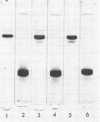
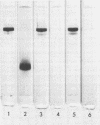
Abstract
Ribulose 1,5-diphosphate carboxylase was isolated from Euglena gracilis Klebs strain Z Pringsheim, Chlorella fusca var. vacuolata, and Chlamydobotrys stellata, and the subunits from each enzyme were separated and purified by gel filtration on Sephadex G-200 in the presence of sodium dodecyl sulfate. Rabbit antibody was elicited against purified Euglena ribulose 1,5-diphosphate carboxylase whole enzyme and the isolated large and small subunits. Euglena ribulose 1,5-diphosphate carboxylase showed partial immunological identity on Ouchterlony gels with the Chlorella and Chlamydobotrys carboxylases. Analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of immunoprecipitates between antibody to the Euglena large subunit and the isolated large subunits of the Chlorella and Chlamydobotrys enzymes showed this was due to determinants on the large subunit. There was no serological affinity between the small subunits of the Euglena, Chlorella, and Chlamydobotrys carboxylases, and NH2-terminal amino acid analyses provided further evidence of variability in the structure of the small subunits.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casola L., Di Matteo G., Di Prisco G., Cervone F. Dansyl chloride binding to proteins. Quantitative estimation of N-terminal, lysyl, and tyrosyl residues by the radioactive reagent. Anal Biochem. 1974 Jan;57(1):38–45. doi: 10.1016/0003-2697(74)90047-5. [DOI] [PubMed] [Google Scholar]
- Chan P. H., Wildman S. G. Chloroplast DNA codes for the primary structure of the large subunit of fraction I protein. Biochim Biophys Acta. 1972 Sep 14;277(3):677–680. doi: 10.1016/0005-2787(72)90115-3. [DOI] [PubMed] [Google Scholar]
- Davis B., Merrett M. J. The glycolate pathway and photosynthetic competence in euglena. Plant Physiol. 1975 Jan;55(1):30–34. doi: 10.1104/pp.55.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givan A. L., Criddle R. S. Ribulosediphosphate carboxylase from Chlamydomonas reinhardi: purification, properties and its mode of synthesis in the cell. Arch Biochem Biophys. 1972 Mar;149(1):153–163. doi: 10.1016/0003-9861(72)90309-8. [DOI] [PubMed] [Google Scholar]
- Gray J. C., Kerwick R. G. An immunological investigation of the structure and function of ribulose 1,5-bisphosphate carboxylase. Eur J Biochem. 1974 May 15;44(2):481–489. doi: 10.1111/j.1432-1033.1974.tb03506.x. [DOI] [PubMed] [Google Scholar]
- Kawashima N., Kwok S. Y., Wildman S. G. Studies on fraction-I protein. 3. Comparison of the primary structure of the large and small subunits obtained from five species of Nicotiana. Biochim Biophys Acta. 1971 Jun 29;236(3):578–586. doi: 10.1016/0005-2795(71)90242-x. [DOI] [PubMed] [Google Scholar]
- Kawashima N., Wildman S. G. Studies on fraction I protein. IV. Mode of inheritance of primary structure in relation to whether chloroplast or nuclear DNA contains the code for a chloroplast protein. Biochim Biophys Acta. 1972 Feb 23;262(1):42–49. doi: 10.1016/0005-2787(72)90217-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lord J. M., Armitage T. L., Merrett M. J. Ribulose 1,5-Diphosphate Carboxylase Synthesis in Euglena: II. Effect of Inhibitors on Enzyme Synthesis during Regreening and Subsequent Transfer to Darkness. Plant Physiol. 1975 Nov;56(5):600–604. doi: 10.1104/pp.56.5.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Brown R. H. Purification and Some Properties of Chlorella fusca Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1975 Feb;55(2):360–364. doi: 10.1104/pp.55.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Merrett M. J. Ribulose diphosphate carboxylase synthesis in euglena: increased enzyme activity after transferring regreening cells to darkness. Plant Physiol. 1975 May;55(5):890–892. doi: 10.1104/pp.55.5.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden B. A. Autotrophic CO2 assimilation and the evolution of ribulose diphosphate carboxylase. Bacteriol Rev. 1973 Sep;37(3):289–319. doi: 10.1128/br.37.3.289-319.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden B. A., Lord J. M., Rowe A., Dilks S. Composition, quaternary structure, and catalytic properties of D-ribulose-1, 5-bisphosphate carboxylase from Euglena gracilis. Eur J Biochem. 1975 May;54(1):195–206. doi: 10.1111/j.1432-1033.1975.tb04129.x. [DOI] [PubMed] [Google Scholar]
- Merrett M. J. Enzyme levels in relation to obligate phototrophy in chlamydobotrys. Plant Physiol. 1976 Aug;58(2):179–181. doi: 10.1104/pp.58.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz H., Reisfeld A., Sagher D., Edelman M. Ribulose Diphosphate Carboxylase from Autotrophic Euglena gracilis. Plant Physiol. 1975 Sep;56(3):345–350. doi: 10.1104/pp.56.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramshaw J. A., Thompson E. W., Boulter D. The amino acid sequence of Helianthus annuus L. (sunflower) cyrochrome c deduced from chymotryptic peptides. Biochem J. 1970 Sep;119(3):535–539. doi: 10.1042/bj1190535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutner A. C., Lane M. D. Nonidentical subunits of ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1967 Aug 23;28(4):531–537. doi: 10.1016/0006-291x(67)90346-4. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Ito T., Akazawa T. Subunit structure of ribulose 1,5-diphosphate carboxylase from Chlorella ellipsoidea. Biochemistry. 1971 Aug 31;10(18):3406–3411. doi: 10.1021/bi00794a014. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]