Abstract
AIM
To evaluate the advantages of endoscopic ultrasound (EUS) in the assessment of detailed structures of pancreatic cystic neoplasms (PCNs) compared to computed tomography (CT) and magnetic resonance imaging (MRI).
METHODS
All patients with indeterminate PCNs underwent CT, MRI, and EUS. The detailed information, including size, number, the presence of a papilla/nodule, the presence of a septum, and the morphology of the pancreatic duct of PCNs were compared among the three imaging modalities. The size of each PCN was determined using the largest diameter measured. A cyst consisting of several small cysts was referred to as a mother-daughter cyst. Disagreement among the three imaging modalities regarding the total number of mother cysts resulted in the assumption that the correct number was the one in which the majority of imaging modalities indicated.
RESULTS
A total of 52 females and 16 males were evaluated. The median size of the cysts was 42.5 mm by EUS, 42.0 mm by CT and 38.0 mm by MRI; there was no significant difference in size as assessed among the three imaging techniques. The diagnostic sensitivity and ability of EUS to classify PCNs were 98.5% (67/68) and 92.6% (63/68), respectively. These percentages were higher than those of CT (73.1%, P < 0.001; 17.1%, P < 0.001) and MRI (81.3%, P = 0.001; 20.3%, P < 0.001). EUS was also able to better assess the number of daughter cysts in mother cysts than CT (P = 0.003); however, there was no significant difference between EUS and MRI in assessing mother-daughter cysts (P = 0.254). The papilla/nodule detection rate by EUS was 35.3% (24/68), much higher than those by CT (5.8%, 3/52) and MRI (6.3%, 4/64). The detection rate of the septum by EUS was 60.3% (41/68), which was higher than those by CT (34.6%, 18/52) and by MRI (46.9%, 30/64); the difference between EUS and CT was significant (P = 0.02). The rate of visualizing the pancreatic duct using EUS was 100%, whereas using CT and MRI it was less than 10%.
CONCLUSION
EUS helps visualize the detailed structures of PCNs and has many advantages over CT and MRI. EUS is valuable in the diagnosis and assessment of PCNs.
Keywords: Endoscopic ultrasound, Detailed structures, Computed tomography, Magnetic resonance imaging, Pancreatic cystic neoplasms
Core tip: This study was designed to evaluate the advantages of endoscopic ultrasound (EUS) in the assessment of the detailed structures of pancreatic cystic neoplasms (PCNs) compared with computed tomography (CT) and MRI. Previous studies showed the advantages of EUS in the diagnosis of PCNs. Multiple published studies compared the diagnostic value of EUS, CT, and MRI in patients with PCNs. However, few studies compared the abilities of the three modalities to evaluate the detailed structures of PCNs. There are many studies about the diagnostic value of imaging modalities in pancreatic cystic lesions (PCLs), and several studies assessed their ability to differentiate PCNs from other PCLs. Several studies compared the abilities of imaging modalities in demonstrating daughter cysts.
INTRODUCTION
Pancreatic cystic lesions (PCLs) include true cysts, pseudocysts and cystic neoplasms. True cysts, most of which are congenital, and pseudocysts, which are primarily caused by inflammation or injury, are non-neoplastic cysts and are regarded as benign whereas cystic neoplasms have the potential to be malignant. Approximately 60% of PCLs are cystic tumors, followed by inflammation and trauma-related pseudocysts, which account for 30%[1]. The prevalence of pancreatic cystic neoplasms (PCNs) has been reported to range widely, from 0.21%-24.3%[2-4]. There are four main types of PCNs: serous cystic neoplasms (SCNs), mucinous cystic neoplasms (MCNs), intraductal papillary mucinous neoplasms (IPMNs), and solid pseudopapillary neoplasms (SPNs). Two additional, but relatively uncommon, types of PCNs include cystic neuroendocrine neoplasms (NENs) and cystadenocarcinomas. Due to the malignant potential of PCNs, differentiating them from non-neoplastic cysts and benign PCNs is critical. Obtaining accurate diagnostic images of detailed anatomic structures will be useful in the classification of PCNs. Using such imaging modalities may aid in the appropriate diagnosis and help determine subsequent treatments[5].
In order to evaluate PCLs, various imaging modalities have been used, including ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), endoscopic ultrasound (EUS), MR cholangiopancreatography (MRCP), positron emission computed tomography (PET) and endoscopic retrograde cholangiopancreatography (ERCP)[6-11]. CT and MRI have been the most frequently used imaging techniques in the diagnosis of PCLs[12]. However, using EUS has an advantage over using CT or MRI in distinguishing between benign PCLs and malignant or potentially malignant lesions[13,14]. The images produced using EUS are higher in resolution[15,16], which we speculate may be related to its ability to show the detailed structures of PCNs.
There are multiple published studies comparing the diagnostic value of EUS, CT, and MRI in patients with PCNs. However, few studies have compared the capability of CT, MRI, and EUS to evaluate the detailed structures of PCNs. In this study, we investigated the capacity of EUS to evaluate cyst size, number, papillae/nodules, septum, and the morphology of the pancreatic duct in patients with PCNs and compared these results with those obtained using CT and MRI.
MATERIALS AND METHODS
This study was approved by the Ethical Committee of Chinese People’s Liberation Army General Hospital.
Patients
All patients who presented to the Chinese PLA General Hospital in Beijing with suspected PCNs between April 2015 and November 2016 (n = 143) were prospectively evaluated for possible study enrollment. Of these, 73 patients had undergone surgery. The eligibility criteria for inclusion in this study included (1) being at least 18 years of age; (2) having the ability to consent to and undergo EUS examination with or without CT and MRI prior to surgery; and (3) pathologically diagnosed with PCNs. Patients were excluded if they could not independently provide informed consent, could not undergo anaesthesia or endoscopic examination, had active acute pancreatitis or pancreatic necrosis, or had a coagulopathy. Patients were also excluded if they were pathologically diagnosed with non-neoplastic cystic lesions. A total of 68 patients who were pathologically diagnosed with PCNs were enrolled in the study.
Study design
Any patient who was suspected of having PCNs using imaging techniques was suggested to undergo CT, MRI, and EUS. CT and MRI scans were individually read by radiologists with more than 10 years of experience and by digestive physicians with more than 3 years of experience. The final report was determined after both the radiologist and digestive physician had separately analyzed the images and then come to an agreement. The agreements correlated well for both CT (κ = 0.797) and MRI (κ = 0.836). EUS procedures were performed by experts with at least 5 years of experience. Patients suspected of having PCNs with a malignant potential using imaging techniques, or those who refused to undergo EUS-guided ablation or imaging for surveillance, were recommended to undergo surgery. Once all of the confirmed PCNs were studied, the effectiveness of using EUS, CT, and MRI to evaluate the cysts was assessed.
Definitions
The largest diameter of each PCN was used to indicate its size. The following rules were used when comparing the ability of the different imaging modalities to assess cyst number and size: only the sizes of cysts that were visualized using all of the imaging techniques were analyzed. A single tumor with unilocular or multilocular cysts (a cyst in a cyst) and cysts that formed a cluster around a central scar (a cyst on a cyst) were both referred to as a mother-daughter cyst. The mother-daughter cyst was regarded as a cyst; however, the total number of daughter cysts was also collected and used as an outcome measurement. When there was a discrepancy among the 3 imaging techniques regarding the number of mother cysts, the correct number was considered to be what the majority of imaging modalities indicated. Papilla and nodule were considered to be one type of structure due to the difficulty in differentiating between the two using the three imaging modalities. Pathology was regarded as the gold standard for a definitive diagnosis of different types of PCNs.
Statistical analysis
All of the calculations were performed using SPSS 17.0. Quantitative data, such as cystic size, are expressed as the mean or median; differences were tested using a t-test or a nonparametric test. Count data, such as the number of daughter cysts, and PCNs, were tested using Fisher’s exact test or χ2. A P value < 0.05 was considered statistically significant.
RESULTS
Of the 73 patients who underwent surgery, 68 had confirmed PCNs and 5 had non-neoplastic cystic lesions. According to pathologic diagnoses, 27 patients had SCNs, 23 had MCNs, 6 had IPMNs, 9 had SPNs, 1 had NEN, and 2 had cystadenocarcinomas.
The baseline characteristics of the 68 enrolled patients, of whom 52 were female and 16 were male, are provided in Table 1. Twenty-four cysts were located in the head/uncinate of the pancreas and 43 in the body/tail; one patient had multiple cysts. All 68 enrolled patients underwent EUS, whereas only 52 patients underwent CT and 64 patients underwent MRI.
Table 1.
Baseline characteristics of the 68 enrolled patients
| Characteristic | No. of patients (n = 68) | |
| Sex | Male | 52 |
| Female | 16 | |
| Age, mean ± SD, yr | 46.3 ± 14.6 | |
| Location | Head/uncinate | 24 |
| Body/tail | 43 | |
| Others | 1 | |
| Examination | CT | 52 |
| MRI | 64 | |
| EUS | 68 | |
| Pathological diagnosis | SCNs | 27 |
| MCNs | 23 | |
| IPMNs | 6 | |
| SPNs | 9 | |
| NEN | 1 | |
| Cystadenocarcinomas | 2 |
Comparison of diagnostic value of the three imaging modalities in PCNs
In the 68 PCNs confirmed by pathology, CT was able to differentiate PCNs from other PCLs with a sensitivity of 73.1% (38/52) and a diagnostic rate of 17.3% (9/52) for differentiating the specific type of PCN; MRI was able to differentiate PCNs from other PCLs with a sensitivity of 81.3% (52/64) and a diagnostic rate of 20.3% (13/24) for differentiating the specific type of PCN; and EUS was able to differentiate PCNs from other PCLs with a sensitivity of 98.5% (67/68) and a diagnostic rate of 92.6% (63/68) for differentiating the specific type of PCN. The diagnostic sensitivity of EUS was higher than those of both CT (P < 0.001) and MRI (P = 0.001). The ability of EUS to specifically classify PCNs was also better than those of both CT (P < 0.001) and MRI (P < 0.001) (Figure 1).
Figure 1.
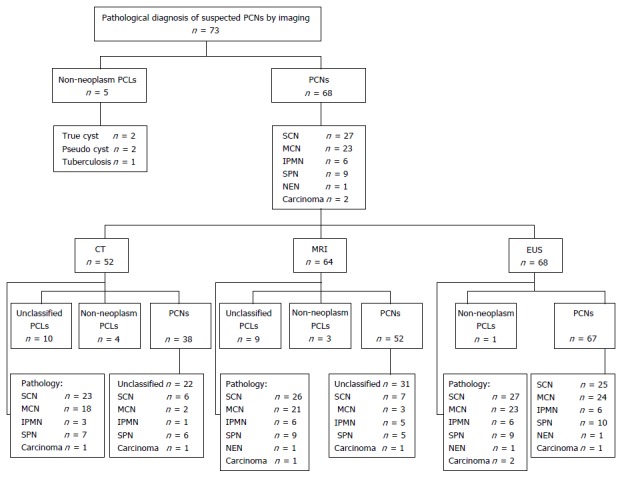
Diagnosis of pancreatic cystic neoplasms by pathology and imaging.
PCN size
Cyst size was assessed by CT for 52 cysts, by MRI for 65 cysts, and by EUS for 69 cysts. One patient had two separate cysts shown using both MRI and EUS. The mean size was 42.0 mm (14.0-97.0 mm) by CT, 38.0 mm (13.0-128.0 mm) by MRI, and 42.5 mm (13.0-100.0 mm) by EUS. There was no statistically significant difference in the evaluation of cyst size using EUS and CT (P = 0.646) or MRI (P = 0.587).
Number of PCNs
The number of PCNs diagnosed by EUS and CT was in concordance in 96.2% (50/52) of the cases among the 52 patients who underwent CT plus EUS. Among the 64 patients who underwent EUS in addition to MRI, the diagnostic concordance rate was 100.0% (64/64). A mother cyst might consist of several daughter cysts. The patient with multiple cysts was excluded when evaluating the ability of there examinations in demonstration of daughter cyst. EUS was shown to be better than CT in evaluating the number of daughter cysts in the mother cysts (P = 0.003). There was no difference between EUS and MRI in evaluating the presence of mother-daughter cysts (P = 0.254); however, EUS showed daughter cysts more clearly and more accurately assessed the number of daughter cysts (Table 2 and Figure 2).
Table 2.
Comparison of the number of daughter cysts visualized by endoscopic ultrasound, computed tomography and magnetic resonance imaging
| Number of daughter cysts | 1 | 2-3 | ≥ 4 | Total |
| CT | 36 | 2 | 13 | 51 |
| MRI | 36 | 9 | 18 | 63 |
| EUS | 29 | 15 | 23 | 67 |
EUS: Endoscopic ultrasound; CT: Computed tomography; MRI: Magnetic resonance imaging.
Figure 2.
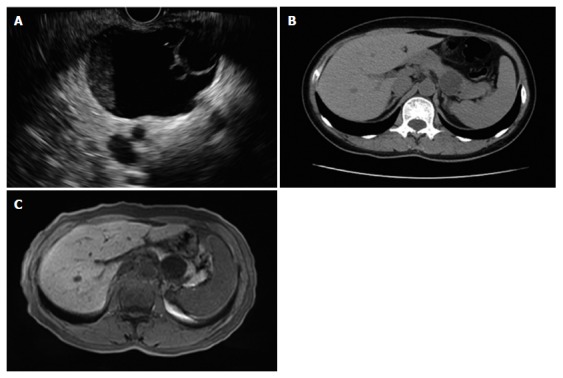
Endoscopic ultrasound, computed tomography and magnetic resonance imaging. A: EUS view. The mother cyst of 4.6 cm × 3.8 cm in the pancreatic tail consisted of two daughter cysts. B: CT image. No daughter cyst was revealed by CT in the same patient. C: MRI image. No daughter cyst was revealed by MRI in the same patient. EUS: Endoscopic ultrasound; CT: Computed tomography; MRI: Magnetic resonance imaging.
Detection rates of papilla/nodule, septum, and pancreatic cystic dilatation in PCNs
The papilla/nodule detection rate by EUS was as high as 35.3% (24/68), which was much higher compared to the detection rates of CT (5.8%, 3/52) and MRI (6.3%, 4/64). The detection rate of septum by EUS (60.3%, 41/68) was higher than those of both CT (34.6%, 18/52) and MRI (46.9%, 30/64). The detection rate of pancreatic cystic dilatation by EUS was 11.7% (8/68), compared with 21.2% (11/52) detected by CT and 21.9% (14/64) by MRI; however, there was no significant difference in the pancreatic cystic dilatation detection rate between EUS and CT (P = 0.163) or MRI (P = 0.119). EUS was able to reveal a pancreatic duct even when it was not dilated. The visualization rate of EUS for a normal pancreatic duct was 100.0%, whereas the visualization rate for a normal pancreatic duct using either CT or MRI were less than 10%. The comparison of detailed PCN characteristics as shown by CT, MRI, and EUS is shown in Table 3.
Table 3.
Comparison of detection rates of papilla/nodule, septum, and pancreatic cystic dilatation in pancreatic cystic neoplasms by computed tomography, magnetic resonance imaging, and endoscopic ultrasound
| CT | MRI | EUS |
P value |
||
| EUS vs CT | EUS vs MRI | ||||
| Papilla/nodule detection rate | 5.80% (3/52) | 6.30% (4/64) | 35.30% (24/68) | 0.000a | 0.000a |
| Septum detection rate | 34.60% (18/52) | 46.90% (30/64) | 60.30% (41/68) | 0.005a | 0.122 |
| Dilated pancreatic duct detection rate | 21.20% (11/52) | 21.90% (14/64) | 11.70% (8/68) | 0.163 | 0.119 |
P < 0.05, there is statistical significance between each other. EUS: Endoscopic ultrasound; CT: Computed tomography; MRI: Magnetic resonance imaging.
Comparison of characteristics between SCNs and MCNs by EUS
The papilla/nodule detection rate, septum detection rate, and dilated pancreatic duct detection rate of SCNs by EUS were 14.8% (4/27), 81.5% (22/27), and 14.8% (4/27), respectively. For MCNs, the papilla/nodule detection rate, septum detection rate, and dilated pancreatic duct detection rate were 47.8% (11/23), 56.5% (13/23), and 13.0% (3/23), respectively (Table 4). The presence of papillae/nodules seemed to be more common in MCNs than in SCNs (P = 0.011). There was no difference in the detection rates of a septum or a dilated pancreatic duct between SCNs and MCNs using EUS.
Table 4.
Comparison of the characteristics of pancreatic cystic neoplasms between serous cystic neoplasms and mucinous cystic neoplasms by endoscopic ultrasound
| EUS | SCNs | MCNs | P value |
| Papilla/nodule | 14.80% (4/27) | 47.80% (11/23) | 0.011a |
| Septum | 81.50% (22/27) | 56.50% (13/23) | 0.055 |
| Duct dilatation | 13.80% (4/27) | 13.0% (3/23) | 0.857 |
P < 0.05, there is statistical significance between each other. EUS: Endoscopic ultrasound; SCNs: Serous cystic neoplasms; MCNs: Mucinous cystic neoplasms.
DISCUSSION
The overall five-year survival rate of pancreatic cancer is approximately 5%[17], however, the prognoses of different types of PCNs vary. SCNs are regarded as benign with a rare possibility of malignant transformation, whereas MCNs and IPMNs are considered to have a malignant potential[18-20]. SPNs are low grade malignant lesions[21]. Therefore, timely and accurate diagnosis of PCNs is particularly important to prevent the progression of cystic neoplasms to cancer. Whether or not EUS imaging provides accurate diagnostic information regarding pancreatic cysts is controversial[7,11,22-29].
The results of our study indicate that EUS was the optimal diagnostic method in distinguishing non-neoplastic cysts from PCNs and in characterizing the PCNs, outperforming both CT and MRI. Similarly, Lu et al[23] found that EUS was able to identify PCNs better when compared to using CT and MRI. In our study, the sensitivity of EUS for diagnosing PCNs and the accuracy for characterizing PCNs were higher than those previously reported[23,30,31]. In our study, almost all of the patients had undergone CT or MRI prior to undergoing EUS; it is possible that the MRI or CT imaging reports may have influenced the diagnosis by EUS. Additionally, although we did not take the results of cystic fluid evaluation into consideration, the final diagnosis was made after fine needle aspiration (FNA) following EUS evaluation. The color and viscosity of the aspirate may have influenced the EUS report. Lastly, the endoscopists in the present study were experienced in EUS, particularly in PCNs, which could have had an effect on the final diagnosis by EUS.
In agreement with Leeds et al[32], our study showed that there were no significant differences between EUS and CT, or between EUS and MRI, in evaluating PCN size. However, this result is inconsistent with the results published by Maimone et al[33], who demonstrated that there was considerable variation in size estimates of pancreatic cysts among the imaging techniques and suggested that these discrepancies should be taken into account when making management decisions. Another previous study by Lee et al[11] indicated that CT was the best imaging modality to use, in comparison to MRI and EUS, with regard to evaluating PCN size. The discrepancy between our results and previously published results could be due to the experience of the operator; EUS requires experienced and skilled operators.
Some of the PCNs were mother-daughter cysts. While the images seen on CT and MRI are static, EUS allows for dynamic observation and high spatial resolution, which results in a more accurate estimation of the total number of daughter cysts in a mother cyst. In our study, EUS, CT, and MRI performed similarly in their ability to evaluate the number of PCNs; however, EUS was clearly more capable in determining the number of daughter cysts when compared to using CT. The detection of daughter cysts using MRI was similar to that of EUS. However, when there were more than four daughter cysts, it was more difficult to accurately count them with MRI, whereas EUS did well when counting between four and ten daughter cysts.
The papilla/nodule detection rate by EUS in our study was shown to be much higher than those by both CT and MRI. Previously, Lu et al[23] also showed that EUS was valuable in the characterization of mural nodules. The ability to detect a papilla/nodule is an important factor in the differentiation of an IPMN and its possible malignant potential. A previous study suggested that EUS had no advantages over MRI in identifying mural nodules, with a detection rate of 23% by pathology[34]. However, this study focused on PCLs and included 5% of pseudocysts. Additionally, this study only took mural nodules into consideration. These reasons may contribute to the different results. EUS demonstrated an advantage over CT in septum detection in our study, which is supported by a previous study[23]. Detection of a septum helps to differentiate a cystic neoplasm from a true cyst and a pseudocyst. No differences were found in the ability of EUS, CT, and MRI to detect pancreatic cystic dilatation. However, the rate of visualization of a normal pancreatic duct by EUS was 100%, while it was less than 10% using CT or MRI. The low detection rate of pancreatic cystic dilatation by EUS may be due to the fact that we did not measure the maximum diameter of the pancreatic duct during dynamic observation. In the field of vision in which the view of a cyst was the best, the pancreatic duct may not be clear or its diameter might not be at its maximum.
The septum detection rate and the cystic dilatation detection rate in SCNs were similar to those in MCNs. Previous reports also suggested that there were no significant differences between SCNs and MCNs in terms of mural nodules; however, only a small number of patients were included in these studies[35], which could explain why the results of our research suggest otherwise.
Our study suggests that EUS has some advantages in the diagnosis and classification of PCNs. Its ability to provide a clear, dynamic view of PCNs with high spatial resolution may contribute to its diagnostic value. EUS can also be used to guide FNA to collect cystic fluid and cystic tissue for biochemical analysis, cytology analysis, and pathological examination, which may also contribute to the diagnosis and the classification of PCNs[36,37]. EUS-guided SpyGlass can aid in the diagnosis and classification of PCNs[38]. EUS-guided ethanol and radiofrequency ablation are promising and minimally invasive treatments for patients with PCNs[39-45]. However, there are limitations to EUS. It is operator-dependent, which could result in inconsistent results among different operators[46]. EUS may also not be readily available in some hospitals. EUS is also an invasive examination that poses a higher risk than CT and MRI. Although this prospective study provides a relatively large sampling of patients, it is not without its limitations. Not all of the patients underwent all three imaging examinations. Additionally, very few patients were diagnosed with IPMNs and SPNs; additional patients are needed to provide more generalizable information regarding the use of EUS in these subtypes.
EUS was shown in this study to differentiate PCNs from other PCLs and characterize the PCN subtype far better than either CT or MRI. The measurement of cystic size using EUS was shown to be no different than when using CT and MRI. However, EUS was more accurate when determining the number of daughter cysts in mother cysts and had a higher detection rate of septum when compared to CT. The papilla/nodule detection rate and the visualization rate for a normal pancreatic duct by EUS were both superior when compared to using the other two diagnostic methods. These results indicate that EUS can be used to gain structural information regarding PCNs and is a valuable diagnostic tool.
COMMENTS
Background
About 60% of pancreatic cystic lesions (PCLs) are cystic neoplasms which have the potential to be malignant. The prevalence of pancreatic cystic neoplasms (PCNs) has been reported to range widely, from 0.21%-24.3%. Because some PCNs have a malignant potential, it is important to differentiate them from other non-neoplastic cysts and benign PCNs. Appropriate diagnosis with imaging modalities may help decide subsequent treatments and is thus extremely important.
Research frontiers
Computed tomography (CT) and magnetic resonance imaging (MRI) are most frequently used in the diagnosis of PCLs. However, endoscopic ultrasound (EUS) has an advantage over CT and MRI in distinguishing between benign pancreatic cystic lesions and malignant or potentially malignant ones. In our study, the inner detailed structures were well studied among three imaging modalities, which have not been reported in other studies.
Innovations and breakthroughs
The authors found that EUS did best in distinguishing non-neoplastic cysts from PCNs and characterize PCNs. There are many studied about the diagnostic value of imaging in PCLs, several studies are about its ability of differentiating PCNs from other PCLs. The measurement of cystic size of EUS has no difference from CT and MRI but EUS does better on judging the number of son-cyst in mother-cyst and has higher detection rates of septum than CT. Several studies compared imaging’s abilities of demonstrating son cysts. The papilla/nodule detection rate and visual rate for normal pancreatic duct of EUS is best among three diagnostic methods. We also found that papilla/nodule seemed to be more common in MCN than serous cystic neoplasms.
Applications
EUS is a valuable tool to help diagnose PCNs and to detail their structures. The patients suspected of having PCNs should be suggested to undergo EUS. This study provides additional evidence supporting the advantages of EUS compared with CT and MRI.
Terminology
A single tumor with unilocular or multilocular cysts (cyst in cyst) and cysts forming a cluster around a central scar (cyst on cyst) were both called a mother-daughter cyst. The mother-daughter cyst was regarded as a cyst.
Peer-review
This study evaluated the advantages of EUS in the assessment of detailed structures of pancreatic cystic neoplasms compared with CT and MRI. The manuscript is interesting.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was reviewed and approved by the Committee of Medical Ethics of Chinese People’s Liberation Army General Hospital.
Clinical trial registration statement: The study was registered at http://www.chictr.org.cn/showproj.aspx?proj=10604. The registration identification number is ChiCTR-OOC-15006118.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: There are no conflicts of interest in relation to this manuscript.
Data sharing statement: There are no additional data available in relation to this manuscript.
Peer-review started: January 5, 2017
First decision: February 9, 2017
Article in press: March 15, 2017
P- Reviewer: Kandulski A, Nusrat S S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang FF
References
- 1.Yoon WJ, Brugge WR. Endoscopic ultrasound and pancreatic cystic lesions-diagnostic and therapeutic applications. Endosc Ultrasound. 2012;1:75–79. doi: 10.7178/eus.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079–2084. doi: 10.1038/ajg.2010.122. [DOI] [PubMed] [Google Scholar]
- 4.Zhang XM, Mitchell DG, Dohke M, Holland GA, Parker L. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547–553. doi: 10.1148/radiol.2232010815. [DOI] [PubMed] [Google Scholar]
- 5.Vege SS, Ziring B, Jain R, Moayyedi P. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819–822; quize 12-13. doi: 10.1053/j.gastro.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Soroida Y, Sato M, Hikita H, Hagiwara S, Sato M, Gotoh H, Kato S, Iwai T, Yamazaki T, Yatomi Y, et al. Pancreatic cysts in general population on ultrasonography: Prevalence and development of risk score. J Gastroenterol. 2016;51:1133–1140. doi: 10.1007/s00535-016-1196-y. [DOI] [PubMed] [Google Scholar]
- 7.Kim JH, Eun HW, Park HJ, Hong SS, Kim YJ. Diagnostic performance of MRI and EUS in the differentiation of benign from malignant pancreatic cyst and cyst communication with the main duct. Eur J Radiol. 2012;81:2927–2935. doi: 10.1016/j.ejrad.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Rastegar N, Matteoni-Athayde LG, Eng J, Takahashi N, Tamm EP, Mortele KJ, Syngal S, Margolis D, Lennon AM, Wolfgang CL, et al. Incremental value of secretin-enhanced magnetic resonance cholangiopancreatography in detecting ductal communication in a population with high prevalence of small pancreatic cysts. Eur J Radiol. 2015;84:575–580. doi: 10.1016/j.ejrad.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kauhanen S, Rinta-Kiikka I, Kemppainen J, Grönroos J, Kajander S, Seppänen M, Alanen K, Gullichsen R, Nuutila P, Ovaska J. Accuracy of 18F-FDG PET/CT, Multidetector CT, and MR Imaging in the Diagnosis of Pancreatic Cysts: A Prospective Single-Center Study. J Nucl Med. 2015;56:1163–1168. doi: 10.2967/jnumed.114.148940. [DOI] [PubMed] [Google Scholar]
- 10.Yoshioka T, Shigekawa M, Yamai T, Suda T, Kegasawa T, Iwahashi K, Ikezawa K, Sakamori R, Yakushijin T, Hiramatsu N, et al. The safety and benefit of pancreatic juice cytology under ERCP in IPMN patients. Pancreatology. 2016;16:1020–1027. doi: 10.1016/j.pan.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Lee YS, Paik KH, Kim HW, Lee JC, Kim J, Hwang JH. Comparison of Endoscopic Ultrasonography, Computed Tomography, and Magnetic Resonance Imaging for Pancreas Cystic Lesions. Medicine (Baltimore) 2015;94:e1666. doi: 10.1097/MD.0000000000001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jani N, Bani Hani M, Schulick RD, Hruban RH, Cunningham SC. Diagnosis and management of cystic lesions of the pancreas. Diagn Ther Endosc. 2011;2011:478913. doi: 10.1155/2011/478913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones MJ, Buchanan AS, Neal CP, Dennison AR, Metcalfe MS, Garcea G. Imaging of indeterminate pancreatic cystic lesions: a systematic review. Pancreatology. 2013;13:436–442. doi: 10.1016/j.pan.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Brugge WR. Diagnosis and management of cystic lesions of the pancreas. J Gastrointest Oncol. 2015;6:375–388. doi: 10.3978/j.issn.2078-6891.2015.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsujino T, Yan-Lin Huang J, Nakai Y, Samarasena JB, Lee JG, Chang KJ. In vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy. Best Pract Res Clin Gastroenterol. 2015;29:601–610. doi: 10.1016/j.bpg.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Bhutani MS. Role of endoscopic ultrasound for pancreatic cystic lesions: Past, present, and future! Endosc Ultrasound. 2015;4:273–275. doi: 10.4103/2303-9027.170400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falasca M, Kim M, Casari I. Pancreatic cancer: Current research and future directions. Biochim Biophys Acta. 2016;1865:123–132. doi: 10.1016/j.bbcan.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:824–848.e22. doi: 10.1053/j.gastro.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Farrell JJ, Fernández-del Castillo C. Pancreatic cystic neoplasms: management and unanswered questions. Gastroenterology. 2013;144:1303–1315. doi: 10.1053/j.gastro.2013.01.073. [DOI] [PubMed] [Google Scholar]
- 21.Cho HW, Choi JY, Kim MJ, Park MS, Lim JS, Chung YE, Kim KW. Pancreatic tumors: emphasis on CT findings and pathologic classification. Korean J Radiol. 2011;12:731–739. doi: 10.3348/kjr.2011.12.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakai Y, Isayama H, Itoi T, Yamamoto N, Kogure H, Sasaki T, Hirano K, Tada M, Koike K. Role of endoscopic ultrasonography in pancreatic cystic neoplasms: where do we stand and where will we go? Dig Endosc. 2014;26:135–143. doi: 10.1111/den.12202. [DOI] [PubMed] [Google Scholar]
- 23.Lu X, Zhang S, Ma C, Peng C, Lv Y, Zou X. The diagnostic value of EUS in pancreatic cystic neoplasms compared with CT and MRI. Endosc Ultrasound. 2015;4:324–329. doi: 10.4103/2303-9027.170425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright GP, Morrow JB, Shaheen M, Goslin BJ, Baatenburg L, Chung MH. Accuracy of endoscopic ultrasound in the evaluation of cystic pancreatic neoplasms: a community hospital experience. Pancreas. 2014;43:465–469. doi: 10.1097/MPA.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 25.Arshad HM, Bharmal S, Duman DG, Liangpunsakul S, Turner BG. Advanced endoscopic ultrasound management techniques for preneoplastic pancreatic cystic lesions. J Investig Med. 2017;65:7–14. doi: 10.1136/jim-2016-000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, Topazian M, Takahashi N, Fletcher J, Petersen G, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804; quiz e14-e15. doi: 10.1053/j.gastro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hijioka S, Hara K, Mizuno N, Imaoka H, Bhatia V, Yamao K. Morphological differentiation and follow-up of pancreatic cystic neoplasms using endoscopic ultrasound. Endosc Ultrasound. 2015;4:312–318. doi: 10.4103/2303-9027.170423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adimoolam V, Sanchez MJ, Siddiqui UD, Yu S, Dzuira JD, Padda MS, Aslanian HR. Endoscopic ultrasound identifies synchronous pancreas cystic lesions not seen on initial cross-sectional imaging. Pancreas. 2011;40:1070–1072. doi: 10.1097/MPA.0b013e31821f65e3. [DOI] [PubMed] [Google Scholar]
- 29.Gerke H, Jaffe TA, Mitchell RM, Byrne MF, Stiffler HL, Branch MS, Baillie J, Jowell PS. Endoscopic ultrasound and computer tomography are inaccurate methods of classifying cystic pancreatic lesions. Dig Liver Dis. 2006;38:39–44. doi: 10.1016/j.dld.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Wamsteker EJ, Gauger PG, Thompson NW, Scheiman JM. EUS detection of pancreatic endocrine tumors in asymptomatic patients with type 1 multiple endocrine neoplasia. Gastrointest Endosc. 2003;58:531–535. doi: 10.1067/s0016-5107(03)01965-5. [DOI] [PubMed] [Google Scholar]
- 32.Leeds JS, Nayar MN, Dawwas M, Scott J, Anderson K, Haugk B, Oppong KW. Comparison of endoscopic ultrasound and computed tomography in the assessment of pancreatic cyst size using pathology as the gold standard. Pancreatology. 2013;13:263–266. doi: 10.1016/j.pan.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Maimone S, Agrawal D, Pollack MJ, Wong RC, Willis J, Faulx AL, Isenberg GA, Chak A. Variability in measurements of pancreatic cyst size among EUS, CT, and magnetic resonance imaging modalities. Gastrointest Endosc. 2010;71:945–950. doi: 10.1016/j.gie.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 34.Duconseil P, Turrini O, Ewald J, Soussan J, Sarran A, Gasmi M, Moutardier V, Delpero JR. ‘Peripheric’ pancreatic cysts: performance of CT scan, MRI and endoscopy according to final pathological examination. HPB (Oxford) 2015;17:485–489. doi: 10.1111/hpb.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SY, Lee JM, Kim SH, Shin KS, Kim YJ, An SK, Han CJ, Han JK, Choi BI. Macrocystic neoplasms of the pancreas: CT differentiation of serous oligocystic adenoma from mucinous cystadenoma and intraductal papillary mucinous tumor. AJR Am J Roentgenol. 2006;187:1192–1198. doi: 10.2214/AJR.05.0337. [DOI] [PubMed] [Google Scholar]
- 36.Alkaade S, Chahla E, Levy M. Role of endoscopic ultrasound-guided fine-needle aspiration cytology, viscosity, and carcinoembryonic antigen in pancreatic cyst fluid. Endosc Ultrasound. 2015;4:299–303. doi: 10.4103/2303-9027.170417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon WJ, Brugge WR. The safety of endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions. Endosc Ultrasound. 2015;4:289–292. doi: 10.4103/2303-9027.170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka SA, McKee JD, Conway WC. Intracystic Biopsy and Diagnosis of Intraductal Papillary Mucinous Neoplasm via SpyGlass Pancreatoscopy. Ochsner J. 2015;15:452–454. [PMC free article] [PubMed] [Google Scholar]
- 39.Pai M, Habib N, Senturk H, Lakhtakia S, Reddy N, Cicinnati VR, Kaba I, Beckebaum S, Drymousis P, Kahaleh M, et al. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52–59. doi: 10.4240/wjgs.v7.i4.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caillol F, Poincloux L, Bories E, Cruzille E, Pesenti C, Darcha C, Poizat F, Monges G, Raoul JL, Bommelaer G, et al. Ethanol lavage of 14 mucinous cysts of the pancreas: A retrospective study in two tertiary centers. Endosc Ultrasound. 2012;1:48–52. doi: 10.7178/eus.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park JK, Song BJ, Ryu JK, Paik WH, Park JM, Kim J, Lee SH, Kim YT. Clinical Outcomes of Endoscopic Ultrasonography-Guided Pancreatic Cyst Ablation. Pancreas. 2016;45:889–894. doi: 10.1097/MPA.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 42.Oh HC, Seo DW, Song TJ, Moon SH, Park DH, Soo Lee S, Lee SK, Kim MH, Kim J. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology. 2011;140:172–179. doi: 10.1053/j.gastro.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Gan SI, Thompson CC, Lauwers GY, Bounds BC, Brugge WR. Ethanol lavage of pancreatic cystic lesions: initial pilot study. Gastrointest Endosc. 2005;61:746–752. doi: 10.1016/s0016-5107(05)00320-2. [DOI] [PubMed] [Google Scholar]
- 44.DeWitt J, McGreevy K, Schmidt CM, Brugge WR. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: a randomized, double-blind study. Gastrointest Endosc. 2009;70:710–723. doi: 10.1016/j.gie.2009.03.1173. [DOI] [PubMed] [Google Scholar]
- 45.DeWitt J, DiMaio CJ, Brugge WR. Long-term follow-up of pancreatic cysts that resolve radiologically after EUS-guided ethanol ablation. Gastrointest Endosc. 2010;72:862–866. doi: 10.1016/j.gie.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 46.Ahmad NA, Kochman ML, Brensinger C, Brugge WR, Faigel DO, Gress FG, Kimmey MB, Nickl NJ, Savides TJ, Wallace MB, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest Endosc. 2003;58:59–64. doi: 10.1067/mge.2003.298. [DOI] [PubMed] [Google Scholar]


