Abstract
Alzheimer's disease (AD) has been associated with magnesium ion (Mg2+) deficits and interleukin-1β (IL-1β) elevations in the serum or brains of AD patients. However, the mechanisms regulating IL-1β expression during Mg2+ dyshomeostasis in AD remain unknown. We herein studied the mechanism of IL-1β reduction using a recently developed compound, magnesium-L-threonate (MgT). Using human glioblastoma A172 and mouse brain D1A glial cells as an in vitro model system, we delineated the signaling pathways by which MgT suppressed the expression of IL-1β in glial cells. In detail, we found that MgT incubation stimulated the activity of extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) and peroxisome proliferator-activated receptor gamma (PPARγ) signaling pathways by phosphorylation, which resulted in IL-1β suppression. Simultaneous inhibition of the phosphorylation of ERK1/2 and PPARγ induced IL-1β upregulation in MgT-stimulated glial cells. In accordance with our in vitro data, the intracerebroventricular (i.c.v) injection of MgT into the ventricles of APP/PS1 transgenic mice and treatment of Aβ precursor protein (APP)/PS1 brain slices suppressed the mRNA and protein expression of IL-1β. These in vivo observations were further supported by the oral administration of MgT for 5 months. Importantly, Mg2+ influx into the ventricles of the mice blocked the effects of IL-1β or amyloid β-protein oligomers in the cerebrospinal fluid. This reduced the stimulation of IL-1β expression in the cerebral cortex of APP/PS1 transgenic mice, which potentially contributed to the inhibition of neuroinflammation.
Keywords: Alzheimer's disease, amyloid β-protein, magnesium-L-threonate, PPARγ
Introduction
Alzheimer's disease (AD) is the most common cause of dementia in people aged 65 years and above, and is characterized clinically by cognitive decline and pathologically by the accumulation of amyloid β-protein (Aβ) plaques and the presence of neurofibrillary tangles (NFTs) in the brain.1 It has been suggested that various factors may be involved in the etiology, pathogenesis, and progression of AD.2 Previous studies have shown that brain magnesium ion (Mg2+) levels3 and serum Mg2+ concentrations4 appear to be significantly lower in AD patients compared with age-matched normal subjects. Consequently, the dysregulation of Mg2+ levels may play a key role in the pathogenesis and progression of AD. The present study was initiated to determine Mg2+ homeostasis in AD.
Although the mechanisms regulating Mg2+ concentrations in AD have yet to be defined, there are a few studies concerning the potential roles of Mg2+ in the brain. It has been shown that Mg2+ is associated with the decreased production of cytokines in brain disease. Sugimoto et al.5 reported that MgSO4 exposure reduced the number of monocytes producing tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) in intrapartum women. Moreover, MgSO4 also inhibited cytokine production in toll-like receptor ligand-treated human peripheral and cord blood mononuclear cells, thereby indicating the broad anti-inflammatory activity of Mg2+. More recently, accumulating evidence suggests that neuroinflammatory mechanisms are critical for the progression of AD. For example, superinduction of one of the Mg2+ targets, IL-1β, in the AD brain is directly related to the excessive expression of neuronal Aβ precursor protein (APP) and the induction of astrocytes/microglial cell activation,6 which contribute to the pathogenesis of AD. In addition, blocking IL-1 signaling rescues cognition by attenuating tau phosphorylation in APP/PS1/Tau transgenic mice.7 These changes may, in part, explain the mechanistic link between Mg2+ and AD. However, the mechanisms underlying the role of Mg2+ elevation in suppressing the expression IL-1β during the course of AD development remain unknown.
To understand the functional significance of Mg2+ in the neuroinflammation of AD, we treated glial cells with magnesium-L-threonate (MgT) for 48 h. We found that MgT treatment markedly inhibited the expression of IL-1β via activating extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) and peroxisome proliferator-activated receptor gamma (PPARγ) pathways in microglia and astroglia cells. Consistent with these in vitro data, our in vivo results reinforced the notion that the oral administration or injection (intracerebroventricular (i.c.v)) of MgT to APP/PS1 mice clearly inhibited the expression of IL-1β in the cerebral cortex by reducing the production and deposition of Aβ oligomers. These in vitro and in vivo observations may be instrumental to understanding the roles of Mg2+ elevation in suppressing neuroinflammation, which potentially contributes to regulating the pathogenesis of AD.
Materials and methods
Reagents
Aβ1-42, GW9662, and the inhibitor for ERK1/2, PD98059, were obtained from Sigma-Aldrich Corp (St. Louis, MO, USA). MgT was purchased from Soyoung Biotechnology Company (Shanghai, China). Antibodies against β-actin, ERK1/2, p-ERK1/2 (Thr 202/Tyr 204), PPARγ, Aβ, IL-1β, NeuN, glial fibrillary acidic protein (GFAP), Alexa Fluor 488-labeled, Fluor 555-labeled, and horseradish peroxidase -labeled secondary antibody were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Iba-1, p-PPARγ (Ser 82) antibody was from Merck Millipore (Bedford, MA, USA). ERK1/2, PPARγ, or scramble siRNA was obtained from Cell Signaling Technology, Inc. 4,6-diamidino-2-phenylindole was obtained from Beyotime Institute of Biotechnology (Haimen, JS, China). The IL-1β and IL-1β enzyme immunoassay kits were from Raybiotech, Inc. (Norcross, GA, USA). The Aβ1-42 enzyme immunoassay kits were from Invitrogen (Carlsbad, CA, USA). All reagents for the quantitative real time polymerase chain reaction (qRT-PCR) and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) experiments were purchased from Bio-Rad Laboratories (California, USA). All other reagents were from Invitrogen unless otherwise specified.
Transgenic mice and treatments
The male wild-type (WT) or APP/PS1 transgenic mice (B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/J (Stock Number: 004462)) (Tg) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Genotyping was performed 3–4 weeks after birth. Five mice per cage were housed in a controlled environment under standard room temperature, relative humidity, and 12 h light/dark cycle with free access to food and water. In selected experiments, 4 months old mice were treated with Mg2+ (100 mg/kg/d) in drinking water for 2 or 5 months before determining the expression of IL-1β or the deposition of Aβ plaques. The general health and body weights of animals were monitored everyday. The brains of animals in different groups (n = 6) were collected under anesthesia and perfusion fixed as previously described.8
Cerebrospinal fluid collection
Cerebrospinal fluid (CSF) was collected as previously described.9 In brief, the mice were anesthetized and placed prone on the stereotaxic instrument. A sagittal incision of the skin was made inferior to the occiput. Under the dissection microscope, the subcutaneous tissue and neck muscles through the midline were bluntly separated. A microretractor was used to hold the muscles apart. Next, the mouse was laid down so that the body made a 135° angle with the fixed head. In this angle, the dura and spinal medulla were visible, had a characteristic glistening and clear appearance, and the circulatory pulsation of the medulla (i.e., a blood vessel) and adjacent CSF space could be seen. The dura was then penetrated with a 6-cm-long glass capillary that had a tapered tip with an outer diameter of 0.5 mm. Following a noticeable change in resistance to the capillary insertion, the CSF flows into the capillary. The average volume of CSF obtained was approximately 7 μl. All samples were stored in polypropylene tubes at –80 °C until injection.
Intracerebroventricular injection
MgT, IL-1β, Aβ oligomers, or vehicle (phosphate-buffered saline (PBS) were injected (i.c.v.) into WT or APP/PS1 transgenic mice as previously described.10 In selected experiments, the WT mice were injected (i.c.v.) with the CSF of APP/PS1 in the absence or presence of Aβ antibody. Briefly, stereotaxic injections were placed at the following coordinates from bregma: mediolateral: –1.0 mm; anteroposterior: –0.22 mm; dorsoventral: –2.8 mm. Following injection, each mouse recovered spontaneously on a heated pad. The reliability of injection sites was validated by injecting trypan blue dye (Invitrogen) in separate cohorts of mice and observing staining in the cerebral ventricles; 24 h after injection, mice were killed under anesthesia and perfused as previously described.8
Organotypic slice culture of brain tissue
Parts of brain tissues were freshly collected from 3-month-old WT C57BL/6 mice. Serial sections (400 μm thick) were cut in a chopper without fixation. The slices were immediately cultured in Dulbecco's Modified Eagle's medium/high glucose medium with 10% fetal bovine serum. In a separate set of experiments, the slices were grown in serum-free medium for an additional 24 h before incubation with MgT in the absence or presence of IL-1β or Aβ oligomers, as previously described.11,12 After 24 h, the tissue sections were fixed, and immunostained with IL-1β antibody by immunohistochemistry staining kit (Invitrogen).
Luciferase assays and live animal imaging
1 × 103 D1A cells pre-transfected with IL-1β promoter plasmids were injected in the right cerebral ventricle. MgT or vehicle (PBS) was then injected (i.c.v.) into the left cerebral ventricle. At different time intervals, mice were anesthetized and injected (i.c.v.) with luciferin in the right cerebral ventricle. The scan was performed exactly 5 min following luciferin introduction. All images were analyzed using Bruker in vivo imaging systems (MS FX PRO, Carestream, Billerica, MA, USA).
Two-photon imaging
The mice were anesthetized and placed on a heating pad in order to maintain a body temperature of 37 °C before surgery. Residual fur was removed by shaving and a midline scalp incision was made that extended approximately from the neck region (between the ears) to the frontal portion of the head (between the eyes). The left part of the skull was carefully taken off using a drill and the right part of the skull was left untouched. The skull chamber was adhered on the top of skull. The membrane of the cerebral cortex was permeabilized with 0.3% triton X-100 solution. In selected experiments, MgT was injected (i.c.v.) into the right ventricles of mice brains. Using the skull chamber, the surface of the cerebral cortex was immunostained with IL-1β antibody before two-photon scanning, which was performed using a custom-built two-photon microscope based on a chameleon excitation laser operating at 690–1064 nm. The laser-scanning unit was mounted on an upright microscope equipped with a water-immersion objective (Zeiss; 20× W), and the fluorescence was detected using specific antibody staining. The brain surface was stained and scanned before and after the injection (i.c.v.) of MgT or vehicle (PBS).
Cell culture
Human glioblastoma A172 or mouse astrocytes/microglia D1A cells were grown (37 °C and 5% CO2) on 6 cm tissue culture dishes (106 cells per dish) in appropriate media. In a select set of experiments, the cells were grown in serum-free medium for an additional 24 h before incubation with inhibitors in the absence or presence of MgT, as previously described.11,12 In separate experiments, the astrocytes were separated from the neopallia of the cerebral hemispheres as previously described.13 In distinct experiments, astrocytes and neuron cells were co-cultured using transwell, which seeded astrocytes in upper chamber and neuron cells in lower chamber of transwell. After 24 h, the neuron cells were immunostained with IL-1β.
Quantitative real-time PCR
qRT-PCR assays were performed with the MiniOpticon Real-Time PCR detection system (Bio-Rad) using total RNA and the GoTaq one-step Real-Time PCR kit with SYBR green (Promega, Madison, Wisconsin, USA) and the appropriate primers as previously described.14 The GenBank accession number and forward and reverse primers for human IL-1β12 and GAPDH14 are provided in our previous publications; for mouse IL-1β (NM_008361.3) F-TTCAAATCTCGCAGCAGCAC, R-GTGCAGTTGTCTAATGGGAACG; GAPDH (NM_001289726.1) F-AACTTTGGCATTGTGGAAGG, R-ACACATTGGGGGTAGGAACA. The gene expression values were normalized to those of GAPDH.
Western blot analysis
Tissues or cells were lysed in radio-immune precipitation assay buffer (25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS) containing protease inhibitor cocktail (Pierce Chemical Company, Rockford, Illinois, USA). The protein content of the cell lysates was determined using a bicinchoninic acid protein assay reagent (Pierce Chemical Company). The total cell lysates (4 μg) were subjected to SDS-PAGE, transferred to a membrane, and probed with a panel of specific antibodies. Each membrane was only probed with one antibody. β-actin was used as a loading control. All of the western experiments were performed at least in triplicate using a different cell preparation each time.
Immunohistochemistry
Mouse brains were collected from 3-month-old WT or APP/PS1 Tg mice and immobilized with 4% paraformaldehyde. Serial sections (10 μM thick) were cut using a cryostat (Leica, CM1850, Germany). Slides were first rehydrated in a graded series of ethanol and submerged in 3% hydrogen peroxide to eliminate endogenous peroxidase activity. IL-1β levels were determined using immunohistochemical staining kit, following the manufacturer's instructions (Invitrogen). In selected experiments, the slices from the brains of human and mouse were double-stained with IL-1β (Alexa Fluor 555-labeled secondary IgG) or one of NeuN, Iba-1, or GFAP (Alexa Fluor 488-labeled secondary IgG) antibody as previously described.15
Measurement of the IL-1β and Aβ concentration
The IL-1β and Aβ1–42 levels were determined using IL-1β and Aβ enzyme immunoassay kits following the manufacturer's instructions. The total protein in the medium was used as a loading control, and the results are expressed as pg of IL-1β or Aβ per µg or mg of total protein.
Transfection
Cells were transfected with 100 nM of an ERK1/2 or PPARγ-specific siRNA oligonucleotide. In control experiments, the cells were transfected with 100 nM of scrambled siRNA. The transfected cells were allowed to recover for at least 12 h in growth medium and then incubated overnight in serum-free medium before extraction. For live animal imaging experiments, cells were transfected with IL-1β promoter plasmids for 48 h before screening with puromycin (1 μg/ml). The stable cell lines were then seeded in the ventricles of C57BL/6 mice.
Animal committee
All animals were handled according to the care and use of medical laboratory animals (Ministry of Health, Peoples Republic of China, 1998) and the guidelines of the laboratory animal ethical standards of China Medical University.
Human brain samples
Human brain samples were obtained from New York Brain Bank, serial numbers P535-00 (normal), TT4263 (early stage of AD), T4308 (middle stage of AD), and T4339 and T4304 (late stage of AD).
Statistical analysis
All data are represented as the mean ± SE of at least three independent experiments. The statistical significance of the differences between the means was determined using Student's t-test or one-way analysis of variance where appropriate. If the means were found to be significantly different, multiple pairwise comparisons were performed using Tukey's post hoc test.16
Results
IL-1β is markedly upregulated in APP/PS1 transgenic mice and AD patients
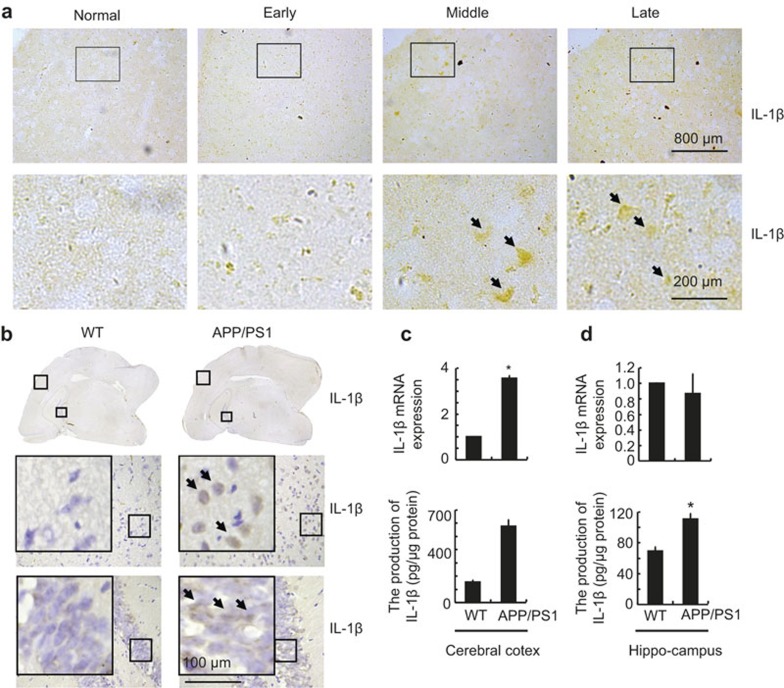
In light of previous studies suggesting critical roles for IL-1β in the pathogenesis of AD,6,17 we evaluated the expression levels of IL-1β in the brains of AD patients and 3-month-old APP/PS1 Tg mice. As shown in Figure 1a, IL-1β immunostaining was evident in the human cerebral cortex, and the positive staining gradually increased with the progression of AD. In accordance with these data, IL-1β immunostaining was also highly enhanced in the cerebral cortex and the dentate gyrus region of the hippocampus in 3-month-old APP/PS1 Tg mice compared with WT C57BL/6 mice (Figure 1b). These data reveal that IL-1β is upregulated with the development/progression of AD. To further confirm this finding, we examined the mRNA and protein levels of IL-1β in the APP/PS1 Tg mice. In agreement with the immunostaining data, our results showed that mRNA levels of IL-1β were upregulated only in the cerebral cortex (Figure 1c and d upper panel), whereas IL-1β protein was upregulated in both the cerebral cortex and the hippocampus of the APP/PS1 mice (Figure 1c and d lower panel). These observations further suggested the potential involvement of IL-1β in AD.
Figure 1.
IL-1β was upregulated in AD patients and APP/PS1 transgenic mice. The tissue blocks of human brains at different stages of AD were collected from the New York Brain Bank at Columbia University. From 40 μm free-floating slices were prepared using a cryostat. (a) In select experiments, the brains of 3-month-old APP/PS1 transgenic mice were collected after anesthesia and perfusion. (b–d) The immunoreactivity of IL-1β was determined by immunohistochemistry using an anti-IL-1β antibody (a and b). These images are representative of six independent experiments, all with similar results. IL-1β mRNA levels were determined by qRT-PCR, and total amounts of GAPDH served as an internal control (c and d upper panels). The production of IL-1β in culture medium was determined using IL-1β enzyme immunoassay kits (c and d lower panels). The data represent the means ± SE of three independent experiments. *P < 0.05 compared with the WT controls.
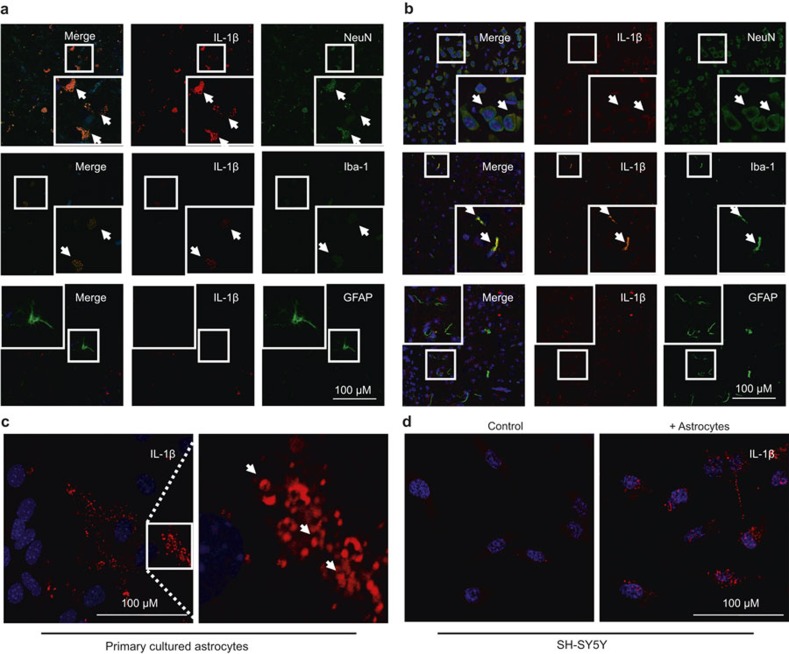
In light of our observations, which showed that only the protein levels of IL-1β were upregulated in the hippocampus of APP/PS1+/– Tg mice, it is easy to speculate that IL-1β may not be transcriptionally synthesized by neuronal cells. As a type of secreted cytokine, it is possible that IL-1β is secreted from other types of cells and exerts its effects on neuronal cells by binding to their receptors. We thus carried out experiments to determine the localization of IL-1β using localization of fluorescence staining. As a first step, we labeled the neurons, microglia, or astrocytes with NeuN, Iba-1, or GFAP antibodies (green), respectively. The brains of humans and C57BL/6 mice were then double-stained with IL-1β antibody (red). The results revealed that IL-1β co-localized with the neuronal and microglia cells, but not with the astrocytes (Figure 2a and b). Nevertheless, we still could not exclude the possibility of IL-1β expression in neuronal cells based on these findings. We thereby further determined the mRNA expression of IL-1β in neuronal and microglial cells. The results demonstrated that IL-1β was highly expressed in both primary cultured astrocytes (Figure 2c) or D1A cells, but not in neuronal cells (Table 1). In addition, both neuronal cells and astrocytes expressed IL-1 receptors, including IL-1 receptor types I and II (Table 1). In view of these observations, we questioned where IL-1β comes from in neuronal cells. To resolve this problem, transwell experiments were carried out to co-culture astrocytes and neurons as described in the Materials and Methods section. Interestingly, by immunostaining neuron cells with IL-1β antibody, the results demonstrated that IL-1β translocated from the astrocytes to the neurons (Figure 2d).
Figure 2.
Localization of IL-1β in the brains of AD patients. The tissue blocks of human brains at a late stage of AD were collected from the New York Brain Bank at Columbia University. From 40 μm free-floating slices were prepared using a cryostat. (a) In select experiments, the brains of 3-month-old APP/PS1 transgenic mice were collected after anesthesia and perfusion. (b) The slices of human (a) or mouse (b) brains were double-stained with NeuN, Iba-1, GFAP (green), or IL-1β (red) antibody before observation using confocal microscopy. In separate experiments, primary cultured astrocytes were immunostained with IL-1β antibody before observation under confocal microscopy. (c) In distinct experiments, the astrocytes were co-cultured with neurons using transwell experiments for 24 h before staining the neurons with IL-1β antibody. The cells were then observed under confocal microscopy. (d) These images are representative of six independent experiments, all with similar results.
Table 1. The enrichment of IL-lβ and IL-l receptors in glia and neuron cells.
| Ct value | IL-lβ | IL-1R1 | IL-1R2 |
|---|---|---|---|
| SH-SY5Y | N/A | N/A | 25.47 |
| A172 | 26.94 | 31.85 | N/A |
| n2a | N/A | 30.46 | 26.98 |
| D1A | 28.94 | 25.11 | 30.82 |
Additionally, our data and observations not only revealed that IL-1β is not produced by neuronal cells but also explained the reason why we could observe IL-1β-positive staining in neuronal cells.
Mg2+ attenuates the synthesis of IL-1β in MgT-treated glial cells
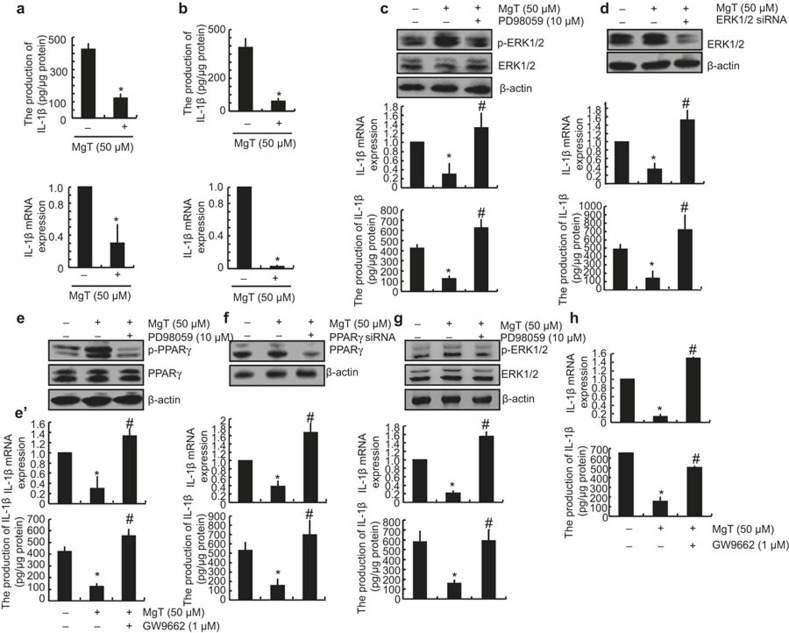
Previous studies have revealed that brain Mg2+ levels3 and serum Mg2+ concentrations4 appear to be significantly lower in AD patients compared with aged-matched normal subjects. These observations suggest a potential protective effect for Mg2+ against AD. Given the essential roles of IL-1β in the pathogenesis and progression of AD,6,17 we initially determined the effects of MgT treatment on the expression of IL-1β in glial cells. Using human A172 cells as a model system for glial cells, we detected the effects of MgT treatment on the expression of IL-1β. As shown in Figure 3a, MgT (50 μM) treatment markedly suppressed the mRNA and protein expression of IL-1β in A172 cells. To further exclude the possibility of cell-specific inhibition of IL-1β by MgT (50 μM), we also carried out similar experiments in mouse astrocytes/microglia D1A cells, and similar results were obtained (Figure 3b). Taken together, our results reveal a key role for Mg2+ in inhibiting the synthesis of IL-1β in glial cells (Figure 3a and b).
Figure 3.
Involvement of ERK1/2 and PPARγ pathways in regulating the expression of IL-1β in MgT-treated A172 or D1A cells. Human glioblastoma A172 (a) or mouse astrocytes/microglia D1A cells (b) were treated with MgT (50 μM) for 48 h. In select experiments, A172 cells were treated with PD98059 (10 μM) in the absence or presence of MgT (50 μM) for 48 h (c and e). In separate experiments, the cells were transfected with ERK1/2 (d) or PPARγ siRNA (f) before incubation with MgT (50 μM) for 48 h. In distinct experiments, A172 cells were treated with GW9662 (1 μM) in the absence or presence of MgT (50 μM) for 48 h (e'). In other experiments, primary cultured astrocytes were treated with MgT (50 μM) in the absence or presence of PD98059 (10 μM) (g) or GW9662 (1 μM) for 48 h (h). Total ERK1/2 (c, d and g upper panel), phosphorylated ERK1/2 levels (c and g upper panel), total PPARγ (e and f upper panel), and phosphorylated PPARγ (e) were detected by immunoblotting using specific Abs. Equal lane loading is demonstrated by the similar intensities of total β-actin. IL-1β protein and mRNA levels were determined by IL-1β enzyme immunoassay kits and qRT-PCR, respectively. The total amounts of protein and GAPDH served as internal controls. The data represent the means ± SE of three independent experiments. *P < 0.05 compared with the vehicle-treated control. #P < 0.05 compared with MgT treatment alone.
Pivotal roles of ERK1/2 and PPARγ signaling pathways in Mg2+-mediated suppression of IL-1β expression in glial cells
We next aimed to elucidate the signaling pathways of IL-1β regulation in MgT-treated glial cells. In light of a previous work suggesting that Mg2+ deprivation inhibits the MEK-ERK signaling cascade,18 we evaluated the effects of exogenous MgT (50 µM) on ERK1/2 activation. In line with these in vivo data, our in vitro results show that MgT treatment induced the phosphorylation of ERK1/2 without altering the total protein levels of ERK1/2 in A172 cells (Figure 3c and d). To further elucidate the potential role of ERK1/2 in the regulation of the expression of IL-1-β we treated A172 cells with the pharmacological ERK1/2 inhibitor PD98059 (10 μM) in the absence or presence of MgT (50 μM). The incubation of A172 cells with PD98059 (10 μM) not only suppressed the MgT-induced phosphorylation of ERK1/2 but also reversed the MgT-dependent decrease of IL-1β synthesis (Figure 3c). To eliminate any potential non-specific effects of the pharmacological ERK1/2 inhibitor PD98059, experiments were carried out using A172 cells transfected with an siRNA oligonucleotide sequence specific for ERK1/2. ERK1/2-knockdown and scramble control cells were treated with MgT (50 μM) or the vehicle control for 48 h. ERK1/2 knockdown markedly reversed the inhibitory effects of MgT on the mRNA and protein expression of IL-1β in A172 cells (Figure 3d).
Treatment of A172 cells with MgT (50 μM) induced PPARγ phosphorylation (Figure 3e). PD98059 (10 μM) abrogated the MgT-induced phosphorylation of PPARγ in A172 cells (Figure 3e). To decipher the role of PPARγ in regulating IL-1β expression, we next treated A172 cells with the PPARγ antagonist GW9662 (1 μM). The results indicate that GW9662 treatment reversed the effects of the MgT-induced suppression of IL-1β expression in A172 cells (Figure 3e'). To eliminate any non-specific effects of the PPARγ antagonist, the experiments were performed using cells transfected with an siRNA oligonucleotide specific for PPARγ. The efficiency of the PPARγ knockdown was demonstrated by assessing the PPARγ mRNA levels in the A172 cells (Figure 3f upper panel). The PPARγ knockdown reversed the inhibitory effects of MgT on the mRNA and protein expression of IL-1β in A172 cells (Figure 3f lower panel). To further confirm the above observations, similar experiments were carried out in primary cultured astrocytes. The results demonstrated that the ERK1/2 and PPARγ signaling pathways are critical for mediating the MgT-dependent downregulation of the expression of IL-1β in primary cultured astrocytes (Figure 3g and h). Taken together, these observations indicate that the activation of the ERK1/2 and PPARγ signaling pathways plays a critical role in regulating MgT-induced suppression of the expression of IL-1β in human glial cells.
Mg2+ elevation inhibits the mRNA and protein expression of IL-1β in APP/PS1 transgenic mice
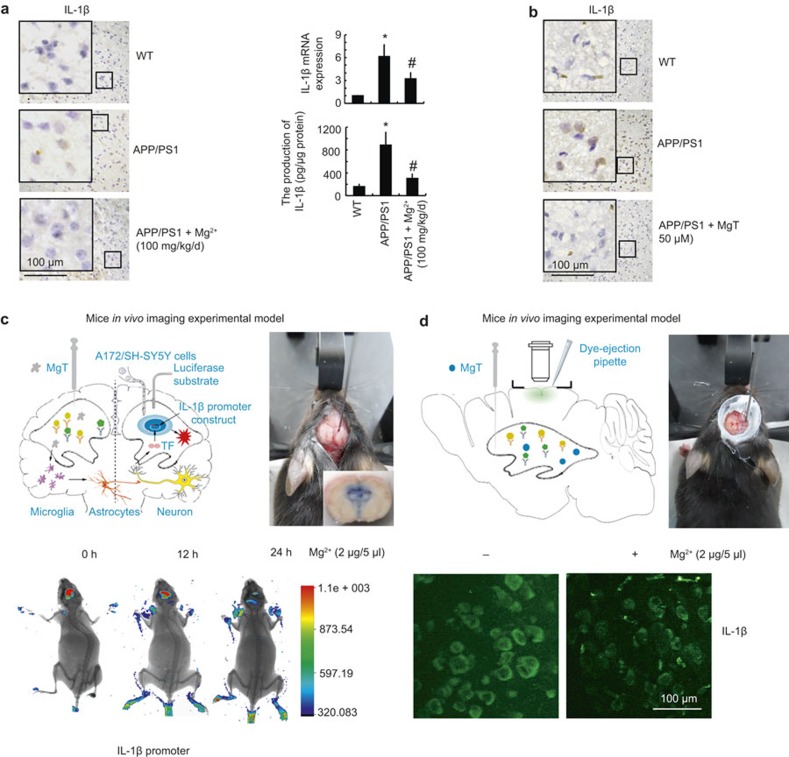
Although Mg2+ elevation suppresses the expression of IL-1β in vitro, little is known about the effects of Mg2+ elevation in the in vivo setting. This is particularly important because Mg2+ homeostasis may be disturbed in AD Tg mice. To delineate the relationship between Mg2+ elevation and the expression of IL-1β in vivo, we first employed immunohistochemistry to evaluate the expression of IL-1β in 3-month-old Tg mice. We found that Mg2+ administration (100 mg/kg/d) for 2 months decreases IL-1β expression in the cerebral cortex of APP/PS1 Tg mice (Figure 4a left panel). In addition, Mg2+ administration (100 mg/kg/d) for 2 months suppressed the mRNA and protein expression of IL-1β in the cerebral cortex (Figure 4a right panel). To validate the inhibitory effects of MgT on the expression of IL-1β, an organotypic slice culture was carried out in the absence or presence of MgT (50 μM) for 24 h. Imunohistochemistry experiments were then carried out to determine the expression of IL-1β after 24 h of culture. The results reveal that MgT (50 μM) treatment abolishes the upregulation of IL-1β in APP/PS1 Tg mice (Figure 4b).
Figure 4.
Elevated levels of Mg2+ in APP/PS1 transgenic mice decrease the expression of IL-1β. The APP/PS1 transgenic mice at the age of 4 months were administered Mg2+ (100 mg/kg/d) for 2 months before collecting the brain (a). In select experiments, the brains of APP/PS1 transgenic mice at the age of 3 months were harvested and sectioned (400 μm) using a cryostat (b). In separate experiments, the left cerebral ventricle was injected with Mg2+ (2 μg/5 μl) or vehicle (PBS) and the right cerebral ventricle was injected (i.c.v.) with D1A cells, which was pre-transfected with IL-1β promoter in the right cerebral ventricle (c). In distinct experiments, the left cerebral ventricle was injected with Mg2+ (2 μg/5 μl) or vehicle (PBS) before staining with IL-1β antibody and scanning under two-photon microscopy (d). The immunoreactivity of IL-1β was determined by immunohistochemistry using an anti-IL-1β antibody (a left panel, b). These images are representative of six independent experiments, all with similar results. IL-1β protein and mRNA levels were determined by qRT-PCR and IL-1β enzyme immunoassay kits, respectively (a right panel). The total amounts of GAPDH and protein served as an internal control. The experimental cartoon and real surgery images are shown (c, d upper panel). Luciferase activities from the different groups of mice were measured using a live animal imaging system (c lower panel). The immunofluorescence of IL-1β was scanned using a two-photon microscope (d lower panel). The data represent the means ± S.E. of three independent experiments. *p < 0.05 compared with wild type mice. #p < 0.05 compared with APP/PS1 transgenic mice.
To further verify the key role of MgT in suppressing the expression of IL-1β in vivo, we combined i.c.v. injection with live animal/two-photon imaging. As described in Figure 4c upper panel, D1A cells transfected with IL-1β promoter constructs were pre-seeded into the right cerebral ventricle, whereas Mg2+ (2 μg/5 μl) was injected into the left cerebral ventricle of 3-month-old APP/PS1 Tg mice. After 24 h, luciferin was injected (i.c.v.) into the right cerebral ventricle of APP/PS1 Tg mice before live animal imaging. The results show that MgT decreased the luciferase activity of the IL-1β promoter (Figure 4c lower panel). In addition, the cerebral cortex of APP/PS1 Tg mice was immunostained with IL-1β before or after injection (i.c.v.) of MgT for 24 h (Figure 4d upper panel). The two-photon scanning results revealed that Mg2+ (2 μg/5 μl) injection (i.c.v.) decreases the immunofluorescence of IL-1β on the surface of the cerebral cortex of APP/PS1 Tg mice (Figure 4d lower panel). Collectively, our results demonstrate that Mg2+ influx in vivo transcriptionally suppresses the expression of IL-1β in APP/PS1 Tg mice.
MgT suppresses the effects of the secreted form of IL-1β and Aβ oligomers in the ventricles by inducing the expression of IL-1β in the cerebral cortex
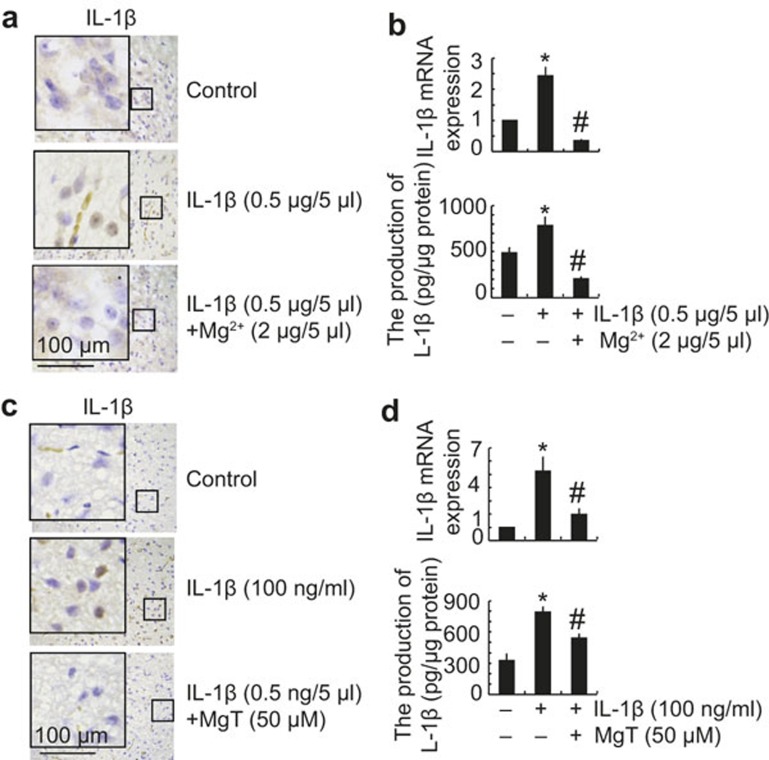
In view of our findings, which showed that MgT incubation decreases the expression of IL-1β in vitro and in vivo, we next examined the potential contribution of the secreted form of IL-1β and Aβ in the pathogenesis and progression of AD. Interestingly, the injection of IL-1β (0.5 μg/5 μl) into the cerebral ventricles of WT C57BL/6 mice for 24 h increased the expression of IL-1β in the cerebral cortex, whereas the addition of Mg2+ (2 μg/5 μl) effects the IL-1β in the ventricles by inducing the expression of IL-1β in the cerebral cortex (Figure 5a and b). Similar results were observed in the organotypic slice cultures of brain tissue (Figure 5c). These findings are in line with in vitro results showing that incubation with MgT (50 μM) suppressed the expression of IL-1β in IL-1β (100 ng ml–1)-stimulated A172 cells (Figure 5d).
Figure 5.
MgT treatment diminished the effects of IL-1β in the CSF with respect to inducing the expression of IL-1β in the cerebral cortex. WT C57BL/6 mice at the age of 3 months were intracerebroventricularly injected with IL-1β (0.5 μg/5 μl) in the absence or presence of Mg2+ (2 μg/5 μl). The brains were then collected and sectioned after 24 h (a and b). In select experiments, the brains of WT C57BL/6 mice at the age of 3 months were harvested and freshly sectioned (400 μm) before treatment with IL-1β (100 ng ml–1) in the absence or presence of MgT (50 μM) for 24 h (c). In separate experiments, A172 cells were treated with IL-1β (100 ng ml–1) in the absence or presence of MgT (50 μM) for 24 h (d). The immunoreactivity of IL-1β was determined by immunohistochemistry using an anti-IL-1β or -APH-1 antibody (a and c). These images are representative of six independent experiments, all with similar results. IL-1β mRNA and protein levels were determined by qRT-PCR and IL-1β enzyme immunoassay kits, respectively (b and d). The total amounts of GAPDH and protein served as an internal control. The data represent the means ± SE of three independent experiments. *P < 0.05 compared with respect to vehicle-treated controls. #P < 0.05 compared with IL-1β-treated alone.
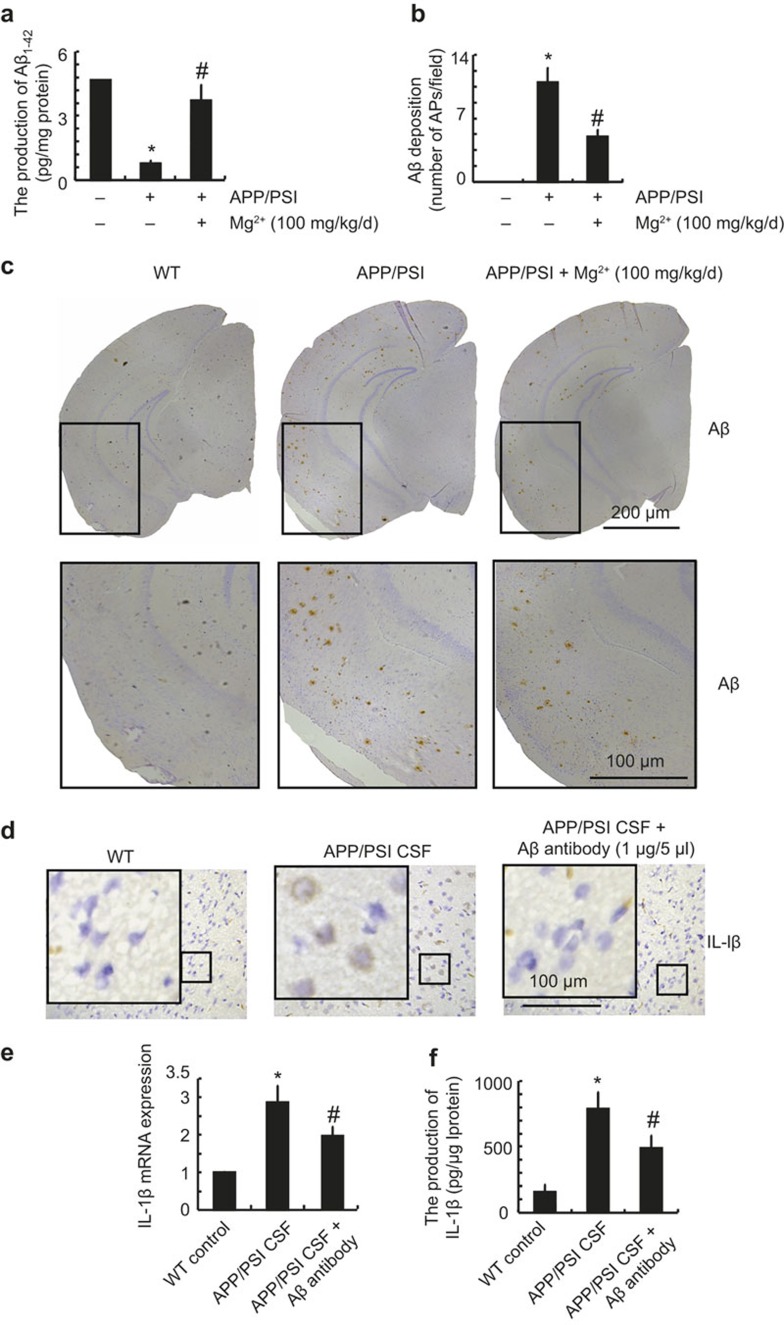
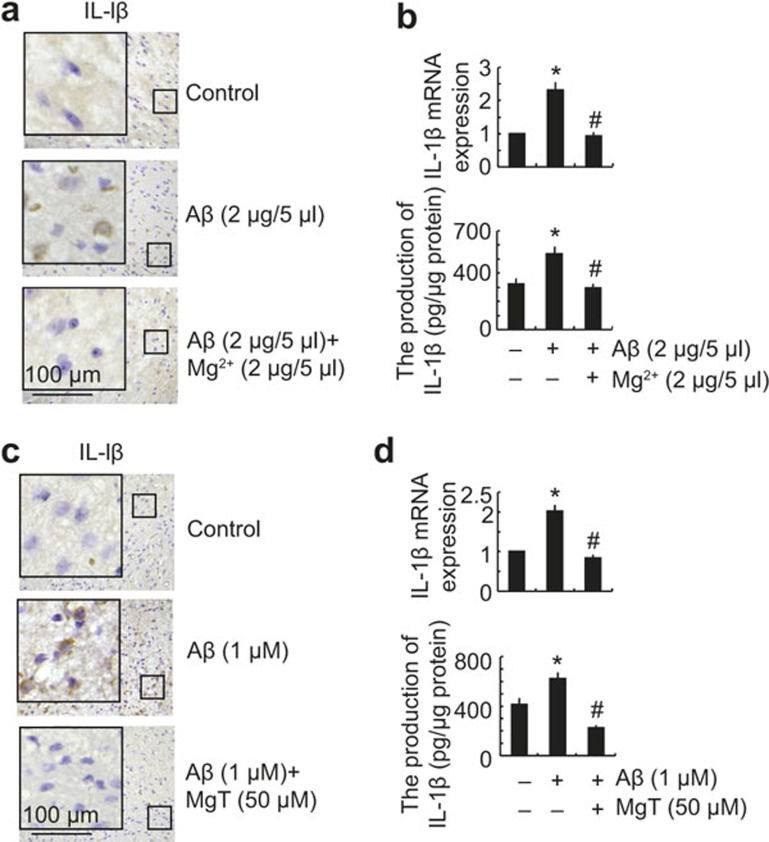
In view of the critical roles of Aβ deposition during neuroinflammation,19,20 we questioned whether Aβ production in APP/PS1 transgenic mice has the ability to regulate the expression of IL-1β. As a first step, we determined the production and deposition of Aβ in APP/PS1 mice. The results demonstrated that the levels of Aβ1-42 in the CSF were decreased in APP/PS1 transgenic mice and restored by MgT treatment (Figure 6a). However, the deposition or aggregation of Aβ1-42 was highly induced and deposited in the APs of 9-month-old APP/PS1 transgenic mice and was suppressed by MgT administration (Figure 6b and c). These observations demonstrated that the majority of Aβ1-42 was progressively aggregated and deposited in the cerebral cortex during the course of AD development. To further evaluate the involvement of Aβ in the regulation of IL-1β expression, in vivo experiments were performed. CSF (5 μl) was collected from APP/PS1 Tg mice and then injected into WT C57BL/6 mice for 2 weeks before killing. Compared with the control subjects, the expression of IL-1β in the cerebral cortex was markedly induced by the injection of APP/PS1 CSF (Figure 6d–f). Moreover, Aβ antibody injection blocked the effects of APP/PS1 CSF with respect to inducing the expression of IL-1β in the cerebral cortex of mice (Figure 6d–f). These observations were clearly in contrast to the total levels of Aβ1-42 in the CSF. In light of the ability of Aβ1-42 aggregation, we suspect that the proportion of aggregated Aβ1-42 was elevated, although the total amount of Aβ1-42 was downregulated in the CSF of APP/PS1 mice. To this end, oligomeric Aβ1-42 (2 μg/5 μl) was injected (i.c.v.) into the ventricles of 3-month-old WT C57BL/6 mice, and the results demonstrated that oligomeric Aβ injection increased the expression of IL-1β in the cerebral cortex (Figure 7a and b). In addition, the upregulation of IL-1β was suppressed by Mg2+ (2 μg/5 μl) injection (i.c.v) in the Aβ oligomers-stimulated C57BL/6 mice (Figure 7a and b). Similar results were obtained by organotypic slice cultures of brain tissue (Figure 7c). These in vivo observations were in concert with in vitro data showing that Aβ (1 μM) treatment stimulated the expression of IL-1β, which was blocked by MgT incubation in glial cells (Figure 7d). Collectively, these data revealed that Aβ1-42 is a downstream molecule of MgT and regulates the expression of IL-1β in APP/PS1 transgenic mice.
Figure 6.
Aβ oligomers in the CSF of APP/PS1 mice have the ability to stimulate the expression of IL-1β in the cerebral cortex of WT mice. The APP/PS1 Tg mice at the age of 4 months were treated with Mg2+ (100 mg/kg/d) for 5 months before collecting the CSF and brains (a-c). Cerebrospinal fluid (CSF) was then injected into the wild type C57BL/6 mice in the absence or presence of Aβ antibody (1 μg/5 μl) for two weeks before scarifice (d-f). The production of Aβ1-42 was determined by ELISA kits, and the total amount of protein served as an internal control (a). The immunoreactivity of Aβ and IL-1β was determined by immunohistochemistry using an anti-Aβ or IL-1β antibody (c, d). These images are representative of six independent experiments, all with similar results. The number of AP/fields was calculated according to the images of IHC (b). IL-1β mRNA and protein levels were determined by qRT-PCR and IL-1β enzyme immunoassay kits, respectively (e, f). The total amounts of GAPDH and protein served as an internal control. *p < 0.05 compared with respect to wild type mice. #p < 0.05 compared with APP/PS1 alone.
Figure 7.
MgT treatment attenuated the effects of Aβ oligomers in the CSF for inducing the expression of IL-1β in the cerebral cortex. The WT C57BL/6 mice at the age of 3 months were injected (i.c.v) with Aβ oligomers (2 μg/5 μl) in the absence or presence of Mg2+ (2 μg/5 μl). The brains were then collected and sectioned after 24 h (a and b). In select experiments, the brains of WT C57BL/6 mice at the age of 3 months were harvested and freshly sectioned (400 μm) before treatment with Aβ oligomers (1 μM) in the absence or presence of MgT (50 μM) for 24 h (c). In distinct experiments, A172 cells were treated with Aβ oligomers (1 μM) in the absence or presence of MgT (50 μM) for 24 h (d). The immunoreactivity of IL-1β was determined by immunohistochemistry using an anti-IL-1β antibody (a and c). These images are representative of six independent experiments, all with similar results. IL-1β mRNA and protein levels were determined by qRT-PCR and IL-1β enzyme immunoassay kits, respectively (b and d). The total amounts of GAPDH and protein served as an internal control. The data represent the means ± SE of three independent experiments. *P < 0.05 compared with respect to vehicle-treated controls. #P < 0.05 compared with Aβ-treated alone.
Discussion
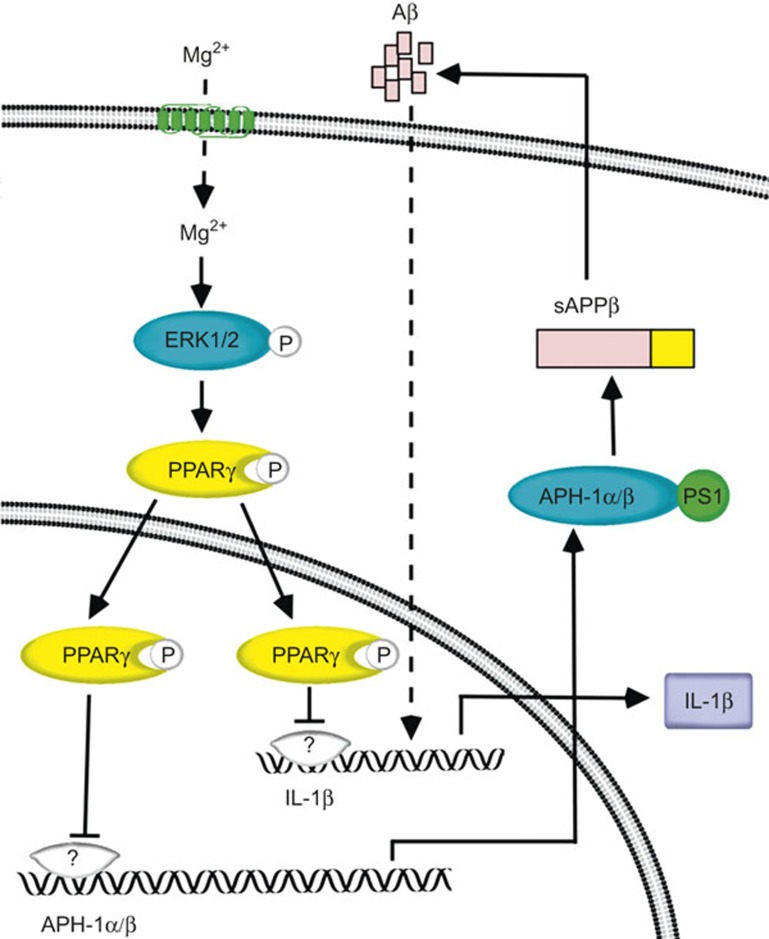
AD is pathologically characterized by a marked induction of neuroinflammation.21 Although Mg2+ has been implicated in reducing neuroinflammation during the course of AD development,22,23 the underlying mechanisms between Mg2+ and IL-1β expression are still elusive. Thus, this study aimed to decipher the suppressive effects of Mg2+ on IL-1β expression. The major findings of this work were as follows: (i) the elevation of Mg2+ in microglia cells suppressed the synthesis of IL-1β; (ii) the ERK1/2 and PPARγ pathways are involved in mediating the suppressive effects of Mg2+ on IL-1β expression in microglia cells; (iii) the secreted form of IL-1β in the CSF is critical for self-induction of IL-1β in the cerebral cortex of APP/PS1 transgenic mice; and (iv) the Aβ oligomers in the CSF mediated the effects of MgT on suppressing the expression of IL-1β in the cerebral cortex of APP/PS1 transgenic mice, which potentially improved the neuroinflammation of AD (Figure 8).
Figure 8.
Proposed cascade of the signaling events regulating the expression of IL-1β by MgT. In detail, attenuated levels of Mg2+ in APP/PS1 transgenic mice will elevate the production of IL-1β and Aβ via ERK1/2- and PPARγ-dependent pathways in glial cells of the APP/PS1 transgenic mouse brain, which in turn will potentially regulate the pathogenesis of AD. Interestingly, the highly secreted IL-1β and Aβ in the CSF are able to regulate the synthesis of IL-1β in the cerebral cortex of APP/PS1 mice. These observations might be instrumental for understanding the roles of Mg2+ in suppressing the neuroinflammation of AD.
Mg2+ is tightly regulated and kept at basal levels under physiological conditions. Accumulating evidence suggests that Mg2+ is markedly downregulated during the course of AD progression.3,4 Indeed, MgT administration prevents synapse loss and reverses memory deficits in aged rats.24 These observations suggest that Mg2+ elevation in the brain may exert neuroprotective effects against the onset of AD. In addition, Lipinski et al.25 also reported that Mg2+ has the ability to disrupt the aggregation between red blood cells and fibrillar Aβ1-42, which protects the brain from the risk of AD. Along these lines, MgSO4 treatment decreases maternal and fetal inflammation following lipopolysaccharide (LPS) injection.26 On the other hand, magnesium deficiency results in cardiac dysfunction and inflammation, as evidenced by the marked induction of cytokine production in rats.27,28,29 Moreover, MgSO4 also decreases inflammation-associated brain injury in fetal mice,30 supporting a link between magnesium, inflammation, and neurological injury in rodents. This evidence prompted us to hypothesize that MgT exerts its neuroprotective effect by downregulating inflammatory cytokine production. Indeed, our findings reveal that MgT treatment obviously suppressed the synthesis of IL-1β both in vitro and in vivo. In line with our data, Sugimoto et al.5 reported that Mg2+ reduces the expression of IL-1β in human monocytic cells. Others31 have found that the administration of MgSO4 to pre-eclamptic placentas resulted in an attenuation of the increased secretion of IL-1β into the maternal circulation and in a reduction of IL-1Ra.
In light of the suppressive effects of MgT on IL-1β expression, we were prompted to reveal the mechanisms. In agreement with our data, Li et al.32 reported that pioglitazone, a PPARγ agonist, suppresses the expression of IL-1β in LPS-stimulated microglia cells. In addition, Valles et al.33 revealed that increased PPARγ expression prevents Aβ-induced IL-1β expression in cultured astrocytes. More specifically, the PPARγ agonist, 1,1-bis(3'-indolyl)-1-(p-trifluoromethylphenyl) methane suppresses Mg2+-induced production of nitric oxide in astrocytes.34 We extended the prior works to ERK1/2, which is responsible for PPARγ phosphorylation. Our data were supported by the study showing that active PPARγ was recruited to phosphorylated ERK1/2, which in turn improves the cognitive decline in AD patients or Tg2576 mice.35 Moreover, Denner et al.36 found a significant overlap between PPARγ target genes and ERK1/2-regulated, cAMP response elements containing target genes in Tg2576 mice. Although they did not extend their study to find the regulatory relationship between ERK1/2 and PPARγ, these prior works have implied the important roles of ERK1/2 and PPARγ signaling pathways in AD development. Along these lines, our data provide a mechanistic understanding of the previous phenomenological studies and establish the key roles of ERK1/2 and PPARγ pathways in mediating IL-1β suppression by MgT exposure.
Because AD is characterized by the production and aggregation of Aβ, we further found that Aβ oligomers in the CSF have the ability to stimulate the expression of IL-1β in the cerebral cortex of APP/PS1 transgenic mice. In line with our data, it has been recently reported that misfolded and aggregated Aβ and tau bind to pattern recognition receptors on microglia and astroglia, which potentially contribute to neuroinflammation by releasing inflammatory cytokines (pro-IL-1β, IL-6, and TNFα).19,20 Others37 have suggested that Aβ oligomeric clusters exacerbate the processing of pro-IL-1β into mature IL-1β in microglial cells, which in turn enhances microglia neurotoxicity and the progression of AD. In addition, Lindberg et al.38 revealed that both oligomer Aβ1-42 peptides and Aβ1-40 are able to upregulate the secretion of IL-1β in primary cultures of microglia from the rat neonatal cerebral cortex. All of these observations revealed a relationship between IL-1β and Aβ during the course of AD progression.
In addition, neuroinflammation is not a passive system activated by the formation of AP and NFTs but instead exacerbates the pathogenesis of AD by inducing Aβ deposition and tau phosphorylation.39 For example, IL-1β has been implicated in promoting the synthesis and amyloidogenic accumulation of APP in thyroid cells and skeletal muscle.40,41 Grilli et al.42 further identified nuclear factor-κB as a critical transcriptional factor of the APP promoter that drives the synthesis of APP in response to IL-1β stimulation in primary mouse neurons. In line with these observations, IL-1β stimulates γ-secretase-mediated cleavage of APP, which in turn controls Aβ production in HEK293 cells.43 All of these observations pointed to the possible roles of IL-1β in accelerating Aβ formation. However, some studies have revealed that IL-1β treatment also increases α-secretase-dependent APP processing,44,45,46 which in turn decreases Aβ deposition in APP/PS1+/– transgenic mice.47 Thus, the ratio of α-, β-, or γ-secretase alteration in response to IL-1β treatment in various microenvironments would be a critical factor in determining the effects of IL-1β on Aβ deposition or AD development.
Although we still could not completely determine the roles of IL-1β in Aβ deposition, especially during the course of AD development, IL-1β has been regarded as a biomarker of AD. Cacabelos et al.48 first reported that IL-1β levels were significantly higher in the CSF of AD patients than in multi-infarct dementia, normal pressure hydrocephalus, and multiple sclerosis patients. Because IL-1β was found to be elevated in the brain of AD patients,48 the pathological role of IL-1β in AD development has been extensively investigated over the past two decades. Three theories about the mechanisms by which IL-1β affects AD progression have emerged as follows: (i) neuroinflammation,49 (ii) tau; (Ghosh et al. 2013;50 Kitazawa et al. 20117), and (iii) cell death.51 Unfortunately, most of these studies were phenomenological in nature. Novel components or drugs need to be developed elevate the levels of Mg2+ in AD brains, which may result in improved treatment options for AD patients.
Using MgT as a model drug, we revealed that Mg2+ elevation suppresses the expression and production of IL-1β in glial cells and the cerebral cortex of APP/PS1. In addition, the phosphorylation of ERK1/2 and PPARγ by MgT-stimulation suppresses the expression of IL-1β in glial cells. Importantly, the production of IL-1β and Aβ oligomers in the CSF is critical for stimulating the expression of IL-1β in the cerebral cortex of APP/PS1 transgenic mice. Based on these novel mechanistic findings, it may be easier to monitor the effects of MgT on combating AD via suppressing the expression of IL-1β in AD patients.
Acknowledgments
This work was supported in part or in whole by the National Natural Science Foundation of China (CN) (31571064, 81500934, 31300777, and 31371091), the National Natural Science Foundation of Liaoning, China (2015020662) the Fundamental Research Funds of China (N142004002 and N130120002) and the Liaoning Provincial Talent Support Program (LJQ2013029). We would like to acknowledge Andrew C. McCourt for critical reading and linguistic revision of the manuscript.
Footnotes
The authors declare no competing financial interests.
References
- Shoghi-Jadid K, Small GW, Agdeppa ED, Kepe V, Ercoli LM, Siddarth P et al. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry 2002; 10: 24–35. [PubMed] [Google Scholar]
- Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol 2010; 6: 131–144. [DOI] [PubMed] [Google Scholar]
- Andrasi E, Pali N, Molnar Z, Kosel S. Brain aluminum, magnesium and phosphorus contents of control and Alzheimer-diseased patients. J Alzheimers Dis 2005; 7: 273–284. [DOI] [PubMed] [Google Scholar]
- Cilliler AE, Ozturk S, Ozbakir S. Serum magnesium level and clinical deterioration in Alzheimer's disease. Gerontology 2007; 53: 419–422. [DOI] [PubMed] [Google Scholar]
- Sugimoto J, Romani AM, Valentin-Torres AM, Luciano AA, Ramirez Kitchen CM, Funderburg N et al. Magnesium decreases inflammatory cytokine production: a novel innate immunomodulatory mechanism. J Immunol 2012; 188: 6338–6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Interleukin-1, neuroinflammation, and Alzheimer's disease. Neurobiol Aging 2001; 22: 903–908. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Cheng D, Tsukamoto MR, Koike MA, Wes PD, Vasilevko V et al. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal beta-catenin pathway function in an Alzheimer's disease model. J Immunol 2011; 187: 6539–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zheng W, Xie JW, Wang T, Wang SL, Teng WP et al. Insulin deficiency exacerbates cerebral amyloidosis and behavioral deficits in an Alzheimer transgenic mouse model. Mol Neurodegener 2010; 5: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Herukka SK, Minkeviciene R, van Groen T, Tanila H. Longitudinal observation on CSF Abeta42 levels in young to middle-aged amyloid precursor protein/presenilin-1 doubly transgenic mice. Neurobiol Dis 2004; 17: 516–523. [DOI] [PubMed] [Google Scholar]
- Piermartiri TC, Figueiredo CP, Rial D, Duarte FS, Bezerra SC, Mancini G et al. Atorvastatin prevents hippocampal cell death, neuroinflammation and oxidative stress following amyloid-beta(1-40) administration in mice: evidence for dissociation between cognitive deficits and neuronal damage. Exp Neurol 2010; 226: 274–284. [DOI] [PubMed] [Google Scholar]
- Wang P, Zhu F, Konstantopoulos K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-kappaB activation. Am J Physiol Cell Physiol 2010; 298: C1445–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhu F, Konstantopoulos K. The antagonistic actions of endogenous interleukin-1beta and 15-deoxy-delta12,14-prostaglandin J2 regulate the temporal synthesis of matrix metalloproteinase-9 in sheared chondrocytes. J Biol Chem 2012; 287: 31877–31893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Song D, Bai Q, Zhou L, Cai L, Hertz L et al. Role of glycogenolysis in stimulation of ATP release from cultured mouse astrocytes by transmitters and high K+ concentrations. ASN Neuro 2014; 6: e00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhu F, Lee NH, Konstantopoulos K. Shear-induced interleukin-6 synthesis in chondrocytes: roles of E prostanoid (EP) 2 and EP3 in cAMP/protein kinase A- and PI3-K/Akt-dependent NF-kappa B activation. J Biol Chem 2010; 285: 24793–24804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhu F, Tong Z, Konstantopoulos K. Response of chondrocytes to shear stress: antagonistic effects of the binding partners toll-like receptor 4 and caveolin-1. FASEB J 2011; 25: 3401–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Guan PP, Guo C, Zhu F, Konstantopoulos K, Wang ZY. Fluid shear stress-induced osteoarthritis: roles of cyclooxygenase-2 and its metabolic products in inducing the expression of proinflammatory cytokines and matrix metalloproteinases. FASEB J 2013; 27: 4664–4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-secretase complex. Neuron 2003; 38: 9–12. [DOI] [PubMed] [Google Scholar]
- Ikari A, Atomi K, Kinjo K, Sasaki Y, Sugatani J. Magnesium deprivation inhibits a MEK-ERK cascade and cell proliferation in renal epithelial Madin-Darby canine kidney cells. Life Sci 2010; 86: 766–773. [DOI] [PubMed] [Google Scholar]
- Lue LF, Rydel R, Brigham EF, Yang LB, Hampel H, Murphy GMJr, et al. Inflammatory repertoire of Alzheimer's disease and nondemented elderly microglia in vitro. Glia 2001; 35: 72–79. [DOI] [PubMed] [Google Scholar]
- Patel NS, Paris D, Mathura V, Quadros AN, Crawford FC, Mullan MJ. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer's disease. J Neuroinflammation 2005; 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol 2015; 14: 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Ding B, Zhou L, Gao X, Guo H, Xu H. Magnesium sulfate provides neuroprotection in lipopolysaccharide-activated primary microglia by inhibiting NF-kappaB pathway. J Surg Res 2013; 184: 944–950. [DOI] [PubMed] [Google Scholar]
- Johnson AC, Tremble SM, Chan SL, Moseley J, LaMarca B, Nagle KJ et al. Magnesium sulfate treatment reverses seizure susceptibility and decreases neuroinflammation in a rat model of severe preeclampsia. PLoS One 2014; 9: e113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky I, Abumaria N, Wu LJ, Huang C, Zhang L, Li B et al. Enhancement of learning and memory by elevating brain magnesium. Neuron 2010; 65: 165–177. [DOI] [PubMed] [Google Scholar]
- Lipinski B, Pretorius E. The role of iron-induced fibrin in the pathogenesis of Alzheimer's disease and the protective role of magnesium. Front Hum Neurosci 2013; 7: 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam HB, Dowling O, Xue X, Lewis D, Rochelson B, Metz CN. Magnesium sulfate ameliorates maternal and fetal inflammation in a rat model of maternal infection. Am J Obstet Gynecol 2011; 204: 364 e361–368. [DOI] [PubMed] [Google Scholar]
- Malpuech-Brugere C, Nowacki W, Rock E, Gueux E, Mazur A, Rayssiguier Y. Enhanced tumor necrosis factor-alpha production following endotoxin challenge in rats is an early event during magnesium deficiency. Biochim Biophys Acta 1999; 1453: 35–40. [DOI] [PubMed] [Google Scholar]
- Shogi T, Oono H, Nakagawa M, Miyamoto A, Ishiguro S, Nishio A. Effects of a low extracellular magnesium concentration and endotoxin on IL-1beta and TNF-alpha release from, and mRNA levels in, isolated rat alveolar macrophages. Magnes Res 2002; 15: 147–152. [PubMed] [Google Scholar]
- Weglicki WB, Phillips TM, Freedman AM, Cassidy MM, Dickens BF. Magnesium-deficiency elevates circulating levels of inflammatory cytokines and endothelin. Mol Cell Biochem 1992; 110: 169–173. [DOI] [PubMed] [Google Scholar]
- Burd I, Breen K, Friedman A, Chai J, Elovitz MA. Magnesium sulfate reduces inflammation-associated brain injury in fetal mice. Am J Obstet Gynecol 2010; 202: 292 e291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amash A, Holcberg G, Sapir O, Huleihel M. Placental secretion of interleukin-1 and interleukin-1 receptor antagonist in preeclampsia: effect of magnesium sulfate. J Interferon Cytokine Res 2012; 32: 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Wang H, Zhang F, Li X, Xiang L, Aiguo S. PPARgamma agonist pioglitazone inhibits microglia inflammation by blocking p38 mitogen-activated protein kinase signaling pathways. Inflamm Res 2010; 59: 921–929. [DOI] [PubMed] [Google Scholar]
- Valles SL, Dolz-Gaiton P, Gambini J, Borras C, Lloret A, Pallardo FV et al. Estradiol or genistein prevent Alzheimer's disease-associated inflammation correlating with an increase PPAR gamma expression in cultured astrocytes. Brain Res 2010; 1312: 138–144. [DOI] [PubMed] [Google Scholar]
- Tjalkens RB, Liu X, Mohl B, Wright T, Moreno JA, Carbone DL et al. The peroxisome proliferator-activated receptor-gamma agonist 1,1-bis(3'-indolyl)-1-(p-trifluoromethylphenyl)methane suppresses manganese-induced production of nitric oxide in astrocytes and inhibits apoptosis in cocultured PC12 cells. J Neurosci Res 2008; 86: 618–629. [DOI] [PubMed] [Google Scholar]
- Jahrling JB, Hernandez CM, Denner L, Dineley KT. PPARgamma recruitment to active ERK during memory consolidation is required for Alzheimer's disease-related cognitive enhancement. J Neurosci 2014; 34: 4054–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denner LA, Rodriguez-Rivera J, Haidacher SJ, Jahrling JB, Carmical JR, Hernandez CM et al. Cognitive enhancement with rosiglitazone links the hippocampal PPARgamma and ERK MAPK signaling pathways. J Neurosci 2012; 32: 16725–16735a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parajuli B, Sonobe Y, Horiuchi H, Takeuchi H, Mizuno T, Suzum A. Oligomeric amyloid beta induces IL-1beta processing via production of ROS: implication in Alzheimer's disease. Cell Death Dis 2013; 4: e975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg C, Selenica ML, Westlind-Danielsson A, Schultzberg M. Beta-amyloid protein structure determines the nature of cytokine release from rat microglia. J Mol Neurosci 2005; 27: 1–12. [DOI] [PubMed] [Google Scholar]
- Zhang B, Gaiteri C, Bodea LG, Wang Z, McElwee J, Podtelezhnikov AA et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer's disease. Cell 2013; 153; 707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J, Barthel K, Wrede A, Salajegheh M, Bähr M, Dalakas MC. Interrelation of inflammation and APP in sIBM: IL-1 beta induces accumulation of beta-amyloid in skeletal muscle. Brain 2008; 131: 1228–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt TL, Steiner E, Klinger P, Sztankay A, Grubeck-Loebenstein B. The production of an amyloidogenic metabolite of the Alzheimer amyloid beta precursor protein (APP) in thyroid cells is stimulated by interleukin 1 beta, but inhibited by interferon gamma. J Clin Endocrinol Metab 1996; 81: 1666–1669. [DOI] [PubMed] [Google Scholar]
- Grilli M, Goffi F, Memo M, Spano P. Interleukin-1beta and glutamate activate the NF-kappaB/Rel binding site from the regulatory region of the amyloid precursor protein gene in primary neuronal cultures. J Biol Chem 1996; 271: 15002–15007. [DOI] [PubMed] [Google Scholar]
- Liao YF, Wang BJ, Cheng HT, Kuo LH, Wolfe MS. Tumor necrosis factor-alpha, interleukin-1beta, and interferon-gamma stimulate gamma-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J Biol Chem 2004; 279: 49523–49532. [DOI] [PubMed] [Google Scholar]
- Kong Q, Peterson TS, Baker O, Stanley E, Camden J, Seye CI et al. Interleukin-1beta enhances nucleotide-induced and alpha-secretase-dependent amyloid precursor protein processing in rat primary cortical neurons via up-regulation of the P2Y(2) receptor. J Neurochem 2009; 109: 1300–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G, Chen S, Wang X, Ba M, Yang H, Lu G. Short-term interleukin-1(beta) increases the release of secreted APP(alpha) via MEK1/2-dependent and JNK-dependent alpha-secretase cleavage in neuroglioma U251 cells. J Neurosci Res 2005; 80: 683–692. [DOI] [PubMed] [Google Scholar]
- Tachida Y, Nakagawa K, Saito T, Saido TC, Honda T, Saito Y et al. Interleukin-1 beta up-regulates TACE to enhance alpha-cleavage of APP in neurons: resulting decrease in Abeta production. J Neurochem 2008; 104: 1387–1393. [DOI] [PubMed] [Google Scholar]
- Matousek SB, Ghosh S, Shaftel SS, Kyrkanides S, Olschowka JA, O' Banion MK et al. Chronic IL-1beta-mediated neuroinflammation mitigates amyloid pathology in a mouse model of Alzheimer's disease without inducing overt neurodegeneration. J Neuroimmune Pharmacol 2012; 7: 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacabelos R, Barquero M, Garcia P, Alvarez XA, Varela de Seijas E. Cerebrospinal fluid interleukin-1 beta (IL-1 beta) in Alzheimer's disease and neurological disorders. Methods Find Exp Clin Pharmacol 1991; 13: 455–458. [PubMed] [Google Scholar]
- Forlenza OV, Diniz BS, Talib LL, Mendonça VA, Ojopi EB, Gattaz WF et al. Increased serum IL-1beta level in Alzheimer's disease and mild cognitive impairment. Dement Geriatr Cogn Disord 2009; 28: 507–512. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Wu MD, Shaftel SS, Kyrkanides S, LaFerla FM, Olschowka JA et al. Sustained interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer's mouse model. The Journal of Neuroscience 2013; 33(11): 5053–5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacabelos R, Alvarez XA, Fernández-Novoa L, Franco A, Mangues R, Pellicer A et al. Brain interleukin-1 beta in Alzheimer's disease and vascular dementia. Methods Find Exp Clin Pharmacol 1994; 16: 141–151. [PubMed] [Google Scholar]