs may be difficult to diagnose, particularly if hypotension is the main feature, and may be mistaken for anaesthesia‐induced hypotension, haemorrhage from arterial puncture or pneumothorax. Anaphylaxis induced by chlorhexidine has been confused with cardiogenic shock and sepsis 15, 16. Interestingly, the efficacy of chlorhexidine‐coated CVCs in preventing infection outside intensive care units has also been questioned by a Cochrane review 17.
In an observational series such as this, and in the absence of a gold standard challenge procedure, only limited conclusions can be made concerning the performance of individual tests.
Clearly, the vast majority of diagnoses were supported utilizing positivity in one of the two favoured tests (SPT or sIgE). As a result, those patients who had multiple tests were either negative in the screening test or were selected in some other way for multiple testing. True performance indices require unselected testing of all patients utilizing all modalities. Where multiple tests were used, the majority of cases were positive in at least two tests. However, nine of 104 (8·7%) of the whole cohort were only positive in a single test representing nine of 25 (36%) of the cases where multiple tests were applied. IDT appeared to be most sensitive as second‐line testing. We cannot estimate specificity in routine practice, as we have not included individuals who definitely do not have chlorhexidine allergy 7, 10. However, high sensitivity in testing for chlorhexidine allergy is arguably more important than specificity, as a false positive will result only in chlorhexidine being avoided, while a false negative could lead to repeat exposure and anaphylaxis.
The basophil activation test was only ever positive in the presence of both sIgE and SPT, but is not available in most centres.
One possible explanation for differences in test positivity favouring IDT when multiple tests are used is that chlorhexidine sIgE reactivity is lost over time, and sIgE and the tests reported here may have been performed several months after the clinical reaction 3, 18. However, there were cases where sIgE and SPT were positive in the absence of IDT. Table 3 shows clearly that IDT (and indeed SPT and sIgE) can be positive on its own and where screening tests are negative, thus further investigations should include IDT. We cannot address the issue of whether the isolated positive IDT (or SPT or sIgE) might be ‘false positive’, nor can any previous series, as we have no definitive challenge data. UK clinics used 5 mg/l chlorhexidine for IDT, while Opstrup used 2 mg/l 7. More prosaically, we will have excluded patients who had a positive SPT or sIgE and did not go on to have IDT in our sensitivity estimates in this subgroup analysis. Thus, Table 3 estimates more closely the results of performing all test modalities where the screening test is negative, and shows that IDT clearly has a potential diagnostic advantage in this setting.
In addition, our IDT testing differed slightly from that used previously to validate IDT testing, in using the forearm rather than the back 7. This variation is true of all skin tests in clinical practice, and argues strongly for the adoption of harmonized approaches to skin testing.
A combination of SPT and sIgE has been recommended as a high sensitivity strategy for testing for chlorhexidine allergy 7, based on data from a cohort of patients in whom testing ‘usually took place 2–4 months after the allergic reaction’. Our data may support this observation, as SPT and sIgE dominate the positive investigations in the whole cohort, but IDT dominates once these single test positives have been screened out. It is noteworthy that, in seven of our cohort of 104 patients, the only positive test was IDT. Had IDT not been performed after finding negative SPT and sIgE, sensitization to chlorhexidine in these patients may not have been revealed.
One logical approach would be to offer sIgE and SPT to all patients, but always to go on to perform IDT if these tests are negative, and there remains a high index of suspicion of chlorhexidine allergy.
Positivity to other potential culprits in a third of our cases is important. Multiple sensitizations to drugs were common. Twenty‐eight of 104 patients had other positive allergy tests, confirming the finding of multiple reactivity in similar proportions to other cohorts of chlorhexidine‐allergic patients 3, 7. However, our patients mainly had reactivity to NMBA, as opposed to the latex, opiates and beta lactams in the other reports.
In our series, multiple reactivity occurred in patients with most combinations of chlorhexidine allergy tests, but was associated most closely with a positive chlorhexidine sIgE test.
High total IgE (above 1500 kUA/l) is a frequent cause of multiple reactivity in other settings. However, high total IgE is not thought to drive false positive chlorhexidine sIgE 18. Until neutralizing and blocking experiments are reported it remains unclear whether this multiple reactivity reflects cross‐reactivity, for example, to quaternary amide groups.
Twelve of the 25 patients who underwent three different chlorhexidine allergy tests also showed evidence of allergy to other substances (Table 2), including seven patients with evidence of NMBA allergy. Of these seven patients with evidence of NMBA allergy, six had positive chlorhexidine sIgE or IDT, while only one had positive chlorhexidine SPT. This suggests that extended panels of allergen testing may be required routinely to ensure that all potential triggers are assessed for clinical relevance. It may also suggest that perioperative allergic reactions associate with multiple drug exposure or procedures.
In summary, we report on the largest series yet described of patients diagnosed with chlorhexidine allergy. We confirm that these reactions are frequently severe. Specific IgE and SPT are reasonable first‐line tests for chlorhexidine allergy, but IDT should be added if these are negative, particularly if referral to the allergy clinic is delayed or if the index of suspicion is high. False negativity in screening tests is not uncommon, and may affect 7% of our series. Multiple sIgE reactivity is relatively common and, until further data are available on its cause and significance, should lead to specialist allergy assessment that looks for sensitization to all the potential drug triggers, and an imputibility assessment for each potential trigger to avoid misdiagnosis. Hidden exposure to chlorhexidine is common in healthcare environments, and we suggest that awareness of the potential allergenicity of chlorhexidine should be part of the training of all health‐care professionals. Chlorhexidine‐coated CVCs were not a common trigger of anaphylaxis in UK cohorts, but appeared to be associated with severe reactions.
Author contributions
R. S. and W. E. designed and initiated the collaborative survey. R. S., W. E., N. H., T. G., M. H., S. S., L. S., E. E. and L. N. saw the patients and collected the data. K. S. carried out in‐vitro BAT tests. M. H. and W. E. analysed the data and drafted the paper. N. H., R. S., W. E., K. S., E. E. and T. G. contributed to revising the paper.
Disclosure
No disclosures.
References
- 1. Krishna MT, York M, Chin T et al Multi‐centre retrospective analysis of anaphylaxis during general anaesthesia in the United Kingdom: aetiology and diagnostic performance of acute serum tryptase. Clin Exp Immunol 2014; 178:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Low AE, McEwan JC, Karanam S, North J, Kong KL. Anaesthesia‐associated hypersensitivity reactions: seven years’ data from a British bi‐specialty clinic. Anaesthesia 2016; 71:76–84. [DOI] [PubMed] [Google Scholar]
- 3. Garvey LH, Kroigaard M, Poulsen LK et al IgE‐mediated allergy to chlorhexidine. J Allergy Clin Immunol 2007; 120:409–15. [DOI] [PubMed] [Google Scholar]
- 4. Sharp G, Green S, Rose M. Chlorhexidine‐induced anaphylaxis in surgical patients: a review of the literature. Aust NZ J Surg 2016; 86:237–43. [DOI] [PubMed] [Google Scholar]
- 5. Savic LC, Kaura V, Yusaf M et al Incidence of suspected perioperative anaphylaxis: a multicenter snapshot study. J Allergy Clin Immunol Pract 2015; 3:454–5 e1. [DOI] [PubMed] [Google Scholar]
- 6. Mertes PM, Malinovsky JM, Jouffroy L et al Reducing the risk of anaphylaxis during anesthesia: 2011 updated guidelines for clinical practice. J Investig Allergol Clin Immunol 2011; 21:442–53. [PubMed] [Google Scholar]
- 7. Opstrup MS, Malling HJ, Kroigaard M et al Standardized testing with chlorhexidine in perioperative allergy – a large single‐centre evaluation. Allergy 2014; 69:1390–6. [DOI] [PubMed] [Google Scholar]
- 8. McGowan EC, Saini S. Update on the performance and application of basophil activation tests. Curr Allergy Asthma Rep 2013; 13:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simons FE, Ardusso LR, Bilo MB et al 2012 Update: World Allergy Organization Guidelines for the assessment and management of anaphylaxis. Curr Opin Allergy Clin Immunol 2012; 12:389–99. [DOI] [PubMed] [Google Scholar]
- 10. Leysen J, Bridts CH, De Clerck LS et al Allergy to rocuronium: from clinical suspicion to correct diagnosis. Allergy 2011; 66:1014–9. [DOI] [PubMed] [Google Scholar]
- 11. Egner W, Sargur R, Shrimpton A, York M, Green K. A 17‐year experience in perioperative anaphylaxis 1998–2015: harmonizing optimal detection of mast cell mediator release. Clin Exp Allergy 2016; 46:1465–73. [DOI] [PubMed] [Google Scholar]
- 12. Bousquet PJ, Kvedariene V, Co‐Minh HB et al Clinical presentation and time course in hypersensitivity reactions to beta‐lactams. Allergy 2007; 62:872–6. [DOI] [PubMed] [Google Scholar]
- 13. Rueff F, Przybilla B, Bilo MB et al Predictors of severe systemic anaphylactic reactions in patients with Hymenoptera venom allergy: importance of baseline serum tryptase. J Allergy Clin Immunol 2009; 124:1047–54. [DOI] [PubMed] [Google Scholar]
- 14. Pemberton MN, Gibson J. Chlorhexidine and hypersensitivity reactions in dentistry. Br Dent J 2012; 213:547–50. [DOI] [PubMed] [Google Scholar]
- 15. Rutkowski K, Wagner A. Chlorhexidine: a new latex? Eur Urol 2015; 68:345–7. [DOI] [PubMed] [Google Scholar]
- 16. Hong CC, Wang SM, Nather A, Tan JH, Tay SH, Poon KH. Chlorhexidine anaphylaxis masquerading as septic shock. Int Arch Allergy Immunol 2015; 167:16–20. [DOI] [PubMed] [Google Scholar]
- 17. Lai NM, Chaiyakunapruk N, Lai NA, O'Riordan E, Pau WS, Saint S. Catheter impregnation, coating or bonding for reducing central venous catheter‐related infections in adults. Cochrane Database Syst Rev 2016; 3:CD007878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Opstrup MS, Poulsen LK, Malling HJ, Jensen BM, Garvey LH. Dynamics of plasma levels of specific IgE in chlorhexidine allergic patients with and without accidental re‐exposure. Clin Exp Allergy 2016; 46:1090–8. [DOI] [PubMed] [Google Scholar]


