Abstract
Antithymocyte globulin (ATG) is often included in the conditioning regimen to prevent graft vs. host disease in allogeneic hematopoietic stem cell (HSC) transplantation. However, because ATG contains antibodies targeting a wide range of antigens on human cells, its potential off-target effects remain a concern. Here, we explored this question in humanized mice that permit the analysis of human cell depletion in tissues. We showed that ATG binds to almost all lineages of human hematopoietic cells including HSCs, and accordingly it is capable of depleting almost all human hematopoietic cells. Interestingly, the efficacy of ATG was highly variable depending on the tissue of residence, with human cells in bone marrow significantly less susceptible than those in the blood and spleen. Recovery of multilineage human lymphohematopoietic reconstitution in humanized mice that received ATG 3 weeks after HSC transplantation indicates that ATG had a minimal effect on human HSCs that have settled in bone marrow niches. However, efficient human HSC depletion and engraftment failure were seen in mice receiving ATG at the time of transplantation. Our data indicate that the efficacy of ATG is tissue-dependent, and suggest a potential risk of impairing donor hematopoietic engraftment when ATG is used in preparative conditioning regimens.
Keywords: ATG, transplantation, hematopoietic stem cell, humanized mice
Introduction
Rabbit antithymocyte globulin (ATG), a polyclonal antibody produced by immunization of rabbits with human thymocytes or T cell line cells, has been used to prevent renal allograft rejection for more than 30 years.1,2,3 The use of ATG in conditioning regimens for allogeneic hematopoietic cell transplantation has been reported to reduce the risk of graft vs. host diseases.4,5,6,7 Immunosuppression by ATG is primarily mediated through the recognition of a series of antigens expressed on human lymphohematopoietic cells, such as CD2, CD3, CD4, and CD8 expressed on T cells; CD19 and CD20 expressed on B cells; CD16 and CD56 expressed on natural killer (NK) cells; and CD11b, CD80 and CD86 expressed on dendritic cells.8,9,10 Indeed, the ability of ATG to recognize human CD45 indicates its capability of binding all lineages of human hematopoietic cells, including hematopoietic progenitor/stem cells (HPCs/HSCs)8,11,12 and thus, raising the possibility that ATG treatment might be deleterious to donor HSC engraftment. However, this question is very difficult to address in patients receiving ATG, and as such the results from in vitro studies remain conflicting. It has been shown that, unlike lymphocytes, human CD34+ HSCs are highly resistant to ATG-induced apoptosis in vitro,12 but others reported that in vitro ATG-treated human CD34+ HSCs cannot engraft in immunodeficient mice.13
Using a humanized mouse model, herein we show that human hematopoietic cells in bone marrow were significantly less susceptible to depletion by ATG than those in peripheral blood and spleen. Furthermore, ATG had minimal effects on human HSCs that have settled in bone marrow niches, but caused human HSC engraftment failure if administered at the time of transplantation.
Materials and methods
Construction of humanized mice and treatment with rabbit ATG
Humanized mice were made by transplantation of human fetal liver-derived CD34+ cells (intravenous (i.v.); 1–5 × 105 per mouse) and fetal thymic tissue fragment (approximately 1 mm3 in size; under the recipient kidney capsule) from the same fetal donor into 2 Gy-irradiated NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice (females; 6–8 weeks of age), as previously described.14,15 Discarded human fetal tissues with gestational age of 17–20 weeks were obtained from Advanced Bioscience Resource (Alameda, CA, USA) and the First Hospital of Jilin University. The humanized mice were treated (i.v.) with rabbit ATG at the indicated time points, and human cell depletion in various tissues were examined. Two rabbit ATG preparations, ATG-Genzyme (ATG-G; Thymoglobulin, Genzyme, Boston, MA, USA) and ATG-Fresenius (ATG-F; Fresenius Biotech GmbH, Gräfelfing, Germany), were used in the current study, but only one product was used for each individual experiment. Protocols involving the use of human tissues and animals were approved by the Institutional Review Board and Institutional Animal Care and Use Committee.
Flow cytometric analysis
Levels of human hematopoietic cells in humanized mice were determined by flow cytometric (FCM) analysis using various combinations of the following monoclonal antibodies: anti-human CD45, CD19, CD3, CD14, CD33, CD34, and isotype controls (all purchased from BD Pharmingen, San Diego, CA, USA); anti-mouse CD45 (BD Pharmingen) and Ter119 (Biolegend, San Diego, CA, USA); and AF488-conjugated goat anti-rabbit immunoglobulin G (IgG; for detecting ATG-binding cells; Invitrogen, Ann Arbor, MI, USA). Peripheral blood was collected from tail vein into heparinized tubes, and mononuclear cells were purified by density gradient centrifugation with Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA). All samples were analyzed on a fluorescence-activated cell sorting (FACS; Fortessa, Becton Dickinson, Mountain View, CA, USA), and dead cells were excluded from the analysis by gating propidium iodide negative cells.
Statistical analysis
The level of significant differences in group means was determined by Student's t-test for parametric data sets. All statistical analysis was performed using Prism (Version 6 GraphPad Software). A P value ≤ 0.05 was considered significant in all analyses herein.
Results
ATG administration efficiently depletes human T and B cells in humanized mice, but its efficacy is largely tissue-dependent
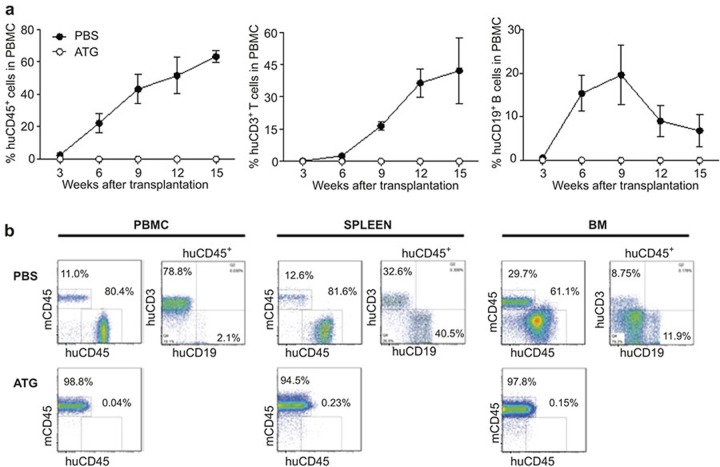
Humanized mice were treated with phosphate-buffered saline (PBS) (as controls) or ATG (three injections, with 2-day intervals) 13 weeks after human thymus/CD34+ cell transplantation, when significant human hematopoietic cell reconstitution was confirmed, and human cell depletion was examined 7 days after the last injection of ATG. Compared with the pretreatment level (measured 3 weeks prior to the first injection of ATG), PBS-treated control mice showed a trend of increase in the level of human CD45+ hematopoietic chimerism (predominantly CD3+ T cells) in the peripheral blood (Figure 1a), which is consistent with previous studies.14,16 In contrast, ATG-treated humanized mice showed a marked decrease in human CD45+ and CD3+ cells (Figure 1a). ATG treatment also efficiently depleted human cells in the spleen (including both T and B cell lineages; Figure 1b), and human thymocytes in the thymic grafts (Figure 1c; Supplementary Figure S1). However, bone marrow-resident human cells were highly resistant to ATG-mediated depletion. ATG treatment failed to induce a significant reduction in any of the human cell lineages measured, including CD3+ T, CD19+ B, and CD34+ HSC/HPC-enriched cell populations (Figure 1d and e). These results were confirmed in similar but independent experiment, in which a significant depletion of human cells was detected in the blood and spleen, but not bone marrow in humanized mice that were treated with ATG 17 weeks after human thymus/CD34+ cell transplantation (Supplementary Figure S2). The fact that ATG recognizes antigens expressed by multilineage human hematopoietic cells8,17 may explain the observed efficient depletion of both human T and B cells in ATG-treated humanized mice. However, human T cells seemed to be more sensitive than B cells to depletion by ATG, as ATG-treated mice showed a significant reduction in peripheral blood human CD3+ T cells, but not CD19+ B cells (Figure 1a and Supplementary Figure S2a), and had a markedly reduced ratio of CD3+ to CD19+ cells in the blood and spleen compared to PBS-treated humanized mice (Supplementary Figure S2e).
Figure 1.
Human cell depletion by ATG in established humanized mice. Humanized mice were constructed by transplantation of human fetal thymus and CD34+ cells, and 13 weeks later received 3 injections i.v. of ATG-G (3 mg/kg−1 per injection at day 0 and day 2, and 30 mg/kg−1 at day 4) or PBS. (a) Levels (%) of human CD45+, CD3+ and CD19+ cells in peripheral blood measured 3 weeks prior to the first injection and 7 days after last injection of PBS or ATG. (b) Percentages of human CD45+, CD3+ and CD19+ cells in the spleen at day 7-post-last injection of PBS or ATG. (c) Percentages of live (PI-negative) human thymocytes in the human thymic grafts at day 7 post-last injection of PBS or ATG. (d–e) Percentages of human CD45+, CD3+ and CD19+ cells (d) and of human CD34+ cells (e) in bone marrow at day 7 post-last injection of PBS or ATG. Data presented are mean ± SEMs (n = 3–4 per group). *P < 0.05; **P < 0.01; ***P < 0.001. Similar results were obtained from an independent experiment presented in Figure S2.
Conditioning treatment with ATG results in donor HSC depletion and engraftment failure
Binding of ATG to human peripheral blood mononuclear cells (PBMCs) and purified CD34+ cells were compared by FCM analysis. As shown in Figure 2, ATG bound to human CD34+ cells to a similar extent as its binding to human T and B cells, raising the possibility that use of ATG as a conditioning therapy may inhibit donor hematopoietic engraftment in patients receiving allogeneic HSC transplantation. To address this question, we compared human hematopoietic engraftment in immunodeficient mice that were conditioned with sublethal (2 Gy) total body irradiation alone, or along with ATG administration (10 mg kg−1) 1 day after CD34+ cell transplantation. Surprisingly, human CD45+ cells were almost undetectable (ranging between 0% and 0.19%) in the peripheral blood from ATG-conditioned mice, while all mice conditioned without ATG showed durable human hematopoietic reconstitution, including both T and B cells (Figure 3a). High levels of human hematopoietic chimerism were also detected in the spleen and bone marrow from mice conditioned without ATG 19 weeks after CD34+ cell transplantation; however, human cells were almost undetectable in both tissues from ATG-conditioned mice (Figure 3b). These results provide direct evidence that using ATG as part of a preparative conditioning regimen for HSC transplantation may impair donor hematopoietic engraftment.
Figure 2.
Direct binding of ATG to human lymphohematopoietic cells. Shown are representative FACS profiles illustrating the binding of ATG-G to human CD3+ and CD19+ PBMCs, bone marrow cells (BMC), and purified CD34+ fetal liver cells (FLC). Binding of ATG was detected by fluorochrome-conjugated goat anti-rabbit antibody.
Figure 3.
ATG administration at the time of human HSC transplantation induces engraftment failure. NSG mice received transplantation of human fetal thymus and CD34+ cells, followed one day later by injection i.v. of PBS or ATG-G (10 mg/kg−1). (a) Percentages of human CD45+, CD3+ and CD19+ cells in PBMCs at the indicated times after human thymus/CD34+ cell transplantation (n = 4). (b) Representative FACS profiles showing human CD45+, CD3+ and CD19+ cell chimerism in PBMCs, spleen and bone marrow at week 19.
ATG treatment does not deplete human HSCs that have settled in bone marrow niches
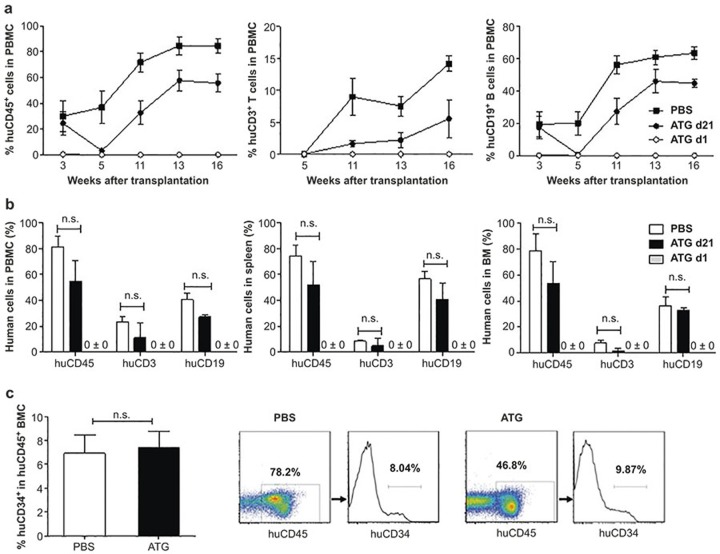
The fact that ATG treatment did not induce significant depletion of human CD34+ BMCs in established humanized mice (Figure 1e and Supplementary Figure S2d) suggests that human HSCs that have settled in bone marrow niches might be resistant to depletion by ATG. To confirm this possibility, we compared human hematopoietic chimerism in humanized mice that were treated with ATG 1 or 21 days after human HSC transplantation. Again, human cells were almost undetectable throughout the observation period of 17 weeks in mice treated with ATG 1 day after human HSC transplantation (Figure 4a). However, despite a marked reduction of human cell chimerism within 2 weeks following ATG administration, the levels of human cell chimerism in peripheral blood increased overtime thereafter in mice treated with ATG 21 days after HSC transplantation (Figure 4a). Serum analysis revealed that ATG remained biologically active for 1–2 weeks in mice (Supplementary Figure S3), correlating with the kinetics of human cell depletion in ATG-treated mice. All mice were killed at week 17 for analysis of human hematopoietic chimerism in the spleen and bone marrow. Humanized mice treated with ATG 21 days after HSC transplantation showed high levels of human hematopoietic reconstitution in spleen and bone marrow, while human cells were not detected in these tissues from mice treated with ATG 1 day after HSC transplantation (Figure 4b and Supplementary Figure S4). Furthermore, the ratio of CD34+ cells in human BMCs was comparable between humanized mice treated with ATG 21 days after HSC transplantation and PBS-treated controls (Figure 4c).
Figure 4.
ATG administration depletes donor HSCs prior to their settling in the bone marrow. NSG mice received transplantation of human fetal thymus and CD34+ cells, followed one day later by injection i.v. of ATG-F (10 mg/kg−1; ATG d1), or 21 days later by injection i.v. of ATG-F (30 mg/kg−1; ATG d21) or PBS. (a) Percentages of human CD45+, CD3+, and CD19+ cells in PBMCs at the indicated times. (b) Percentages of human CD45+, CD3+, and CD19+ cells in PBMCs, spleen and bone marrow (BM) at week 17. Representative staining profiles are presented in Figure S4. (c) Percentages of human CD34+ cells in bone marrow and representative staining profiles from ATG d21 and PBS control groups at week 17. N = 4 per group; N.S. indicates not significant.
These results were confirmed by a separate experiment. Because ATG, as a part of the conditioning regimen, is also given prior to transplantation, in this experiment we included a group of mice that were treated with ATG 3 days prior to human HSC transplantation (ATG d-3; Figure 5). ATG treatment at d-3 and d1 both resulted in a complete depletion of human cells in peripheral blood (Figure 5a) and bone marrow (Figure 5b). However, significant depletion of human cells was detected only in blood, but not in bone marrow in mice treated with ATG at day 21. In these mice, the levels of human chimerism in blood were markedly decreased by week 2 post-ATG treatment (week 5 post-HSC transplantation; Figure 5a), whereas the levels of CD34+ cells in bone marrow remained comparable to those in control mice treated with rabbit IgG or PBS (Figure 5b). These results indicate that human HSCs settled in bone marrow niches are resistant to depletion by ATG. We also measured ATG binding to human BMCs in mice treated with ATG 21 days after HSC transplantation. As shown in Figure 6, all human BMCs, including CD34+ cells, isolated 1 day after ATG treatment were coated by ATG, indicating that the observed poor depletion of human BMCs is unlikely due to inefficient binding of ATG to human cells in the bone marrow.
Figure 5.
Comparison of human cell depletion in peripheral blood and bone marrow in ATG-treated mice. ATG-F (10 mg/kg−1; i.v.) was administrated to NOD/SCID mice 3 days prior to (ATG d-3), or 1 (ATG d1) or 21 (ATG d21) days after human HSC transplantation. Mice in the control groups received PBS or polyclonal rabbit IgG antibodies (10 mg/kg−1) 1 day after human HSC transplantation. (a) Percentages of human CD45+ cells in PBMCs at week 3 (measured right before ATG injection in the ATG d21 group) and week 5. (b) Percentages of CD34+ human cells in bone marrow at week 5. Each group contained 3–4 mice; group means and representative FACS profiles are shown in the top and bottom panels, respectively.
Figure 6.
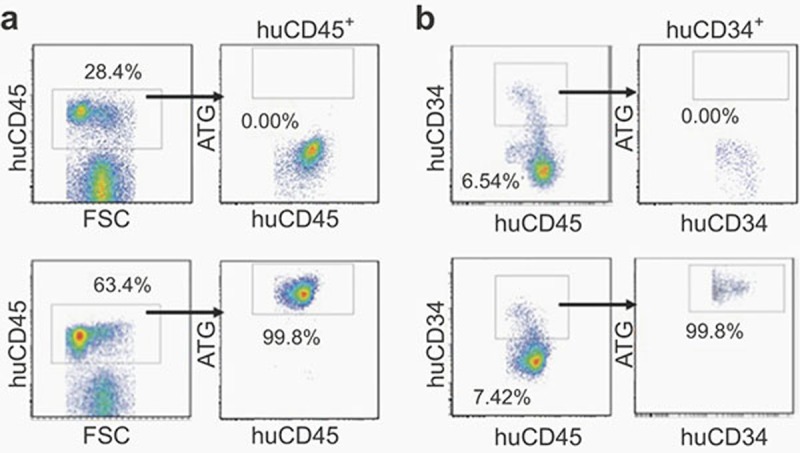
NSG mice were treated with PBS (control; top panels) or ATG-F (30 mg/kg; bottom panels) 3 weeks after human CD34+ cell transplantation. Bone marrow cells were prepared one day later and analyzed for ATG binding to human cells by FACS using fluorochrome-conjugated goat anti-rabbit antibody. (a) ATG binding to human CD45+ cells. (b) ATG binding to CD34+ cells. Shown are representative FACS profiles and the number in each figure indicates the percentage of cells in the gate.
Discussion
The efficacy of ATG-mediated lymphocyte depletion in patients has mainly been evaluated by measuring peripheral blood cells. Previous studies using rabbit anti-mouse thymocyte globulin (mATG) showed that T cells in the spleen are significantly less sensitive to depletion by mATG than those in peripheral blood.18 However, susceptibility of human lymphocytes in tissues to depletion by ATG remains largely unknown. We therefore seek to use humanized mice to investigate the efficacy of ATG to deplete human hematopoietic and lymphoid cells in different tissues. Our data indicate that ATG can deplete multilineage human hematopoietic cells, including T cells, B cells, and HSCs, but its efficacy is highly variable depending on the tissue of residence, with human cells in bone marrow significantly less susceptible than those in the blood and spleen.
Previous studies have shown that memory T cells are less susceptible to ATG19,20,21 and bone marrow serves as an important reservoir for memory T cells,22,23,24 suggesting that the lower level of bone marrow cell depletion by ATG might be due to its enrichment by memory T cells. However, this is contrary to the observation that not only T cells, but other bone marrow human cells (e.g., CD19+ cells) were also significantly less sensitive to depletion by ATG compared to their counterpart cell populations in peripheral blood and spleen. It has been reported that macrophages in bone marrow are significantly less phagocytic compared to those in other tissues (e.g., spleen and liver),25,26 which may be partially responsible for the poor depletion of human BMCs by ATG. Together, these results suggest that the bone marrow microenvironment is likely protective against ATG-mediated depletion.
ATG recognizes CD45, a marker expressed by almost all hematopoietic cells, including HSCs.9,11 Consistently, we observed that ATG bounds to human CD34+ cells to a similar extent as its binding to human T and B cells. Given the fact that in cell culture ATG induces apoptosis in various human cell populations, such as T cells, B cells, NK cells, and monocytes, but not in HSCs, ATG treatment has been suggested to improve hematopoietic engraftment.12 However, in vitro treatment of human HSCs with ATG at a very high concentration prior to transplantation diminished their potential to engraft in immunodeficient mice.13 These studies suggest that ATG-coated HSCs can be eliminated in vivo, likely via mechanisms other than direct induction of apoptosis. Interestingly, we observed that ATG depletes HSCs prior to their settling in the bone marrow niches, but minimally affects HSC function in established humanized mice. We acknowledge that a limitation of the current study is the use of fetal CD34+ cells. However, the fact that there is virtually no difference in binding to ATG between human bone marrow- and fetal liver-derived CD34+ cells (Figure 2) suggests that these findings are likely to apply to adult HSC transplantation. Together, these data indicate that the effect of ATG on HSCs can be largely prevented by the bone marrow niche microenvironment, and that the timing of ATG administration determines its effect on donor hematopoietic engraftment. We suggest that ATG administration immediately before or after HSC transplantation may potentially result in engraftment failure and therefore, caution should be taken when including ATG in preparative conditioning regimens for allogeneic HSC transplantation.
Conflict of interest
The authors declare no competing financial interests.
Acknowledgments
The authors thank Ms. Meifang Wang and Mr. Zhifu Gan for their excellent animal care. This work was supported by grants from Chinese MOST (973 Program 2015CB964400 and 2013CB966900; 863 Program 2014AA021601), NSFC (81200397, 81273334), National Science and Technology Major Project of China (2013ZX10002008), Chinese Ministry of Education (IRT1133), and NIH (P01AI045897). Supplementary information of this article can be found on the Cellular & Molecular Immunology's website (http://www.nature.com/cmi).
Footnotes
Supplementary information of this article can be found on the Cellular & Molecular Immunology's website (http://www.nature.com/cmi).
Supplementary Information
References
- Brennan DC, Schnitzler MA. Long-term results of rabbit antithymocyte globulin and basiliximab induction. N Engl J Med 2008; 359: 1736–1738. [DOI] [PubMed] [Google Scholar]
- Hardinger KL, Schnitzler MA, Miller B, Lowell JA, Shenoy S, Koch MJ et al. Five-year follow up of thymoglobulin versus ATGAM induction in adult renal transplantation. Transplantation 2004; 78: 136–141. [DOI] [PubMed] [Google Scholar]
- Cantarovich D, Rostaing L, Kamar N, Ducloux D, Saint-Hillier Y, Mourad G et al. Early corticosteroid avoidance in kidney transplant recipients receiving ATG-F induction: 5-year actual results of a prospective and randomized study. Am J Transplant 2014; 14: 2556–2564. [DOI] [PubMed] [Google Scholar]
- Lindemans CA, Chiesa R, Amrolia PJ, Rao K, Nikolajeva O, de Wildt A et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood 2014; 123: 126–132. [DOI] [PubMed] [Google Scholar]
- Appelbaum FR, Bacigalupo A, Soiffer R. Anti-T cell antibodies as part of the preparative regimen in hematopoietic cell transplantation-a debate. Biol Blood Marrow Transplant 2012; 18: S111–S115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood 2001; 98: 2942–2947. [DOI] [PubMed] [Google Scholar]
- Storek J. Impact of serotherapy on immune reconstitution and survival outcomes after stem cell transplantations in children: thymoglobulin versus alemtuzumab. Biol Blood Marrow Transplant 2015; 21: 385–386. [DOI] [PubMed] [Google Scholar]
- Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia 2007; 21: 1387–1394. [DOI] [PubMed] [Google Scholar]
- Storek J, Mohty M, Boelens JJ. Rabbit anti-T cell globulin in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2015; 21: 959–970. [DOI] [PubMed] [Google Scholar]
- Leitner J, Grabmeier-Pfistershammer K, Majdic O, Zlabinger G, Steinberger P. Interaction of antithymocyte globulins with dendritic cell antigens. Am J Transplant 2011; 11: 138–145. [DOI] [PubMed] [Google Scholar]
- Flynn J, Cox CV, Rizzo S, Foukaneli T, Rice K, Murphy M et al. Direct binding of antithymoctye globulin to haemopoietic progenitor cells in aplastic anaemia. Br J Haematol 2003; 122: 289–297. [DOI] [PubMed] [Google Scholar]
- Grullich C, Ziegler C, Finke J. Rabbit anti T-lymphocyte globulin induces apoptosis in peripheral blood mononuclear cell compartments and leukemia cells, while hematopoetic stem cells are apoptosis resistant. Biol Blood Marrow Transplant 2009; 15: 173–182. [DOI] [PubMed] [Google Scholar]
- Wunderlich M, Brooks RA, Panchal R, Rhyasen GW, Danet-Desnoyers G, Mulloy JC. OKT3 prevents xenogeneic GVHD and allows for reliable initiation of xenografts from unfractionated human hematopoietic tissues. Blood 2014; 123: e134–e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 2006; 108: 487–492. [DOI] [PubMed] [Google Scholar]
- Hu Z, Van Rooijen N, Yang YG. Macrophages prevent human red blood cell reconstitution in immunodeficient mice. Blood 2011; 118: 5938–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan P, Wang L, Diouf B, Eguchi H, Su H, Bronson R et al. Induction of human T-cell tolerance to porcine xenoantigens through mixed hematopoietic chimerism. Blood 2004; 103: 3964–3969. [DOI] [PubMed] [Google Scholar]
- Bosch M, Dhadda M, Hoegh-Petersen M, Liu Y, Hagel LM, Podgorny P et al. Immune reconstitution after anti-thymocyte globulin-conditioned hematopoietic cell transplantation. Cytotherapy 2012; 14: 1258–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CQ, Chernatynskaya AV, Wasserfall CH, Wan S, Looney BM, Eisenbeis S et al. Anti-thymocyte globulin (ATG) differentially depletes naive and memory T cells and permits memory-type regulatory T cells in nonobese diabetic mice. BMC Immunol 2012; 13: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama I, Nadazdin O, Boskovic S, Ochiai T, Smith RN, Sykes M et al. Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant 2007; 7: 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant 2005; 5: 465–474. [DOI] [PubMed] [Google Scholar]
- Do JS, Valujskikh A, Vignali DA, Fairchild RL, Min B. Unexpected role for MHC II-peptide complexes in shaping CD8 T-cell expansion and differentiation in vivo. Proc Natl Acad Sci U S A 2012; 109: 12698–12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol 2005; 26: 360–366. [DOI] [PubMed] [Google Scholar]
- Mazo IB, Honczarenko M, Leung H, Cavanagh LL, Bonasio R, Weninger W et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity 2005; 22: 259–270. [DOI] [PubMed] [Google Scholar]
- Tokoyoda K, Zehentmeier S, Hegazy AN, Albrecht I, Grün JR, Löhning M et al. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity 2009; 30: 721–730. [DOI] [PubMed] [Google Scholar]
- Oldenborg P-A, Zheleznyak A, Fang Y-F, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science 2000; 288: 2051–2054. [DOI] [PubMed] [Google Scholar]
- Abe M, Cheng J, Qi J, Glaser RM, Thall AD, Sykes M et al. Elimination of porcine hemopoietic cells by macrophages in mice. J Immunol 2002; 168: 621–628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.