Chronic inflammation is regarded as an important factor in cancer progression. In addition to the immune surveillance function in the early stage of tumorigenesis, inflammation is also known as one of the hallmarks of cancer and can supply the tumor microenvironment with bioactive molecules and favor the development of other hallmarks of cancer, such as genetic instability and angiogenesis. Moreover, inflammation contributes to the changing tumor microenvironment by altering stromal cell turnover rates and polarizing immune cell immunosuppressive capabilities.1 In addition, many tumor suppressor genes have been reported to be involved in inflammation modulation.2 However, the types of molecules and the underlying mechanisms involved in inflammation-associated cancer need to be further investigated, which may provide new targets for the control of cancer.
Recognition of danger-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) is the key step that modulates inflammation. Five major PRR families have been reported to be involved in tumorigenesis, including the Toll-like receptors (TLRs), C-type lectin receptors (CLRs), RIG-I-like receptors (RLRs), absent-in-melanoma (AIM)-like receptors (ALRs) and the nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs). TLRs and CLRs are located in the plasma membranes, whereas RLRs, ALRs and NLRs are intracellular PRRs.3 Unlike other families that have been shown to bind their specific cognate ligands, the distinct ligands for NLRs are still unknown. In fact, mounting evidence suggests that NLRs function as cytoplasmic sensors and participate in modulating TLR, RLR and CLR signaling pathways.4 NLRs can sense stimulatory signals from pathogens, host cells and environmental sources, although the interaction patterns are still unclear.3 Importantly, dysfunction of NLRs leads to various inflammatory diseases, autoimmune diseases and inflammation-associated carcinogenesis.5 Given the vital roles of NLRs in inflammation and disease progression, research is now focused on elucidating their roles in the pathogenesis of diseases such as cancer, with the aim of finding new therapeutic targets.
NLRs contain three conserved functional domains: a C-terminal leucine-rich repeat for ligand recognition, a central NACHT domain essential for oligomerization and an N-terminal protein-protein interaction domain. According to the types of N-terminal domains, mammalian NLRs are classified into NLRA, NLRB, NLRC and NLRP subfamilies with acidic transactivation domains, baculoviral inhibitory repeat (BIR)-like domains, caspase recruitment domains and pyrin domains.4, 6 Since the first NLR was discovered in early 2000,7 at least 23 distinct NLR and NLR-like proteins have been identified in humans.4 NLRs, including NLRP1, NLRP3, NLRP6, NLPR7, NLRP12, NLRC4 and NAIP, can function in an inflammasome-dependent manner to produce interleukin-1β (IL-1β), IL-18 and caspase-1, thus inducing inflammation and regulating cell pyroptosis. By contrast, other NLRs such NLRP10, NOD1, NOD2, NLRC3, NLRC5, NLRX1 and CIITA function independent of the inflammasome and can regulate NF-κB and MAPK pathways or act as transcriptional regulators.6 Interestingly, the functional patterns of NLRs are not fixed. Recently, NLRP3 has been reported to regulate STAT5 and IL-2,8 and NAIP was found to regulate the STAT3 pathway independent of inflammasome formation.9
The AOM/DSS model is the most popular model used to study the function of NLRs in inflammation-associated carcinogenesis. Inflammasomes initiated by NLRs or AIM2 have been widely reported to participate in the maintenance of intestinal homeostasis.10, 11 Nlrp3-/-, Nlrp6-/-, Nlrc4-/-, Nlrp1-/-, Nlrx1-/- and Nlrp12-/- mice are more susceptible to AOM/DSS-induced colorectal cancer than wild-type (WT) mice.11 Loss of IL-18 may partially account for the enhanced tumorigenesis in the abovementioned mice. IL-18 production is mainly regulated by inflammasomes and can stimulate regeneration and repair in the colon.4 Molecules that can regulate the functions of the inflammasome are currently gaining attention and are potential beneficial treatments for inflammasome-associated disorders.12 Moreover, deficiency of NLRX1, NOD1 or NOD2, which are non-inflammasome NLRs, results in increased susceptibility to colorectal cancer. NLRX1 sensitizes cells to apoptotic cell death in response to DSS treatment and promotes epithelial proliferation. NLRX1 is a negative regulator of AKT and NF-κB. NOD1 participates in the maintenance of the intestinal epithelial barrier permeability and balances inflammatory responses under AOM/DSS stimulation, whereas NOD2 regulates apoptosis and the NF-κB and MAPK signaling pathways.11 The functions of other non-inflammasome NLRs in inflammation-associated carcinogenesis are unknown. Recently, Dr. Rajendra Karki's research group published a paper in Nature that described the function of a poorly researched non-inflammasome-forming NLR, NLRC3, in colitis-associated carcinogenesis.13
NLRC3 was first identified in 2005 and found to be highly expressed in T lymphocytes. NLRC3 has been reported to suppress the signaling pathways involved in T cell activation by influencing the transcription factors NF-κB, NFAT and AP1.14 When stimulated by virus infection, NLRC3 negatively regulates the STING pathway in bone marrow-derived macrophages by directly associating with STING and blocking STING trafficking to puncta, thus inhibiting interferon-β expression.15 Reports on the functions of NLRC3 in carcinogenesis are rare. Karki et al.13 demonstrated that NLRC3 expression is significantly reduced in colorectal cancer tissues in mice; then, the authors established Nlrc3-/- mice and treated them with AOM/DSS. As expected, the Nlrc3-/- mice were more susceptible to the induction of colorectal cancer and exhibited greater weight loss, more tumor nodules, larger tumor sizes and higher histological scores. These findings indicate that NLRC3 acts as a suppressor in the development of colorectal cancer.
Establishment of an inflammatory state is crucial for colitis-associated carcinogenesis.16 To assess the inflammatory state of the Nlrc3-/- mice treated with AOM/DSS, Karki et al.13 examined the expression of inflammatory cytokines, inflammatory signaling pathways and immune cell infiltration in colon tissues at days 80 and 14, which was 3 days after the first round of DSS treatment. There were no significant differences at day 80 between the Nlrc3-/- mice and WT mice. At day 14, pro-inflammatory cytokines, including IL-1β, IL-6, tumor necrosis factor, granulocyte colony stimulating factor, chemokine (C-X-C motif) ligand 1, monocyte chemoattractant protein 1 and macrophage inflammatory protein 1α, were greatly increased in the Nlrc3-/- mice, whereas IL-18 remained unchanged. The NF-κB and STAT3 signaling pathways were activated at day 14, whereas the ERK signal pathway was unaltered. The number of innate immune cells, including macrophages, neutrophils and natural killer cells, increased, whereas the numbers of B cells and T cells remained unchanged, and the number of dendritic cells decreased. These data demonstrate the hyper-inflammatory state of the Nlrc3-/- mice when treated with AOM/DSS, which possibly accounts for the hypersensitivity to carcinogenesis. Next, to further determine whether bone marrow-derived hematopoietic cells or stromal cells play a dominant role in the carcinogenesis of the Nlrc3-/- mice, Karki et al.13 established bone marrow chimeras and found that radioresistant stromal cells were more important than hematopoietic cells during carcinogenesis, because cancer progression was faster in Nlrc3-/- mice that received bone marrow from WT mice than in WT mice that received bone marrow from Nlrc3-/- mice. The results from conditional knockout mice further indicated that Nlrc3-/- intestinal epithelial cells were critical during carcinogenesis. Immunohistochemical assessment of PCNA and Ki67 in colon tissues and an in vitro culture of colonic epithelial stem cells both demonstrated the high proliferation ability of colon epithelial cells when Nlrc3 was conditionally knocked out. Then, Karki et al.13 found that the PI3K/mTOR pathway was aberrantly activated in the Nlrc3-/- epithelial cells. Activation of the PI3K/mTOR pathway has been shown to occur on day 8 in this model, which is prior to the increase in inflammatory cytokines and immune cells. Co-immunoprecipitation experiments showed that NLRC3 could bind to PI3K to reduce the activity of p85 and disrupt the association between the PI3K p85 and p110α subunits. All three domains of NLRC3 were shown to participate in this interaction. These results indicate that NLRC3 protects epithelial cells from aberrant proliferation by interacting with the PI3K/mTOR pathway and reducing the role of inflammation in colonic carcinogenesis.
The study by Karki et al.13 illustrates an essential protective role of NLRC3 in colonic epithelial cells during colitis-induced colorectal carcinogenesis and expands our understanding of functional mechanisms of NLRC3 (Figure 1). A recent paper published in the Journal of Experimental Medicine has revealed that another NLR member, NLR family apoptosis inhibitory proteins (NAIPs), protects the colonic epithelium against tumorigenesis. Unlike NLRC3, NAIP knockout mice exhibit a decreased inflammatory response despite the increased colorectal carcinogenesis. NAIP knockout mice exhibit activation of the STAT3 pathway and increased anti-apoptotic and proliferation-related gene expression in the colonic epithelial cells. Interestingly, NAIPs regulate the STAT3 pathway in an inflammasome-independent manner, which is inconsistent with the traditional view that NAIPs form an inflammasome with NLRC4.9 In addition, NLRP6 has also been found to be expressed in colonic epithelial cells and can assist in protecting epithelial cells in an inflammasome-dependent manner. Aberrant NLRP6 inflammasomes have been demonstrated to induce altered microbiota and boost tumorigenesis.17 These studies indicate the critical roles of NLRs in regulating epithelial cells during inflammation-associated carcinogenesis and reveal the different regulatory patterns of the various NLRs co-existing in a distinct cell type. However, it is unknown whether the functions of these NLRs are redundant, or whether they are differently stimulated by different ligands under various disease contexts. Additional studies are needed to address these questions.
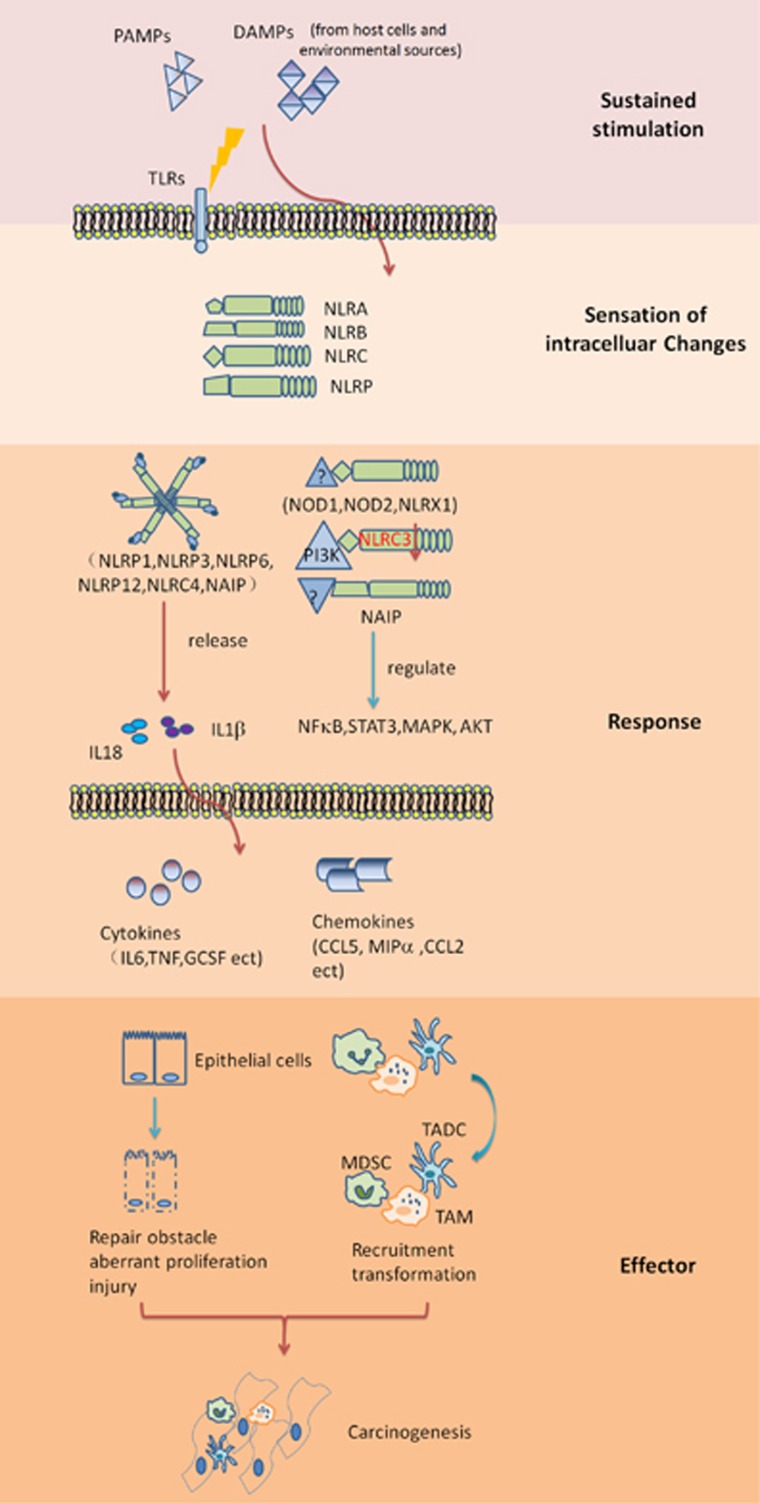
Figure 1.
Functions of NLRs in inflammation-associated carcinogenesis. Under sustained stimulation from pathogens, host cells or environmental sources, NLRs sense the intracellular changes caused by infiltrating extracellular molecules or activation of the downstream pathways of other PRRs and work to preserve cytosolic sanctity. NLRs function by associating with other proteins and through many interacting adaptors; however, details are still unclear. NLRs can form inflammasomes to release IL-18 and IL-1β, which are pro-inflammatory cytokines and related to repair and regeneration. In addition, NLRs can regulate NF-κB, STAT3, MAPK, and AKT signaling pathways in an inflammasome-independent manner. Overwhelming sustained stimulation will dysregulate the expression of NLRs, which have unknown mechanisms. Karki et al.13 recently reported that NLRC3 is downregulated in AOM/DSS-induced colorectal tumor tissue, and the ability of NLRC3 to associate with PI3K and inhibit PI3K/mTOR is impaired, which results in aberrant proliferation of intestinal epithelial cells. Dysregulated NLRs will boost inflammation by promoting the production of pro-inflammatory cytokines and chemokines and then influence the function of epithelial cells and immune cells, which favors a pro-tumor environment. DAMP, danger-associated molecular pattern; IL-18, interleukin-18; MDSC, myeloid-derived suppressing cell; NLR, nucleotide-binding and oligomerization domain-like receptor; PAMP, pathogen-associated molecular pattern; PRR, pattern recognition receptor; TADC, tumor-associated dendritic cell; TAM, tumor-associated macrophage; TLR, Toll-like receptor.
The findings of Karki et al.13 revealed the importance of the cellular location of the NLRs. As we know, the tumor microenvironment consists of multiple cell types. NLRs in different cell types will exert different functions. For instance, NLRP3 plays a multi-faceted role in carcinogenesis and is not restricted by cell type. The NLRP3 inflammasome is implicated in T-cell priming, MDSC accumulation and cancer cell invasion.11 NLRP3 derived from the hematopoietic compartment, rather than from the intestinal epithelial cells or stromal cells, is responsible for protecting against increased colonic inflammation and tumorigenesis. In the context of acute colitis induced by DSS, NLRP3 expression in the non-hematopoietic compartment appears to be more important.18 Furthermore, different NLRs may possess different cell type preferences. NOD1 is ubiquitously expressed, whereas NOD2 is highly expressed in hematopoietic cells.19 NLRC3 is highly expressed in T cells but also functions in epithelial cells, and the role of NLRC3 in immune cells still needs to be explored. Different NLRs can interact with each other or regulate the same or similar signaling pathways. NLRP12, NLRP6, NLRP2 and NLRP4 can inhibit the NF-κB pathway, whereas NOD1 and NOD2 promote the NF-κB pathway.3 Although the knowledge of the sensing and regulatory mechanisms of NLRs has expanded in the most recent decade,20, 21, 22 the direct interaction of NLRs with other proteins or ligands has not been extensively investigated. The study of Karki et al.13 is a good first step toward characterizing these interactions. Regarding the complex and sophisticated tumor–host communication, the inflammatory regulatory mechanism in tumor-associated immune cells and stromal cells may be different from those in acute infection mediated by viruses and bacteria. More efforts are needed to determine the function of NLRs in tumor-associated immune cells, stromal cells and cancer cells. The functions of the NLRs in different stages of inflammation-associated carcinogenesis have been further characterized by more investigations. In addition to the AOM/DSS model, which imitates ulcerative colitis-associated colorectal cancer, other inflammation-associated carcinogenesis should also be investigated, such as hepatitis-related hepatocellular carcinoma. Exploring the interplay between NLRs or between other PRRs under different contexts will provide insight into the functional network in cancer. Resolution of these conflicting findings will help reveal the role of NLR members in inflammation-associated carcinogenesis and identify new cancer therapeutic targets.
Footnotes
The authors declare no conflict of interest.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- Zhao R, Liu Y, Wang H, Yang J, Niu W, Fan S et al. BRD7 plays an anti-inflammatory role during early acute inflammation by inhibiting activation of the NF-small ka, CyrillicB signaling pathway. Cell Mol Immunol 2016; 13: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Shin JS, Nahm MH. NOD-like receptors in infection, immunity, and diseases. Yonsei Med J 2016; 57: 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen IC. Non-Inflammasome Forming NLRs in Inflammation and Tumorigenesis. Front Immunol 2014; 5: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Kinio A, Saleh M. Functions of NOD-Like Receptors in Human Diseases. Front Immunol 2013; 4: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe F, Douglas T, Saleh M. Advances in Nod-like receptors (NLR) biology. Cytokine Growth Factor Rev 2014; 25: 681–697. [DOI] [PubMed] [Google Scholar]
- Ting JP, Kastner DL, Hoffman HM. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol 2006; 6: 183–195. [DOI] [PubMed] [Google Scholar]
- Bruchard M, Rebe C, Derangere V, Togbé D, Ryffel B, Boidot R et al. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat Immunol 2015; 16: 859–870. [DOI] [PubMed] [Google Scholar]
- Allam R, Maillard MH, Tardivel A, Chennupati V, Bega H, Yu CW et al. Epithelial NAIPs protect against colonic tumorigenesis. J Exp Med 2015; 212: 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratsimandresy RA, Indramohan M, Dorfleutner A, Stehlik C. The AIM2 inflammasome is a central regulator of intestinal homeostasis through the IL-18/IL-22/STAT3 pathway. Cell Mol Immunol 2017; 14: 127–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Jha S. NLR-regulated pathways in cancer: opportunities and obstacles for therapeutic interventions. Cell Mol Life Sci 2016; 73: 1741–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn H, Kang SG, Yoon SI, Kim PH, Kim D, Lee GS. Poly-gamma-glutamic acid from Bacillus subtilis upregulates pro-inflammatory cytokines while inhibiting NLRP3, NLRC4 and AIM2 inflammasome activation. Cell Mol Immunol 2016; 13: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R, Man SM, Malireddi RK, Kesavardhana S, Zhu Q, Burton AR et al. NLRC3 is an inhibitory sensor of PI3K-mTOR pathways in cancer. Nature 2016; 540: 583–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti BJ, Davis BK, Zhang J, O'connor W Jr, Williams KL, Ting JP. CATERPILLER 16.2 (CLR16.2), a novel NBD/LRR family member that negatively regulates T cell function. J Biol Chem 2005; 280: 18375–18385. [DOI] [PubMed] [Google Scholar]
- Zhang L, Mo J, Swanson KV, Wen H, Petrucelli A, Gregory SM et al. NLRC3, a member of the NLR family of proteins, is a negative regulator of innate immune signaling induced by the DNA sensor STING. Immunity 2014; 40: 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Wu X, Ma Z, Tan W, Wang L, Na D et al. Expression of the novel adipokine C1qTNF-related protein 4 (CTRP4) suppresses colitis and colitis-associated colorectal cancer in mice. Cell Mol Immunol 2016; 13: 688–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambetti LP, Mortellaro A. NLRPs, microbiota, and gut homeostasis: unravelling the connection. J Pathol 2014; 233: 321–330. [DOI] [PubMed] [Google Scholar]
- Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 2011; 29: 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol 2014; 14: 9–23. [DOI] [PubMed] [Google Scholar]
- Motta V, Soares F, Sun T, Philpott DJ. NOD-like receptors: versatile cytosolic sentinels. Physiol Rev 2015; 95: 149–178. [DOI] [PubMed] [Google Scholar]
- Jones JD, Vance RE, Dangl JL. Intracellular innate immune surveillance devices in plants and animals. Science 2016; 354: 1117–1126. [DOI] [PubMed] [Google Scholar]
- Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol 2015; 33: 257–290. [DOI] [PMC free article] [PubMed] [Google Scholar]