Abstract
Background and objective: Umbilical cord (UC)-derived mesenchymal stem cells (MSCs) have shown immunoregulation of various immune cells. The aim of this study was to investigate the mechanism of UC MSCs in the regulation of peripheral regulatory T cells (Treg) and T helper 17 (Th17) cells in patients with systemic lupus erythematosus (SLE). Methods: Thirty patients with active SLE, refractory to conventional therapies, were given UC MSCs infusions. The percentages of peripheral blood CD4+CD25+Foxp3+ regulatory T cells (Treg) and CD3+CD8-IL17A+ Th17 cells and the mean fluorescence intensities (MFI) of Foxp3 and IL-17 were measured at 1 week, 1 month, 3 months, 6 months, and 12 months after MSCs transplantation (MSCT). Serum cytokines, including transforming growth factor beta (TGF-β), tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and IL-17A were detected using ELISA. Peripheral blood mononuclear cells from patients were collected and co-cultured with UC MSCs at ratios of 1:1, 10:1, and 50:1, respectively, for 72 h to detect the proportions of Treg and Th17 cells and the MFIs of Foxp3 and IL-17 were determined by flow cytometry. The cytokines in the supernatant solution were detected using ELISA. Inhibitors targeting TGF-β, IL-6, indoleamine 2,3-dioxygenase (IDO), and prostaglandin E2 were added to the co-culture system, and the percentages of Treg and Th17 cells were observed. Results: The percentage of peripheral Treg and Foxp3 MFI increased 1 week, 1 month, and 3 months after UC MSCs transplantation, while the Th17 proportion and MFI of IL-17 decreased 3 months, 6 months, and 12 months after the treatment, along with an increase in serum TGF-β at 1 week, 3 months, and 12 months and a decrease in serum TNF-α beginning at 1 week. There were no alterations in serums IL-6 and IL-17A before or after MSCT. In vitro studies showed that the UC MSCs dose-dependently up-regulated peripheral Treg proportion in SLE patients, which was not depended on cell–cell contact. However, the down-regulation of Th17 cells was not dose-dependently and also not depended on cell–cell contact. Supernatant TGF-β and IL-6 levels significantly increased, TNF-α significantly decreased, but IL-17A had no change after the co-culture. The addition of anti-TGF-β antibody significantly abrogated the up-regulation of Treg, and the addition of PGE2 inhibitor significantly abrogated the down-regulation of Th17 cells. Both anti-IL-6 antibody and IDO inhibitor had no effects on Treg and Th17 cells. Conclusions: UC MSCs up-regulate Treg and down-regulate Th17 cells through the regulation of TGF-β and PGE2 in lupus patients.
Keywords: mesenchymal stem cells, regulatory T cells, systemic lupus erythematosus, T helper 17 cells, umbilical cord
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by polyclonal activation of B and T lymphocytes. In addition to Th1 and Th2 cells imbalance, the roles of regulatory T cells (Treg) and T helper 17 (Th17) cells were found played important roles in the pathogenesis of SLE.1 Either quantitative and/or qualitative deficiencies of Treg were found in active lupus patients, and which were correlated with clinical disease activity as well as organ involvement.2,3 Furthermore, the number of Th17 cells, as well as Th17-related cytokines such as interleukin 17 (IL-17), IL-6, were found increased in SLE patients.4 Moreover, the induction of SLE by pristane in IL-17 sufficient wild-type mice did not occur in IL-17 deficient mice, which were protected from development of lupus autoantibodies and glomerulonephritis,5 suggesting that Th17 cells participated in lupus pathogenesis.
Mesenchymal stem cells (MSCs) are a heterogeneous population of fibroblast-like, multipotent cells characterized by their ability to differentiate in vitro and in vivo into tissues of the mesodermal lineage. Nowadays, it is well established that human MSCs possess immunomodulatory properties on T cells, B cells, dendritic cells, macrophages, and natural killer cells,6 which makes MSCs an attractive tool for cell-based therapies in graft vs. host disease (GVHD) and autoimmune diseases.7,8 Recently, several key mechanisms have been described contributing to the regulation of MSCs on Treg and Th17 cells. Human MSCs induced the trimethylation of histone H3K4 at the promoter of the Foxp3 gene locus, whereas it suppressed trimethylation of the corresponding region in the RORC gene in mixed lymphocyte reactions. These epigenetic changes were associated with the induction of Foxp3+ Treg and inhibition of Th17 cells, which was enhanced when MSCs were pre-incubated with interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α).9 However, the regulation of MSCs on Treg/Th17 cells balance in an autoimmune-diseased environment is unknown.
In the present study, we found that in lupus microenvironment, umbilical cord (UC)-derived MSCs could secrete some functional factors to rebalance the aberrant Treg- and Th17-cell proportions.
Materials and methods
Lupus patients and healthy subjects
Totally, 51 SLE patients and 39 healthy subjects were included in this study. All the subjects were given informed consents for the collection of peripheral blood. Clinical study of UC mesenchymal stem cell transplantation (MSCT) for lupus patients was registered in ClinicalTrial. gov (identifier: NCT01741857). Thirty patients underwent MSCs transplantation as previously described.10,11 This study was approved by the Ethics Committee at The Affiliated Drum Tower Hospital of Nanjing University Medical School and was conducted in accordance with the principle set forth under the 1989 Declaration of Helsinki.
Antibodies and reagents
The following antibodies (to human) were from BD Biosciences (BD Pharmingen, Fremont, CA, USA): fluorescein isothiocyanate (FITC)-conjugated anti-human CD3 (OKT3), anti-CD4 (11830), phycoerythrin (PE)-conjugated anti-human CD4 (11830), IL-17A, allophycocyanin (APC)-conjugated anti-human CD8 (RPA-T8), CD25 (M-A251), and their respectively isotype-matched control antibodies (mouse IgG1, mouse IgG2a). The following antibodies were from eBioscience (San Diego, CA, USA): PE-Cy7-conjugated Foxp3 (PCH101), FITC-conjugated anti-human CD29, CD73, CD90, PE-conjugated anti-human CD45, CD34, CD14 anti-CD79, and APC-conjugated anti-human CD105 and anti-HLA-DR. Purified anti-human CD3 (OKT3), CD28 (CD28.2) (no azide and low endotoxin), anti-human TGF-β and IL-6 antibodies were all from R&D Systems (Minneapolis, MN, USA). PGE2 inhibitor, CAY10404, was from Cayman Chemical. 1-Methyl-DL-tryptophan (1-MT, indoleamine 2,3-dioxygenase (IDO) inhibitor) was from Sigma-Aldrich (St. Louis, MO, USA). Human TGF-β1, TNF-α, IL-6, IL-17 ELISA kits were from BioLegend (San Diego, CA, USA).
Isolation and culture of umbilical cord-derived MSCs
Fresh UCs were obtained from informed consents and healthy mothers in local maternity hospitals after normal deliveries. The cords were rinsed by phosphate-buffered saline (PBS), and cord blood was removed. The washed cords were cut into 1 mm2-sized pieces and subsequently incubated at 37 °C in humid air with 5% CO2 in Dulbecco's modified Eagle's medium with low glucose containing 10% fetal bovine serum (FBS). Non-adherent cells were removed by washing. After 10 days, fibroblast-like cells appeared and were trypsinised and passaged into a new flask for further expansion. Cell surface markers were assessed by flow cytometric analysis showing that CD29, CD73, CD90, and CD105 expression was >95%, in parallel with CD45, CD34, CD14, CD79, and HLA-DR expression <2%. Moreover, the capacity of MSC that differentiate along adipogenic and osteogenic lineages was also assayed in vitro.
Isolation and culture of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were isolated from active lupus patients and healthy controls at the same time point, then were co-cultured with UC-MSCs for 72 h at ratios of 1:1, 10:1, and 50:1, respectively. In some experiments, anti-TGF-β antibody, anti-IL-6 antibody, PGE2 inhibitor (CAY10404), and/or IDO inhibitor (1-MT) were added to neutralize cytokine, and transwell system (0.4 μM pore size, Millipore) was used to block cell–cell contact. After 3-day culture, cells were harvested for examining by flow cytometry.
Flow cytometry analysis and ELISA
PBMCs were resuspended in PBS containing 1% bovine serum albumin and 0.1% sodium azide. For the staining of surface antigens, cells were incubated with FITC-, PE-, PE-Cy7-, or APC-conjugated monoclonal antibodies or their negative control antibodies as indicated for 30 min on ice. Intracellular staining of Foxp3 and IL-17A was performed. We detected the amounts of TGF-β1, TNF-α, IL-6, and IL-17 in the conditioned media and/or human serum with ELISA Kits (eBioscience or BioLegend) according to the manufacturer's instructions.
Quantitative real-time polymerase chain reaction
PBMCs from healthy controls (control) or lupus patients pre- and 1 week post-MSC transplantation were collected. Complimentary DNA (cDNA) was synthesized from TRIzol-isolated total RNA by use of the SuperScript III First-Strand Synthesis SuperMix for quantitative reverse-transcribed polymerase chain reaction (qRT-PCR, Takara). For real-time PCR experiments, reactions containing the SYBR Premix EX Taq (Takara Dalian, China), ROX Reference Dye (50×, Takara), cDNA, and gene primers were run on the StepOnePlus Real-Time PCR Systems and analyzed with StepOne Software V2.1 (Applied Biosystems New York, USA). Gene primers were listed in Table 1. The relative gene quantification was done using the 2−ΔΔCt method following normalization to glyceraldehyde-3-phosphate dehydrogenase of healthy control group.
Table 1. Primers for real-time PCR.
| Gene | Forward | Reverse |
|---|---|---|
| TGF-β | 5′-AGCGACTCGCCAGAGTGGTTA-3′ | 5′-GCAGTGTGTTATCCCTGCTGTCA-3′ |
| COX-2 | 5′-TGACCAGAGCAGGCAGATGAA-3′ | 5′-CCACAGCATCGATGTCACCATAG-3′ |
| IL-6 | 5′-AAGCCAGAGCTGTGCAGATGAGTA-3′ | 5′-TGTCCTGCAGCCACTGGTTC-3′ |
| IDO | 5′-GAATGGCACACGCTATGGAA-3′ | 5′-CAGACTCTATGAGATCAGGCAGATG-3′ |
| RORC | 5′-CTGCAAGACTCATCGCCAAAG-3′ | 5′-TTTCCACATGCTGGCTACACA-3′ |
| IL23R | 5′-GCAAACGCACTAGGCATGGA-3′ | 5′-TGGTCTTGGGCACTGTAGCATTTA-3′ |
| Foxp3 | 5′-CTCCTACCCACTGCTGGCAAAT-3′ | 5′-CCCTGCCCTTCTCATCCAGA-3′ |
| GAPDH | 5′-GCACCGTCAAGGCTGAGAAC-3′ | 5′-TGGTGAAGACGCCAGTGGA-3′ |
TGF-β spliced leader RNA silencing
The expression of TGF-β by MSCs was down-regulated by RNA interference technique. TGF-β spliced leader RNA (siRNA) and non-target control were synthesized by Thermo Dharmacon Scientific (Waltham, MA, USA) (TGF-β siRNA sequence: AUUGAGGGCUUUCGCCUUA-CCGAGAAGCGGUACCUGAA-GCAGAGUACACACAGCAUA-GGACUAUCCACCUGCAAGA, non-target control sequence: UGGUUUACAUGUCGACUAA-UGGUUUACAUGUUGUGUGA-UGGUUUACAUGUUUUCUGA-UGGUUUACAUGUUUUCCUA). SiRNAs were transfected into cells with Lipofectamine 2000 (Invitrogen Carlsbad, CA, USA) according to the instruction and the interference effect was examined by real-time PCR.
Statistical analysis
We used the t-test for statistical analysis for parametric data and the Mann–Whitney U test for non-parametric data. One-way analysis of variance was used when there were more than two groups, and then followed by Bonferroni test among different groups. We performed statistical analyses with SPSS16.0 software and GraphPad Prism 4.3 and considered a P value less than 0.05 as significant. Data are shown as means ± standard error of mean.
Results
Patients information
Thirty SLE patients who were refractory to conventional therapies underwent UC MSCT, in which 26 patients were reported previously.11 Another 21 lupus patients with active disease were enrolled for the collection of peripheral blood for in vitro experiments. The clinical features of the SLE patients as well as healthy controls were listed in Table 2.
Table 2. Clinical features of lupus patients and healthy controls.
| SLE patients | Healthy controls | |
|---|---|---|
| Number | 51 | 39 |
| Gender (female/male) | 48/3 | 37/2 |
| Age (years) | 17–56 (mean 48 ± 11) | 21–55 (mean 45 ± 6) |
| Disease duration (month) | 6–108 (mean 32) | NA |
| Baseline SLEDAI score | 9–20 (mean 11.5) | NA |
NA, not available.
UC MSCs modulate Treg and Th17 cells in human SLE in vivo
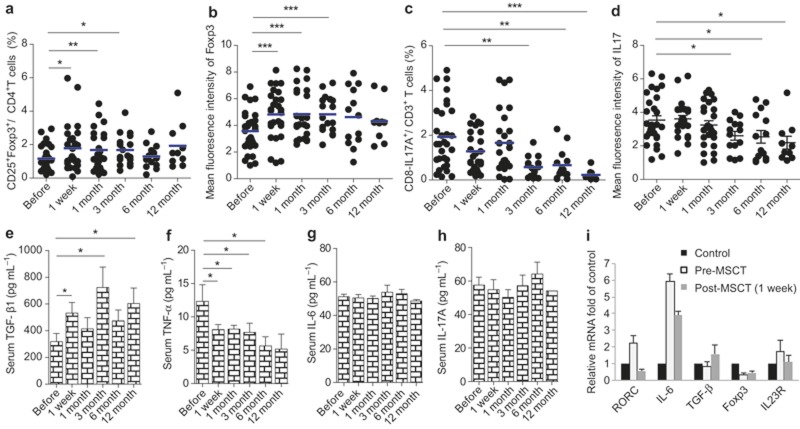
The peripheral blood CD25+Foxp3+/CD4+ T cells as well as CD8-IL17A+/CD3+ T cells percentages were analyzed before MSCT and 1 week, 1 month, 3 months, 6 months, and 12 months after MSCT. We found that both the percentage of CD25+Foxp3+/CD4+ T cells and the mean fluorescence intensities (MFI) of Foxp3 significantly increased at 1 week, 1 month, and 3 month after MSCs treatment (Figure 1a and b, Supplementary Figure S1a), while the percentage of CD8-IL17A+/CD3+T cells and the MFI of IL-17 decreased, with statistical differences at 3-, 6-, and 12-month follow-ups (Figure 1c and d, Supplementary Figure S1b). We also assessed the changes of Treg and Th17 cells in responders and non-responders in those patients who underwent UC MSCs transplantation, and found similar changes between two groups (Supplementary Figure S2). Serum different cytokines were detected and we found serum TGF-β increased at 1week, 3-month, and 12-month visits (Figure 1e). Serum TNF-α levels significantly decreased since 1 week after UC MSCs treatment (Figure 1f), while serum IL-6 and IL-17A had no changes after MSCT (Figure 1g and h). We also analyzed regulatory T cells and Th17 cells-related transcriptional factors, including RORC, Foxp3, IL-6, TGF-β, and IL23R pre- and post-MSC treatments by real-time PCR, and found that the gene expression of RORC decreased, while TGF-β increased 1 week after MSCT (Figure 1i). These data suggested that UC MSCs transplantation modulated Treg and Th17 cells, as well as their related cytokines, in drug-resistant lupus patients.
Figure 1.
UC MSCs up-regulate Treg and down-regulate Th17 cell in lupus patients in vivo. Thirty refractory lupus patients were given UC MSCs transplantation. The peripheral CD4+CD25+Foxp3+ Treg percentage (a), the mean fluorescence of Foxp3 (b), the CD3+CD8-IL-17A+ Th17 cell percentage (c), and the mean fluorescence of IL-17 (d) were assessed by flow cytometry at different visit times. The serum levels of TGF-β (e), TNF-α (f), IL-6 (g), and IL-17A (h) were determined using ELISA. cDNA was synthesized from the PBMC of patients pre- and post-MSCT, and the relative mRNA expression of RORC, IL-6, TGF-β, Foxp3, and IL-23R was assessed by real-time PCR. PBMCs from healthy individuals were used as the control (I). *P < 0.05, **P < 0.01, ***P < 0.001.
The role of UC MSCs on Treg and Th17 cells in vitro in SLE
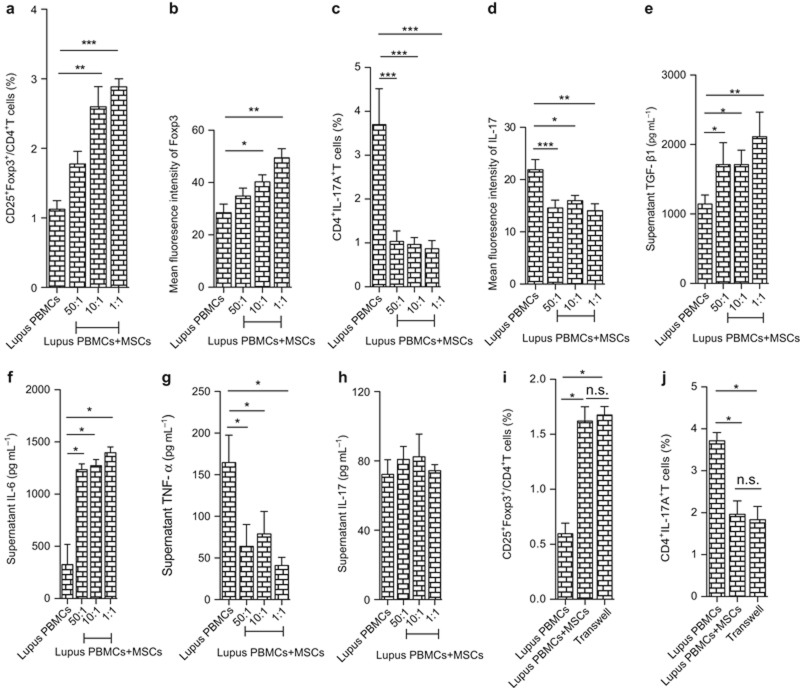
Next, we wanted to verify the regulatory effects on Treg and Th17 cells by MSCs in vitro. PBMCs were isolated and co-cultured with UC MSCs at different ratios (1:1, 10:1, 50:1) in vitro for 72 h. Our data showed that UC MSCs could significantly up-regulate the CD25+Foxp3+/CD4+ Treg percentage (Figure 2a, Supplementary Figure S3a), as well as the MFI of Foxp3 (Figure 2b), while down-regulating the CD4+IL17+ T-cell percentage (Figure 2c, Supplementary Figure S3b), and the MFI of IL-17 (Figure 2d) in lupus PBMC, in which the up-regulation of Treg cells occurred in a dose-dependent manner (Figure 2a and b). TGF-β and IL-6 in the culture supernatant increased, while TNF-α decreased in the presence of UC MSCs and IL-17 showed no change (Figure 2e–h). Moreover, the modulation of both Treg and Th17 cells was not dependent on cell–cell contact (Figure 2g and h). These data suggested that certain soluble factors mediated the regulatory effects of UC MSCs on Treg and Th17 cells in lupus PBMC.
Figure 2.
UC MSCs up-regulate Treg and down-regulate Th17 cell in lupus patients in vitro. PBMCs from lupus patients were co-cultured with UC MSCs at different ratios (50:1, 10:1, 1:1); 72 h later, the percentages of CD4+CD25+Foxp3+ Treg (a) and CD4+IL17A+ Th17 (b) were assessed by flow cytometry. The culture supernatant levels of TGF-β (c), IL-6 (d), TNF-α (e), and IL-17A (f) were determined using ELISA. In the co-cultures of lupus PBMC and UC MSCs (10:1), transwells were used, and 72 h later, the percentages of CD4+CD25+Foxp3+ Treg (g) and CD4+IL17A+ Th17 (h) were assessed by flow cytometry. *P < 0.05, **P < 0.01, ***P < 0.001, n.s., no significance.
Soluble factors increase in the lupus microenvironment
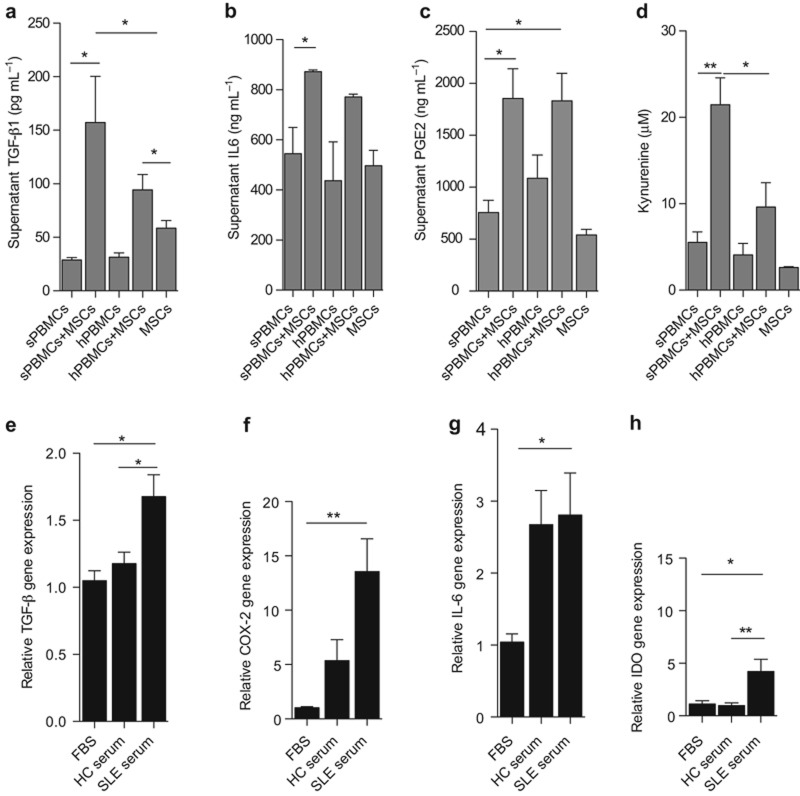
Based on the transwell culture system, we know that soluble factors may mediate the regulation of Treg and Th17 cells in lupus patients by UC MSCs. The currently reported factors mostly involve TGF-β, IL-6, PGE2, and IDO, so next we wanted to discover whether these factors increased in the co-culture of UC MSCs with lupus PBMC and healthy PBMC. After 72 h, the culture supernatant levels of TGF-β, IL-6, and PEG2 were assessed using ELISA. The IDO enzyme activity that was evaluated by kynurenine levels was detected by high-performance liquid chromatography (HPLC), as previously reported. Our results showed that all of these molecules increased in the culture supernatants when UC MSCs were co-cultured with lupus PBMC (Figure 3a–d). The production of these functional factors was mediated by the interactions between lupus PBMC and UC MSCs because neither patient PBMC nor UC MSCs secreted large levels of these cytokines alone. These data underscored the finding that the lupus microenvironment promotes UC MSCs to produce functional factors.
Figure 3.
SLE patients' PBMC and sera enhance UC MSCs to produce functional factors. PBMC from active lupus patients (sPBMC) and healthy individuals (hPBMC) were co-cultured with UC MSCs (10:1); 72 h later, the culture supernatants were collected and the levels of TGF-β (a), IL-6 (b), and PGE2 (c) were assessed by ELISA. The culture supernatant kynurenine levels were assessed by HPLC (d). Sera from healthy controls and active SLE patients were collected and used to stimulate UC MSCs in vitro at a concentration of 10%. FBS was used as control. After 48 h, the mRNA levels of TGF-β (e), COX-2 (f), IL-6 (g), and IDO (h) were analyzed by real-time PCR. *P < 0.05, **P < 0.01.
To confirm our hypothesis, serum from both active lupus patients and healthy subjects was used to treat UC MSCs, and FBS was used as a control. After 48 h, the production of TGF-β, IL-6, PGE2, and IDO was detected using real-time PCR. Consistent with the above results, lupus serum promoted UC MSCs to secrete higher levels of TGF-β, COX-2, IL-6, and IDO compared with FBS, where both TGF-β and IDO significantly increased when stimulated by lupus serum compared with healthy serum-stimulated cultures (Figure 3e–h).
TGF-β produced by MSCs mediates the up-regulation of Treg
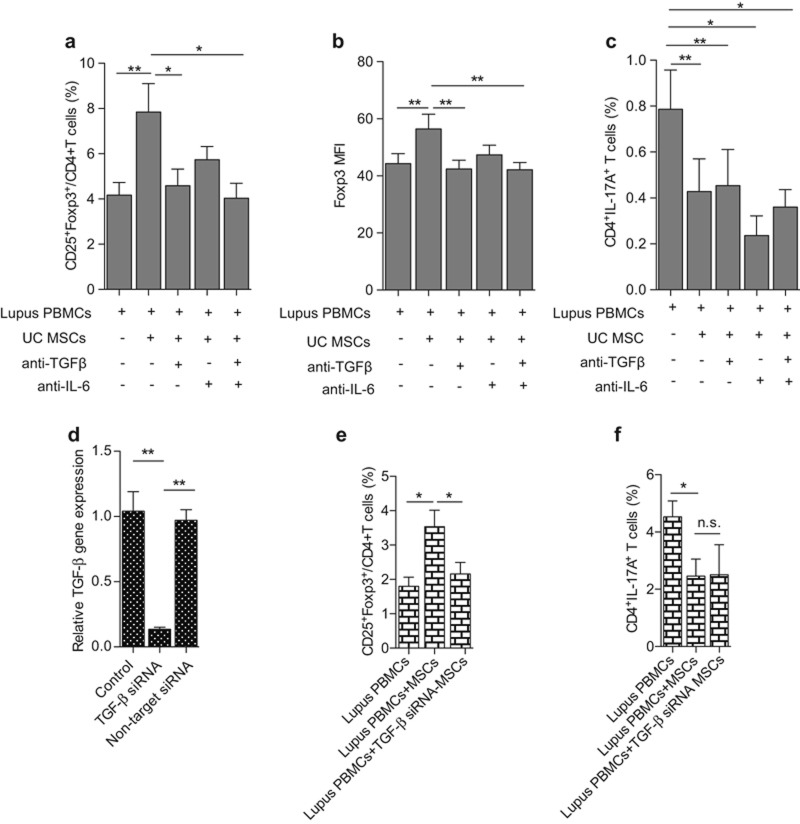
First, we wanted to know which factor mediates the regulation of Treg by MSCs in SLE. Two antibodies that target TGF-β and IL-6 were used. In the co-culture of UC MSCs and lupus PBMC, TGF-β, or IL-6 antibodies (5 µg mL−1 and 10 µg mL−1, respectively) was added. After 72 h, we found that the addition of anti-TGF-β antibody could significantly abrogate the up-regulation of Treg by MSCs (PBMC: 4.17% ± 1.59%, PBMC + MSCs: 7.85% ± 3.54%, PBMC + MSCs + anti-TGFβ: 4.58% ± 2.10%, PBMC + MSCs + anti-IL6: 5.74% ± 1.65%, PBMC + MSCs + anti-TGFβ + anti-IL6: 4.03% ± 1.87%; PBMC vs. PBMC + MSCs P = 0.0022, PBMC + MSCs vs. PBMC + MSCs + anti-TGFβ P = 0.026, PBMC + MSCs vs. PBMC + MSCs + anti-TGFβ + anti-IL6 P = 0.0166) (Figure 4a, Supplementary Figure S4a). Moreover, the Foxp3 MFI also markedly decreased in the presence of the anti-TGF-β antibody (PBMC: 44.26 ± 9.86, PBMC + MSCs: 56.40 ± 14.61, PBMC + MSCs + anti-TGFβ: 42.42 ± 8.64, PBMC + MSCs + anti-IL6: 47.35 ± 9.54, PBMC + MSCs + anti-TGFβ + anti-IL6: 42.16 ± 7.16; PBMC vs. PBMC + MSCs P = 0.0041, PBMC + MSCs vs. PBMC + MSCs + anti-TGFβ P = 0.0049, PBMC + MSCs vs. PBMC + MSCs + anti-TGFβ + anti-IL6 P = 0.0036) (Figure 4b). However, neither the anti-TGF-β nor the anti-IL6 antibody reversed the effect on the down-regulation of Th17 cells by UC MSCs (PBMC: 0.79% ± 0.38%, PBMC + MSCs: 0.43% ± 0.32%, PBMC + MSCs + anti-TGFβ: 0.45% ± 0.35%, PBMC + MSCs + anti-L6: 0.23% ± 0.19%, PBMC + MSCs + anti-TGFβ + anti-IL6: 0.36% ± 0.17%; PBMC vs. PBMC + MSCs P = 0.0029, PBMC vs. PBMC + MSCs + anti-TGFβ P = 0.0035, PBMC vs. PBMC + MSCs + anti-IL6 P = 0.0112, PBMC vs. PBMC + MSCs + anti-TGFβ + anti-IL6 P = 0.0246) (Figure 4c, Supplementary Figure S4b). To confirm the effect of TGF-β on modulating Treg in lupus patients, TGF-β siRNA was used to down-regulate TGF-β expression on UC MSCs (Figure 4d). The results revealed that TGF-β siRNA-UC MSCs lost the ability to up-regulate Treg cells (PBMC: 1.80% ± 0.65%, PBMC + MSCs: 3.53% ± 1.17%, PBMC + TGFβsiRNA-MSCs: 2.16% ± 0.82%; PBMC vs. PBMC + MSCs P = 0.0100, PBMC + MSCs vs. PBMC + TGFβsiRNA − MSCs P = 0.0405) (Figure 4e) but did not affect Th17 cells (PBMC: 4.53% ± 0.96%, PBMC + MSCs: 2.46% ± 1.03%, PBMC + TGFβsiRNA − MSCs: 2.50% ± 1.82%) (Figure 4f). These data suggested that TGF-β-mediated Treg modulation by UC MSCs in lupus patients.
Figure 4.
TGF-β mediates the up-regulation on Treg cells in lupus patients. In the co-culture of lupus patients PBMC and UC MSCs (10:1), an anti-TGF-β antibody and an anti-IL6 antibody, alone or in combination, was added; 72 h later, the percentages of CD4+CD25+Foxp3+ Treg (a), CD4+IL17A+ Th17 (c), and the MFI of Foxp3 (b) were analyzed by flow cytometry. TGF-β siRNA-UC MSCs were used to down-regulate TGF-β expression on UC MSCs (d), and the percentages of CD4+CD25+Foxp3+ Treg (e) and CD4+IL17A+ Th17 (f) were assessed by flow cytometry. *P < 0.05, **P < 0.01.
PGE2 produced by MSCs mediates the down-regulation of Th17 cells
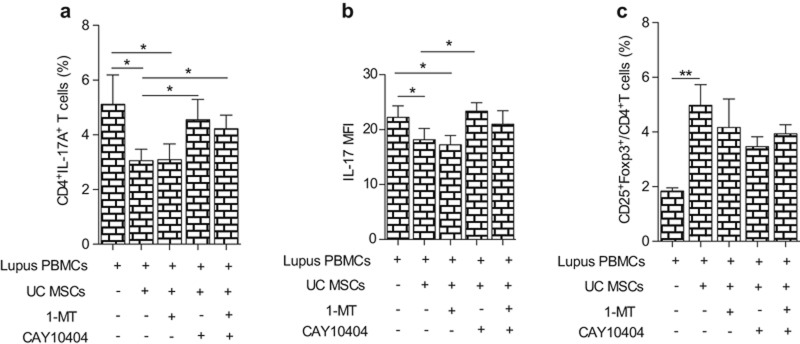
Next, we wanted to know which factor(s) mediated the regulation of Th17 cells in lupus. The IDO inhibitor, 1-MT, or the PGE2 inhibitor, CAY10404, alone or in combination, was added in the co-culture of UC MSCs and lupus PBMC. After 72 h, we found that CAY10404, but not 1-MT, significantly reversed the inhibition of the Th17 cell percentage by UC MSCs (PBMC: 5.11% ± 2.85%, PBMC + MSCs: 3.06% ± 1.10%, PBMC + MSCs + 1 MT: 3.10% ± 1.52%, PBMC + MSCs + CAY10404: 4.55% ± 1.96%, PBMC + MSCs + 1 MT + CAY10404: 4.22% ± 1.32%; PBMC vs. PBMC + MSCs P = 0.0382, PBMC vs. PBMC + MSCs+1 MT P = 0.041, PBMC + MSCs vs. PBMC + MSCs + CAY10404 P = 0.0394, PBMC + MSCs vs. PBMC + MSCs + 1 MT + CAY10404 P = 0.044) (Figure 5a, Supplementary Figure S5a). In addition, the MFI of IL-17 showed a similar change (Figure 5b). Neither inhibitor had an effect on the modulation of Treg by UC MSCs (PBMC: 1.84% ± 0.34%, PBMC + MSCs: 4.98% ± 2.26%, PBMC + MSCs + 1 MT: 4.17% ± 2.76%, PBMC + MSCs + CAY10404: 3.47% ± 1.01%, PBMC + MSCs + 1 MT + CAY10404: 3.93% ± 0.88%; PBMC vs. PBMC + MSCs P = 0.0024, PBMC vs. PBMC+MSCs + 1 MT P = 0.0438, PBMC vs. PBMC + MSCs + CAY10404 P = 0.0016, PBMC vs. PBMC + MSCs + 1 MT + CAY10404: P = 0.0011) (Figure 5c, Supplementary Figure S5b). These data revealed that PGE2, secreted by UC MSCs, mediated the inhibition of Th17 cells.
Figure 5.
PGE2 mediates the down-regulation on Th17 cell in lupus patients. In the co-culture of lupus patients PBMC and UC MSCs (10:1), a PGE2 inhibitor (CAY10404) and an IDO inhibitor (1-MT), alone or in combination, was added; 72 h later, the percentage of CD4+IL17A+Th17 (a), MFI of IL17 (b) and the percentage of CD4+CD25+Foxp3+ Treg (c) were analyzed by flow cytometry. *P < 0.05, **P < 0.01.
Discussion
The number and function of Treg cells are found to be defective in lupus patients, and Treg alterations reflect changes in SLE disease activity index (SLEDAI) with a high sensitivity (87.7%).12 Moreover, the frequency of Th17 cells, as well as the related cytokines in the serum, including IL-6, IL-1β, and IL-23, and signal transducer and activator of transcription 3 activity, was higher in SLE patients and down-regulated by disease treatment.13 Breaking the Th17/Treg balance in peripheral blood may play an important role in the development of SLE and could be responsible for an increased pro-inflammatory response, especially in the active form of the disease.14 However, an IL-17 inhibitor has not been considered for the clinical treatment of lupus patients.15An IL-6 receptor antagonist, tocilizumab, induced short-term disease remission in some cases of lupus nephritis16,17 but with high rate of relapse after long-term follow-up,18 suggesting that directly inhibiting Th17 cells and its related cytokines was not appropriate for lupus treatment. A recent study by Schmidt et al. showed that IL-17A deficiency did not affect the morphological or functional parameters in MRL/lpr mice with lupus nephritis, nor did IL-17A neutralization affect the clinical course of nephritis in NZB/NZW mice, suggesting that the Th17/IL-17A immune response plays no major role in the immunopathogenesis of lupus nephritis in MRL/lpr and NZB/NZW mice.19 In vitro, both autologous and allogeneic Treg cells can be satisfactorily isolated, expanded, and successfully adoptively transfused into patients with chronic GVHD.20 However, the long-term follow-up revealed some adverse events, such as malignant melanoma or skin cancer. Moreover, the expense of Treg expansion in vitro is much higher for a wider clinical application, so there are only several clinical studies considering the infusion of Treg for treatment in patients, and the direct infusion of Treg cells or inhibiting Th17 cells was not considered appropriate as a clinical application.
MSCT has been used in the clinic for many diseases including GVHD, autoimmune diseases, ischemic diseases, and decompensated liver cirrhosis. Our phase 1, phase 2, and multi-center studies have all showed satisfactory safety profiles and clinical effects of UC MSCs transplantation for lupus patients10,11,21; however, the underlying therapeutic mechanisms must be emphasized. In our previous short-term follow-up data, we showed that allogeneic MSCs transplantation significant ameliorated disease activity in lupus patients and upregulated peripheral blood Treg and serum TGF-β simultaneously.10 In the present study, we also showed that UC MSCs up-regulated TGF-β and Treg both in vivo and in vitro in lupus patients. TGF-β played an important role in the maintenance and survival of both naturally occurring Treg and induced Treg cells,22,23 and we demonstrated that anti-TGF-β antibody or TGF-β siRNA could abrogate the up-regulation of Treg by MSCs. Moreover, in the stimulation of the lupus microenvironment, MSCs produced higher levels of TGF-β than in normal circumstances. These data underscored that MSCs could interact with the lupus environment and secret functional molecules to induce immune-tolerance. In another autoimmune disease model of systemic sclerosis, we found that allogeneic MSCs induced T-cell apoptosis by Fas/FasL, stimulating macrophages to secret TGF-β, then up-regulated Treg in vivo and in vitro.24 These results also revealed that TGF-β played a very important role in Treg regulation. However, in lupus patients, whether the increased TGF-β was produced by only MSCs or by other immune cells such as macrophages needs further investigation.
In normal individuals, the effect of MSCs on Th17 cells has been reported to be different. IFN-γ and TNF-α synergistically enhanced the expression of CD54 by MSCs, enabling the CCR6 chemokine ligand CCL20 to induce the in vitro adhesion of Th17 cells to MSCs. MSCs prevented the in vitro differentiation of naive CD4+ T cells into Th17 cells and inhibited the production of IL-17, IL-22, IFN-γ, and TNF-α by fully differentiated Th17 cells.25 Another study also confirmed that MSCs can potently inhibit Th17 differentiation, and this suppression requires cell-contact-dependent COX-2 induction, resulting in the direct Th17 inhibition by PGE2 via EP4.26 However, the study by Eljaafari et al. indicated that interaction of PBMC with bone marrow MSCs promoted Th17 cell expansion as early as 24 h. IL-17A production was also increased in PBMC stimulated with anti-CD3 plus anti-CD28 antibodies.27 Later, Carrión et al. revealed that the state of CD4+ T-cell activation directed the effect of MSCs on Th17 cells. Early addition (day 0) of MSCs suppressed the Th17 cell lineage, but addition at day 3 markedly increased IL-17 production by Th17 polarized cells by 50%,28 which may partly explain the different conclusions in different studies regarding the effects of MSCs on Th17 cells.
In the present study, we showed that the inhibition of Th17 cells by UC MSCs was mediated by PGE2, but not IDO, IL-6, or TGF-β. However, the addition of PGE2 inhibitor did not completely abrogate the inhibitory effect on Th17 cells, which indicated that other unknown factors are also involved in the regulation on Th17 cells by MSCs. For example, in mouse models, Qu et al. showed the IL-10-mediated inhibition of Th17 cell production, which depended on STAT5 activation.29 Thus, other functional molecules including IL-10, HLA-G, hepatocyte growth factor, and heme oxygenase 1 could be assessed in future studies.
It is well established that MSCs and the immune system interact closely. During an immune response, the inflammatory cytokines, such as TNF-α, IFN-γ, IL-1β, and IL-1α, which are produced by T cells and antigen-presenting cells, modulate the function of MSCs, leading to the release of immunosuppressive factors, the altered expression of surface molecules or the production of growth factors. These factors/molecules are crucial components for immunoregulation and tissue repair by MSCs.30 In multiple sclerosis, human bone marrow MSCs increased Th17 responses. However, pretreating MSCs with the proinflammatory cytokine IL-1 accentuated these effects and caused decreases in the Th17 subset.31 Our recent work also showed that lupus patient-derived CD8+ T cells that produced IFN-γ could stimulate MSCs to express large amount of IDO, which mediated the inhibition of responsive T-cell proliferation.32 Here, we showed that when co-cultured with lupus patient-derived PBMC or stimulated by the serum of lupus patients, MSCs expressed higher levels of IDO, IL-6, TGF-β, and PGE2, in which TGF-β and PGE2 mediated the modulation of Treg and Th17 cell subsets, respectively. However, which molecule stimulated the function of MSCs is unknown and requires more studies.
In conclusion, in the present study we showed for the first time that UC MSCs interact with the lupus patient environment and secrete TGF-β to up-regulate Treg and PGE2 to down-regulate Th17 cells.
Competing interests
The study was supported by the Major International (Regional) Joint Research Project (Grant NO 81120108021 to Dr. Sun), the National Natural Science Foundation of China (Grant NO. 81273304 to Dr. Sun; Grant NO. 81401347 to Dr. Wang; Grant NO. 81202333 to Dr. Liang), the Jiangsu Provincial Natural Science Foundation (BK20140098 to Dr. Wang), the Jiangsu Provincial Health Department Foundation (Q201411 to Dr. Wang) and the Scientific Research Project of the Nanjing Municipal Health Bureau (to Dr. Wang). The authors have no financial or other relationships such as consultancies, employment, providing expert testimony, honoraria, speakers' bureaus, retainers, stock options, or ownership.
Footnotes
Supplementary information of this article can be found on the Cellular & Molecular Immunology's website (http://www.nature.com/cmi).
Supplementary Information
References
- Yang J, Chu Y, Yang X, Gao D, Zhu L, Yang X et al. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum 2009; 60: 1472–1483. [DOI] [PubMed] [Google Scholar]
- Lee HY, Hong YK, Yun HJ, Kim YM, Kim JR, Yoo WH. Altered frequency and migration capacity of CD4+CD25+ regulatory T cells in systemic lupus erythematosus. Rheumatology (Oxford) 2008; 47: 789–794. [DOI] [PubMed] [Google Scholar]
- Scheinecker C, Bonelli M, Smolen JS. Pathogenetic aspects of systemic lupus erythematosus with an emphasis on regulatory T cells. J Autoimmun 2010; 35: 269–275. [DOI] [PubMed] [Google Scholar]
- Martin JC, Baeten DL, Josien R. Emerging role of IL-17 and Th17 cells in systemic lupus erythematosus. Clin Immunol 2014; 154: 1–12. [DOI] [PubMed] [Google Scholar]
- Amarilyo G, Lourenço EV, Shi FD, La Cava A. IL-17 promotes murine lupus. J Immunol 2014; 193: 540–543. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol 2012; 12: 383–396. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008; 371: 1579–1586. [DOI] [PubMed] [Google Scholar]
- Tyndall A. Mesenchymal stem cell treatments in rheumatology:a glass half full? Nat Rev Rheumatol 2014; 10: 117–124. [DOI] [PubMed] [Google Scholar]
- Mougiakakos D, Jitschin R, Johansson CC, Okita R, Kiessling R, Le Blanc K. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood 2011; 117: 4826–4835. [DOI] [PubMed] [Google Scholar]
- Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum 2010; 62: 2467–2475. [DOI] [PubMed] [Google Scholar]
- Wang D, Li J, Zhang Y, Zhang M, Chen J, Li X et al. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res Ther 2014; 16: R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tselios K, Sarantopoulos A, Gkougkourelas I, Boura P. CD4+CD25 highFOXP3+ T regulatory cells as a biomarker of disease activity in systemic lupus erythematosus: a prospective study. Clin Exp Rheumatol 2014; 32: 630–639. [PubMed] [Google Scholar]
- Wen Z, Xu L, Xu W, Xiong S. Detection of dynamic frequencies of Th17 cells and their associations with clinical parameters in patients with systemic lupus erythematosus receiving standard therapy. Clin Rheumatol 2014; 33: 1451–1458. [DOI] [PubMed] [Google Scholar]
- Talaat RM, Mohamed SF, Bassyouni IH, Raouf AA. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: correlation with disease activity. Cytokine 2015; 72: 146–153. [DOI] [PubMed] [Google Scholar]
- Reichert JM. Antibodies to watch in 2015. MAbs 2015; 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai A, Naniwa T, Tamechika S, Maeda S. Short-term add-on tocilizumab and intravenous cyclophosphamide exhibited a remission-inducing effect in a patient with systemic lupus erythematosus with refractory multiorgan involvements including massive pericarditis and glomerulonephritis. Mod Rheumatol 2014; 30: 1–4. [DOI] [PubMed] [Google Scholar]
- Thanarajasingam U, Niewold TB. Sirukumab: a novel therapy for lupus nephritis? Expert Opin Investig Drugs 2014; 23: 1449–1455. [DOI] [PubMed] [Google Scholar]
- Jüptner M, Zeuner R, Schreiber S, Laudes M, Schröder JO. Successful application of belimumab in two patients with systemic lupus erythematosus experiencing a flare during tocilizumab treatment. Lupus 2014; 23: 428–430. [DOI] [PubMed] [Google Scholar]
- Schmidt T, Paust HJ, Krebs CF, Krebs CF, Turner JE, Kaffke A et al. Function of the Th17/interleukin-17A immune respon se in murine lupus nephritis. Arthritis Rheumatol 2015; 67: 475–487. [DOI] [PubMed] [Google Scholar]
- Theil A, Tuve S, Oelschlägel U, Maiwald A, Döhler D, Oßmann D et al. Adoptive transfer of allogeneic regulatory T cells into patients with chronic graft-versus-host disease. Cytotherapy 2015; 17: 473–486. [DOI] [PubMed] [Google Scholar]
- Wang D, Zhang H, Liang J, Li X, Feng X, Wang H et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant 2013; 22: 2267–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003; 198: 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang P, Chen W, Li J, Kulkarni AB, Perruche S. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol 2008; 9: 632–640. [DOI] [PubMed] [Google Scholar]
- Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T et al. Mesenchymal stem cell induced immunoregulation involves FAS-Ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 2012; 10: 544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannam S, Pène J, Moquet-Torcy G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol 2010; 185: 302–312. [DOI] [PubMed] [Google Scholar]
- Duffy MM, Pindjakova J, Hanley SA, McCarthy C, Weidhofer GA, Sweeney EM et al. Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol 2011; 41: 2840–2851. [DOI] [PubMed] [Google Scholar]
- Eljaafari A, Tartelin ML, Aissaoui H, Chevrel G, Osta B, Lavocat F et al. Bone marrow-derived and synovium-derived mesenchymal cells promote Th17 cell expansion and activation through caspase 1 activation: contribution to the chronicity of rheumatoid arthritis. Arthritis Rheum 2012; 64: 2147–2157. [DOI] [PubMed] [Google Scholar]
- Carrión F, Nova E, Luz P, Apablaza F, Figueroa F. Opposing effect of mesenchymal stem cells on Th1 and Th17 cell polarization according to the state of CD4+ T cell activation. Immunol Lett 2011; 135: 10–16. [DOI] [PubMed] [Google Scholar]
- Qu X, Liu X, Cheng K, Yang R, Zhao RC. Mesenchymal stem cells inhibit Th17 cell differentiation by IL-10 secretion. Exp Hematol 2012; 40: 761–770. [DOI] [PubMed] [Google Scholar]
- Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol 2012; 33: 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington PJ, Boivin MN, Renoux C, François M, Galipeau J, Freedman MS. Reciprocal Th1 and Th17 regulation by mesenchymal stem cells: implication for multiple sclerosis. Ann Neurol 2010; 68: 540–545. [DOI] [PubMed] [Google Scholar]
- Wang D, Feng X, Lu L, Konkel JE, Zhang H, Chen Z et al. A CD8 T cell-IDO axis is required for mesenchymal stem cell suppression of human SLE. Arthritis Rheumatol 2014; 66: 2234–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.