Visual Abstract

Key Words: cardiac conditioning, cardiac metabolism, left ventricular assist device
Abbreviations and Acronyms: GC-MS, gas chromatography–mass spectroscopy; HF, heart failure; LV, left ventricular; LVAD, left ventricular assist device; TCA, tricarboxylic acid
Highlights
-
•
LVAD unloading reverses several but not all aspects of myocardial remodeling and usually leads to incomplete cardiac recovery in a subset of patients with advanced HF.
-
•
We performed metabolomic analysis and mitochondrial structural and functional characterization in paired human myocardial tissue procured from 31 patients with advanced HF at LVAD implant and at heart transplant plus tissue from 11 normal donors.
-
•
LVAD unloading induces glycolysis up-regulation without a corresponding increase in glucose oxidation.
-
•
Lack of post-LVAD improvement in mitochondrial function and volume density could explain the glycolysis-glucose oxidation mismatch.
-
•
Therapeutic interventions, such as myocardial conditioning, that are known to improve mitochondrial biogenesis, structure, and function might further improve cardiac metabolism and energy production and thereby enhance cardiac recovery with LVAD-induced unloading.
Summary
This study sought to investigate the effects of mechanical unloading on myocardial energetics and the metabolic perturbation of heart failure (HF) in an effort to identify potential new therapeutic targets that could enhance the unloading-induced cardiac recovery. The authors prospectively examined paired human myocardial tissue procured from 31 advanced HF patients at left ventricular assist device (LVAD) implant and at heart transplant plus tissue from 11 normal donors. They identified increased post-LVAD glycolytic metabolites without a coordinate increase in early, tricarboxylic acid (TCA) cycle intermediates. The increased pyruvate was not directed toward the mitochondria and the TCA cycle for complete oxidation, but instead, was mainly converted to cytosolic lactate. Increased nucleotide concentrations were present, potentially indicating increased flux through the pentose phosphate pathway. Evaluation of mitochondrial function and structure revealed a lack of post-LVAD improvement in mitochondrial oxidative functional capacity, mitochondrial volume density, and deoxyribonucleic acid content. Finally, post-LVAD unloading, amino acid levels were found to be increased and could represent a compensatory mechanism and an alternative energy source that could fuel the TCA cycle by anaplerosis. In summary, the authors report evidence that LVAD unloading induces glycolysis in concert with pyruvate mitochondrial oxidation mismatch, most likely as a result of persistent mitochondrial dysfunction. These findings suggest that interventions known to improve mitochondrial biogenesis, structure, and function, such as controlled cardiac reloading and conditioning, warrant further investigation to enhance unloading-induced reverse remodeling and cardiac recovery.
Heart failure (HF) is a global epidemic with a prevalence of 2% to 3% in the United States and Europe, and it is a huge cost to the health care systems (1). Despite advances in pharmacological therapies, implantable defibrillators, cardiac resynchronization, and stem cell/regenerative therapies, patients with advanced HF experience mortality rates in excess of 50% per year (1). Mechanical unloading through a left ventricular assist device (LVAD) is a therapeutic intervention that leads to substantial improvement in cardiac function in a subgroup of patients with end-stage HF 2, 3.
The functional response of the failing human heart to mechanical unloading is not an “all or none” phenomenon, and the mechanisms guiding this response are the subject of investigation (4). On the basis of previous human and animal studies, left ventricular (LV) pressure and volume overload alters metabolic substrate utilization, decreases mitochondrial function, and reduces energy production in the failing heart (5). Mechanical unloading has the potential to reverse these deleterious metabolic adaptations of the failing heart and even activate cellular pathways of cardioprotection and cardiac repair 6, 7. On the other hand, excessive or prolonged unloading could decrease myocardial energy demand, leading to myocardial hibernation and suppression of cellular repair.
We have previously shown that LVAD unloading may lead to hypertrophy regression through disruption of the equilibrium between protein synthesis and degradation (8). This altered equilibrium could also affect the intracellular pool of amino acids. An elevation in amino acid levels could provide alternative building blocks for protein synthesis and could supplement the Krebs tricarboxylic acid (TCA) cycle with intermediates through anaplerosis. Up-regulation of anaplerosis has previously been shown to play a cardioprotective role in the failing heart (9).
In this study we investigate how the mechanical unloading induced by LVAD affects the bioenergetic adaptation and metabolic perturbation in the chronically failing human heart.
Methods
Study population
We prospectively enrolled 31 consecutive patients with clinical characteristics consistent with dilated cardiomyopathy and chronic advanced HF who required circulatory support with continuous flow LVAD as bridge to transplantation. Patients who required LVAD support due to acute HF (acute myocardial infarction, acute myocarditis, post-cardiotomy cardiogenic shock, and so on) were prospectively excluded. Eleven normal donor hearts, not allocated for heart transplantation due to noncardiac reasons (size, infection, and so on) were used as normal controls. The study was approved by the institutional review board of the participating institutions, and informed consent was provided by all patients (see the Supplemental Appendix for details).
Myocardial tissue acquisition
Myocardial tissue was prospectively collected from the LV apical core at LVAD implantation. At the time of cardiac transplantation, the tissue was obtained from the apex at least 1.5 to 2 cm distant from the LVAD inflow cannula site to avoid any reactive foreign body inflammatory changes. Control samples were acquired from hearts that were not transplanted due to noncardiac reasons. Normal donor LV apical tissue was harvested and processed the same way as the failing hearts.
Metabolomic analysis
The levels of metabolites in the cardiac tissue were measured by gas chromatography–mass spectroscopy (GC-MS) analysis. For details about tissue extraction and GC-MS analysis, please see the Supplemental Appendix.
Electron microscopy
Epon-embedded tissue blocks were subject to ultramicrotomy and osmium/uranyl acetate–based staining for electron microscopy. Imaging was performed on a JEM 1010 electron microscope (Jeol, Peabody, Massachusetts). A total of 5 optical fields/heart were examined at 11,000× magnification. The number of mitochondria in each size range was calculated as the percentage of the total mitochondria count on the same amount of area in each representative sampling. Mitochondria volume density was estimated as previously described (10).
Relative mitochondrial deoxyribonucleic acid levels
The mitochondrial deoxyribonucleic acid (DNA) content was quantified with real-time polymerase chain reaction using SYBR Green PCR master mix (Thermo Fisher Scientific, Waltham, Massachusetts) and the primers that targeted a stable site in mitochondrial DNA minimal arc and single-copy nuclear DNA within the β2M gene (11). The relative mitochondrial DNA content was calculated from the ratio of mitochondrial DNA copy number to nuclear DNA copy number, and the relative fold change was normalized to normal donors (nonfailing heart) using the ΔΔCT method (see the Supplemental Appendix for details).
Mitochondrial respiratory flux
Mitochondrial respiration was assessed as previously described (12). For details, please see the Supplemental Appendix.
Statistical analysis
Statistical analysis was performed as previously described 8, 13. Values are expressed as mean ± SEM. The paired samples Student t test was used for comparisons between pre- and post-LVAD unloading. The independent samples t test was used for comparisons between the failing and the normal hearts. Prism 6 (GraphPad, San Diego, California) was used for statistical analysis. Significance was determined as p < 0.05.
Results
Study population and hemodynamic data
The clinical characteristics of the study population are presented in Table 1. Hemodynamic data before and 2 months after LVAD implantation are shown in Table 2. Left- and right-sided filling pressures were decreased after LVAD implantation, indicating that LVAD effectively induces left and right ventricular unloading. The duration of LVAD support was 243 ± 29.8 days.
Table 1.
Baseline Clinical Characteristics of the Study Population Before LVAD Implantation (N = 31)
| Men | 30 (97) |
| Age, yrs | 48 ± 2 |
| Etiology | |
| Ischemic cardiomyopathy | 11 (35) |
| Nonischemic cardiomyopathy | 20 (65) |
| New York Heart Association functional class | |
| III | 10 (32) |
| IV | 21 (68) |
| Duration of heart failure history, yrs | 5.2 ± 0.6 |
| Systolic arterial pressure, mm Hg | 97.0 ± 1.5 |
| Diastolic arterial pressure, mm Hg | 66.0 ± 1.4 |
| Serum concentrations | |
| Brain natriuretic peptide, pg/ml | 1,568 ± 157 |
| Creatinine, mg/dl | 1.40 ± 0.05 |
| Sodium, mmol/l | 133.0 ± 0.7 |
| Hemoglobin, g/dl | 12.1 ± 0.3 |
Values are n (percentile of the total population) or mean ± SEM.
LVAD = left ventricular assist device.
Table 2.
Hemodynamic Measurements Before and 2 Months After LVAD Implantation (N = 16)∗
| Before LVAD Implantation | 2 Months After LVAD Implantation | p Value | |
|---|---|---|---|
| Mean right atrial, mm Hg | 12.5 ± 1.4 | 8.6 ± 1.1 | 0.007 |
| Pulmonary capillary wedge, mm Hg | 25.0 ± 2.4 | 12.0 ± 1.6 | 0.0002 |
| Systolic pulmonary arterial, mm Hg | 53.6 ± 3.8 | 37.5 ± 3.1 | 0.001 |
| Diastolic pulmonary arterial, mm Hg | 28.3 ± 2.3 | 14.7 ± 1.1 | <0.0001 |
| Pulmonary vascular resistance, Woods Units | 4.1 ± 0.6 | 2.8 ± 0.4 | 0.03 |
| Cardiac index, l·m−2·min−1 | 1.9 ± 0.2 | 2.4 ± 0.2 | 0.046 |
Values are mean ± SEM.
LVAD = left ventricular assist device.
Right heart catheterization at 2 months post-implant was not performed in patients that had thrombotic or bleeding complications or difficulties with their anticoagulation management.
Metabolomic data
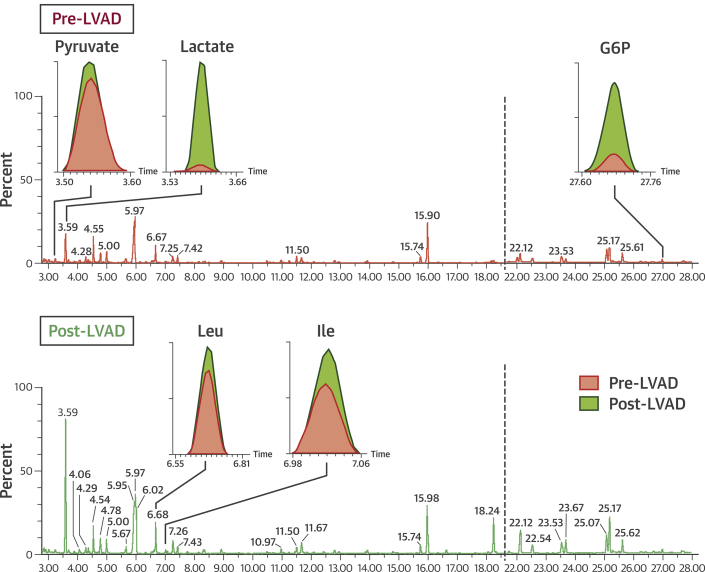
A total of 30 paired myocardial tissue samples obtained at the time of LVAD implantation and again at the time of heart transplantation together with 11 nonfailing heart samples were examined with GC-MS. Figure 1 depicts the GC-MS findings of a representative myocardial sample before and after LVAD unloading.
Figure 1.
Gas Chromatography–Mass Spectroscopy of a Representative Myocardial Sample Before and After LVAD Unloading
(Top) Before left ventricular assist device (LVAD) unloading (red); (bottom) after LVAD unloading (green). The insets show the area under the curve ratio of metabolite signals at pre- (pink) and post-LVAD (green) time points. The glycolysis intermediates glucose-6-phosphate (G6P), pyruvate, and lactate increased significantly post-LVAD. Similarly, the branched chain amino acids leucine (leu) and isoleucine (ile) also increased in the post-LVAD unloading sample.
Glycolysis, krebs (TCA) cycle intermediates, and anaplerosis
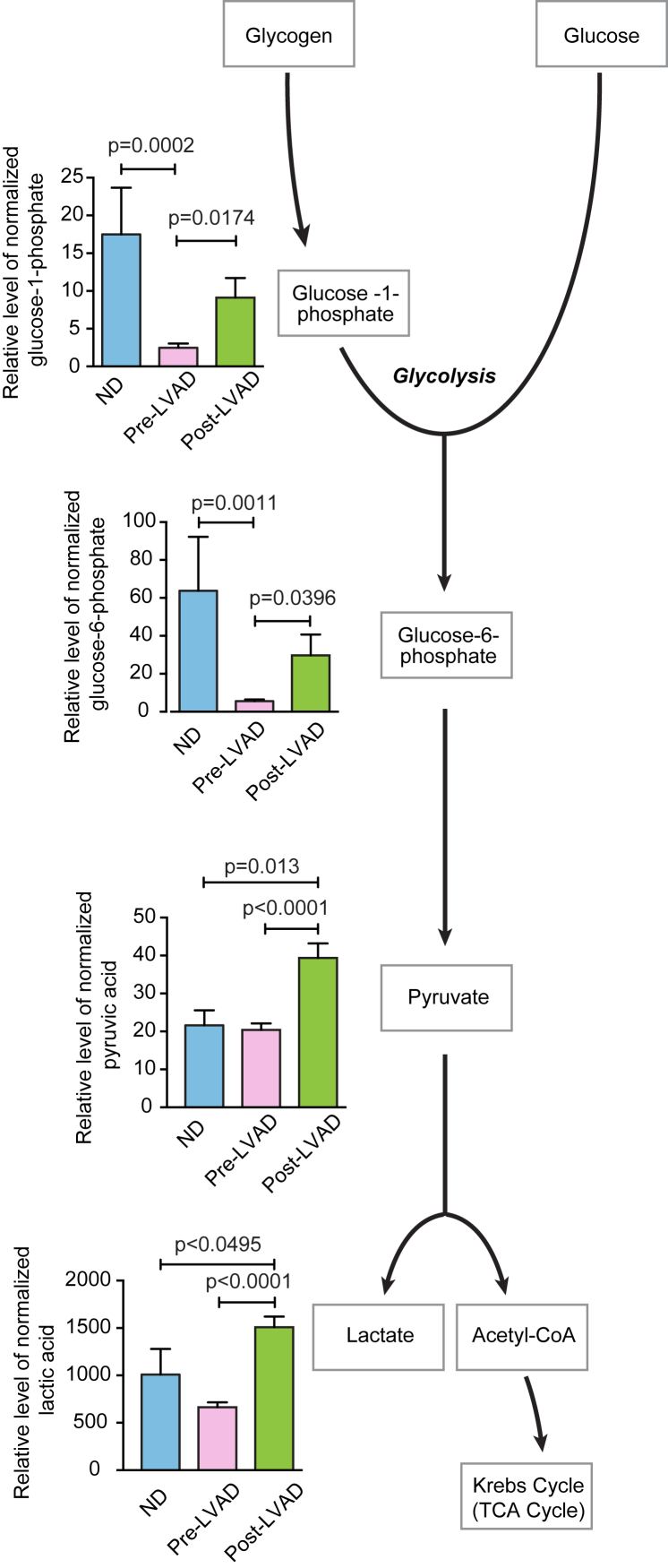
Glucose-1-phosphate, which is a product of glycogenolysis, increased after LVAD unloading (9.12 ± 2.60 vs. 2.47 ± 0.56; p = 0.02), suggesting increased utilization of glycogen during mechanical unloading (Figure 2). Glucose-6 phosphate, which is the product of the first reaction of glycolysis, increased after LVAD unloading (29.68 ± 11.00 vs. 5.55 ± 0.87; p = 0.04) (Figure 2). Along the same pathway, pyruvate (the final product of glycolysis before mitochondrial oxidation) was also increased post-LVAD (39.4 ± 4.0 vs. 20.1 ± 2.0; p < 0.0001), suggesting a glycolytic shift during LVAD-induced mechanical unloading. Post-LVAD pyruvate levels were also significantly higher relative to the normal donor heart (39.4 ± 4.0 vs. 21.6 ± 4.0; p = 0.013) (Figure 2). Interestingly, we observed increased post-LVAD levels of lactate relative to both the pre-LVAD myocardial tissue sample (1,509 ± 112 vs. 665 ± 52; p < 0.0001) and the normal donor heart (1,509 ± 112 vs. 1,010 ± 269; p = 0.049) (Figure 2).
Figure 2.
Glycolytic Metabolites in the Nonfailing Donor Heart and the Failing Heart Before and After LVAD Unloading
The diagram depicts glycolytic metabolism of glucose derived from glycogen or extracellular glucose. Glycolytic intermediates are increased following left ventricular assist device (LVAD) unloading as exemplified by glucose 1 phosphate, glucose 6 phosphate, pyruvate, and also increased levels of lactate. CoA = coenzyme A; ND = nonfailing donor heart; TCA = tricarboxylic acid.
The early Krebs (TCA) cycle intermediates, that is, citrate and a-ketoglutarate, were decreased in failing hearts compared with normal donor hearts (2,804 ± 361 vs. 5,989 ± 2,355; p = 0.04; and 0.40 ± 0.06 vs. 0.70 ± 0.14; p = 0.008) (Figure 3). Interestingly, despite increased pyruvate post-LVAD, these TCA cycle intermediates remained at low levels after LVAD unloading. Taken together, these findings suggest increased glycolysis and a defect in mitochondrial oxidation leading to increased cytosolic lactate rather than the increased pyruvate entering the TCA cycle in the mitochondria.
Figure 3.
Krebs (TCA) Cycle Intermediates
The center diagram depicts the TCA cycle following pyruvate oxidation, and the graphs show the quantitative comparisons of TCA intermediates in cardiac tissue of NDs and at the pre- and post-LVAD time points. Despite the post-LVAD increase of pyruvate, early TCA cycle intermediates, that is, citrate and α-ketoglutarate, remained unaltered in the post-LVAD unloaded human heart, whereas levels of succinate, fumarate and malate are increased. Abbreviations as in Figure 2.
By contrast, the subsequent TCA cycle intermediates after the succinyl CoA anaplerotic access point increased significantly after LVAD unloading (succinate: 941 ± 84 vs. 225 ± 25; p < 0.0001; fumarate: 75.0 ± 7.6 vs. 50.0 ± 9.7; p = 0.032; and malate: 225 ± 25 vs. 101 ± 12; p = 0.0002) (Figure 3). These findings suggest altered anaplerosis and cataplerosis during LVAD mechanical unloading of the failing human heart (Figure 3). Along the same lines, Figure 4 shows that most amino acids were decreased in the failing human hearts compared with the normal donor hearts, and after LVAD unloading, their levels were restored or even further increased. Of note, the amino acids isoleucine, methionine, and leucine that are precursors for the TCA cycle anaplerotic entry point at the level of succinyl CoA were markedly elevated, even higher than the levels of the normal donor hearts (Figure 4). In addition, phenylalanine and tyrosine, which are precursors for the TCA cycle anaplerotic entry point at the level of fumarate, were also significantly elevated during LVAD mechanical unloading (Figure 4).
Figure 4.
Amino Acids Were Decreased in the Failing Human Heart Compared With the Normal Heart
Left ventricular assist device (LVAD) unloading restored amino acid levels. #Significant difference in comparison to the normal donor heart; p < 0.05. *Significant difference in comparison to the pre-LVAD time point; p < 0.05.
Mitochondria oxidative phosphorylation capacity
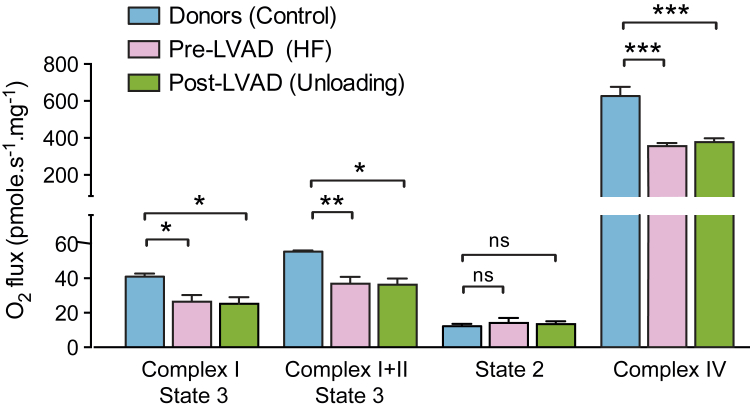
To investigate the alterations of mitochondrial function between nonfailing donor hearts and advanced failing hearts before and after LVAD unloading, we assessed mitochondrial respiratory flux of the collected myocardial tissue. State 3 complex I and state 3 complex I + II respiration demonstrated a significant reduction in the failing human heart before LVAD implantation relative to normal donor hearts; however, the state 2 showed no significant difference among all the groups (Figure 5). State 3 complex I and state 3 complex I + II respiration were unchanged after LVAD unloading (Figure 5). The respiratory control ratio and maximum respiration induced by TMPD and ascobate (complex IV) were also significantly higher in normal donor hearts than in the failing human hearts before and after LVAD unloading (Figure 5). The respiratory control ratio was 4.7 ± 0.5 for normal donor hearts, and 3.4 ± 0.8 and 2.8 ± 0.3 for failing hearts at pre- and post-LVAD, respectively. Thus LVAD-induced mechanical unloading did not reverse the impaired oxidative capacity of the mitochondria of the failing human heart.
Figure 5.
Oxidative Function of Mitochondria in the Nonfailing Donor Heart and in the Advanced Failing Heart at Pre- and Post-LVAD Unloading Time Points
Complex I, complex I + II, and complex IV respiration of isolated permeabilized myofibers from failing hearts was significantly lower compared with the nonfailing heart. Mitochondrial oxidative capacity remained significantly reduced after left ventricular assist device (LVAD) unloading. *p < 0.05; **p < 0.01; ***p < 0.001.
Mitochondria density and structure
Mitochondrial volume density in failing hearts, assessed by electron microscopy, was reduced compared with the normal heart (Figures 6A to 6C). Consistent with this observation, mitochondrial DNA content was also found to be decreased in the failing heart compared with the normal donor hearts (Figure 6D). The mitochondria volume density and mitochondrial DNA levels were not significantly altered during LVAD unloading (Figures 6A to 6D). Moreover, the ultrastructural morphology (as assessed by electron microscopy) revealed that cardiac mitochondria at the time of LVAD implant were smaller (Figure 6E) and characterized by disorganized cristae and decreased matrix density versus those of normal donor hearts. However, mitochondria after LVAD unloading consistently revealed significant but variable improvements in some of these specific morphological characteristics (Figures 6A, 6B, and 6E). We did not have enough subjects to adequately explore whether the subgroup of patients with significant myocardial functional improvement after LVAD unloading (i.e., cardiac recovery “responders”) exhibited improved mitochondrial oxidative capacity and increased mitochondrial density and DNA content to a greater extent than the cardiac recovery “nonresponders.” However, our limited data did show a trend toward greater mitochondrial improvement in the patients with the greatest favorable cardiac functional and structural response after LVAD mechanical unloading (data not shown).
Figure 6.
Ultrastructural Morphology, Mitochondrial Volume Density, and Mitochondrial DNA Content in the Normal Donor Heart and in the Failing Human Heart Before and After LVAD Unloading
(A) Transmission electron microscopy photomicrographs depicting longitudinal rows of mitochondria located in parallel with the sarcomeres/contractile apparatus. The magnification is 15,000×, and the scale bar indicates the length of 2 μm. (B) Higher magnification of mitochondrial ultrastructure at 50,000×. Compared with the normal donor heart, the mitochondria at the pre-LVAD time point were smaller, had disorganized cristae, and had decreased matrix density, whereas the mitochondria after LVAD unloading consistently showed improvement in these specific morphological characteristics. The scale bar indicates the length of 1 μm. Mitochondrial volume density (C) and mitochondrial DNA (mtDNA) content (D) were lower in the failing human heart compared with the nonfailing donor heart. mtDNA levels and volume density remained unchanged after LVAD unloading. The number of large mitochondria in the normal control group were higher than the failing human heart (E). The average size of mitochondria improved slightly after LVAD unloading. *Significant difference in comparison to the normal donor heart; p < 0.05. +Significant difference in comparison to the pre-LVAD time point. Abbreviations as in Figure 2.
Nucleic acids
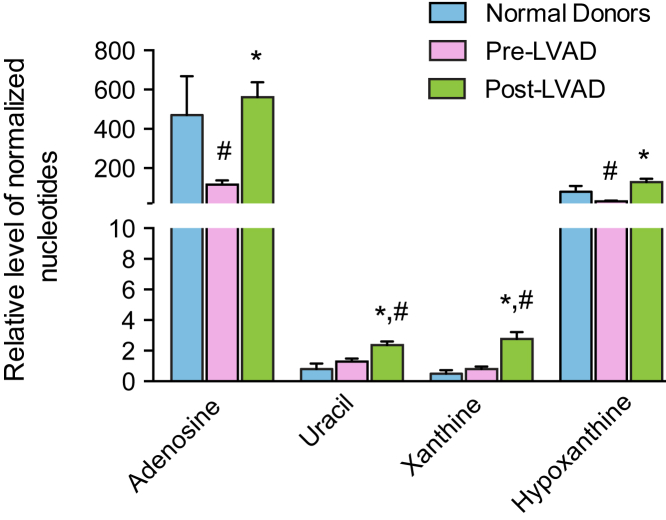
To assess changes in DNA synthesis with LVAD therapy, purines and pyrimidines were measured in the myocardial samples. Cardiac levels of the purine intermediates, xanthine and hypoxanthine, were increased after LVAD unloading (2.8 ± 0.5 vs. 0.8 ± 0.2; p < 0.0001; and 128 ± 17 vs. 31 ± 4; p < 0.0001, respectively) (Figure 7). Similarly, uracil, a pyrimidine product, was also elevated after LVAD unloading (2.5 ± 0.2 vs. 1.3 ± 0.2; p < 0.0001). Finally, adenosine levels increased post-LVAD unloading and reached the levels of the normal heart (561 ± 77 vs. 116 ± 21; p < 0.0001).
Figure 7.
Nucleotide Levels in Normal Donor Heart and Advanced Failing Heart Before and After LVAD Unloading
The myocardial tissue post-LVAD unloading showed a significant increase in nucleotide levels. #Significant difference in comparison to the normal donor heart; p < 0.05. *Significant difference in comparison to the pre-LVAD time point; p < 0.05.
Discussion
Our report integrates static and dynamic/functional cardiac metabolism studies performed in a large number of paired human myocardial tissue samples. Our findings indicate a post-LVAD induction of all measured glycolytic metabolites, including pyruvate, but without a corresponding increase in the initial intermediates in the Krebs cycle. This finding, in combination with the post-LVAD increased lactate levels, is consistent with decreased mitochondrial utilization of pyruvate generated from enhanced glycolysis leading to increased cytosolic lactate. Thus the post-LVAD heart is associated with a glycolysis-pyruvate mitochondrial oxidation “mismatch.” This conclusion is supported by our evaluation of mitochondrial function and structure: 1) the lack of post-LVAD improvement in mitochondrial oxidative capacity of the failing human heart; and 2) the persistent reduction in mitochondrial volume density and DNA content. We did not have an adequate number of subjects to perform a robust subgroup analysis to convincingly address whether the smaller subgroup of patients with HF who had improved cardiac function and structure after LVAD unloading (i.e., cardiac recovery responders) might exhibit improved mitochondrial function and structure. However, our preliminary data did show a trend toward such mitochondrial improvement in the patients with the greater cardiac functional and structural response after LVAD mechanical unloading. Furthermore, the ultrastructural morphology as assessed with electron microscopy revealed that compared with the normal donor heart, cardiac mitochondria at the pre-LVAD time point had smaller size, disorganized cristae, and decreased matrix density, whereas the mitochondria after LVAD unloading consistently showed significant improvement in these specific morphological characteristics. Together, these findings suggest that LVAD support induces myocardial “remission” rather than complete myocardial recovery. Importantly, demonstration of mitochondrial “health” could be used as a “signature” of true recovery. Furthermore, therapeutic interventions, such as myocardial conditioning, that are known to improve mitochondrial biogenesis, structure, and function might be useful to further improve cardiac metabolism and energy production and thereby enhance myocardial reverse remodeling and cardiac recovery with LVAD-facilitated mechanical unloading.
We also found increased levels of amino acids following LVAD implantation. Amino acids can serve as an alternative energy source by supplying the Krebs cycle with intermediate metabolites through the anaplerosis cycle. Indeed, we found specific intermediate metabolites at the Krebs cycle anaplerosis entry points to be significantly increased post-LVAD unloading. These post-LVAD increased nucleic amino acids could play an important role as alternative building blocks for protein synthesis and cellular biogenesis to facilitate LVAD-induced reverse remodeling of the failing human heart. Also, these increased nucleotide concentrations could potentially indicate increased flux through the pentose phosphate pathway.
Glycolysis-pyruvate mitochondrial oxidation mismatch
Glucose represents a more efficient energy source requiring less oxygen consumption per unit of adenosine triphosphate (ATP) generated relative to fatty acids 14, 15. Positron emission tomography studies have shown increased glucose and decreased fatty acid uptake from the failing heart, suggesting that glucose is the main metabolic substrate of the failing heart, whereas the normal heart relies more on fatty acids (16). Whether this “metabolic switch” from fatty acids to glucose plays a beneficial or detrimental role in the progression of the disease and whether LVAD unloading could favorably affect the metabolic phenotype of the failing heart is a matter of investigation (17). Weitzel et al. (18) investigated 8 patients with LVADs and showed that both glucose and lactate are reduced in the failing compared with the normal heart and that LVAD unloading restored their levels. Of note, that study did not investigate the response of glucose oxidation after mechanical unloading. Animal and human data from 2 recently published studies from Aubert et al. (19) and Bedi Jr. et al. (20) showed decreased levels of TCA cycle intermediates with parallel up-regulation of ketone oxidation in the failing heart. In agreement with the aforementioned studies, we observed a significant reduction of metabolites involved in glycolysis and glucose oxidation in the failing heart at the pre-LVAD implantation time point. This metabolic pattern combined with the reduced levels of other metabolic substrates (i.e., fatty acid and amino acids) and the decreased oxidative phosphorylation capacity represent a decreased metabolic state consistent with severe energetic impairment of the failing heart (5).
The failing heart is characterized by impaired mitochondrial biogenesis, as well as structural and functional mitochondrial defects 21, 22. A recent study from Cordero-Reyes et al. (23) surprisingly showed that isolated mitochondria from failing human hearts demonstrate preserved respiration; the authors concluded that impaired energy metabolism of the failing heart might be due to decreased mitochondria number or defects in substrate delivery. In another study by Scheubel et al. (24), decreased complex I activity of the failing human heart was not associated with mitochondrial DNA damage or suppressed mitochondrial gene expression. The absence of mitochondrial DNA defects could serve as a good prognostic factor, providing the mitochondria of the failing human heart with the potential of recovery under appropriate therapeutic interventions (24). Whether LVAD unloading could lead to improvement of mitochondrial function is a matter of controversy. Lee et al. (25) have shown improved state 2 and 4 mitochondrial respiration of the unloaded compared with the nonunloaded failing heart. By contrast, Mital et al. (26) did not observe differences in the baseline mitochondrial respiration, but the mitochondria of the unloaded heart had a favorable response to NO. These studies are limited by the absence of control normal hearts and by the fact that the comparisons of mitochondrial function were not made with paired pre- and post-LVAD myocardial tissue samples but were instead between unloaded and nonunloaded hearts from different patients.
In our study, we also found evidence indicating a post-LVAD glycolysis-pyruvate oxidation mismatch. Although glycolysis was induced after LVAD unloading leading to increased pyruvate production, the latter was not directed into the TCA cycle and the mitochondria for complete oxidation. Instead, we provided evidence that the pyruvate was converted into lactic acid in the cytoplasm. This phenomenon might be explained by the decreased energy demands of the unloaded heart that makes glycolysis a sufficient energy source to cover ATP requirements. Also, because intramitochondrial glucose oxidation is the major source for reactive oxygen species production in the respiratory chain, the observed glycolysis-pyruvate oxidation mismatch could represent a protective mechanism leading to oxidative stress reduction.
Amino acids, anaplerosis, and interactions with the TCA cycle and myocardial repair mechanisms
A transcriptomic analysis by Lai et al. (27) showed a reduction in the expression of a subset of genes involved in amino acid degradation in the failing heart. We did not observe elevated levels of amino acids in the failing human heart before LVAD unloading compared with the normal heart. However, we identified increased levels of amino acids after LVAD unloading. We hypothesize that amino acids may serve as an alternative energy source by supplying the TCA cycle with metabolic intermediates through anaplerosis. The balance of anaplerosis and cataplerosis is essential for the function of the TCA cycle, but the effect of changes in the anaplerosis/cataplerosis flux in the energetic and metabolic adaptations of chronic HF and during mechanical unloading is not well understood. The prevailing view in cardiovascular disease is that increased anaplerotic flux might be beneficial and may play an important role in regulating bioenergetic adaptations to ATP demand (28). Another collateral metabolic pathway involving anaplerosis/catabolism is the biosynthesis of building blocks (nonessential amino acids, fatty acid, and nucleotides) necessary for repair mechanisms of the failing heart. In support of this hypothesis, we found increased nucleic acid levels, which could be consistent with induction of nucleotide synthesis or salvage pathways in the unloaded heart (Figure 7). In addition to their putative role in protein synthesis, we believe that amino acids are also used as metabolic substrates in TCA cycle anaplerosis or by being transformed to fatty acids through removal (cataplerosis) of TCA cycle intermediates (29). In our study, we found increased levels post-LVAD of succinate and of subsequent TCA cycle intermediates such as fumarate and malate, whereas preceding intermediates such as ketoglutarate remained unaltered. These findings, in conjunction with the increased isoleucine and methionine post-LVAD, suggest that succinate could serve as the entry point of the increased amino acids into the TCA cycle of the LVAD unloaded heart. Also, elevated levels of fumarate, phenylalanine, and tyrosine suggest that the deamination of phenylalanine and tyrosine to fumarate could be another entry point to the TCA cycle. Finally, elevated levels of malate could be explained by either the conversion of fumarate to malate through the TCA cycle or by the up-regulation of the pyruvate-malate shuttle and the subsequent conversion of pyruvate to malate (30). The supplementation of the TCA cycle with intermediates ensures continued function of the cycle and continued production of nicotinamide adenine dinucleotide (NADH) that is directed to the respiratory chain for ATP production. Interestingly, we observed a significant increase of malate after LVAD unloading. Malate plays an important role in cardiac metabolism because it can be transformed to pyruvate and glucose, thereby augmenting glycolytic metabolism via the malate/aspartate shuttle (31). Increased activity of the shuttle leads to oxidation of NADH to NAD+ in the cytosol and transportation of NADH in the mitochondria (32). Cytosolic NAD+ is important because it is used to promote glycolysis by the transformation of glyceraldehyde-3-phosphate to 1,3-bisphosphoglyceraldehyde. Mitochondrial NADH is led to the electron transport chain (ETC) for ATP production. Thus, up-regulation of the malate/aspartate shuttle ensures continued glycolysis and NADH flux to the electron transport chain for ATP production.
Mitochondrial density and function, cardiac reloading/conditioning, and clinical research implications
Our data indicate that the impairment in mitochondrial density and function post-LVAD could represent an underlying mechanism for the glycolysis-pyruvate oxidation mismatch by limiting subsequent pyruvate oxidation. Furthermore, our preliminary data also revealed that the subset of patients with the greater myocardial functional response after LVAD mechanical unloading also showed improved post-LVAD mitochondrial function and structure. On the basis of our observations, interventions that are known to further enhance mitochondria number, function, and structure could promote the favorable cardiac functional and structural response indicative of myocardial recovery in LVAD patients 4, 33. Prior studies have shown that exercise and conditioning promotes mitochondria biogenesis and improves glucose metabolism 34, 35. Importantly, Kajimoto et al. (36) showed that myocardial reloading after a period of unloading increases TCA intermediates, suggesting increased anaplerosis and mitochondrial oxidative capacity. On the basis of these results, exercise and controlled cardiac reloading could be additional therapeutic interventions that could enhance LVAD-induced reverse remodeling and myocardial recovery and warrant further investigation. A controlled cardiac reloading and myocardial conditioning strategy has been recently attempted in LVAD patients by our group and others with promising results 37, 38.
Study limitations
Our study design does not allow multiple serial tissue evaluations. This could have provided useful information about dynamic changes of substrate utilization by the failing heart during chronic mechanical unloading. Also, our experimental setup did not allow us to measure insulin resistance and myocardial glucose uptake. These measurements might have provided useful information as to whether the induction of glycolysis is secondary to increased glucose uptake or to increased activity of glycolytic enzymes. Finally, our data represent static metabolic measurements at the pre- and post-LVAD unloading time points. Thus, conclusions regarding dynamic metabolic changes should be made cautiously. Future pulse chase studies with stable isotope-labeled metabolites are warranted to confirm the metabolic intracellular flux.
Conclusions
LVAD-induced mechanical unloading of the failing human heart induces up-regulation of the glycolysis without a parallel increase in pyruvate utilization for mitochondrial oxidation. Amino acid levels increased significantly during LVAD unloading, potentially acting as a compensatory mechanism and an alternative energy source through the TCA cycle anaplerosis entry points while also serving as potential building blocks for cardiac repair. The reduced mitochondrial density, structure, and function after LVAD unloading could represent a potential mechanism for the glycolysis-pyruvate oxidation mismatch and could provide a signature for myocardial recovery. Therefore, interventions that enhance mitochondrial biogenesis, function, and structure, such as exercise and controlled cardiac reloading and conditioning, should be further investigated as a strategy that may enhance LVAD-induced myocardial reverse remodeling and cardiac recovery.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Mechanical unloading reverses several, but not all, aspects of myocardial remodeling. Our study describes increased post-LVAD glycolysis, but reduced mitochondrial density and suppressed mitochondrial function, in the failing human heart that is not corrected by LVAD unloading. The identification of nonreversible features of cardiac remodeling could guide the application of adjunct pharmacological or other physiological interventions in an effort to enhance unloading induced cardiac recovery. Furthermore, demonstration of mitochondrial “health” could be used a “signature” of true recovery.
TRANSLATIONAL OUTLOOK: The described persistent mitochondrial defect might be the underlying cause of the limitations observed with LVAD-induced myocardial recovery. The next important step will be to determine whether therapeutic interventions, such as myocardial conditioning and controlled cardiac reloading, that are known to improve mitochondrial biogenesis, structure, and function will enhance the favorable cardiac functional response in the patient with an LVAD.
Acknowledgment
The GC-MS experiments were performed by the University of Utah’s Metabolomics Core.
Footnotes
This work was funded by the American Heart Association CVGPS Discovery Grant 15CVGPSD27690000 (to Dr. Drakos); the Doris Duke Foundation Clinical Scientist Development Grant 7/2013 (to Dr. Drakos); National Institutes of Health National Center for Research Resources grant that supports the Center Clinical Translational Sciences UL1-RR025764 and C06-RR11234, Deseret Foundation #00571 (to Drs. Drakos and Kfoury); a VA Merit Review Award, Clinical Science Research and Development/1I01CX000710-01A1 (to Drs. Stehlik and Drakos); National Heart, Lung, and Blood Institute (NHLBI) grant 4R01 HL089592-03 (to Dr. Selzman); the Howard Hughes Medical Institute and National Institutes of Health (to Dr. Rutter); National Institutes of Health/NHLBI (to Dr. Abel); the NHLBI, National Institute of Allergy and Infectious Diseases, Juvenile Diabetes Research Foundation, and the Department of Defense (to Dr. Li); and the International Society for Heart and Lung Transplantation Research Fellowship Award (to Dr. Diakos). Dr. Deberardinis has served on the scientific advisory boards of Agios Pharmaceuticals and Peloton Therapeutics. Dr. Stehlik has received research funding from St. Jude; and has received speaker honoraria from St. Jude and HeartWare. Dr. Drakos has received research support from Abiomed Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Appendix
References
- 1.Hunt S.A., Abraham W.T., Chin M.H. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Birks E.J., Tansley P.D., Hardy J. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355:1873–1884. doi: 10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]
- 3.Birks E.J., George R.S., Hedger M. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: a prospective study. Circulation. 2011;123:381–390. doi: 10.1161/CIRCULATIONAHA.109.933960. [DOI] [PubMed] [Google Scholar]
- 4.Drakos S.G., Kfoury A.G., Stehlik J. Bridge to recovery: understanding the disconnect between clinical and biological outcomes. Circulation. 2012;126:230–241. doi: 10.1161/CIRCULATIONAHA.111.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neubauer S. The failing heart—an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 6.Chokshi A., Drosatos K., Cheema F.H. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation. 2012;125:2844–2853. doi: 10.1161/CIRCULATIONAHA.111.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupte A.A., Hamilton D.J., Cordero-Reyes A.M. Mechanical unloading promotes myocardial energy recovery in human heart failure. Circ Cardiovasc Genet. 2014;7:266–276. doi: 10.1161/CIRCGENETICS.113.000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diakos N.A., Selzman C.H., Sachse F.B. Myocardial atrophy and chronic mechanical unloading of the failing human heart: implications for cardiac assist device-induced myocardial recovery. J Am Coll Cardiol. 2014;64:1602–1612. doi: 10.1016/j.jacc.2014.05.073. [DOI] [PubMed] [Google Scholar]
- 9.Russell R.R., 3rd, Taegtmeyer H. Changes in citric acid cycle flux and anaplerosis antedate the functional decline in isolated rat hearts utilizing acetoacetate. J Clin Invest. 1991;87:384–390. doi: 10.1172/JCI115008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boudina S., Sena S., Theobald H. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 11.Phillips N.R., Sprouse M.L., Roby R.K. Simultaneous quantification of mitochondrial DNA copy number and deletion ratio: a multiplex real-time PCR assay. Sci Rep. 2014;4:3887. doi: 10.1038/srep03887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park S.Y., Gifford J.R., Andtbacka R.H. Cardiac, skeletal, and smooth muscle mitochondrial respiration: are all mitochondria created equal? Am J Physiol Heart Circ Physiol. 2014;307:H346–H352. doi: 10.1152/ajpheart.00227.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drakos S.G., Kfoury A.G., Hammond E.H. Impact of mechanical unloading on microvasculature and associated central remodeling features of the failing human heart. J Am Coll Cardiol. 2010;56:382–391. doi: 10.1016/j.jacc.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanley W.C., Recchia F.A., Lopaschuk G.D. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 15.Stanley W.C., Chandler M.P. Energy metabolism in the normal and failing heart: potential for therapeutic interventions. Heart Fail Rev. 2002;7:115–130. doi: 10.1023/a:1015320423577. [DOI] [PubMed] [Google Scholar]
- 16.Davila-Roman V.G., Vedala G., Herrero P. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:271–277. doi: 10.1016/s0735-1097(02)01967-8. [DOI] [PubMed] [Google Scholar]
- 17.van Bilsen M., van Nieuwenhoven F.A., van der Vusse G.J. Metabolic remodelling of the failing heart: beneficial or detrimental? Cardiovasc Res. 2009;81:420–428. doi: 10.1093/cvr/cvn282. [DOI] [PubMed] [Google Scholar]
- 18.Weitzel L.B., Ambardekar A.V., Brieke A. Left ventricular assist device effects on metabolic substrates in the failing heart. PloS One. 2013;8:e60292. doi: 10.1371/journal.pone.0060292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aubert G., Martin O.J., Horton J.L. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133:698–705. doi: 10.1161/CIRCULATIONAHA.115.017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedi K.C., Jr., Snyder N.W., Brandimarto J. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016;133:706–716. doi: 10.1161/CIRCULATIONAHA.115.017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karamanlidis G., Nascimben L., Couper G.S., Shekar P.S., del Monte F., Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res. 2010;106:1541–1548. doi: 10.1161/CIRCRESAHA.109.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarreta D., Orus J., Barrientos A. Mitochondrial function in heart muscle from patients with idiopathic dilated cardiomyopathy. Cardiovasc Res. 2000;45:860–865. doi: 10.1016/s0008-6363(99)00388-0. [DOI] [PubMed] [Google Scholar]
- 23.Cordero-Reyes A.M., Gupte A.A., Youker K.A. Freshly isolated mitochondria from failing human hearts exhibit preserved respiratory function. J Mol Cell Cardiol. 2014;68:98–105. doi: 10.1016/j.yjmcc.2013.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheubel R.J., Tostlebe M., Simm A. Dysfunction of mitochondrial respiratory chain complex I in human failing myocardium is not due to disturbed mitochondrial gene expression. J Am Coll Cardiol. 2002;40:2174–2181. doi: 10.1016/s0735-1097(02)02600-1. [DOI] [PubMed] [Google Scholar]
- 25.Lee S.H., Doliba N., Osbakken M., Oz M., Mancini D. Improvement of myocardial mitochondrial function after hemodynamic support with left ventricular assist devices in patients with heart failure. J Thorac Cardiovasc Surg. 1998;116:344–349. doi: 10.1016/s0022-5223(98)70136-9. [DOI] [PubMed] [Google Scholar]
- 26.Mital S., Loke K.E., Addonizio L.J., Oz M.C., Hintze T.H. Left ventricular assist device implantation augments nitric oxide dependent control of mitochondrial respiration in failing human hearts. J Am Coll Cardiol. 2000;36:1897–1902. doi: 10.1016/s0735-1097(00)00948-7. [DOI] [PubMed] [Google Scholar]
- 27.Lai L., Leone T.C., Keller M.P. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: a multisystems approach. Circ Heart Fail. 2014;7:1022–1031. doi: 10.1161/CIRCHEARTFAILURE.114.001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Des Rosiers C., Labarthe F., Lloyd S.G., Chatham J.C. Cardiac anaplerosis in health and disease: food for thought. Cardiovasc Res. 2011;90:210–219. doi: 10.1093/cvr/cvr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owen O.E., Kalhan S.C., Hanson R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 30.Pound K.M., Sorokina N., Ballal K. Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circ Res. 2009;104:805–812. doi: 10.1161/CIRCRESAHA.108.189951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen T.T., Stottrup N.B., Lofgren B., Botker H.E. Metabolic fingerprint of ischaemic cardioprotection: importance of the malate-aspartate shuttle. Cardiovasc Res. 2011;91:382–391. doi: 10.1093/cvr/cvr051. [DOI] [PubMed] [Google Scholar]
- 32.Rupert B.E., Segar J.L., Schutte B.C., Scholz T.D. Metabolic adaptation of the hypertrophied heart: role of the malate/aspartate and alpha-glycerophosphate shuttles. J Mol Cell Cardiol. 2000;32:2287–2297. doi: 10.1006/jmcc.2000.1257. [DOI] [PubMed] [Google Scholar]
- 33.Drakos S.G., Wever-Pinzon O., Selzman C.H. Magnitude and time course of changes induced by continuous-flow left ventricular assist device unloading in chronic heart failure: insights into cardiac recovery. J Am Coll Cardiol. 2013;61:1985–1994. doi: 10.1016/j.jacc.2013.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H., Bei Y., Lu Y. Exercise prevents cardiac injury and improves mitochondrial biogenesis in advanced diabetic cardiomyopathy with PGC-1alpha and Akt activation. Cell Physiol Biochem. 2015;35:2159–2168. doi: 10.1159/000374021. [DOI] [PubMed] [Google Scholar]
- 35.Vettor R., Valerio A., Ragni M. Exercise training boosts eNOS-dependent mitochondrial biogenesis in mouse heart: role in adaptation of glucose metabolism. Am J Physiol Endocrinol Metab. 2014;306:E519–E528. doi: 10.1152/ajpendo.00617.2013. [DOI] [PubMed] [Google Scholar]
- 36.Kajimoto M., O'Kelly Priddy C.M., Ledee D.R. Myocardial reloading after extracorporeal membrane oxygenation alters substrate metabolism while promoting protein synthesis. J Am Heart Assoc. 2013;2:e000106. doi: 10.1161/JAHA.113.000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Healy A.H., Koliopoulou A., Drakos S.G., McKellar S.H., Stehlik J., Selzman C.H. Patient-controlled conditioning for left ventricular assist device-induced myocardial recovery. Ann Thorac Surg. 2015;99:1794–1796. doi: 10.1016/j.athoracsur.2014.07.058. [DOI] [PubMed] [Google Scholar]
- 38.Frazier O.H., Baldwin A.C., Demirozu Z.T. Ventricular reconditioning and pump explantation in patients supported by continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2015;34:766–772. doi: 10.1016/j.healun.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.