Summary
Chronic infection with norovirus is emerging as a significant risk for patients with immunodeficiency – either primary or secondary to therapeutic immunosuppression. Patients with primary immunodeficiency present a range of pathological responses to norovirus infection. Asymptomatic infections occur and differentiating viral carriage or prolonged viral shedding after self‐limiting infection from infection causing protracted diarrhoea can be challenging, due to relatively mild pathological changes that may mimic other causes of diarrhoea in such patients (for instance pathogenic bacteria or parasites or graft‐versus‐host disease). However, a subset of patients with common variable immunodeficiency (CVID) experience a severe norovirus‐associated enteropathy leading to intestinal villous atrophy and malabsorption. Symptomatic infection of up to 8 years has been demonstrated with clinical and histological recovery on viral clearance. Although oral immunoglobulins and nitazoxanide have been used to treat noroviral infections associated with immunosuppression, ribavirin is the only agent to date that has been linked to viral clearance in the Noroviral enteropathy associated with CVID.
Keywords: enteropathy, immunodeficiency, norovirus, ribavirin
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Clinical challenges in the management of patients with B cell immunodeficiencies. Clinical and Experimental Immunology 2017, 188: 323–5.
The role of genomics in common variable immunodeficiency disorders. Clinical and Experimental Immunology 2017, 188: 326–32.
When to initiate immunoglobulin replacement therapy (IGRT) in antibody deficiency: a practical approach. Clinical and Experimental Immunology 2017, 188: 333–41.
Progressive multi‐focal leucoencephalopathy – driven from rarity to clinical mainstream by iatrogenic immunodeficiency. Clinical and Experimental Immunology 2017, 188: 342–52.
Considerations for dosing immunoglobulin in obese patients. Clinical and Experimental Immunology 2017, 188: 353–62.
Norovirus
Norovirus is an unenveloped, positive stranded RNA virus, a member of the Caliciviridae 1. It was first identified in stool isolates from an outbreak of ‘winter vomiting disease’ in Norwalk, Ohio in 1968 2, where the majority of patients experienced nausea and vomiting and approximately a third also had profuse diarrhoea 3. Norovirus is thought to be responsible for approximately 21 million cases of acute gastroenteritis in the United States every year, constituting approximately 60% of all cases with a known cause 4. High viral loads in stool and vomit combined with a low infective dose 5 and short incubation period result in a high attack rate, averaging 50% (9–78%) in close environments such as health‐care facilities or cruise ships 6. Food (particularly fruit, oysters and other food eaten raw) and water‐borne outbreaks are reported 7, 8.
The viral genome encodes two capsid proteins and six non‐structural proteins organized in three open reading frames (ORF 1–3) 9. There is enormous diversity within and between strains, with six broad genotypes identified (G I–VI) based on the amino acid sequence of the VP1 capsid protein (which differs as much as 38% between GI and GII isolates) 10, 11. Of these, G II (predominantly G II.4) is responsible for the majority of human infections, followed by G I and G IV 12. Specific strains affect animals (cows, pigs, dogs, rodents) and antibodies to these strains can be detected in asymptomatic humans, raising the possibility of norovirus being at least partially zoonotic 13.
Our understanding of norovirus biology is limited by the lack of a suitable ex‐vivo culture for the human virus, and is dependent upon animal models 14. The attachment receptor for virus entry into the cell is possibly a carbohydrate moiety in view of the protection afforded by a common null mutation of FUT2 (encoding fucosyl transferase) 15, 16, although this is strain‐specific, with some strains capable of infecting the resultant ‘non‐secretor’ phenotype 17, 18. While transcytosis may occur through enterocytes, epitheliotrophism is difficult to demonstrate, and current evidence favours intestinal immune cells of the lamina propria as the cellular target in which viruses replicate – including dendritic cells, macrophages and Peyer's patch B cells 19, 20, 21, 22. In murine infection, norovirus can be detected along the length of the gastrointestinal tract from stomach to colon 14 and dissemination to other organs such as spleen and liver may occur, possibly as a result of viraemia or via dendritic cells 23, 24, 25.
Norovirus infection in the immunocompetent
In most individuals norovirus infection results in a short‐lived intense gastroenteritis, with two‐thirds of patients experiencing nausea and vomiting, and approximately three‐quarters have profuse diarrhoea 26. Abdominal pain, fever and leucocytosis are reported, but unusual 27. Following an average 48‐h incubation period 28, symptoms last for 2–3 days but can be more prolonged and serious in elderly or very young people. One study reported a 30‐day mortality rate of 7% in patients of a median age of 77 years 29. Central nervous system involvement has been reported, and infants appear to be particularly at risk of (afebrile) seizures associated with norovirus gastroenteritis 30. Norovirus continues to be shed for prolonged periods following resolution of symptoms – a recent study revealed 47% of individuals excreting virus for 21 days or more 31.
Similarly, asymptomatic infection is common, with high proportions of stool samples from unaffected individuals revealing the presence of norovirus 32.
Despite the severity of the symptoms, pathological changes are sparse. One human study has demonstrated epithelial tight junction dysfunction and increased permeability along with stimulated anion flux. There were minimal changes in villous architecture but a doubling of enterocyte apoptosis, related to an increase of cytotoxic intraepithelial lymphocytes 33.
Postinfective functional gastrointestinal symptoms have been described 34.
Immune responses to norovirus
Experimental rechallenge of volunteers has demonstrated that short‐lived strain‐specific immunity occurs (waning between 6 months and 2 years) 35 and mathematical modelling based on infection rates has suggested postinfective immunity to last for 4·1–8·7 years 36. Humoral immunity is key as viral protection coincides with the detection of immunoglobulin (Ig)A antibodies in saliva, and passive immunity is transferred to infants by maternal antibody in breast milk 37. Furthermore, humoral immunity drives selection with emerging strains developing mutations induced at codons that lead to amino acid changes at known antibody binding epitopes in capsid proteins 38.
The extent to which murine experimental studies can be extrapolated to humans (and indeed even between different human‐infecting genotypes) is unclear 14. Mice deficient in type I interferon receptors or downstream signalling pathways [signal transducer and activator of transcription 1 (STAT‐1)] succumb to a multi‐system infection with severe diarrhoea, gastric bloating and extra‐intestinal pathology 39. Recombination activating gene (RAG) knock‐out mice are unable to clear the virus, but the infective load is reduced substantially following transfer of immune serum, B cells, CD4+ T cells or CD8+ T cells 40, which suggests that both antibody and cytotoxic T cell responses play a part in viral clearance.
Norovirus in immunosuppressed individuals
Immunosuppression changes the course of norovirus infection significantly, leading to prolonged symptoms and viral shedding 41, 42. An initial report of two renal transplant recipients in 2009 demonstrated persistent norovirus excretion 43. One case was asymptomatic, but shed virus for more than 7 months before spontaneous clearance, and the other showed features of severe enteritis with diarrhoea, fever and weight loss and required a reduction of immunosuppression to clear the infection. A subsequent report from Paris found norovirus (or sapovirus, a related calicivirus) infection to be responsible for 80% of cases of otherwise unexplained diarrhoea in renal transplant recipients, with significant consequences of graft failure (in 81%) or rejection following reduction of immunosuppression (in five patients). Norovirus excretion was detected in stool for up to 581 days 44.
A series of nine renal transplant recipients reported from Zurich with chronic symptomatic noroviral infection reported chronic intermittent diarrhoea 45. Molecular analysis of viral isolates showed the majority to be separate strains of GII.4, indicating sporadic infection rather than nosocomial transmission from a common source. Significant intrahost evolution occurred, with one virus mutating at least 25 capsid protein amino acid residues during an 898‐day period of viral shedding.
Chronic noroviral infection has also been reported in recipients of other solid organs, including heart 46, lung 47, pancreas 48 and intestine 49, and also after bone marrow transplantation 50, 51, 52, 53, where it presents a particular challenge as it may be misdiagnosed as intestinal graft‐versus‐host disease (GVHD) and lead to intensification of immunosuppression rather than reduction. Histological features of the two conditions overlap and there is no certainty that ongoing viral excretion represents symptomatic infection; furthermore, noroviral infection may accompany GVHD as a result of treatment with increased immunosuppression.
A report from London included 12 patients with chronic noroviral infection following allogeneic haemopoeitic stem cell transplantation (HSCT) in whom diarrhoea persisted for a median of 3 months, and six patients required artificial nutrition support 50. Two patients died, one of whom was related directly to malnutrition associated with the infection. Similarly, two of 10 patients with noroviral enteritis died following HSCT in a Japanese centre, with a duration of symptoms up to 135 days (median 41). Infection was found to be more common after a second graft 51.
In Hong Kong, eight of 55 children undergoing HSCT developed chronic noroviral infection with time to clear the virus of up to 263 days (median 145 days) 52. Six children required parenteral nutrition support and three died. All six patients who engrafted successfully with allogeneic cells developed acute GVHD, although not statistically significant, but there was an association with chronic GVHD in these patients. The intensity of the conditioning regimen (with fludarabine or almetuzumab in particular) and the use of peripheral or cord blood stem cells (that require heavier immunosuppression than bone marrow‐derived cells) were risk factors for development of noroviral infection. The recovery of donor T cells in peripheral blood was strikingly associated with clearance of infection in 13 children from Manchester, even when delayed to 13 months after transplant 53. Artificial nutritional support was required in all patients, with two patients requiring parenteral nutrition for almost a year, stopped on symptomatic recovery only after viral clearance.
Reports of severe complications associated with chronic noroviral infection after transplant, including agranulocytosis 54 and haemophagocytic lymphohistiocytosis 55, are difficult to link directly to the infection itself in view of the clinical context, but may hint at the development of extra‐intestinal pathology in heavily immunosuppressed individuals.
Despite the relatively small number of case reports and series, the overall extent of the problem posed by chronic norovirus infection is demonstrated by a report from Texas of 22% of all solid organ and bone marrow transplant recipients developing the infection, more than 50% of whom required hospitalization, and in this institution it was the most common enteropathogen 56. It is now apparent that chronic noroviral infection poses a substantial risk to transplant recipients, either from sporadic or nosocomial infection.
Common variable immunodeficiency (CVID) enteropathy
CVID is the most common identified symptomatic primary antibody deficiency in Europeans, affecting approximately 1 : 25 000. The immunopathology is variable, and probably relates to defects in a variety of different pathways that lead to terminal B cell differentiation 57, 58. A description of the different attempts to classify CVID subtypes is beyond the scope of this paper; however, a subtype associated with T cell defects that occurs in approximately 5% of cases appears to present with granulomatous disease, splenomegaly, gastrointestinal involvement and lymphoma and carries a worse prognosis 59. A number of single gene defects have been identified in CVID but, together, only amount to approximately 2–10% of cases 60. A subtype of CVID is associated strongly with autoimmune conditions either through a shared genetic predisposition that includes human leucocyte antigen (HLA) or mechanistically due to associated immune dysregulation 61.
Gastrointestinal disturbances are common in CVID, with diarrhoea being the most common symptom reported 62, 63, 64, 65, 66, 67, 68. Common causes include enteropathogens such as Giardia, Salmonella, Campylobacter and Cryptosporidium, with a variety of case reports of other common or unusual organisms 63, 66, 67, 69, 70, 71. However, a range of inflammatory gastrointestinal pathologies are described 63, 72. In rare cases, histological appearances and clinical progression can mimic ulcerative colitis or Crohn's disease and respond similarly to immunosuppressive medication. However, a clearly defined entity of malabsorption and villous atrophy of unknown cause (including non‐responsiveness to gluten withdrawal) known as ‘CVID enteropathy’ appears to be peculiar to this condition 62.
CVID enteropathy occurs in approximately 5% of patients with CVID, and appears to be associated with the subtype of patients found to have defective T cell function 65. The clinical presentation resembles that of coeliac disease, with diarrhoea, malabsorption and villous atrophy (Fig. 1) on mucosal biopsy of the proximal duodenum 65, 66. Symptoms may be profound, with fat malabsorption sufficient to cause clinical steatorrhoea, protein‐losing enteropathy and malnutrition sufficient to require parenteral nutrition support. Nausea and vomiting are associated frequently and the nausea can be persistent and troublesome. Histological features are described as resembling the appearance of acute intestinal GVHD, with mild to severe villous atrophy. Enterocytes become flattened and vacuolated and there is increased epithelial apoptosis. Chronic inflammatory infiltrates in the lamina propria lack plasma cells and may include neutrophils that may also infiltrate the epithelium (Fig. 2a). The only features that distinguish CVID enteropathy clearly from coeliac disease are the presence of lymphoid follicular hyperplasia and the lack of plasma cells; however, these are underlying features of the CVID mucosa itself, and they do not permit discrimination of CVID enteropathy from coeliac disease co‐existent with CVID 65. A significant degree of intestinal mucosal enhancement and wall thickening may be seen on cross‐sectional imaging with computerized tomography (CT) or magnetic resonance imaging (MRI) demonstrating a pan‐enteritis (Fig. 3). Diagnosis of CVID enteropathy requires an exhaustive search for known pathogens and exclusion of coeliac disease. This can be challenging (as serological tests are unhelpful), and depends upon demonstration of symptomatic and histological response to gluten withdrawal in patients with the susceptibility HLA haplotypes (HLA‐DQ2 or 8). Histological recovery to gluten withdrawal in coeliac disease may take a year or more 73, and there is a learning curve for patients to be able to exclude effectively all gluten from the diet, therefore characterization of the response to gluten withdrawal may be prolonged. While GVHD and coeliac disease remain the most difficult to differentiate histologically from CVID enteropathy, other pathogens may cause a milder form of enteropathy in CVID. Giardia may lead to villous blunting but without severe inflammation, although excess eosinophils may be present in the lamina propria, and the parasites are clearly detectable on routine histopathological specimens and in stool 74. Bacterial infections such as Campylobacter are more likely to lead to an ulcerative distal enterocolitis and the clinicopathological features of co‐existent inflammatory bowel disease are generally sufficiently distinct to distinguish from CVID enteropathy. A recent review of viruses associated with primary immunodeficiencies identified a number that are shed over prolonged time‐periods and may have been associated with exacerbation of underlying inflammatory enteropathy 75, and stool samples should be examined to exclude agents such as enteroviruses, rotavirus, astrovirus and adenovirus.
Figure 1.
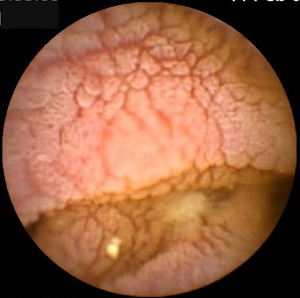
Image from small intestine taken by video capsule enteroscopy in a patient with longstanding chronic norovirus infection with common variable immunodeficiency (CVID). Note the oedematous thickened folds with fissuring and scalloping and lack of surface villi.
Figure 2.
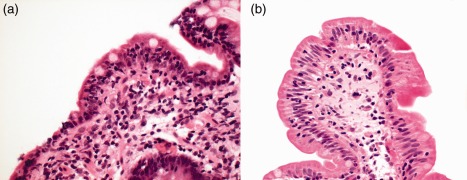
(a) Close‐up of duodenal biopsy in patient with common variable immunodeficiency and chronic norovirus infection [haematoxylin and eosin (H&E) stain, ×40]. Norovirus RNA was detected by polymerase chain reaction (PCR) in stool and also from the duodenal biopsy specimen. Marked villous atrophy is apparent with a chronic inflammatory infiltrate in the lamina propria (lacking plasma cells in view of the immunodeficiency). The enterocytes are reduced in height and vacuolated. (b) Close‐up of duodenal biopsy in the same patient following norovirus clearance with a prolonged course of ribavirin (H&E stain, ×40). Note that the inflammatory infiltrate in the lamina propria has resolved, there is restitution of villous architecture and the enterocytes are columnar.
Figure 3.
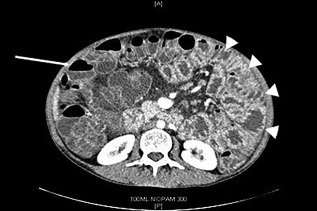
Abdominal cross‐sectional contrast‐enhanced computerized tomography (CT image) from a patient with longstanding chronic norovirus infection with common variable immunodeficiency (CVID). Small bowel loops appear thickened with hyperenhancement of the mucosa (arrowheads), representing a pan‐enteritis of the small intestine in contrast to the normal appearance of the colon (arrow).
The cause of CVID enteropathy is currently unknown, but in the setting of CVID, infective or autoimmune pathologies have been considered. The diagnosis requires exclusion of known enteric pathogens and treatment to date has largely comprised immunosuppressive therapies, including steroids, thiopurines, cyclosporin A and anti‐tumour necrosis factor (TNF)‐α monoclonal antibodies 65, 76. Partial symptom responses (with improvement in nausea more than diarrhoea) have been demonstrated by such an approach but without resolution of intestinal inflammation or villous atrophy, and patients usually remain dependent upon nutritional support.
Norovirus infection and CVID enteropathy
As many of the pathological features of CVID enteropathy are in keeping with an infective origin, and following the coincidental finding of prolonged norovirus excretion in one of our patients, we investigated the possibility of chronic norovirus as a cause of CVID enteropathy in our patients 77. A previous review of chronic and persistent viral infection in immunodeficiency noted the possibility that enteropathy might be triggered by a viral infection, such as is known to be the case with childhood rotavirus in infection and coeliac disease, but were unable to identify any reports of a viral association with CVID enteropathy 75.
All eight of our patients with CVID enteropathy demonstrated persistent norovirus excretion for periods of up to 1200 days, and none of the asymptomatic controls that were tested had norovirus present in stool. No other known enteric pathogens or enteroviruses were present in the patients with enteropathy. Sequencing of the isolates demonstrated separate strains of GII.4 in all cases. Three patients had a compatible haplotype for coeliac disease, but two made no symptomatic response and the other had only a short‐lived benefit from gluten withdrawal. Five patients required intravenous nutritional support. Reverse transcription–polymerase chain reaction (RT–PCR) of archived paraffin‐embedded intestinal biopsies demonstrated the presence of norovirus RNA sequences in the duodenum in seven of eight cases. Norovirus was detected in all intestinal biopsies in two patients during the entire 5‐ or 8‐year course, respectively, of their symptomatic presentation with CVID enteropathy. Furthermore, sequence analysis demonstrated that in one case the same virus had been present throughout the course of the infection, rather than the occurrence of intermittent reinfection with different strains. Clearance of norovirus (in two cases following prolonged treatment with oral ribavirin and in one case spontaneously) was accompanied by rapid resolution of symptoms, resolution of intestinal inflammation and restitution of duodenal villous morphology (Fig. 2a,b). In addition, serum immunoglobulin levels were maintained with normal therapeutic dosage, whereas previously high‐dose replacement was required, due presumably to enteric loss. Both patients receiving parenteral nutritional support were able to recommence full oral nutrition.
In our series, the evidence is strongly suggestive of chronic noroviral infection being the predominant cause of CVID enteropathy, and since publication of our report we have become aware of a large number of cases of CVID enteropathy associated with noroviral infection from different centres worldwide. However, other enteroviruses, such as parechovirus, have been detected in this setting 78 and it remains to be seen whether norovirus is just one of several viruses that can result in this entity. The clinical introduction of new technologies such as chip‐based rapid PCR for multiple organisms may shed new light upon the associated viral infections with CVID enteropathy in the near future.
The spectrum of norovirus infection in immunodeficiency
A survey from Paris 79 of 62 children (median age 3·5 years) suffering from a range of inherited primary immunodeficiency conditions found norovirus excretion in stools in 24 patients (38·7%). Thirteen of these were asymptomatic; however, symptomatic presentation was twice as common in those with norovirus infection as those without. Norovirus excretion was three times as common in symptomatic patients as those without symptoms. Viraemia was identified in two children. Of 10 patients with undefined ‘enteropathy’ in this study, five had no virus identified and three had norovirus, although the histological features described in two patients were mild. CVID is not diagnosed in children below the age of 4 years, and the findings of this study cannot be extrapolated to adults, where CVID is the most common form of primary immunodeficiency.
There is clearly a wide spectrum of responses to norovirus that include acute self‐limiting infection, asymptomatic carriage, prolonged or persistent shedding following acute infection, a severe sprue‐like enteropathy and even viraemia, leading to the possibility of extra‐intestinal organ involvement. Given that the majority of infections are caused by the same genotype – II.4 – it is unlikely that the range of responses relates to separate viral strains of differing virulence, and therefore most probably reflects altered host immune responses. Immunoglobulin replacement in patients with antibody deficiency appears insufficient to clear norovirus, which suggests that secretory IgA is required. To date, prolonged viral shedding has not been described in isolated IgA deficiency, which may relate to residual secretory IgA or compensation with IgM at mucosal surfaces. Prolonged asymptomatic viral shedding in immunodeficient patients may act potentially as a reservoir of strains for longer than specific herd immunity persists and has implications for recurrent community outbreaks 14.
The enteropathy associated with CVID – and with norovirus infection in all patients in our cohort – appears not to occur in other forms of primary immunodeficiency, where enteritis is rare and phenotypically distinct 80. Notably, the severity of the symptomatic and histopathological features of norovirus enteropathy in CVID (resulting in subtotal villous atrophy and malabsorption) is far greater than is seen in patients with acute infection or chronic infection associated with immunosuppression. This could be due simply to a greater burden of viral infection; however, patients with even more profound combined immunodeficiency do not demonstrate such features and it has been difficult to demonstrate viral cytopathic effects in enterocytes. It is more likely that the unique noroviral enteropathy associated with CVID results from a dysregulated host response, which may account for the similarity of the lesion to that seen in coeliac disease. This possibility is supported by the host response to Helicobacter pylori in patients with CVID, where infection appears to lead to more severe gastritis, atrophy and a higher risk of gastric adenocarcinoma than in other forms of immunodeficiency or in immunocompetent individuals 81. Recent evidence from the CVID transcriptome would also suggest the possibility of higher baseline inflammatory responses 82.
Treatment of norovirus infection in immunodeficiency
It is only with the recent identification of chronic noroviral infection and asymptomatic carriage that efforts have been directed towards treatment. There are no anti‐virals effective against norovirus that are approved currently for human use, and experience is limited to case reports.
In view of the apparent requirement for luminal antibody for viral clearance, oral immunoglobulin administration has been employed in cases of persistent norovirus excretion in immunodeficient patients post‐transplant. Two days of administration appeared to clear the virus in 11 of 12 patients with norovirus gastroenteritis following lung transplantation 83, and was successful in a subsequent case report 84, but failed to clear the virus in one patient treated with alemtuzumab for chronic lymphocytic leukaemia 85. There are no studies or reports of the successful use of oral immunoglobulin in norovirus infection associated with CVID.
Nitazoxanide was commenced in one patient with a 14‐day history of symptomatic norovirus infection following haemopoeitic stem cell transplantation, with symptomatic improvement within 24 h and complete resolution within 4 days 86.
On the basis of in‐vitro studies 87, we evaluated ribavirin in our patients with CVID enteropathy and chronic norovirus infection 77. Viral clearance occurred in two patients, with a treatment duration of up to 6 months. Notably, therapeutic drug level monitoring was required in view of reduced mucosal absorption. Three further patients failed to achieve viral clearance, despite the use of additional pegylated interferon‐α in two. There were no clear differences that could discriminate responders from non‐responders, and we hypothesize that a slow attainment of adequate plasma levels may lead to anti‐viral resistance. Therefore, the efficacy of ribavirin with or without interferon in this setting requires further investigation; however, the hope is that newer, less toxic, approaches will be forthcoming as a result of new advances in targeted molecular therapeutics and a greater knowledge of norovirus biology from murine infections 88. Until such time as anti‐noroviral therapies are developed successfully, understanding of the risk posed by norovirus to patients with immunodeficiency (particularly in the setting of a subset of CVID) should lead to heightened awareness and precautions to prevent infection and secondary transmission.
Disclosure
The authors report no disclosures.
References
- 1. Robilotti E, Deresinski S, Pinsky BA. Norovirus. Clin Microbiol Rev 2015; 28:134–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kapikiam AZ, Wyatt RG, Dolin R et al Visualization by immune electron microscopy of a 27‐nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol 1972; 10:1075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adler JL, Zickl R. Winter vomiting disease. J Infect Disease 1969; 119:668–73. [DOI] [PubMed] [Google Scholar]
- 4. Patel MM, Hall AJ, Vinje J, Parashar UD. Noroviruses: a comprehensive review. J Clin Virol 2009; 44:1–8. [DOI] [PubMed] [Google Scholar]
- 5. Teunis PF, Moe CL, Liu P et al Norwalk virus: how infectious is it? J Med Virol 2008; 80:1468–76. [DOI] [PubMed] [Google Scholar]
- 6. Harris JP, Lopman BA, O'Brien SJ. Infection control measures for Norovirus: a systematic review of outbreaks in semi‐enclosed settings. J Hosp Infect 2010; 74:1–9. [DOI] [PubMed] [Google Scholar]
- 7. Kukkula M, Maunula L, Silvennoinen E, von Bonsdorff CH. Outbreak of viral gastroenteritis due to drinking water contaminated by Norwalk‐like viruses. J Infect Dis 1999; 180:1771–6. [DOI] [PubMed] [Google Scholar]
- 8. Alfano‐Sobsey E, Sweat D, Hall A et al Norovirus outbreak associated with undercooked oysters and secondary household transmission. Epidemiol Infect 2012; 140:276–82. [DOI] [PubMed] [Google Scholar]
- 9. Lambden PR, Caul EO, Ashley CR, Clarke IN. Sequence and genome organisation of a human small round structured (Norwalk‐like) virus. Science 1993; 259:516–9. [DOI] [PubMed] [Google Scholar]
- 10. Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. Norovirus classification and proposed strain nomenclature. Virology 2006; 346:312–23. [DOI] [PubMed] [Google Scholar]
- 11. Kroneman A, Vega E, Vennema H et al Proposal for a unified norovirus nomenclature and genotyping. Arch Virol 2013; 158:2059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Noel JS, Fankhauser RL, Ando T, Monroe SS, Glass RI. Identification of a distinct common strain of ‘Norwalk‐like viruses’ having a global distribution. J Infect Dis 1999; 179:1334–44.] [DOI] [PubMed] [Google Scholar]
- 13. Widdowson MA, Rockx B, Schepp R et al Detection of serum antibodies to bovine norovirus in veterinarians and the general population in the Netherlands. J Med Virol 2005; 76:119–28. [DOI] [PubMed] [Google Scholar]
- 14. Karst SM, Wobus CE, Goodfellow IG, Green KY, Virgin HW. Advances in Norovirus biology. Cell Host Microbe 2014; 15:668–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marionneau S, Airaud F, Bovin NV, Le Pendu J, Ruvoen‐Clouet N. Influence of the combined ABO, FUT2 and FUT3 polymorphism on susceptibility to Norwalk virus attachment. J Infect Dis 2005; 192:1071–7. [DOI] [PubMed] [Google Scholar]
- 16. Harrington PR, Lindesmith L, Yount B, Moe CL, Baric RS. Binding of Norwalk virus‐like particles to ABH histo‐blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice. J Virol 2002; 76:12335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carlsson B, Kindberg E, Buesa J et al The G428A nonsense mutation in FUT2 provides strong but not absolute protection against symptomatic GII.4 norovirus infection. PLOS ONE 2009; 4:e5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nordgren J, Kindberg E, Lindgren PE, Matussek A, Svensson L. Norovirus gastroenteritis outbreak with a secretor‐independent susceptibility patter. Emerg Infect Dis 2010; 16:81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duizer E, Schwab KJ, Neill FH, Atmar RL, Koopmans MPG, Estes MK. Laboratory efforts to cultivate noroviruses. J Gen Virol 2004; 85:79–87. [DOI] [PubMed] [Google Scholar]
- 20. Chan MCW, Ho WS, Sung JJY. In vitro whole‐virus binding of a Norovirus genogroup II.4 strain to cells of the lamina propria and Brunner's glands in the human duodenum. J Virol 2011; 85:8427−30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lay MK, Atmar RL, Guix S et al Norwalk virus does not replicate in human macrophages or dendritic cells derived from the peripheral blood of susceptible humans. Virology 2010; 406:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Basic M, Keubler LM, Buettner M et al Norovirus triggered microbiota‐driven mucosal inflammation in Interleukin 10 deficient mice. Inflamm Bowel Dis 2014; 20:431–43. [DOI] [PubMed] [Google Scholar]
- 23. Shortland A, Chettle J, Archer J et al Pathology caused by persistent murine norovirus infection. J Gen Virol 2014; 95:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mumphrey SM, Changotra H, Moore TN et al Murine Norovirus 1 infection is associated with histopathological changes in immunocompetent hosts, but clinical disease is prevented by STAT‐1dependent interferon responses. J Virol 2007; 81:3251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takanashi S, Hashira S, Matsunaga T et al Detection, genetic characterisation and quantification of norovirus RNA from sera of children with gastroenteritis. J Clin Virol 2009; 44:161–3. [DOI] [PubMed] [Google Scholar]
- 26. Lopman BA, Reacher MH, Vipond IB, sarangi J, Brown DW. Clinical manifestation of norovirus gastroenteritis in healthcare settings. Clin Infect Dis 2004; 39:318–24. [DOI] [PubMed] [Google Scholar]
- 27. Arness MK, Feighner BH, Canham ML et al Norwalk‐like viral gastroenteritis outbreak in US army trainees. Emerg Infect Dis 2000; 6:204–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee RM, Lessler J, Lee RA et al Incubation periods of viral gastroenteritis: a systematic review. BMC Infect Dis 2013; 13:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gustavsson L, Andersson LM, Brink M, Lindh M, Westin J. Venous lactate levels can be used to identify patients with poor outcome following community‐onset norovirus enteritis. Scand J Infect Dis 2012; 44:782–7. [DOI] [PubMed] [Google Scholar]
- 30. Chan CM, Chan CW, Ma CK, Chan HB. Norovirus as cause of benign convulsion associated with gastro‐enteritis. J Paediatr Child Health 2011; 47:373–7. [DOI] [PubMed] [Google Scholar]
- 31. Costantini VP, Cooper EM, Hardaker HL et al Epidemiologic, virologic and host genetic factors of norovirus outbreaks in long‐term care facilities. Clin Infect Dis 2016; 62:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Phillips G, Tam CC, Rodrigues LC, Lopman B. Prevalence and characteristics of asymptomatic norovirus infection in the community and England. Epidemiol Infect 2010; 138:1454–8. [DOI] [PubMed] [Google Scholar]
- 33. Troeger H, Loddenkemper C, Schneider T et al Structural and functional changes of the duodenum in human norovirus infection. Gut 2009; 58:1070–7. [DOI] [PubMed] [Google Scholar]
- 34. Porter CK, Faix DJ, Shiau D, Espiritu J, Espinosa BJ, Riddle MS. Postinfectious gastrointestinal disorders following norovirus outbreaks. Clin Infect Dis 2012; 55:915–22. [DOI] [PubMed] [Google Scholar]
- 35. Johnson PC, Mathewson JJ, DuPont HL, Greenberg HB. Multiple‐challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J Infect Dis 1990; 161:18–21. [DOI] [PubMed] [Google Scholar]
- 36. Simmons K, Gambhir M, Leon J, Lopman B. Duration of immunity to norovirus gastroenteritis. Emerg Infect Dis 2013; 19:1260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saito M, Goel‐Apaza S, Espetia S et al Norovirus working group in Peru. Multiple norovirus infections in a birth cohort in a Peruvian periurban community. Clin Infect Dis 2014; 58:483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Debbink K, Lindesmith LC, Donaldson EF et al Emergence of new pandemic GII.4 Sydney norovirus strain correlates with escape from herd immunity. J Infect Dis 2013; 208:1877–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thackray LB, Duan E, Lazear HM et al Critical role for interferon regulatory factor 3 (IRF‐3) and IRF‐7 in type 1 interferon‐mediated control of murine norovirus replication. J Virol 2012; 86:13515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chachu KA, LoBue AD, Strong DW, Baric RS, Virgin HW. Immune mechanisms responsible for vaccination against and clearance of mucosal and lymphatic norovirus infection. PLoS Pathog 2008; 4:e1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bok K, Green KY. Norovirus gastroenteritis in immunocompromised patients. N Engl J Med 2012; 367:2126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Green KY. Norovirus infection in immunocompromised hosts. Clin Microbiol Infect 2014; 20:717–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Westhoff TH, Vergoulidou M, Loddenkemper C et al Chronic norovirus infection in renal transplant recipients. Nephrol Dial Transplant 2009; 24:1051–3. [DOI] [PubMed] [Google Scholar]
- 44. Roos‐Weil D, Ambert‐Balay K, Lanternier F et al Impact of norovirus/sapovirus‐related diarrhea in renal transplant recipients hospitalised for diarrhea. Transplantation 2011; 92:61–9. [DOI] [PubMed] [Google Scholar]
- 45. Schorn R, Hohne M, Meerbach A et al Chronic norovirus infection after kidney transplantation: molecular evidence for immune‐driven viral evolution. Clin Infect Dis 2010; 51:307–14. [DOI] [PubMed] [Google Scholar]
- 46. Ebdrup L, Böttiger B, Mølgaard H, Laursen AL. Devastating diarrhoea in a heart‐transplanted patient J Clin Virol 2011; 50:263–5. [DOI] [PubMed] [Google Scholar]
- 47. Boillat Blanco N, Kuonen R, Bellini C et al Chronic norovirus gastroenteritis in a double hematopoietic stem cell and lung transplant recipient. Transpl Infect Dis 2011; 13:213–5. [DOI] [PubMed] [Google Scholar]
- 48. Echenique IA, Stosor V, Gallon L, Kaufman D, Qi C, Zembower TR. Prolonged norovirus infection after pancreas transplantation: a case report and review of chronic norovirus. Transpl Infect Dis 2016; 18:98–104. [DOI] [PubMed] [Google Scholar]
- 49. Kaufman SS, Chatterjee NK, Fushino ME et al Calicivirus enteritis in an intestinal transplant recipient. Am J Transp 2003; 3:764–8. [DOI] [PubMed] [Google Scholar]
- 50. Roddie C, Paul JP, Benjamin R et al Allogeneic hematopoietic stem cell transplantation and norovirus gastroenteritis: a previously unrecognized cause of morbidity. Clin Infect Dis 2009; 49:1061–8. [DOI] [PubMed] [Google Scholar]
- 51. Ueda R, Fuji S, Mori S et al Characteristics and outcomes of patients diagnosed with norovirus gastroenteritis after allogeneic hematopoietic stem cell transplantation based on immunochromatography. Int J Hematol 2015; 102:121–8. [DOI] [PubMed] [Google Scholar]
- 52. Robles JD, Cheuk DK, Ha SY, Chiang AK, Chan GC. Norovirus infection in pediatric hematopoietic stem cell transplantation recipients: incidence, risk factors, and outcome. Biol Blood Marrow Transplant 2012; 18:1883–9. [DOI] [PubMed] [Google Scholar]
- 53. Saif MA, Bonney DK, Bigger B et al Chronic norovirus infection in pediatric hematopoietic stem cell transplant recipients: a cause of prolonged intestinal failure requiring intensive nutritional support. Pediatr Transplant 2011; 15:505–9. [DOI] [PubMed] [Google Scholar]
- 54. Chehade H, Girardin E, Delich V, Pascual MA, Venetz JP, Cachat F. Acute norovirus‐induced agranulocytosis in a pediatric kidney transplant recipient. Transpl Infect Dis 2012; 14:E27–9. [DOI] [PubMed] [Google Scholar]
- 55. Salvador C, Meister B, Larcher H, Crazzolara R, Kropshofer G. Hemophagocytic lymphohistiocytosis after allogeneic bone marrow transplantation during chronic norovirus infection. Hematol Oncol 2014; 32:102–6. [DOI] [PubMed] [Google Scholar]
- 56. Ye X, Van JN, Munoz FM et al Noroviruses as a cause of diarrhea in immunocompromised pediatric hematopoietic stem cell and solid organ transplant recipients. Am J Transplant 2015; 15:1874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chapel H, Lucas M, Lee M et al Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood 2008; 112:277–86. [DOI] [PubMed] [Google Scholar]
- 58. Salzer U, Warnatz K, Peter HH. Common variable immunodeficiency – an update. Arth Res Ther 2012; 14:223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ardeniz O, Cunninghma‐Rundles C. Granulomatous disease in common variable immunodeficiency. Clin Immunol 2009; 133:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bogaert DJ, Dullaers M, Lambrecht BN, Vermaelen KY, de Baere E, Haerynck F. Genes associated with common variable immunodeficiency: one diagnosis to rule them all? J Med Genet 2016; 53:575–90. [DOI] [PubMed] [Google Scholar]
- 61. Resnick ES, Moshier EL, Godbold JH, Cunningham‐Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood 2011; 119:1650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Teahon K, Webster AD, Price AB et al Studies on the enteropathy associated with primary hypogammaglobulinaemia. Gut 1994; 35:1244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Daniels JA, Lederman HM, Maitra A et al Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID): a clinicopathologic study and review. Am J Surg Pathol 2007; 31:1800–12. [DOI] [PubMed] [Google Scholar]
- 64. Khodadad A, Aghamohammadi A, Parvaneh N et al Gastrointestinal manifestations in patients with common variable immunodeficiency. Dig Dis Sci 2007; 52:2977–83. [DOI] [PubMed] [Google Scholar]
- 65. Malamut G, Verkarre V, Suarez F et al The enteropathy associated with common variable immunodeficiency: the delineated frontiers with celiac disease. Am J Gastroenterol 2010; 105:2262–75. [DOI] [PubMed] [Google Scholar]
- 66. Agarwal S, Mayer L. Diagnosis and treatment of gastrointestinal disorders in patients with primary immunodeficiency. Clin Gastroenterol Hepatol 2013; 11:1050–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Oksenhendler E, Gerard L, Fieschi C et al Infections in 252 patients with common variable immunodeficiency. Clin Infect Dis 2008; 46:1547–54. [DOI] [PubMed] [Google Scholar]
- 68. Quinti I, Soresina A, Spadaro G et al Long term follow up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol 2007; 27:308–16. [DOI] [PubMed] [Google Scholar]
- 69. Silva GB, Fernandes KP, Segundo GR. Common variable immunodeficiency and isosporiasis: first report case. Rev Soc Bras Med Trop 2012; 45:768–9. [DOI] [PubMed] [Google Scholar]
- 70. Giavina‐Bianchi P, Silva Fde S, Toledo‐Barros M, Birolini D, Kalil J, Rizzo LV. A rare intestinal manifestation in a patient with common variable immunodeficiency and strongyloidiasis. Int Arch Allergy Immunol 2006; 140:199–204. [DOI] [PubMed] [Google Scholar]
- 71. Wagner DK, Wright JJ, Ansher AF, Gill VJ. Dysgonic fermenter 3‐associated gastrointestinal disease in a patient with common variable hypogammaglobulinemia. Am J Med 1988; 84:315–8. [DOI] [PubMed] [Google Scholar]
- 72. Washington K, Stenzel TT, Buckley RH, Gottfried MR. Gastrointestinal pathology in patients with common variable immunodeficiency and X‐linked agammaglobulinemia. Am J Surg Pathol 1996; 20:1240–52. [DOI] [PubMed] [Google Scholar]
- 73. Sharkey LM, Corbett G, Currie E, Lee J, Sweeney N, Woodward JM. Optimising delivery of care in coeliac disease – comparison of the benefits of repeat biopsy and serological follow up. Aliment Pharmacol Ther 2013; 38:1278–91. [DOI] [PubMed] [Google Scholar]
- 74. Biagi F, Bianchi PA, Zilli A et al The significance of duodenal mucosal atrophy in patients with common variable immunodeficiency. A clinical and histopathologic study. Am J Clin Pathol 2012; 138:185–9. [DOI] [PubMed] [Google Scholar]
- 75. Van de Ven AAJM, van Konijnenburg DPH, Wensing AMJ, van Montfrans JM. The role of prolonged viral gastrointestinal infections in the development of immunodeficiency‐related enteropathy. Clinic Rev Allerg Immunol 2012; 42:79–91. [DOI] [PubMed] [Google Scholar]
- 76. Chua I, Standish R, Lear S et al Anti‐tumour necrosis factor‐alpha therapy for severe enteropathy in patients with common variable immunodeficiency (CVID). Clin Exp Immunol 2007; 150:306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Woodward JM, Gkrania‐Klotsas E, Cordero‐Ng AY et al The role of chronic norovirus infection in the enteropathy associated with common variable immunodeficiency. Am J Gastroenterol 2015; 110:320–7. [DOI] [PubMed] [Google Scholar]
- 78. Van der Ven AA, Douma JW, RAdemaker C et al Pleconaril‐resistant parechovirus‐associated enteropathy in agammaglobulinaemia. Antivir Ther 2011; 16:611–4. [DOI] [PubMed] [Google Scholar]
- 79. Frange P, Touzot F, Debre M et al Prevalence and clinical impact of norovirus fecal shedding in children with inherited immune deficiencies. J Infect Dis 2012; 206:1269–74. [DOI] [PubMed] [Google Scholar]
- 80. Hernandes‐Trujillo VP, Scalchunes C, Cunningham‐Rundles C et al Autoimmunity and inflammation in X‐linked agammaglobulinaemia. J Clin Immunol 2014; 34:627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zullo A, Romiti A, Rinaldi V et al Gastric pathology in patients with common variable immunodeficiency. Gut 1999; 45:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Park J, Munagala I, Xu H et al Interferon signature in inflammatory common variable immune deficiency. PLOS ONE 2012; 17:e74893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gairard‐Dort AC, Degot T, Hirschi S et al Clinical usefulness of oral immunoglobulins in lung transplant recipients with norovirus gastroenteritis: a case series. Transplant Proc 2014; 46:3603–5. [DOI] [PubMed] [Google Scholar]
- 84. Chagla Z, Quirt J, Woodward K, Neary J, Rutherford C. Chronic norovirus infection in a transplant patient successfully treated with enterally administered immune globulin. J Clin Virol 2013; 58:306–8. [DOI] [PubMed] [Google Scholar]
- 85. Ronchetti AM, Henry B, Ambert‐Balay K et al Norovirus‐related chronic diarrhea in a patient treated with alemtuzumab for chronic lymphocytic leukemia. BMC Infect Dis 2014; 14:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Siddiq DM, Koo HL, Adachi JA, Viola GM. Norovirus gastroenteritis successfully treated with nitazoxanide. J Infect 2011; 63:394–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chang KO, George DW. Interferons and ribavirin effectively inhibit Norwalk virus replication in replicon‐bearing cells. J Clin Virol 2007; 81:12111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Arias A, Emmott E, Vashist S, Goodfellow I. Progress towards the prevention and treatment of norovirus infections. Future Microbiol 2013; 8:1475–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


