Summary
The programmed cell death 1 (PD‐1) receptor plays a major role in regulating T cell activation. Our aim was to determine how inflammation influences PD‐1‐mediated T cell suppression. Flow cytometry analysis of rheumatoid arthritis (RA) and psoriatic arthritis (PsA) synovial fluid (SF) mononuclear cells showed an increase in the percentage of PD‐1+ cells within the CD4+ and CD8+ T cell compartment compared to paired peripheral blood (PB). Upon in‐vitro T cell receptor (TCR) stimulation of healthy control (HC) CD4+ T cells in the presence of plate‐bound PD‐L1fc chimera, significantly decreased proliferation and interferon (IFN)‐γ secretion was observed. In contrast, CD4+ T cells from RA and PsA PB and SF appeared resistant to such PD‐1‐mediated inhibition. Addition of the proinflammatory cytokines tumour necrosis factor (TNF)α, interleukin (IL)‐6 and IL‐1β, which were increased in RA and PsA SF compared to osteoarthritis (OA) SF, consistently abrogated PD‐1‐mediated suppression in HC CD4+ T cell cultures. This effect was reversed by inhibitors of these cytokines. Soluble PD‐1 (sPD‐1) levels were increased in cell culture supernatants from TNFα and IL‐6‐stimulated cultures compared to untreated controls, and also in RA and PsA, but not in OA, serum and SF. Functionally, addition of sPD‐1fc counteracted PD‐1‐mediated suppression of HC CD4+ T cells, and increased T cell proliferation in HC CD4+ T cell/monocyte co‐cultures. These in‐vitro findings indicate that CD4+ T cells from patients with RA and PsA show increased resistance to PD‐1‐mediated suppression, which may be explained in part by the presence of soluble PD‐1 in the inflammatory environment.
Keywords: inflammatory arthritis, IL‐6, PD‐1, PD‐L1, TNFα
Introduction
The programmed cell death 1 (PD‐1) receptor, a transmembrane protein and member of the B7 family, plays a critical role in T cell regulation 1. PD‐1 is expressed on T cells, where its expression increases within the first 24 h of T cell activation and decreases with antigen clearance 2, 3, 4. Upon ligation of PD‐1 by its ligands (PD‐L1/B7‐H1 and PD‐L2/B7‐DC), T cell responses are down‐regulated 5, 6. PD‐1 ligation leads to inhibition of the phosphatidylinositol 3‐kinase (PI3K) pathway, resulting in reduced Akt (protein kinase B) phosphorylation and reduced expression of transcription factors GATA‐3, T‐bet and Eomes 7, 8. The overall effect of PD‐1 ligation is decreased T cell activation and cytokine production 9, 10, 11, 12. The clinical relevance of PD‐1 in immune regulation is evidenced by the recent success of PD‐1 blockade in the treatment of certain end‐stage cancers, leading to reduced tumour burden and enhanced anti‐tumour immunity in a considerable number of patients 13, 14. Conversely, in inflammatory conditions it has been documented that disruption of the PD‐1 gene (pdcd1) in mice leads to lupus‐like syndrome, proliferative arthritis, diabetes, autoimmune cardiomyopathy and increased susceptibility to collagen‐induced arthritis (CIA) 15, 16, 17, 18, 19, 20. In humans, polymorphisms in the PDCD1 gene have been associated with susceptibility to rheumatoid arthritis (RA), ankylosing spondylitis (AS), systemic lupus erythematosus (SLE), multiple sclerosis (MS) and type 1 diabetes mellitus 21, 22, 23, 24, 25.
Several investigators have shown that frequencies of PD‐1+ CD4+ T cells are increased in RA synovial fluid compared to RA peripheral blood (PB) and healthy control (HC) PB 20, 26, 27. However, despite high levels of this inhibitory receptor at the site of inflammation, the immune system seems unable to regulate persistent T cell activation and cytokine production. This poses the question as to whether the PD‐1 pathway is impaired during inflammation. An indication of a defective PD‐1 pathway in RA comes from a study indicating that RA synovial fluid (SF) CD4+ T cells show reduced PD‐1‐mediated inhibition compared to RA PB cells 20. This suggests that under conditions of chronic inflammation the PD‐1 pathway is modulated. Thus far, little is known regarding the PD‐1/PD‐L1 pathway in the context of psoriatic arthritis (PsA). PsA and RA, while sharing a number of common pathological features, are two distinct diseases with serological, genetic and radiological differences 28. Here we determined the expression of PD‐1 on T cells from PB and SF of patients with RA or PsA, and investigated how inflammatory mediators associated with RA and PsA affect PD‐1‐mediated T cell suppression. Our data indicate that CD4+ T cells from patients with RA and PsA are compromised in their PD‐1‐mediated inhibition and reveal a potential role for soluble PD‐1 (sPD‐1) in the aberrant PD‐1‐mediated regulation in these diseases.
Materials and methods
Patients and healthy volunteers
Heparinized PB and matched SF samples were obtained from patients with RA and PsA recruited from the rheumatology out‐patient clinic at Guy's and St Thomas' Hospital NHS Trust (London, UK). Information on clinical and demographic parameters is provided in Supporting information, Table 1. HC subjects were recruited from among local student and staff volunteers. Written informed consent was received from all participants. Ethics approval was given by the Bromley Research Ethics Committee (approval no. 06/Q0705/20) for HC, RA and PsA and by the Guy's Research Ethics Committee (approval no. 01/05/01) for osteoarthritis (OA). All samples were collected in compliance with the Declaration of Helsinki.
PBMC, SFMC and cell subset isolation
PB mononuclear cells (PBMC) and SF mononuclear cells (SFMC) were isolated by Lymphoprep™ (Axis‐Schield, Oslo, Norway) density‐gradient centrifugation. Cell subsets were isolated by magnetic separation (Miltenyi Biotech, Bergisch Gladbach, Germany and Dynabeads Thermofisher, Paisley, UK) and purity was determined by flow cytometry. CD4+ T cells (purity range 95–99%) were isolated by negative isolation from PBMC or SFMC or from the CD14‐depleted cell fractions following the manufacturers' instructions. CD14+ monocytes (purity range 96–98%) were selected positively using CD14 MicroBeads (Miltenyi Biotec).
Cell culture
Cell subsets were cultured for 5 days in culture medium (RPMI‐1640; Gibco, Paisley, UK), supplemented with 1% penicillin/streptomycin, 1% L‐glutamine (Gibco) and 10% heat‐inactivated fetal calf serum (Gibco) and maintained at 37°C and 5% CO2 atmosphere. Cells were stimulated with either plate‐bound anti‐CD3 monoclonal antibody (mAb) (OKT3; Janssen‐Cilag Ltd, High Wycombe, UK) (1·5 µg/ml) in CD4+ T cell only cultures or with soluble anti‐CD3 mAb (OKT3; Janssen‐Cilag Ltd) (100 ng/ml) in CD4+ T cell/CD14+ monocyte co‐cultures.
Flow cytometry analysis of cell frequency and phenotype
For ex‐vivo analysis of frequency and phenotype of each cell subset, PBMC or SFMC were stained extracellularly for 30 min at 4°C using different combinations of the following antibodies: fluorescein isothiocyanate (FITC)‐conjugated CD279 (PD‐1; BioLegend, Cambridge, UK), phycoerythrin (PE)‐conjugated CD274 (PD‐L1; BD Pharmingen, Oxford, UK), PE/cyanin 7 (Cy7)‐conjugated CD3 (Biolegend), peridinin chlorophyll (PerCP)/Cy5·5‐conjugated CD4 (Biolegend), PacBlue‐conjugated CD8 (Biolegend), allophycocyanin (APC)‐conjugated CD8 (Biolegend) and Vio770‐conjugated CD14 (Miltenyi Biotech). Following surface staining, cells were fixed in 2% paraformaldehyde (PFA) for 15 min at 4°C, washed twice and acquired on a BD fluorescence activated cell sorter (FACS)Calibur or a BD FACSCanto II. Data were analysed using FlowJo software (version 7.6.5; Tree Star, Ashland, OR, USA).
T cell proliferation and PD‐1 ligation assays
96 well flat‐bottomed plates (Costar, Corning Inc., Corning, NY, USA) were coated with anti‐CD3 mAb (OKT3; Janssen‐Cilag Ltd) (1·5 µg/ml) and with either PD‐L1fc or immunoglobulin (Ig)G1fc (R&D Systems, Abingdon, UK) (ranging from 0 to 5 µg/ml according to the experiment) in phosphate‐buffered saline (PBS) solution (Sigma Aldrich, Poole, UK) for 4 h at 37°C and 5% CO2. Plates were washed twice with PBS before cells were added for culture. CD4+ T cells were isolated from cryopreserved HC PBMC, RA and PsA PBMC and RA and PsA SFMC and plated at a concentration of 1 × 105 cells per well in a final volume of 200 μl of culture medium. In some cultures, human recombinant tumour necrosis factor (hrTNF)α, human recombinant interleukin (hrIL)‐6 or hrIL‐1β (all at 10 ng/ml; R&D Systems) were added in the absence or presence of anti‐TNFα drug adalimumab (AbbVie, Chicago, IL, USA), anti‐IL‐6R drug tocilizumab (Roche, Basel, Switzerland) or anti‐IL‐1β mAb (R&D Systems) (all at 1 µg/ml). In some cultures, HC CD4+ T cells were cultured in PD‐L1fc (0, 0·1 and 1 µg/ml)‐coated plates in the presence of soluble PD‐1fc (0·5 and 1 µg/ml; R&D Systems). In other experiments, freshly isolated HC CD4+ T cells and autologous CD14+ monocytes (used as a source of PD‐L1 ligand) were co‐cultured in 96‐well flat‐bottomed plates (Costar, Corning Inc.) at 1 : 1 ratios (total cells per well 1 × 105) in culture medium containing 100 ng/ml soluble anti‐CD3 mAb and soluble PD‐1fc or IgG1fc (0, 0·25, 0·5 and 1 µg/ml). In all assays, at day 4, cells were pulsed with [3H]‐thymidine (0·25 μCi/well) (GE Healthcare, Little Chalfont, UK) and T cell proliferation was assessed after 18 h (on day 5) using a Topcount scintillation counter (Perkin Elmer, Cambridge, UK). Proliferation was determined and expressed as counts per minute (cpm) and as suppression of T cell proliferation (%) according to the following formula: [(medium only condition − PD‐L1fc condition)/medium only condition] × 100.
Detection of soluble cytokines and soluble PD‐1
CD4+ T cell culture supernatants were collected at day 5 and stored at −80°C. IFN‐γ levels were determined by enzyme‐linked immunosorbent assay (ELISA) using the ELISA MAX™ standard set (Biolegend). Levels of sPD‐1 were determined by human PD‐1 DuoSet ELISA (R&D Systems). Serum samples from HC donors and serum and paired cell‐free SF samples from patients with OA, RA or PsA were collected and stored at −80°C until analysed by ELISA (for sPD‐1; R&D Systems) or Bio‐Plex Pro™ (for TNFα, IL‐6 and IL‐1β; Bio‐Rad Laboratories) on the Luminex FlexMap 3D platform (Luminex Corporation, Austin, TX, USA). All assays were performed according to the manufacturers' protocols.
RNA extraction and real‐time–quantitative polymerase chain reaction (RT–qPCR)
Total RNA was isolated using the ReliaPrep™ RNA cell Miniprep System (Promega, Southampton, UK) according to the manufacturer's protocol. cDNA synthesis was performed using a high‐capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) and mRNA expression of PD‐1Δex3 was determined using the SensiMix™ SYBR No‐ROX Kit (Bioline, London, UK). Data were collected and analysed on a Rotor‐Gene Q thermocycler (Qiagen, Hilden, Germany). The β‐actin gene was used as an endogenous control and relative gene expression was expressed as 2–ΔΔCT. PCR primer pairs (IDT, Leuven, Belgium) were as follows: PD‐1Δex3, 5′‐AGGGTGACAGGGACAATAGG‐3′ and 5′‐CCATAGTCCACAGAGAACAC‐3′, β‐actin, 5′‐ATTGGCAATGAGCGGTTC‐3′ and 5′‐CGTGGATGCCACAGGACT −3'.
Statistical analysis
Significance testing was performed with GraphPad Prism software (version 7; GraphPad, La Jolla, CA, USA) using the appropriate statistical tests, as indicated in the figure legends.
Results
PD‐1+ T cell frequencies are increased in RA and PsA synovial fluid compared to peripheral blood
First, we investigated the frequencies of PD‐1+ cells among T cells in PB and paired SF from patients with RA and PsA. Significantly increased percentages of PD‐1+ cells were found within SF CD4+ T cells (identified either by CD3+CD14–CD4+ cells or CD3+CD8– cells) compared to PB (Fig. 1a,b). In addition, increased percentages of PD‐1+ cells were found within the SF CD8+ T cell compartment in both RA and PsA (Fig. 1c,d).
Figure 1.
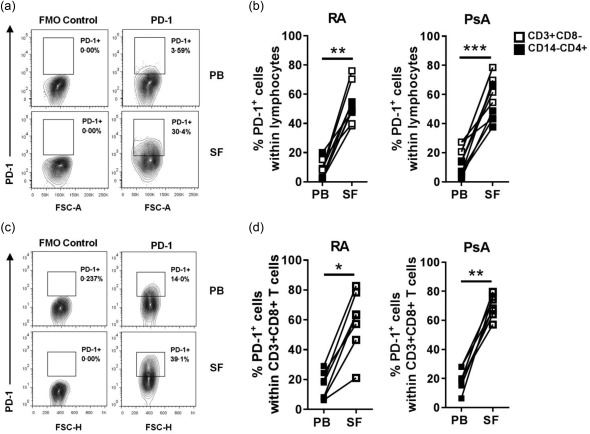
Programmed cell death 1 (PD‐1)+ T cell frequencies are increased in rheumatoid arthritis (RA) and psoriatic arthritis (PsA) synovial fluid compared to peripheral blood. Frequencies of PD‐1+ T cells were analysed ex vivo by flow cytometry in peripheral blood mononuclear cells (PBMC) and synovial fluid mononuclear cells (SFMC) from RA and PsA patients. (a) Contour plot of CD3+CD14–CD4+PD‐1+ cells from paired PBMC and SFMC of one representative PsA donor. (b) Cumulative data showing percentage of PD‐1+ cells within CD3+CD8– or CD3+CD14–CD4+ (RA n = 10; PsA n = 11) PB and SF cell populations. (c) Contour plot of CD3+CD8+PD‐1+ cells from paired PBMC and SFMC of one representative RA donor. (d) Cumulative data showing percentage of PD‐1+ cells within CD3+CD8+ (RA n = 7; PsA n = 8) PB and SF cell populations. Data were analysed by Wilcoxon matched‐pairs signed‐rank test. *P < 0·05, **P < 0·01 and ***P < 0·001. Isotype control staining showed a similar result to fluorescence minus 1 (FMO) staining (Supporting information, Fig. S5).
PD‐1 ligation reduces proliferation of CD4+ T cells from healthy donors, but not CD4+ T cells from patients with RA or PsA
To investigate whether the PD‐1 expression is functional in RA and PsA, we set up a PD‐1 ligation assay using anti‐CD3 and PD‐L1fc (or IgG1fc as control)‐coated plates, based on previously described protocols 9, 29, 30. As expected, PD‐1 ligation resulted in a significant and dose‐dependent reduction of healthy control PB‐derived CD4+ T cell proliferation, while no effect was observed in the presence of IgG1fc control (Fig. 2a,b). IFN‐γ production was also inhibited in a PD‐L1fc dose‐dependent fashion (Supporting information, Fig. S1a). We then compared PD‐1 ligation of PB‐derived CD4+ T cells from healthy donors and patients with RA in parallel experiments. In contrast to the suppressive effects on T cell proliferation observed upon PD‐1 ligation of CD4+ T cells from healthy donors, CD4+ T cells from patients with RA appeared to be resistant to PD‐1‐mediated suppression of T cell proliferation (Fig. 2c,d). Next, we cultured CD4+ T cells from the blood and synovial fluid from patients with RA and PsA with increasing doses of plate‐bound PD‐L1fc. Even at the highest dose of PD‐L1fc (5 µg/ml) we did not detect a decrease in T cell proliferation upon PD‐1 ligation (Fig. 2e,f). Similarly, when RA and PsA cell culture supernatants were tested for IFN‐γ production, we could not detect a consistent decrease in the levels of IFN‐γ (Supporting information, Fig. S1b). These data indicate that CD4+ T cells from the blood and synovial fluid of patients with RA or PsA are resistant to PD‐1 ligation compared to healthy control cells.
Figure 2.
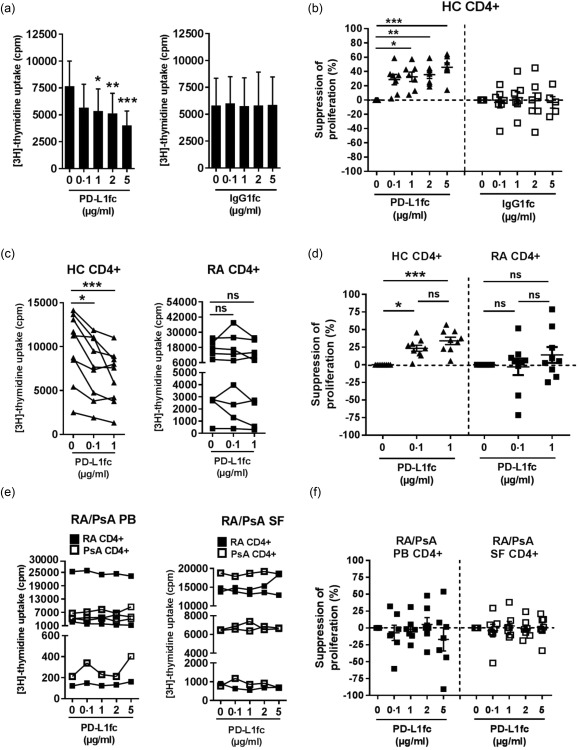
Programmed cell death‐1 (PD‐1) ligation reduces proliferation of CD4+ T cells from healthy donors but not CD4+ T cells from patients with rheumatoid arthritis (RA) or psoriatic arthritis (PsA). (a–f) CD4+ T cells were isolated from healthy control (HC) peripheral blood mononuclear cells (PBMC) and RA and PsA PBMC and synovial fluid mononuclear cells (SFMC) and cultured for 5 days in plates precoated with anti‐CD3 monoclonal antibody (mAb) (OKT3; 1·5 µg/ml) and PD‐L1fc/IgG1fc (0, 0·1, 1, 2 and 5 µg/ml). Proliferation was assessed on day 5 by [3H]‐thymidine incorporation. (a) HC CD4+ T cell proliferation (cpm) and (b) suppression of proliferation following PD‐1 ligation by PD‐L1fc (n = 7) or immunoglobulin (Ig)G1fc (n = 5–7). (c) Cell proliferation (cpm) and (d) suppression of proliferation of CD4+ T cells isolated from HC and RA PBMC (n = 9) in presence of PD‐L1fc. (e) Cell proliferation (cpm) and (f) suppression of proliferation of CD4+ T cells isolated from RA and PsA PBMC and paired SFMC in presence of PD‐L1fc (n = 3 RA PB/SF; n = 4 PsA PB/SF). Data were analysed by Friedman test with Dunn's multiple comparison test. *P < 0·05, **P < 0·01 and ***P < 0·001. Data in (b,d,f) show mean ± standard error of the mean.
TNFα, IL‐6 and IL‐1β counteract PD‐1‐mediated suppression of CD4+ T cell proliferation
Because RA and PsA CD4+ T cells, especially those from the synovial fluid, are derived from a proinflammatory environment, we sought to examine how inflammatory cytokines may influence PD‐1‐mediated T cell suppression. First, we determined the levels of TNFα, IL‐6 and IL‐1β in RA and PsA‐derived sera and paired SF, compared to healthy serum and serum and SF from disease control patients with osteoarthritis (OA). Increased levels of all three cytokines were detected in both RA and PsA SF when compared to HC serum or OA SF (Fig. 3a,b). These data confirm the inflammatory nature of SF in both RA and PsA. In the analysed RA and PsA sera, we only found mild increases of TNFα and IL‐6 compared to healthy or OA control serum.
Figure 3.
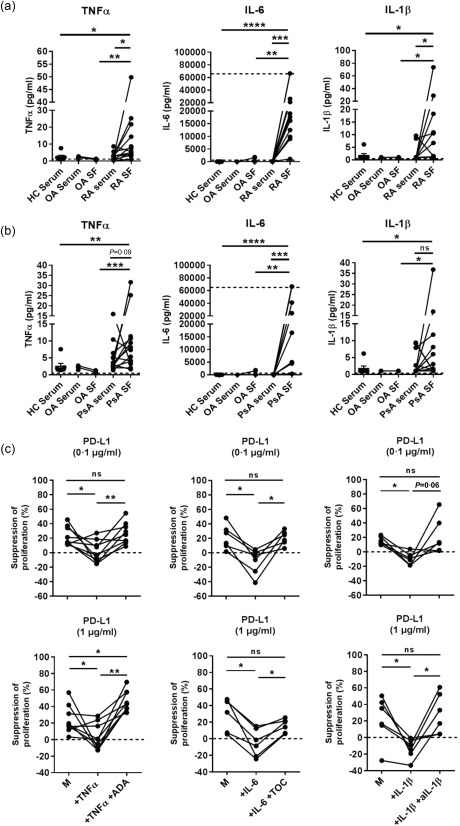
Tumour necrosis factor (TNF)α, interleukin (IL)‐6 and IL‐1β counteract the programmed cell death‐ligand 1 (PD‐L1)‐mediated suppression of healthy control (HC) CD4+ T cell proliferation. (a,b) Levels of proinflammatory cytokines TNFα, IL‐6 and IL‐1β in paired rheumatoid arthritis (RA) and psoriatic arthritis (PsA) serum/synovial fluid (SF) (n = 12), in osteoarthritis (OA) (disease control) serum/SF (n = 3–4) and in HC serum (n = 7). Wilcoxon's matched‐pairs signed‐rank test for RA/PsA serum versus RA/PsA SF and Mann–Whitney test for RA/PsA SF versus HC serum or OA SF. *P < 0·05, **P < 0·01 and ***P < 0·001. (c) Plates were coated with PD‐L1fc at the indicated concentrations, and PD‐L1‐mediated suppression of proliferation by CD4+ T cells from HC PB was assessed in absence (medium, M) or presence of 10 ng/ml of TNFα (n = 9), IL‐6 (n = 5) or IL‐1β (n = 6) ± anti‐TNFα (adalimumab; ADA), anti‐IL‐6R (tocilizumab; TOC) and anti‐IL‐1β (all at 1 µg/ml). Data in (c) were analysed by Wilcoxon matched‐pairs signed‐rank test. *P < 0·05 and **P < 0·01.
Next, we assessed whether the presence of TNFα, IL‐6 or IL‐1β had a functional impact on PD‐1‐mediated suppression of CD4+ T cell proliferation. HC CD4+ T cells were cultured with increasing concentrations of PD‐L1fc (0, 0·1 and 1 µg/ml) in the absence or presence of TNFα, IL‐6 or IL‐1β (10 ng/ml). To block the effect of the cytokines, the anti‐TNFα drug adalimumab, anti‐IL‐6R drug tocilizumab or anti‐IL‐1β mAb (1 µg/ml) were added at the beginning of the culture where indicated. Addition of each individual cytokine was able to abrogate the suppressive effects of PD‐1 ligation on CD4+ T cell proliferation at both 0·1 and 1 µg/ml PD‐L1 concentrations (Fig. 3c). In each sample tested, adalimumab, tocilizumab and anti‐IL‐1β mAb were able to reverse these cytokine‐mediated effects completely. Together, these data indicate that the RA‐ and PsA‐associated inflammatory cytokines TNFα, IL‐6 and IL‐1β can counteract the suppressive effects of PD‐1 ligation on CD4+ T cells, at least in vitro.
Soluble PD‐1 (sPD‐1) is induced in vitro by TNFα and IL‐6 in HC CD4+ T cell cultures and can be detected in serum and SF of both RA and PsA patients
Having demonstrated that TNFα, IL‐6 and IL‐1β can be detected in RA and PsA patients and that each of these cytokines abrogates PD‐L1fc activity in vitro, we sought to identify a possible underlying mechanism. ELISA analysis of HC CD4+ T cell supernatants from cytokine‐stimulated cultures revealed that both TNFα and IL‐6 were able to induce sPD‐1 compared to medium‐only conditions (Fig. 4a,b). No significant increase in sPD‐1 was observed in IL‐1β‐stimulated cultures (Fig. 4c). This increase in sPD‐1 was abrogated in the presence of adalimumab or tocilizumab. Quantitative PCR analysis of HC CD4+ T cells from the same TNFα‐ and IL‐6‐stimulated cultures revealed increases of the PD‐1Δex3 splice variant (Supporting information, Fig. S2). These results are in line with a previous study from another group reporting that TNFα promotes sPD‐1 expression 31 but also identify IL‐6 as an inducer of sPD‐1 and PD‐1Δex3.
Figure 4.
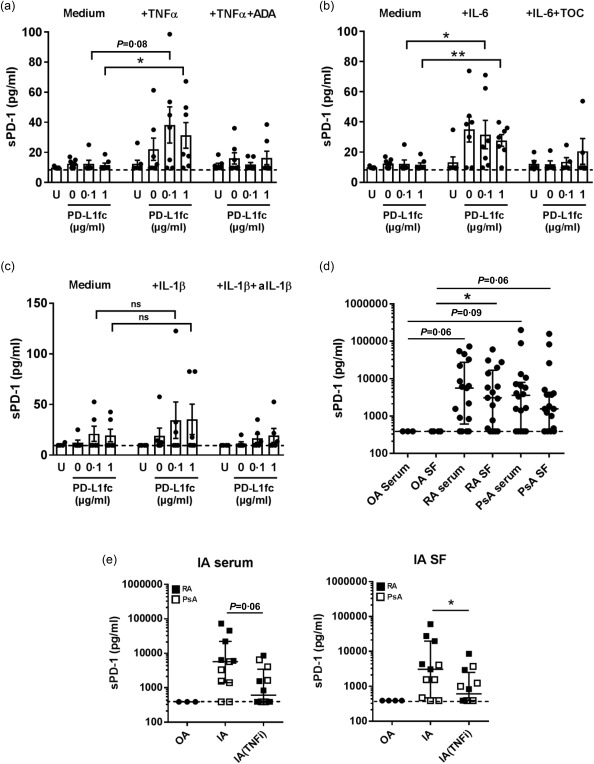
Soluble programmed cell death‐1 (sPD‐1) is induced in vitro by tumour necrosis factor (TNF)α, interleukin (IL)‐6 in HC CD4+ T cell cultures and can be detected in serum and synovial fluid (SF) of rheumatoid arthritis (RA) and psoriatic arthritis (PsA) patients. (a–c) sPD‐1 levels in supernatants of healthy control (HC) CD4+ T cells stimulated with (a) TNFα (10 ng/ml) or TNFα+ adalimumab (ADA; 1 µg/ml) (n = 7), (b) IL‐6 (10 ng/ml) or IL‐6+ tocilizumab (TOC; 1 µg/ml) (n = 5–‐7) and (c) IL‐1β (10 ng/ml) or IL‐1β+ anti‐IL‐1β (anti‐IL‐1β; 1 µg/ml) (n = 5). (d) sPD‐1 levels (median with interquartile range) in RA and PsA paired serum/synovial fluid (SF) and osteoarthritis (OA) serum/SF (OA, n = 3–4; RA, n = 17; PsA, n = 18). (e) sPD‐1 levels (median with interquartile range) in control disease OA (n = 3–4), RA (n = 5) and PsA (n = 6–7) serum and SF of patients treated with TNFi versus non‐TNFi therapy. Data in (a,b,c,e) were analysed by Mann–Whitney test while data in (d) were analysed by Kruskal–Wallis test with Dunn's multiple comparison test. *P < 0·05, **P < 0·01, ***P < 0·001.
In addition, ELISA analysis revealed that sPD‐1 was detectable in none of three OA serum and none of four OA SF samples, while it was detected at high levels in 13 of 17 RA and 13 of 18 PsA serum and SF samples (Fig. 4d). Additionally, cross‐sectional analysis of serum and SF from patients with RA or PsA undergoing TNF‐inhibitor (TNFi) therapy revealed lower levels of sPD‐1 when compared to patients not receiving TNFi therapy (Fig. 4e). These data show that proinflammatory cytokines such as TNFα and IL‐6 can modulate the amount of sPD‐1 in vitro and that TNFi therapy might modulate sPD‐1 levels in the serum and SF of patients with inflammatory arthritis.
sPD‐1 modulates PD‐1‐mediated suppression of HC CD4+ T cells and induces proliferation in CD4+ T cell/CD14+ monocyte co‐cultures
To investigate whether sPD‐1 is able to modulate PD‐1/PD‐L1 interactions, we first tested if sPD‐1fc itself promotes T cell proliferation of anti‐CD3 stimulated HC CD4+ T cells cultured in the absence of PD‐L1fc. In these experimental conditions, sPD‐1fc did not induce any increase in cell proliferation (Supporting information, Fig. S3), suggesting no direct effect on HC CD4+ T cells. We then cultured HC CD4+ T cells in the presence of increasing amounts of PD‐L1fc ligand in the absence or presence of sPD‐1fc chimera (0·5 or 1 µg/ml). In PD‐L1fc precoated plates, addition of sPD‐1fc was able to abrogate the activity of the ligand in a dose‐dependent fashion, resulting in less efficient suppression of T cell proliferation when compared to medium only (Fig. 5a,b). These data indicate that in a CD4+ T cell‐only culture system, sPD‐1 is able to modulate negatively an otherwise functional PD‐1/PD‐L1 interaction.
Figure 5.
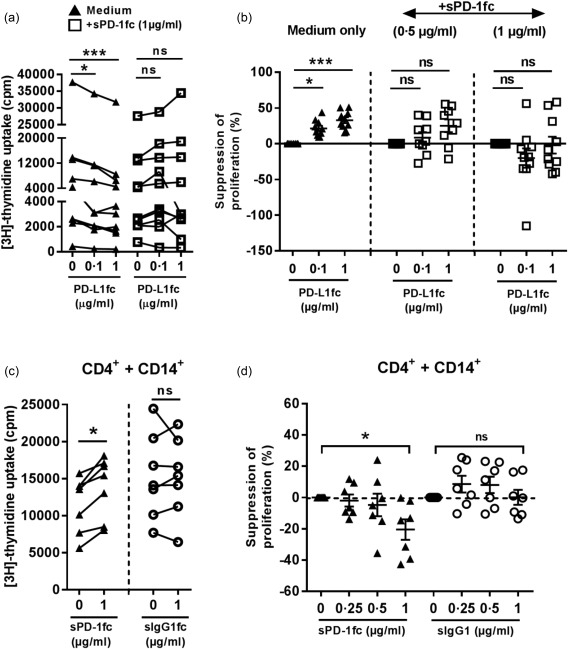
Soluble programmed cell death 1 (sPD‐1) modulates PD‐1‐mediated suppression of healthy control (HC) CD4+ T cells and induces proliferation in CD4+ T cell/CD14+ monocyte co‐cultures. (a) Proliferation and (b) suppression of proliferation of HC CD4+ T cells cultured in anti‐CD3 monoclonal antibody (mAb) (OKT3) and PD‐L1fc pre‐coated plates with or without soluble PD‐1fc (0.5 and 1 µg/ml) (n = 9–10). (c) Proliferation and (d) suppression of proliferation of HC CD4+ T cells cultured with autologous CD14+ monocytes at a 1 : 1 ratio (n = 7) in presence of soluble anti‐CD3 mAb (100 ng/ml) and soluble PD‐1fc/IgG1fc control (0, 0·25, 0·5 and 1 µg/ml). Data were analysed by Friedman's test with Dunn's multiple comparison test (a,b) and Wilcoxon's signed‐rank test (c,d). *P < 0·05 and ***P < 0·001.
To investigate further the ability of sPD‐1 to modulate the PD‐1/PD‐L1 interaction, we set up a co‐culture system using HC CD4+ T cells and autologous CD14+ cells as a source of natural PD‐L1. The ability of HC CD14+ cells to express PD‐L1 was tested by flow cytometry by culturing the cells overnight with 10 ng/ml of IFN‐γ, a known inducer of PD‐L1 27, 32 (Supporting information, Fig. S4). Freshly isolated HC CD4+ T cells and autologous monocytes were cultured at a 1 : 1 ratio with soluble anti‐CD3 mAb (100 ng/ml) in the absence or presence of increasing doses of sPD‐1 or IgG1fc control (0·25, 0·5 and 1 µg/ml). Addition of sPD‐1fc led to a significant dose‐dependent increase in T cell proliferation compared to control‐treated cells (Fig. 5c,d). These data indicate that soluble PD‐1 receptor can modulate PD‐1 ligation in both an artificial system (PD‐L1fc precoated plates) as well as in a more physiological context (in the presence of PD‐L1+ APC).
Discussion
The present study provides evidence for compromised PD‐1 mediated suppression in CD4+ T cells from patients with RA and PsA. Our study identifies the proinflammatory cytokines TNFα, IL‐6 and IL‐1β as negative modulators of PD‐1‐mediated T cell suppression in vitro and demonstrates that sPD‐1 is capable of interfering with effective PD‐1 ligation. To our knowledge, this is the first study to examine PD‐1 function in RA and PsA simultaneously, and to provide evidence that the inflammatory milieu of these two diseases has a role in modulating PD‐1 ligation.
We show that the frequencies of PD‐1+ cells within CD4+ and CD8+ T cell subsets are increased significantly in the synovial fluid from patients with RA or PsA when compared to peripheral blood, thus extending the findings from previous studies focusing only on RA 20, 26, 27, 33. The increase in the frequencies of PD‐1+ T cells suggests that in RA and PsA PD‐1 might have a role in regulating T cell effectors, especially at the site of inflammation. However, despite this increased frequency of PD‐1+ T cells, inflammation persists, suggesting that this pathway is impaired in these diseases.
Thus far, only limited data exist regarding PD‐1 function in inflammatory arthritis. One study using a PD‐L1fc chimera demonstrated that PD‐1 ligation inhibited cell proliferation and IFN‐γ production by CD4+ T cells from peripheral blood of RA patients, but that synovial fluid CD4+ T cells required higher concentrations of PD‐L1fc to achieve similar levels of inhibition 20. The authors speculated that the inflammatory milieu found in the RA joint might be accountable for reduced PD‐1‐mediated suppression, as they showed that addition of cell‐free SF to RA PB CD4+ T cells modulated PD‐1 ligation negatively 20. However, no specific mediator of the effect was identified. It has been shown previously that the common gamma chain cytokines IL‐2, IL‐7 and IL‐15, as well as CD28 co‐stimulation, can interfere with PD‐1 cross‐linking via pSTAT‐5 activation 9, 29. In our study, we show that in both RA and PsA, CD4+ T cells from the blood and synovial fluid are more resistant to PD‐1 ligation in terms of suppression of T cell proliferation and IFN‐γ production when compared to healthy cells. We show that the proinflammatory cytokines TNFα, IL‐6 and IL‐1β are found at increased levels in RA and PsA SF, and can reduce PD‐1‐mediated suppression of proliferation in CD4+ T cells from healthy donors. Inhibitors of these cytokines can counteract this effect. These findings provide further evidence that the presence of certain proinflammatory cytokines can be critical in determining the outcome of PD‐1 engagement during the immune response.
Mechanistically, our data demonstrate that TNFα and IL‐6, but not IL‐1β, induce the secretion of sPD‐1 by CD4+ T cells, and that sPD‐1 levels are increased significantly in the serum and SF of patients with RA or PsA compared to OA. The latter data support and extend two recent studies that showed that sPD‐1 can be detected in the serum and SF from patients with RA 27, 34. In these studies sPD‐1 serum levels correlated positively with the Disease Activity Score (DAS28), the presence of rheumatoid factor, and with levels of TNFα in the RA SF but not the serum 27, 34. Recent studies showed that expression of the PD‐1Δex3 variant is observed in T cells from patients with RA, but only minimally in T cells from patients with OA or from HC 27, 31. PD‐1Δex3 is a splice variant of PD‐1, which lacks the transmembrane domain and whose putative translational product is a soluble form of PD‐1 34, 35. It was shown that TNFα, IL‐17 and IFN‐γ can increase PD‐1Δex3 splice variant mRNA expression in healthy human CD4+ T cells 31. Our data indicate that in addition to those cytokines, sPD‐1 protein expression and PD‐1Δex3 splice variant mRNA expression can also be regulated by IL‐6. Furthermore, preliminary analysis from a cross‐sectional investigation of PsA and RA serum and SF suggests that patients treated with TNFi therapy have lower sPD‐1 levels compared to patients not treated with TNFi therapy. Further longitudinal studies on patients treated with adalimumab or tocilizumab are required, however, before conclusive statements can be made regarding the effect of biologics on sPD‐1 levels.
Our data demonstrate that the inflammatory cytokines TNFα and IL‐6 can lead to increased levels of PD‐1Δex3 splice variant as well as sPD‐1. It is possible that a certain amount of PD‐1 might also be released from the cell membrane via other mechanisms. The presence of high levels of certain metalloproteinases (MMPs) such as MMP‐9 and MMP‐13 has been described previously in inflammatory arthritis 36, 37, and it has been shown recently that expression of the PD‐1 ligands PD‐L1 and PD‐L2 in infant foreskin fibroblasts can be regulated through proteolytic cleavage by MMPs 38. Future studies may reveal whether such an MMP‐mediated proteolytic cleavage may also contribute to generation of soluble PD‐1.
Notably, we show, using a recombinant PD‐1 chimera, that sPD‐1 is functionally able to counteract PD‐L1fc‐mediated suppression of healthy CD4+ T cell proliferation, and to enhance CD4+ T cell proliferation when co‐cultured with CD14+ monocytes that can naturally express PD‐L1. Importantly, recent studies in autoimmune hepatitis 39 and cutaneous systemic sclerosis 40 support the notion that sPD‐1 might interfere with the PD‐1 pathway, thereby disrupting T cell regulation.
Collectively, our data indicate that CD4+ T cells from the PB and SF of patients with chronic RA or PsA are more resistant to PD‐1‐mediated regulation than CD4+ T cells from healthy individuals. We show that the proinflammatory cytokines TNFα, IL‐6 and IL‐1β, which are present at increased levels in the inflamed joints of RA and PsA patients, are capable of negatively modulating PD‐1 ligation in vitro. Finally, we show that TNFα and IL‐6 are capable of inducing sPD‐1 in HC CD4+ T cells and that sPD‐1 modulates T cell proliferation by interfering with PD‐1 ligation. Thus, our findings provide new evidence that the inflammatory environment of the RA and PsA joint compromises PD‐1/PD‐L1‐mediated T cell regulation.
Disclosure
The authors have declared no disclosures relating to this study.
Author contributions
D. B. conceived the study, performed most of the experiments and wrote the manuscript. C. H. performed some of the experiments. V. M. C. and L. S. T conceived and supervised the study and wrote the manuscript.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website.
Supplementary Table 1. Demographic and clinical parameters of the patients included in the study. Some samples were used for flow cytometry or functional assays only, whilst other samples were only used for cytokine detection in serum and SF. Clinical and demographic data are provided, where available. Abbreviations used: DAS28, disease activity score of 28 joints; DMARDs, disease‐modifying anti‐rheumatic drugs.
Supplementary Figure 1. PD‐1 ligation reduces IFN‐γ production by HC CD4+ T cells but not RA or PsA CD4+ T cells. CD4+ T cells from HC PBMC, RA and PsA PBMC and SFMC were cultured in plates pre‐coated with anti‐CD3 mAb (OKT3) and PD‐Llfc/IgGlfc. Supernatants were collected at day 5 and tested by ELISA for IFN‐γ production. (a) IFN‐γ production in HC CD4+ T cell cultures in presence of PD‐L1fc (0, 0·1 and 1 μg/m1 range; n=11 and 0, 0·1, 1, 2 and 5 μg/m1 range; n=4). (b) IFN‐γ production in RA and PsA CD4+ T cell cultures; IA PB (RA n=2; PsA n=3) and IA SF (RA n=2; PsA n=4). Data were analysed by Friedman Test with Dunn's Multiple Comparison test. *P < 0·05, **P < 0·01 and ***P < 0·001.
Supplementary Figure 2. Expression of PD‐1Δex3 transcript in activated HC CD4+ T cells in presence of TNFα and IL‐6. HC CD4+ T cells were cultured in absence (medium, M) or presence of 10 ng/ml of TNFα (n=4) or IL‐6 (n=3) +/‐ anti‐TNFα (adalimumab; ADA) or anti‐IL‐6R (tocilizumab; TOC) (all at 1 µg/ml) for 5 days. PD‐1Δex3 expression was examined by qPCR and normalised to β‐Actin housekeeping gene (mean ± SEM).
Supplementary Figure 3. Proliferation of HC CD4+ T cells in presence of increasing sPD‐lfc concentrations. HC CD4+ T cells (n=9) were cultured with immobilised anti‐CD3 mAb (OKT3) in presence of increasing concentrations of sPD‐lfc (0, 0·5 and 1 µg/ml). Proliferation was assessed at day 5 by [3H]‐thymidine incorporation and displayed as counts per minute (cpm). Data (mean ± SEM) were analysed by Friedman Test with Dunn's Multiple Comparison test. *P < 0·05, **P < 0·01 and ***P <0·001.
Supplementary Figure 4. PD‐L1 expression in CD14+ monocytes following IFN‐γ stimulation. CD14+ monocytes where positively isolated from HC PBMC and cultured overnight at 37°C in medium only or in medium supplemented with IFN‐γ (10 ng/ml). PD‐L1 expression was assessed after 12 hrs by flow cytometry. (a) Representative experiment. Shaded histograms represent the isotype control, open histograms indicate the expression profile for PD‐L1 with/out IFN‐γ stimulation. (b) Cumulative data (n=3).
Supplementary Figure 5. PD 1+ T cell frequencies are increased in synovial fluid compared to peripheral blood. Contour plot of CD3+CD4+PD‐1+ cells from paired PBMC and SFMC of one representative RA donor. FMO controls and isotype controls are shown for the CD3+CD4+populations.
Acknowledgements
This work was supported by Innovative Medicines Initiative grant BTCure (ref. 115142). The authors would like to thank Professor Bruce Kirkham and Dr Estee Chan from the Department of Rheumatology at Guy's & St Thomas' NHS Foundation Trust for their help in collecting patients' samples. The authors would also like to thank all patients and healthy volunteers who donated blood and synovial fluid for the project. The authors acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust.
References
- 1. Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD‐1 and their advantages for clinical application. Nat Immunol 2013; 14:1212–8. [DOI] [PubMed] [Google Scholar]
- 2. Nishimura H, Agata Y, Kawasaki A et al Developmentally regulated expression of the PD‐1 protein on the surface of double‐negative (CD4–CD8–) thymocytes. Int Immunol 1996; 8:773–80. [DOI] [PubMed] [Google Scholar]
- 3. Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP‐1 and SHP‐2 associate with immunoreceptor tyrosine‐based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 2004; 173:945–54. [DOI] [PubMed] [Google Scholar]
- 4. Barber DL, Wherry EJ, Masopust D et al Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439:682–7. [DOI] [PubMed] [Google Scholar]
- 5. Zhang X, Schwartz JC, Guo X et al Structural and functional analysis of the costimulatory receptor programmed death‐1. Immunity 2004; 20:337–47. [DOI] [PubMed] [Google Scholar]
- 6. Keir ME, Liang SC, Guleria I et al Tissue expression of PD‐L1 mediates peripheral T cell tolerance. J Exp Med 2006; 203:883–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parry RV, Chemnitz JM, Frauwirth KA et al CTLA‐4 and PD‐1 receptors inhibit T‐cell activation by distinct mechanisms. Mol Cell Biol 2005; 25:9543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kao C, Oestreich KJ, Paley MA et al Transcription factor T‐bet represses expression of the inhibitory receptor PD‐1 and sustains virus‐specific CD8+ T cell responses during chronic infection. Nat Immunol 2011; 12:663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Freeman GJ, Long AJ, Iwai Y et al Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192:1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Latchman Y, Wood CR, Chernova T et al PD‐L2 is a second ligand for PD‐1 and inhibits T cell activation. Nat Immunol 2001; 2:261–8.] [DOI] [PubMed] [Google Scholar]
- 11. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD‐1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tseng SY, Otsuji M, Gorski K et al B7‐DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med 2001; 193:839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zou W, Wolchok JD, Chen L. PD‐L1 (B7‐H1) and PD‐1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med 2016; 8:328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamanishi J, Mandai M, Matsumura N, Abiko K, Baba T, Konishi I. PD‐1/PD‐L1 blockade in cancer treatment: perspectives and issues. Int J Clin Oncol 2016; 21:462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus‐like autoimmune diseases by disruption of the PD‐1 gene encoding an ITIM motif‐carrying immunoreceptor. Immunity 1999; 11:141–51. [DOI] [PubMed] [Google Scholar]
- 16. Nishimura H, Okazaki T, Tanaka Y et al Autoimmune dilated cardiomyopathy in PD‐1 receptor‐deficient mice. Science 2001; 291:319–22. [DOI] [PubMed] [Google Scholar]
- 17. Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD‐Pdcd1–/– mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci USA 2005; 102:11823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okazaki T, Honjo T. The PD‐1‐PD‐L pathway in immunological tolerance. Trends Immunol 2006; 27:195–201. [DOI] [PubMed] [Google Scholar]
- 19. Okazaki T, Honjo T. PD‐1 and PD‐1 ligands: from discovery to clinical application. Int Immunol 2007; 19:813–24. [DOI] [PubMed] [Google Scholar]
- 20. Raptopoulou AP, Bertsias G, Makrygiannakis D et al The programmed death 1/programmed death ligand 1 inhibitory pathway is up‐regulated in rheumatoid synovium and regulates peripheral T cell responses in human and murine arthritis. Arthritis Rheum 2010; 62:1870–80. [DOI] [PubMed] [Google Scholar]
- 21. Kong EK, Prokunina‐Olsson L, Wong WH et al A new haplotype of PDCD1 is associated with rheumatoid arthritis in Hong Kong Chinese. Arthritis Rheum 2005; 52:1058–62. [DOI] [PubMed] [Google Scholar]
- 22. Lee SH, Lee YA, Woo DH et al Association of the programmed cell death 1 (PDCD1) gene polymorphism with ankylosing spondylitis in the Korean population. Arthritis Res Ther 2006; 8:R163.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bertsias GK, Nakou M, Choulaki C et al Genetic, immunologic, and immunohistochemical analysis of the programmed death 1/programmed death ligand 1 pathway in human systemic lupus erythematosus. Arthritis Rheum 2009; 60:207–18. [DOI] [PubMed] [Google Scholar]
- 24. Huang CH, Wong RH, Wei JC et al Effects of genetic polymorphisms of programmed cell death 1 and its ligands on the development of ankylosing spondylitis. Rheumatology (Oxf) 2011; 50:1809–13. [DOI] [PubMed] [Google Scholar]
- 25. James ES, Harney S, Wordsworth BP, Cookson WO, Davis SJ, Moffatt MF. PDCD1: a tissue‐specific susceptibility locus for inherited inflammatory disorders. Genes Immun 2005; 6:430–7. [DOI] [PubMed] [Google Scholar]
- 26. Hatachi S, Iwai Y, Kawano S et al CD4+ PD‐1+ T cells accumulate as unique anergic cells in rheumatoid arthritis synovial fluid. J Rheumatol 2003; 30:1410–9. [PubMed] [Google Scholar]
- 27. Wan B, Nie H, Liu A et al Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol 2006; 177:8844–50. [DOI] [PubMed] [Google Scholar]
- 28. Veale DJ, Fearon U. What makes psoriatic and rheumatoid arthritis so different? RMD Open 2015; 1:e000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bennett F, Luxenberg D, Ling V et al Program death‐1 engagement upon TCR activation has distinct effects on costimulation and cytokine‐driven proliferation: attenuation of ICOS, IL‐4, and IL‐21, but not CD28, IL‐7, and IL‐15 responses. J Immunol 2003; 170:711–8. [DOI] [PubMed] [Google Scholar]
- 30. Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death‐1 ligand 1 interacts specifically with the B7‐1 costimulatory molecule to inhibit T cell responses. Immunity 2007; 27:111–22.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu C, Jiang J, Gao L et al Soluble PD‐1 aggravates progression of collagen‐induced arthritis through Th1 and Th17 pathways. Arthritis Res Ther 2015; 17:340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dong H, Zhu G, Tamada K, Chen L. B7‐H1, a third member of the B7 family, co‐stimulates T‐cell proliferation and interleukin‐10 secretion. Nat Med 1999; 5:1365–9. [DOI] [PubMed] [Google Scholar]
- 33. Cho BA, Sim JH, Park JA et al Characterization of effector memory CD8+ T cells in the synovial fluid of rheumatoid arthritis. J Clin Immunol 2012; 32:709–20. [DOI] [PubMed] [Google Scholar]
- 34. Greisen SR, Rasmussen TK, Stengaard‐Pedersen K et al Increased soluble programmed death‐1 (sPD‐1) is associated with disease activity and radiographic progression in early rheumatoid arthritis. Scand J Rheumatol 2014; 43:101–8. [DOI] [PubMed] [Google Scholar]
- 35. Nielsen C, Ohm‐Laursen L, Barington T, Husby S, Lillevang ST. Alternative splice variants of the human PD‐1 gene. Cell Immunol 2005; 235:109–16. [DOI] [PubMed] [Google Scholar]
- 36. Lindy O, Konttinen YT, Sorsa T et al Matrix metalloproteinase 13 (collagenase 3) in human rheumatoid synovium. Arthritis Rheum 1997; 40:1391–9. [DOI] [PubMed] [Google Scholar]
- 37. Gruber BL, Sorbi D, French DL et al Markedly elevated serum MMP‐9 (gelatinase B) levels in rheumatoid arthritis: a potentially useful laboratory marker. Clin Immunol Immunopathol 1996; 78:161–71. [DOI] [PubMed] [Google Scholar]
- 38. Dezutter‐Dambuyant C, Durand I, Alberti L et al A novel regulation of PD‐1 ligands on mesenchymal stromal cells through MMP‐mediated proteolytic cleavage. Oncoimmunology 2016; 5:e1091146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aarslev K, Dige A, Greisen SR et al Soluble programmed death‐1 levels are associated with disease activity and treatment response in patients with autoimmune hepatitis. Scand J Gastroenterol 2016; 52 :93–9. [DOI] [PubMed] [Google Scholar]
- 40. Yanaba K, Hayashi M, Yoshihara Y, Nakagawa H. Serum levels of soluble programmed death‐1 and programmed death ligand‐1 in systemic sclerosis: association with extent of skin sclerosis. J Dermatol 2016; 43:954–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website.
Supplementary Table 1. Demographic and clinical parameters of the patients included in the study. Some samples were used for flow cytometry or functional assays only, whilst other samples were only used for cytokine detection in serum and SF. Clinical and demographic data are provided, where available. Abbreviations used: DAS28, disease activity score of 28 joints; DMARDs, disease‐modifying anti‐rheumatic drugs.
Supplementary Figure 1. PD‐1 ligation reduces IFN‐γ production by HC CD4+ T cells but not RA or PsA CD4+ T cells. CD4+ T cells from HC PBMC, RA and PsA PBMC and SFMC were cultured in plates pre‐coated with anti‐CD3 mAb (OKT3) and PD‐Llfc/IgGlfc. Supernatants were collected at day 5 and tested by ELISA for IFN‐γ production. (a) IFN‐γ production in HC CD4+ T cell cultures in presence of PD‐L1fc (0, 0·1 and 1 μg/m1 range; n=11 and 0, 0·1, 1, 2 and 5 μg/m1 range; n=4). (b) IFN‐γ production in RA and PsA CD4+ T cell cultures; IA PB (RA n=2; PsA n=3) and IA SF (RA n=2; PsA n=4). Data were analysed by Friedman Test with Dunn's Multiple Comparison test. *P < 0·05, **P < 0·01 and ***P < 0·001.
Supplementary Figure 2. Expression of PD‐1Δex3 transcript in activated HC CD4+ T cells in presence of TNFα and IL‐6. HC CD4+ T cells were cultured in absence (medium, M) or presence of 10 ng/ml of TNFα (n=4) or IL‐6 (n=3) +/‐ anti‐TNFα (adalimumab; ADA) or anti‐IL‐6R (tocilizumab; TOC) (all at 1 µg/ml) for 5 days. PD‐1Δex3 expression was examined by qPCR and normalised to β‐Actin housekeeping gene (mean ± SEM).
Supplementary Figure 3. Proliferation of HC CD4+ T cells in presence of increasing sPD‐lfc concentrations. HC CD4+ T cells (n=9) were cultured with immobilised anti‐CD3 mAb (OKT3) in presence of increasing concentrations of sPD‐lfc (0, 0·5 and 1 µg/ml). Proliferation was assessed at day 5 by [3H]‐thymidine incorporation and displayed as counts per minute (cpm). Data (mean ± SEM) were analysed by Friedman Test with Dunn's Multiple Comparison test. *P < 0·05, **P < 0·01 and ***P <0·001.
Supplementary Figure 4. PD‐L1 expression in CD14+ monocytes following IFN‐γ stimulation. CD14+ monocytes where positively isolated from HC PBMC and cultured overnight at 37°C in medium only or in medium supplemented with IFN‐γ (10 ng/ml). PD‐L1 expression was assessed after 12 hrs by flow cytometry. (a) Representative experiment. Shaded histograms represent the isotype control, open histograms indicate the expression profile for PD‐L1 with/out IFN‐γ stimulation. (b) Cumulative data (n=3).
Supplementary Figure 5. PD 1+ T cell frequencies are increased in synovial fluid compared to peripheral blood. Contour plot of CD3+CD4+PD‐1+ cells from paired PBMC and SFMC of one representative RA donor. FMO controls and isotype controls are shown for the CD3+CD4+populations.


