Abstract
Genetic mutations in the gene encoding DOCK8 cause an autosomal recessive form of hyper immunoglobulin E syndrome (AR-HIES), referred to as DOCK8 deficiency. DOCK8 deficiency in humans results in the onset of combined immunodeficiency disease (CID), clinically associated with chronic infections with diverse microbial pathogens, and a predisposition to malignancy. It is now becoming clear that DOCK8 regulates the function of diverse immune cell sub-types, particularly lymphocytes, to drive both innate and adaptive immune responses. Early studies demonstrated that DOCK8 is required for lymphocyte survival, migration and immune synapse formation, which translates to poor pathogen control in the absence of DOCK8. However, more recent advances have pointed to a crucial role for DOCK8 in regulating the signal transduction events that control transcriptional activity, cytokine production and functional polarization of immune cells. Here, we summarize recent advances in our understanding of DOCK8 function, paying particular attention to an emerging role as a signaling intermediate to promote immune responses to diverse external stimuli.
Keywords: DOCK8, immunodeficiency, lymphocytes, NK Cells, signal transduction
Introduction
DOCK8 is a member of the DOCK family of proteins.1 These proteins function as guanine nucleotide exchange factors (GEFs) to regulate the activity of Rho GTPases, such as Cdc42 and Rac1.2 Because GTPases act as molecular switches to regulate diverse signaling pathways, DOCK proteins control many cellular processes including migration, phagocytosis and adhesion.2 Unlike classical GEFs, which acquire GEF activity through Dbl homology (DH) and pleckstrin homology (PH) domains, DOCK family members possess a unique DOCK homology region 2 (DHR2) domain with GEF activity. They also have a DHR1 domain that is known to interact with phospholipids,3, 4 and may promote their localization into membranes, and subsequent involvement in signal transduction events.
DOCK8 is expressed predominantly in cells of the immune system (www.biogps.org), and a key link between DOCK8 and the regulation of immunity was discovered in 2009, when two independent studies identified loss-of-function mutations in the DOCK8 gene in patients suffering from CID.5, 6 DOCK8 mutations were also identified in a murine mutagenesis screen for defects in humoral immune responses.7 Patients with DOCK8 deficiency present with clinical characteristics such as eczema, respiratory tract infections, high levels of serum IgE, hyper-eosinophilia, and a failure to clear bacterial, fungal and viral infections (Table 1).5, 8, 9 In particular, they suffer from severe cutaneous viral infections with molluscum contagiosum, papilloma virus and herpes simples virus.8
Table 1. Clinical manifestations of DOCK8 deficiency and possible cellular causes.
| Clinical manifestations of DOCK8 immunodeficiency10 | Possible underlying cellular cause(s) |
|---|---|
| Cutaneous viral infections (predominantly herpes simplex virus, papilloma virus, molluscum contagiosum) | Increased cytothripsis Defective NK cytotoxicity Decreased number of plasmacytoid dendritic cells and interferon gamma |
| Recurrent sinopulmonary infections/bronchiectasis | Defective production of long-term antibody responses Decreased memory B cells |
| Life-threatening bacterial infection | Defective production of long-term antibody responses Decreased memory B cells |
| Eczema/ food allergy | Increased TH2 cells |
| Mucocutaneous Candidiasis | Decreased TH17 cells |
| Malignancies | Cutaneous viral infections with increased epithelial cell turnover Defective NK/ T cell responses |
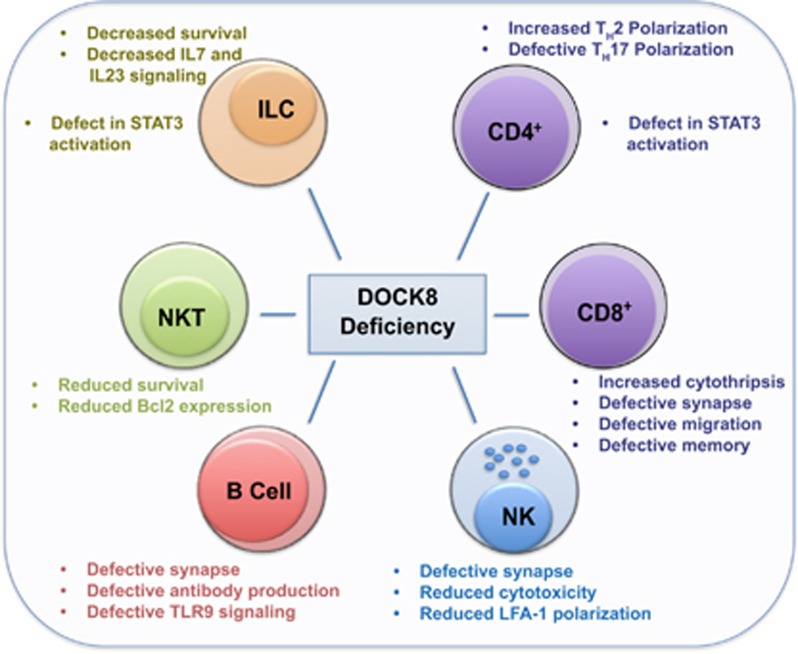
Since the discovery of DOCK8 mutations as a genetic driver of AR-HIES, the clinical manifestations have pointed to a crucial role for DOCK8 in regulating multiple facets of the immune response. The importance of this protein for health is emphasized by the high levels of morbidity and mortality in patients with DOCK8 deficiency, with an overall survival rate of 33% by the age of 30 years.10 Indeed, only 4% of patients reach the age of 30 without at least one life-threatening infection, malignancy or severe cerebral event (Table 1).10 As a result, research in the field has focused on understanding how DOCK8 regulates the function of lymphocytes, particularly T cells, natural killer (NK) cells and B cells. These studies have highlighted the requirement for DOCK8 in the proper function of these immune cells, and the susceptibility to infection in the absence of DOCK8 expression.9 However, until recently, the molecular mechanisms by which DOCK8 drives immunity remained relatively unknown. Here, we review the recent advances in our understanding of how DOCK8 regulates immune cell function (Figure 1). We pay particular attention to emerging evidence that DOCK8 functions as a signaling adaptor to control diverse signaling events in lymphocytes.
Figure 1.
DOCK regulates diverse cellular functions in multiple immune cell types.
Dock8 regulates immune synapse formation
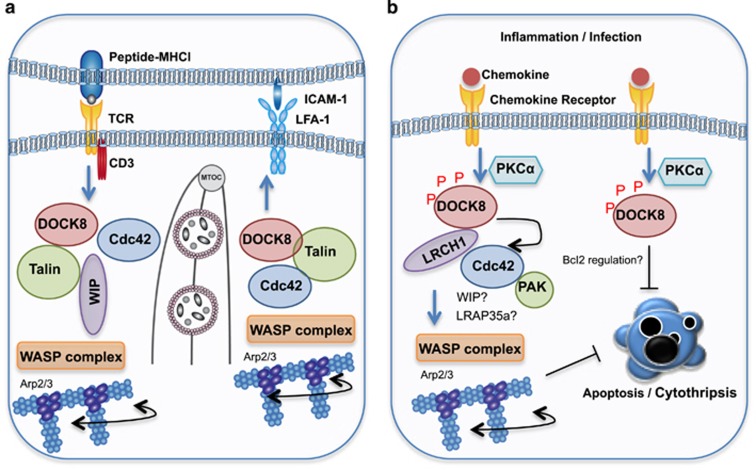
The immune synapse is a specialized structure that forms between the plasma membrane of two immune cells, or the membrane of a cytotoxic lymphocyte with a target cell (Figure 2a).11, 12 Upon synapse formation, a complex set of molecular events occur within cells to promote the signal transduction cascades required for optimal activation, function and homeostasis of immune cells. Therefore, disruption of this process often results in human disease.11, 12, 13 Formation of an immune synapse triggers actin polymerization and cytoskeletal rearrangements that facilitate the spatial re-organization of signaling molecules within supra-molecular activation cluster (SMAC) regions.12, 13, 14 These events are initiated by Cdc42-mediated activation of the cytoskeleton re-modeling proteins, WASp, and are critical for optimal immune responses (Figure 2a).15 Upon synapse formation in T cells, proteins such as protein kinase C theta (PKCθ) and leukocyte-specific protein tyrosine kinase (Lck), become polarized into a region proximal to the synapse contact site called the central SMAC region (cSMAC).12 This initiates the TCR-mediated signaling cascades to drive T-cell activation and effector function. In addition, adhesion, co-stimulatory and docking molecules, such as lymphocyte function-associated antigen 1 (LFA-1) and talin, accumulate in a ring surrounding the cSMAC (called the peripheral SMAC or pSMAC), to enhance TCR activation signals.12
Figure 2.
DOCK8 regulates lymphocyte function. (a) Upon TCR engagement, DOCK8 GEF activity activates Cdc42. DOCK8 also serves as a scaffold molecule to link Cdc42 to the WASP complex via an interaction with WIP and Talin. WASP then drives Arp2/3-dependent actin polymerization. Simultaneously, LFA-1 binding to ICAM-1 results in formation of a complex composed of DOCK8, Talin and WASP which also promotes cytoskeletal changes through WASP activation. Cytoskeletal remodeling facilitates MTOC and cytotoxic granule polarization toward the immune synapse. (b) DOCK8 is sequestered by LRCH1. Upon chemokine receptor engagement, PKCα phosphorylates DOCK8 which enables it to bind and activate Cdc42, driving WASP-dependent cytoskeletal changes. LRAP35a and WIP may facilitate Cdc42-mediated WASP activation in T cells. A Cdc42-PAK signaling axis suppresses cytothripsis, while DOCK8 may directly suppress cell death through regulation of the cell death machinery.
DOCK8 was initially identified as a critical mediator of the immune synapse in B cells.7 B-cell receptor signaling upon antigen recognition triggers signaling cascades that mobilize the integrin LFA-1 to cluster at the pSMAC.16, 17 This facilitates its binding to ICAM-1 on the surface of the antigen expressing cell to promote B-cell adhesion and spreading.16, 17 In the absence of DOCK8, a defect in B-cell LFA-1 polarization leads to impaired synapse formation and consequently, long-lived antibody production.7 Because integrin ligation can serve as a co-stimulatory signal to promote proliferation and inhibit apoptosis, it is likely that DOCK8 can indirectly provide this signal by promoting LFA-1 polarization.18, 19 Similarly, CD8+ T cells fail to optimally polarize LFA-1 to the immune synapse with dendritic cells, resulting in a delay in the first round of T cell division. This, and other intrinsic defects discussed later, may contribute to poor memory responses in vivo.20 Although a separate study found no defect in T-cell division in the absence of DOCK8,21 the poor memory responses found in both studies point to sub-optimal TCR signaling in the absence of DOCK8. The discrepancies may relate to the use of mice bearing different DOCK8 mutations, or the use of CD3/CD28 stimulation versus dendritic cell-presented antigen.
NK cells form an immune synapse with malignant or virally infected cells. NK cell cytotoxicity is regulated by ligands expressed on target cells and activatory/inhibitory receptors expressed on the NK cell. If activation signals dominate inhibitory signals, a cytotoxic synapse is formed, which triggers granule delivery to the synaptic cleft.11, 14 DOCK8-deficient human NK cells also appear to form defective synapses. Independent groups have demonstrated that DOCK8 knockdown or DOCK8-deficient human NK cells have reduced cytotoxic activity due to a failure to optimally polarize LFA-1 and F-actin, and to deliver cytotoxic granules to the target cell.22, 23 Interestingly, DOCK8 was shown to orchestrate talin and WASp accumulation at the synapse, two essential regulators of F-actin reorganization and integrin affinity maturation, respectively (Figure 2a).23
Taken together, these studies clearly demonstrate an essential role for DOCK8 in regulating synapse formation in divergent cell types. However, formation of the synapse involves a complex set of molecular events, including the spatial rearrangement of proteins into SMACs, cytoskeletal rearrangements, and signaling cascades and the exact sequence of events is still not fully understood. Indeed, multiple feed back loops are likely to exist, in which signaling cascades, cytoskeletal rearrangements and SMAC formation is heavily interdependent (Figure 2a). It is still not clear whether DOCK8 regulates one of these processes, or whether DOCK8 is directly involved in several; or indeed, whether DOCK8 is required for synapse formation in other lymphocyte subsets such as CD4+ T cells (Figure 1).21
Dock8 regulates immune cell trafficking
DOCK8-deficient mice develop lymphopenia and have a defect in CD4+ T cell egress,21 a finding supported by a later study demonstrating a role for DOCK8 in the MST kinase signaling pathway, which promotes the egress of single positive thymocytes.24 These observations may explain some of the clinical manifestations associated with immune deficiency in DOCK8-deficient patients (Table 1). Interestingly, the pathology of DOCK8 deficiency in humans often involves microbial invasion of barrier tissues, particularly the skin. In 2014, it was discovered that CD8+ T cells adopted an unusual, elongated morphology in the absence of DOCK8.25 Importantly, these cells lost viability during migration through a gel matrix, which mimics migration from peripheral blood into dense tissue such as skin. This T-cell death, called cytothripsis, was caspase-independent and occurred through an apparent loss of structural integrity (Figure 2b). Cdc42 or PAK knockdown cells phenocopied loss of DOCK8, suggesting a DOCK8–Cdc42–PAK2 axis was implementing cytoskeletal changes required to maintain T-cell viability. Similarly, DOCK8 regulates Cdc42 activation in dendritic cells, which is required for their accumulation in the lymph node for T-cell priming.26, 27, 28 More recently, DOCK8 was also shown to regulate macrophage migration through an interaction with LRAP35, linking Cdc42 to actomyosin dynamics.29 Thus, in this context, DOCK8 can activate Cdc42, but also function as an adaptor/scaffold molecule to link Cdc42 to the cytoskeleton.29
T-cell infiltration into the central nervous system is a major driver of multiple sclerosis, however, little is known about the mechanism driving migration in this context. Xu et al.30 recently identified a protein, LRCH1, that competes with Cdc42 to interact with DOCK8 and restrict T cell migration. They demonstrated that LRCH1 restrains PKCα–DOCK8–Cdc42 module–induced T-cell migration (Figure 2b).30 Importantly, DOCK8 mutant mice or Lrch1 transgenic mice are protected from experimental autoimmune encephalomyelitis (EAE).30 DOCK8 also regulates phagocyte migration during sepsis. Destruction of erythrocytes (hemolysis) during infection is thought to promote bacterial growth by liberating the nutrient heme-iron. Interestingly, heme appears to also inhibit phagocyte recruitment via disruption of DOCK8–Cdc42-mediated cytoskeletal rearrangements.31
Taken together, DOCK8 functions as a key regulator of diverse immune cell subtype migration through activation of Cdc42, but is likely to adopt multiple and complex roles in the signaling events that culminate in this process. In support of this concept, a signaling complex composed of DOCK8, WASp-interacting protein (WIP) and WASp is assembled upon TCR ligation, and drives TCR-mediated cytoskeletal changes.32 Here, it appears that, as well as a Cdc42 activator in this context, DOCK8 facilitates WASp-driven actin polymerization by promoting an interaction with the ARP2/3 complex (Figure 2).32 Indeed, DOCK8 is required for immune synapse formation, actin foci formation, mechanotransduction, T cell transendothelial migration, and homing to lymph nodes in this setting.32
Dock8 in maintenance of lymphocyte persistence
Studies using DOCK8 knockout or mutant mice have determined that DOCK8 expression is required for the maintenance and persistence of many immune cell subsets. DOCK8 mutant B cells are unable to form marginal zone B cells, or to persist in germinal centers.7 Similarly, DOCK8 mutant mice display T-cell lymphopenia, with increased T-cell turnover due to excessive cell death.21 While CD8+ T cells in these mice mount a normal primary response to antigen, the persistence of memory cells is sub-optimal.12, 21 These data point to a critical role for DOCK8 in generating a TCR signal strong enough to ensure long-term memory cell production, or indeed a role in suppression of apoptosis20
DOCK8 also regulates the survival of NKT cells in mice and humans, providing further evidence that DOCK8 may be required to restrain cell death.33 Importantly, NKT cells deficient in DOCK8 expressed reduced levels of the anti-apoptotic protein factor B-cell lymphoma 2 (Bcl2),33 suggesting that DOCK8 may promote immune cell survival by directly regulating the cell death machinery; however, this molecular link remains unexplored (Figure 2b). DOCK8 is also essential for the function and survival of RORγt+ innate lymphoid cells (ILCs).34 The scarce ILCs in DOCK8-deficient mice following Citrobacter rodentium infection are also less responsive to IL-7- and IL-23-induced signaling, leading to defective IL-22 release. Diminished IL-22 release occurred through sub-optimal STAT3 activation in the absence of DOCK8. In addition, DOCK8-deficient ILC3s could not phosphorylate STAT5 as efficiently as wild type cells in response to IL-7.34 These ILCs also had a higher rate of spontaneous apoptosis, again linking DOCK8 to the apoptotic program. It will be interesting to determine if DOCK8 is regulating cell death through the modulation of co-stimulation signals within the immune synapse, leading to enhanced activation-induced cell death, or whether there is a more direct link to cell death induction, as suggested by reduced Bcl2 levels in NKT cells.
Regulation of polarization and cytokine production
While both DOCK8 and STAT3 mutations give rise to a hyper-IgE syndrome, STAT3 mutations are more frequently associated with pulmonary symptoms and are associated with classical skeletal features, while DOCK8-deficient patients more often present with multiple allergies and recurrent viral infections of the skin.35 Nonetheless, this clinical overlap suggests DOCK8 and STAT3 may operate in the same pathway in some circumstances.35, 36 Two recent studies found that CD4+ T cells from DOCK8-deficient patients are preferentially polarized to a TH2 effector phenotype, with defective ability to polarize towards a TH17 cytokine producing state.37, 38 Mechanistically, DOCK8 constitutively associates with STAT3 in CD4+ T cells in a GEF-independent manner, but promotes STAT3 activation through a GEF-dependent mechanism upon IL-6 or IL-21 stimulation.38 Indeed, mutant cells that lack DOCK8 GEF activity, but retain protein expression, had defective TH17 polarization. Intriguingly, DOCK8 was required for STAT3 translocation to the nucleus, where it drives transcription of TH17-associated genes. These data support the finding that STAT3 is required for IL-22 production in response to IL23 in ILCs.34 Similarly, a recent study has also implicated DOCK8 in the production of IL-31 in CD4+ T cells by controlling the transcription factor EPAS1.39 Thus, DOCK8 can directly regulate signal transduction events that are required for downstream cytokine production (Figure 1). Furthermore, these experiments support the concept that DOCK8 has multiple functions, simultaneously catalyzing enzymatic reactions while serving as a molecular scaffold within signaling networks.
Further evidence that DOCK8 acts as a signaling intermediate in immune cells came from an elegant study by Geha and colleagues, which found that DOCK8 functions as a signaling adaptor in B cells, linking Toll-like receptor (TLR) 9 activation to STAT3 transcription factor-mediated proliferation and differentiation.40 DOCK8 also constitutively associates with the TLR adaptor, MYD88 and the kinase, Pyk2. Upon TLR9 ligation, Pyk2 phosphorylates DOCK8, allowing it to bind the Src kinase Lyn. Lyn then activates Syk to drive STAT3 translocation to the nucleus. Thus, DOCK8 has an essential role in linking external signals to Src-dependent signaling cascades in a GEF activity-independent manner. As discussed above, DOCK8 is also required for optimal cytokine-induced STAT3 activation in T cells.38 However, IL-6- and IL-21-triggered STAT3 remained intact in DOCK8-deficient B cells.40 This suggests that the role of DOCK8 can be divergent in different cell types upon activation with the same extracellular stimuli.
Conclusion
With the discovery in 2009 that mutations in the Dock8 gene leads to combined immunodeficiency disease in humans, DOCK8 recaptured the interest of immunologists and cell biologists worldwide. A flurry of elegant studies have ensued since then, demonstrating that DOCK8 is critical for proper immune cell function, with the absence of DOCK8 in humans leading to impaired immunity and susceptibility to a host of infections of viral, bacterial and fungal origin. The studies described here have determined that DOCK8 plays a critical role in establishing the scaffolds required for immune cell migration, synapse formation and signal transduction events. Although still to be unraveled, an intriguing role for DOCK8 in regulating cell death may also be revealed in the future.
Given the large number of guanine exchange factors known to activate various Rho GTPases,41, 42 it is also intriguing that the loss of just one guanine exchange factor for Cdc42, namely DOCK8, has such severe and varied effects on the immune system (Figure 1). This raises the possibility that the specific role of DOCK8 in activating Cdc42 in different immune subsets may be antigen-specific, or dependent on the stage of the immune response. Equally, the variety of interacting proteins and downstream targets of DOCK8 described above are likely to give further specificity, perhaps restricting its activity to certain immune cells, or for regulating the response to specific types of signals. It is hoped that future studies will be able to unravel this complexity. Whether DOCK8 regulates signal transduction in other immune cell subsets, and clarification on how DOCK8 drives optimal signaling to influence critical aspects of the immune response, such as memory, are also questions that remain to be answered. In addition, how other Cdc42-specific GEFs expressed in immune cells (such as DOCK1143) function, and how their activity affects DOCK8 will be important to understand.
More broadly, a better understanding of the specific differences between the role of DOCK8 in mice and humans, and the roles of other DOCK proteins in immunity and disease is required. Until we develop a greater understanding of the diverse roles of DOCK8, and begin to elucidate the molecular pathways involved, it will be difficult to provide any targeted treatment for DOCK8-deficient patients, for whom the current treatment options are limited, and, due to the poor prognosis, will need to continue to undergo high-risk hematopoietic stem cell transplantation.
Footnotes
The authors declare no conflict of interest.
References
- Cote JF, Vuori K. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J Cell Sci 2002; 115: 4901–4913. [DOI] [PubMed] [Google Scholar]
- Meller N, Merlot S, Guda C. CZH proteins: a new family of Rho-GEFs. J Cell Sci 2005; 118: 4937–4946. [DOI] [PubMed] [Google Scholar]
- Cote JF, Motoyama AB, Bush JA, Vuori K. A novel and evolutionarily conserved PtdIns(3,4,5)P3-binding domain is necessary for DOCK180 signalling. Nat Cell Biol 2005; 7: 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara S, Kiyokawa E, Iemura S, Natsume T, Wassmer T, Cullen PJ et al. The DHR1 domain of DOCK180 binds to SNX5 and regulates cation-independent mannose 6-phosphate receptor transport. Mol Biol Cell 2008; 19: 3823–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med 2009; 361: 2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol 2009; 124: 1289–302 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall KL, Lambe T, Johnson AL, Treanor B, Kucharska E, Domaschenz H et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat Immunol 2009; 10: 1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt KR, Gertz ME, Keles S, Schaffer AA, Sigmund EC, Glocker C et al. The extended clinical phenotype of 64 patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol 2015; 136: 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HC. Dedicator of cytokinesis 8 (DOCK8) deficiency. Curr Opin Allergy Clin Immunol 2010; 10: 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin SE, Kilic SS, Aytekin C, Kumar A, Porras O, Kainulainen L et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options—a review of 136 patients. J Clin Immunol 2015; 35: 189–198. [DOI] [PubMed] [Google Scholar]
- Kearney CJ, Brennan AJ, Darcy PK, Oliaro J. The role of the immunological synapse formed by cytotoxic lymphocytes in immunodeficiency and anti-tumor immunity. Crit Rev Immunol 2015; 35: 325–347. [DOI] [PubMed] [Google Scholar]
- Dustin ML. The immunological synapse. Cancer Immunol Res 2014; 2: 1023–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML. Membrane domains and the immunological synapse: keeping T cells resting and ready. J Clin Invest 2002; 109: 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol 2008; 8: 713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Ho HY, Kirschner MW. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J Cell Biol 2000; 150: 1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco YR, Fleire SJ, Cameron T, Dustin ML, Batista FD. LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity 2004; 20: 589–599. [DOI] [PubMed] [Google Scholar]
- Yuseff MI, Pierobon P, Reversat A, Lennon-Dumenil AM. How B cells capture, process and present antigens: a crucial role for cell polarity. Nat Rev Immunol 2013; 13: 475–486. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang H. Integrin signalling and function in immune cells. Immunology 2012; 135: 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arana E, Harwood NE, Batista FD. Regulation of integrin activation through the B-cell receptor. J Cell Sci 2008; 121: 2279–2286. [DOI] [PubMed] [Google Scholar]
- Randall KL, Chan SS, Ma CS, Fung I, Mei Y, Yabas M et al. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med 2011; 208: 2305–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe T, Crawford G, Johnson AL, Crockford TL, Bouriez-Jones T, Smyth AM et al. DOCK8 is essential for T-cell survival and the maintenance of CD8+ T-cell memory. Eur J Immunol 2011; 41: 3423–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizesko MC, Banerjee PP, Monaco-Shawver L, Mace EM, Bernal WE, Sawalle-Belohradsky J et al. Defective actin accumulation impairs human natural killer cell function in patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol 2013; 131: 840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham H, Guerrier S, Kim J, Schoon RA, Anderson EL, Hamann MJ et al. Dedicator of cytokinesis 8 interacts with talin and Wiskott-Aldrich syndrome protein to regulate NK cell cytotoxicity. J Immunol 2013; 190: 3661–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou F, Praskova M, Xia F, Van Buren D, Hock H, Avruch J et al. The Mst1 and Mst2 kinases control activation of rho family GTPases and thymic egress of mature thymocytes. J Exp Med 2012; 209: 741–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Dove CG, Hor JL, Murdock HM, Strauss-Albee DM, Garcia JA et al. DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med 2014; 211: 2549–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T et al. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood 2012; 119: 4451–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaswamy JK, Singh A, Gowthaman U, Wu R, Gorrepati P, Sales Nascimento M et al. Coincidental loss of DOCK8 function in NLRP10-deficient and C3H/HeJ mice results in defective dendritic cell migration. Proc Natl Acad Sci USA 2015; 112: 3056–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabro S, Liu D, Gallman A, Nascimento MS, Yu Z, Zhang TT et al. Differential Intrasplenic Migration of Dendritic Cell Subsets Tailors Adaptive Immunity. Cell Rep 2016; 16: 2472–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi A, Uruno T, Sanematsu F, Ushijima M, Sakata D, Hara T et al. DOCK8 Regulates Macrophage Migration through Cdc42 Activation and LRAP35a Interaction. J Biol Chem 2016; 292: 2191–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Han L, Zhao G, Xue S, Gao Y, Xiao J et al. LRCH1 interferes with DOCK8-Cdc42-induced T cell migration and ameliorates experimental autoimmune encephalomyelitis. J Exp Med 2017; 214: 209–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins R, Maier J, Gorki AD, Huber KV, Sharif O, Starkl P et al. Heme drives hemolysis-induced susceptibility to infection via disruption of phagocyte functions. Nat Immunol 2016; 17: 1361–1372. [DOI] [PubMed] [Google Scholar]
- Janssen E, Tohme M, Hedayat M, Leick M, Kumari S, Ramesh N et al. A DOCK8-WIP-WASp complex links T cell receptors to the actin cytoskeleton. J Clin Invest 2016; 126: 3837–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford G, Enders A, Gileadi U, Stankovic S, Zhang Q, Lambe T et al. DOCK8 is critical for the survival and function of NKT cells. Blood 2013; 122: 2052–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Eken A, Fry M, Bettelli E, Oukka M. DOCK8 regulates protective immunity by controlling the function and survival of RORgammat+ ILCs. Nat Commun 2014; 5: 4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen TH. STAT3 and the Hyper-IgE syndrome: clinical presentation, genetic origin, pathogenesis, novel findings and remaining uncertainties. JAKSTAT 2013; 2: e23435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos AC, Hagl B, Schlesinger A, Halm BE, Ballenberger N, Pinarci M et al. Atopic dermatitis, STAT3- and DOCK8-hyper-IgE syndromes differ in IgE-based sensitization pattern. Allergy 2014; 69: 943–953. [DOI] [PubMed] [Google Scholar]
- Tangye SG, Pillay B, Randall KL, Avery DT, Phan TG, Gray P et al. Dedicator of cytokinesis 8-deficient CD4+ T cells are biased to a TH2 effector fate at the expense of TH1 and TH17 cells. J Allergy Clin Immunol 2016. [DOI] [PMC free article] [PubMed]
- Keles S, Charbonnier LM, Kabaleeswaran V, Reisli I, Genel F, Gulez N et al. Dedicator of cytokinesis 8 regulates signal transducer and activator of transcription 3 activation and promotes TH17 cell differentiation. J Allergy Clin Immunol 2016; 138: 1384–1394 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura K, Uruno T, Shiraishi A, Tanaka Y, Ushijima M, Nakahara T et al. The transcription factor EPAS1 links DOCK8 deficiency to atopic skin inflammation via IL-31 induction. Nat Commun 2017; 8: 13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabara HH, McDonald DR, Janssen E, Massaad MJ, Ramesh N, Borzutzky A et al. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat Immunol 2012; 13: 612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni K, Yang J, Zhang Z, Barford D. Multiple factors confer specific Cdc42 and Rac protein activation by dedicator of cytokinesis (DOCK) nucleotide exchange factors. J Biol Chem 2011; 286: 25341–25351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DR, Rossman KL, Der CJ. Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease. Oncogene 2014; 33: 4021–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikimi A, Meller N, Uekawa N, Isobe K, Schwartz MA, Maruyama M. Zizimin2: a novel, DOCK180-related Cdc42 guanine nucleotide exchange factor expressed predominantly in lymphocytes. FEBS Lett 2005; 579: 1039–1046. [DOI] [PubMed] [Google Scholar]