Abstract
Introduction:
Rodent studies suggest that nicotine metabolites and minor tobacco alkaloids such as nornicotine and cotinine may promote cigarette smoking by enhancing nicotine rewarding and reinforcing effects. However, there is little information on the effects of these minor tobacco alkaloids on nicotine withdrawal. The present studies were conducted to determine whether the minor tobacco alkaloids nornicotine and cotinine exhibit nicotine-like behavioral effects in a mouse model of spontaneous nicotine withdrawal.
Methods:
Mice were infused with nicotine or saline for 14 days. Experiments were conducted on day 15, 18–24 hours after minipump removal. Ten minutes prior to testing, nicotine-dependent ICR male mice received an acute injection of nicotine (0.05 and 0.5 mg/kg), nornicotine (2.5 and 25 mg/kg), or cotinine (5 and 50 mg/kg) to determine effects on somatic signs, anxiety-like behaviors, and hyperalgesia spontaneous signs of withdrawal.
Results:
Nicotine and the minor tobacco alkaloid nornicotine, but not cotinine, produced dose-dependent reversal of nicotine withdrawal signs in the mouse.
Implications:
The minor tobacco alkaloid and nicotine metabolite nornicotine at high doses have nicotinic like effects that may contribute to tobacco consumption and dependence.
Introduction
Smoking raises the risk of cardiovascular, pulmonary diseases and cancer. Nicotine is the main active ingredient in tobacco smoke that leads to and maintains tobacco addiction.1 However, several nicotine-related alkaloids such as anabasine, nornicotine, and cotinine have also been identified in tobacco.2 The last two are also important nicotine metabolites identified in humans and rodents. Nicotine is extensively metabolized to a number of metabolites by the liver, with cotinine being the most abundant one (70%–80% of nicotine). This transformation is mediated primarily by microsomal cytochrome P450 CYP2A6. Nornicotine is a minor metabolic product of the peripheral and brain metabolism of nicotine.3,4 Nornicotine was reported to bind to and activate neuronal nicotinic receptors (nAChRs) with high affinity and to release dopamine from striatal slices in a variety of in vitro functional tests.5,6 Furthermore, nornicotine exhibited nicotine-like properties in several behavioral assays such as locomotor sensitization, and shares discriminative-stimulus and reinforcing effects with nicotine in rodent and nonhuman primate intravenous (i.v.) self-administration and intracranial self-stimulation models.7–18 In contrast, cotinine was shown to bind with very low affinity to nAChRs and failed to activate or block these receptors in in vitro functional assays.5,6 In addition, cotinine failed to elicit nicotine-like properties in rodent i.v. self-administration, intracranial self-stimulation, and drug discrimination paradigms.14,15,19 However, cotinine has been found to exhibit some neuroprotective effects in some animal disease models.20,21 Collectively, these data suggest that nornicotine, but not cotinine, may contribute to the behavioral and reinforcing effects of tobacco.
The present study was conducted to further evaluate these two nicotine metabolites and tobacco smoke constituents to determine whether they impact nicotine withdrawal, one of the primary causes of high tobacco relapse rates.22 To this end, we evaluated the potential of acute effects of nicotine, nornicotine and cotinine to reverse withdrawal signs in well-established mouse models of nicotine withdrawal.
Methods
Subjects
Male adult ICR mice (20–25 g) obtained from Harlan Laboratories (Indianapolis, IN) were used throughout the study. Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care approved facility in groups of five and had free access to food and water. Experiments were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Drugs
(−)-Nicotine hydrogen tartrate salt [(−)-1-Methyl-2-(3-pyridyl) pyrrolidine (+)-bitartrate salt], (±)-nornicotine, and (−)-cotinine were purchased from Sigma-Aldrich (St. Louis, MO). All drugs were dissolved in physiological saline (0.9% sodium chloride) and injected at a total volume of 1 ml/100 g body weight unless noted otherwise. All doses are expressed as the free base of the drug. Drugs were injected subcutaneously (s.c.).
Chronic Administration of Nicotine
Mice (n = 7/per group) were anesthetized with sodium pentobarbital (45 mg/kg, intraperitoneal or i.p.) and implanted s.c. with Alzet osmotic minipumps ([model 2002]; Durect Corporation, Cupertino, CA). The concentration of nicotine was adjusted according to animal weight and the minipump flow rate to deliver 24 mg/kg/day for 14 days.
Spontaneous Nicotine Withdrawal
To assess the effects of acute nicotine, nornicotine, and cotinine administration on spontaneous withdrawal from nicotine, mice were infused with nicotine or saline for 14 days. On day 14, mice were anesthetized under isoflurane anesthesia and minipumps were removed in the evening. On day 15, 18–24 hours after minipump removal, mice were pretreated s.c. with either vehicle, nicotine (0.05 and 0.5 mg/kg), nornicotine (2.5 and 25 mg/kg), or cotinine (5 and 50 mg/kg), 10 minutes prior to observations for various withdrawal signs. The mice were first evaluated for 5 minutes in the plus maze test for anxiety-related behavior, followed by a 20-minute observation of somatic signs measured as paw and body tremors, head shakes, backing, jumps, curls, and ptosis. Hyperalgesia was evaluated in the hot-plate test (52°C) immediately following the somatic sign observation period. The specific testing sequence was chosen based on our prior studies showing that this order of testing reduced within-group variability and produced the most consistent results.23 All studies were performed by an observer blinded to experimental treatment.
Data Analysis
The data obtained were analyzed using the GraphPad software, version 6.0 (GraphPad Software, Inc., La Jolla, CA) and expressed as the mean ± S.E.M. Statistical analysis was done in three steps. First, the highest doses of nicotine, nornicotine and cotinine used in this study were compared with their vehicles in chronic saline exposed mice using simple t test to assess the responses after their acute injections. Secondly, to test whether chronic nicotine exposure induced withdrawal symptoms as seen in somatic signs, anxiogenic-like effects in the elevated plus maze, and hyperalgesia in the hot plate test, simple t test was used between acute vehicle treated saline and nicotine minipump groups. Finally, possible alleviation of withdrawal signs by nicotine, nornicotine or cotinine treatments was tested using ordinary one-way analysis of variance (ANOVA), followed by the Tukey post hoc correction. Before ANOVA, the data were first assessed for the normality of the residuals and equal variance. Variances were similar between groups and were assessed using either the F‐test or the Brown–Forsythe test and the Bartlett’s test. All data passed these tests. The p-values < .05 were considered significant.
Results
Anxiety-Related Signs of Withdrawal
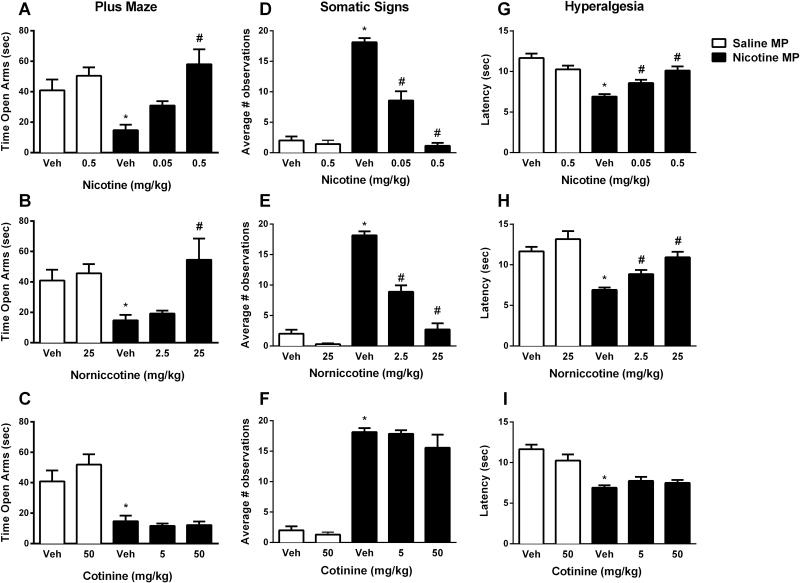
Anxiety-related behavior signs were measured in mice following 18–24 hour withdrawal from chronic nicotine and following acute treatment with nicotine, nornicotine, cotinine or vehicle. Results are shown in Figure 1. Nicotine withdrawal significantly increased anxiety-related behavior in the plus maze (assessed as a decrease in time spent in the open arms) compared to the saline minipump group (t test; p < .01, t = 3.291, df = 12, Figure 1A, B, and C). Acute administration of the highest doses of nicotine, nornicotine, and cotinine alone did not induce anxiety-related behavior when compared with the vehicle saline-treated group ([t test; p = .3133, t = 1.053, df = 12, Figure 1A], [t test; p = .6161, t = 5148, df = 12, Figure 1B] and [t test; p = .2864, t = 1.116, df = 12, Figure 1C], respectively). The one way ANOVA followed by Tukey post hoc revealed that, while nicotine (0.5 mg/kg, s.c.) and nornicotine (25 mg/kg, s.c.) pretreatments significantly reversed anxiety-related behavior [F(2,18) = 12.18, p < .001, Figure 1A and F(2,18) = 6.751, p < .01, Figure 1B, respectively], cotinine failed to alter chronic nicotine-induced signs in the plus maze test [F(2,18) = 0.3861, p = .6852, Figure 1C] at either of the two doses tested. To ensure that the results in the plus maze were not due to changes in locomotor activity, the average total number of arm crosses was analyzed for each group. There were no significant between group differences in this measure (Table 1).
Figure 1.
Effects of nicotine and nicotine metabolites on anxiety-like withdrawal signs in the mouse. Male ICR mice were chronically infused with saline or nicotine (24 mg/kg/day) for 14 days. After mini pumps removal, mice received nicotine (0.05 mg/kg, s.c.) or their respective vehicles. Ten minutes later, (A, B, and C) anxiety-like behaviors (Time spent in the open arm), (D, E, and F) somatic signs, and (G, H, and I) hyperalgesia (latency time) were measured. For statistical analysis, at first, the highest doses of nicotine, nornicotine and cotinine used in this study were compared with their vehicles in chronic saline exposed mice using simple t test to assess the responses after their acute injections. Secondly, to test whether chronic nicotine exposure induced withdrawal symptoms as seen in anxiety-like behaviors, somatic signs, and hyperalgesia, simple t test was used between acute vehicle treated saline and nicotine minipump groups. Finally, possible alleviation of withdrawal signs by nicotine, nornicotine or cotinine treatments was tested using ordinary one-way analysis of variance, followed by the Tukey post hoc correction. Data are presented as the mean ± SEM of 7 animals. *Denotes p < .05 vs. Vehicle treatment in saline minipump group, #Denotes p < .05 vs. Vehicle treatment in nicotine minipump group. MP = Minipumps; Veh = Vehicle.
Table 1.
Nicotine and Its Metabolites Do Not Significantly Alter the Average Number of Arm Crosses in the Plus Maze Assessment
| Treatment | Average number of arm crosses ± SEM |
|---|---|
| Saline MP-vehicle | 7.6 ± 0.6 |
| Saline MP-nicotine (0.5) | 7.4 ± 0.4 |
| Saline MP-nornicotine (2.5) | 7.0 ± 0.7 |
| Saline MP-cotinine (50) | 6.9 ± 0.6 |
| Nicotine MP-vehicle | 6.9 ± 0.6 |
| Nicotine MP-nicotine (0.05) | 7.6 ± 0.3 |
| Nicotine MP-nicotine (0.5) | 7.6 ± 0.4 |
| Nicotine MP-nornicotine (2.5) | 7.6 ± 0.7 |
| Nicotine MP-nornicotine (25) | 6.9 ± 0.6 |
| Nicotine MP-cotinine (5) | 7.6 ± 0.6 |
| Nicotine MP-cotinine (50) | 7.9 ± 0.8 |
Nicotine-withdrawn mice were treated with vehicle or nicotine (0.05 and 0.5 mg/kg), nornicotine (2.5 and 25 mg/kg) and cotinine (5 and 50 mg/kg) and the total number of crosses between the open and closed arms of the plus maze was counted. Numbers are presented as the total average number of arm crosses ± SEM for seven to eight mice per group MP, mini pump.
Somatic Signs of Withdrawal
Following the plus maze test, we assessed the effects of nicotine, nornicotine, and cotinine administration on somatic signs of spontaneous withdrawal. Mice infused with nicotine for 14 days developed somatic signs, indicated by a significant increase in number of observations 18–24 hour after mini pump removal (t test; p < .001, t = 17.23, df = 12, Figure 1D, E, and F). Acute administration of the highest doses of nicotine, nornicotine, and cotinine alone did not induce somatic signs in the saline-control group ([t test; p = .5356, t = 0.6378, df = 12, Figure 1D], [t test; p < .05, t = 2.521, df = 12, Figure 1E] and [t test; p = .3767, t = 0.918, df = 12, Figure 1F], respectively). The one way ANOVA followed by Tukey post hoc revealed that nicotine (0.05 and 0.5 mg/kg, s.c.) and nornicotine (2.5 and 25 mg/kg, s.c.) pretreatments dose-dependently reversed somatic signs [F(2,18) = 72.96, p < .001, Figure 1D and F(2,18) = 71.39, p < .001, Figure 1E, respectively]. However, cotinine at 5 and 50 mg/kg did not significantly reverse somatic signs in nicotine-dependent mice [F(2,18) = 1.101, p = .3541, Figure 1F].
Hyperalgesia Signs of Withdrawal
We evaluated the effects of nicotine and its metabolites on nicotine withdrawal-induced hyperalgesia using the hot plate test. Nicotine, nornicotine, and cotinine administration at the highest doses tested did not affect the latency of animals in the saline-treated group ([t test; p = .079, t = 1.920, df = 12, Figure 1G], [t test; p = .2088, t = 1.328, df = 12, Figure 1H] and [t test; p = .1576, t = 1.507, df = 12, Figure 1I], respectively). Chronic nicotine exposure caused significant hyperalgesia after mini pump removal with a significant decrease in hot plate latency (t test; p < .001, t = 7.522, df = 12, Figure 1). The one way ANOVA followed by Tukey post hoc revealed that nicotine and nornicotine pretreatments significantly reversed hyperalgesic response in nicotine withdrawn mice in a dose-dependent manner [F(2,18) = 15.99, p < .001, Figure 1G and F(2,18) = 14.71, p < .001, Figure 1H, respectively]. In contrast, the nicotine withdrawn animals treated with cotinine displayed no changes in hyperalgesia o response [F(2,18) = 1.18, p = .3299, Figure 1I].
Discussion
In the present study, we evaluated the effects of nicotine metabolites and minor tobacco alkaloids on nicotine withdrawal symptoms in mice. As predicted, nicotine withdrawal significantly increased anxiety-related behaviors, increased expression of somatic withdrawal signs, and decreased response latencies in the hot-plate test. While nicotine and nornicotine alleviated all nicotine spontaneous withdrawal signs, cotinine failed to do so.
The reduction of withdrawal signs by nornicotine is consistent with previous reports of its nicotine and (+) epibatidine-like pharmacological and behavioral effects in rodents and primates.8–10,14,15,24,25 Although this study was the first to systematically investigate the effects of nicotine metabolites on multiple signs of nicotine withdrawal, a previous paper reported failure of nornicotine at a dose of 5.6 mg/kg in reversing a decrease in response rate seen in nicotine-dependent mice after precipitated withdrawal by mecamylamine.9 However, it is likely that the nornicotine dose used by Caine et al. was not high enough to attenuate nicotine withdrawal since at the dose of 2.5 mg/kg, we saw little or no reduction of anxiety-like behaviors and hyperlagesia in our withdrawal model. While doses of nornicotine that produced nicotine-like effects in the present withdrawal study undoubtedly exceed exposure levels achieved through smoking conventional cigarettes, they may still decrease the intensity of nicotine withdrawal syndrome. In addition, previous studies showed that nornicotine might enhance the reinforcing properties of nicotine.8–10,14 Thus, the potential public health benefits of a proposed nicotine reduction policy could be undermined by strategic use of non-nicotine additives by cigarette manufacturers or consumers to initiate and maintain smoking behavior.
The failure of cotinine in reducing nicotine withdrawal signs in our testing is consistent with previous studies that reported lack of nicotine-like effects for cotinine in many dependence-like behaviors in rodents and nonhuman primates.14,16
Conclusion
Our results show that nornicotine, but not cotinine, produced nicotine-like effects in mouse spontaneous withdrawal assays. Our initial results suggest nornicotine as a potential pharmacotherapy for smoking cessation since it can alleviate both physical and affective nicotine withdrawal signs. Our observations extend also the previous findings on the effects of nornicotine and cotinine on nicotine discrimination, reward, and reinforcement to nicotine withdrawal assessment. Collectively, these findings suggest that nicotine reduction strategy in tobacco products could lead to a more manifest role of minor alkaloids such as nornicotine in initiating and maintaining of smoking dependence.
Funding
This work was supported by National Institute on Drug Abuse grant # DA-05274 and DA-032246 (MID).
Declaration of Interests
None declared.
Acknowledgments
SE and DB contributed equally to this work. The authors greatly appreciate the technical assistance of Tie Han.
References
- 1. Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am J Med. 2008;121(4 suppl 1):S3–10. [DOI] [PubMed] [Google Scholar]
- 2. Huang HY, Hsieh SH. Analyses of tobacco alkaloids by cation-selective exhaustive injection sweeping microemulsion electrokinetic chromatography. J Chromatogr A. 2007;1164(1–2):313–319. [DOI] [PubMed] [Google Scholar]
- 3. Crooks PA, Dwoskin LP. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem Pharmacol. 1997;54(7):743–753. [DOI] [PubMed] [Google Scholar]
- 4. Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. [DOI] [PubMed] [Google Scholar]
- 5. Dwoskin LP, Teng LH, Crooks PA. Nornicotine, a nicotine metabolite and tobacco alkaloid: desensitization of nicotinic receptor-stimulated dopamine release from rat striatum. Eur J Pharmacol. 2001;428(1):69–79. [DOI] [PubMed] [Google Scholar]
- 6. Papke RL, Dwoskin LP, Crooks PA. The pharmacological activity of nicotine and nornicotine on nAChRs subtypes: relevance to nicotine dependence and drug discovery. J Neurochem. 2007;101(1):160–167. [DOI] [PubMed] [Google Scholar]
- 7. Bardo MT, Bevins RA, Klebaur JE, Crooks PA, Dwoskin LP. (-)-Nornicotine partially substitutes for (+)-amphetamine in a drug discrimination paradigm in rats. Pharmacol Biochem Behav. 1997;58(4):1083–1087. [DOI] [PubMed] [Google Scholar]
- 8. Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl). 1999;146(3):290–296. [DOI] [PubMed] [Google Scholar]
- 9. Caine SB, Collins GT, Thomsen M, Wright C, Lanier RK, Mello NK. Nicotine-like behavioral effects of the minor tobacco alkaloids nornicotine, anabasine, and anatabine in male rodents. Exp Clin Psychopharmacol. 2014;22(1):9–22. [DOI] [PubMed] [Google Scholar]
- 10. Desai RI, Barber DJ, Terry P. Asymmetric generalization between the discriminative stimulus effects of nicotine and cocaine. Behav Pharmacol. 1999;10(6–7):647–656. [DOI] [PubMed] [Google Scholar]
- 11. Desai RI, Barber DJ, Terry P. Dopaminergic and cholinergic involvement in the discriminative stimulus effects of nicotine and cocaine in rats. Psychopharmacology (Berl). 2003;167(4):335–343. [DOI] [PubMed] [Google Scholar]
- 12. Desai RI, Bergman J. Drug discrimination in methamphetamine-trained rats: effects of cholinergic nicotinic compounds. J Pharmacol Exp Ther. 2010;335(3):807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Desai RI, Bergman J. Methamphetamine-like discriminative-stimulus effects of nicotinic agonists. J Pharmacol Exp Ther. 2014;348(3):478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Desai RI, Doyle MR, Withey SL, Bergman J. Nicotinic effects of tobacco smoke constituents in nonhuman primates. Psychopharmacology (Berl). 2016;233(10):1779–1789. [DOI] [PubMed] [Google Scholar]
- 15. Harris AC, Tally L, Muelken P, et al. Effects of nicotine and minor tobacco alkaloids on intracranial-self-stimulation in rats. Drug Alcohol Depend. 2015;153:330–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoffman AC, Evans SE. Abuse potential of non-nicotine tobacco smoke components: acetaldehyde, nornicotine, cotinine, and anabasine. Nicotine Tob Res. 2013;15(3):622–632. [DOI] [PubMed] [Google Scholar]
- 17. Smith JW, Stolerman IP. Recognising nicotine: the neurobiological basis of nicotine discrimination. Handb Exp Pharmacol. 2009;192:295–333. [DOI] [PubMed] [Google Scholar]
- 18. Takada K, Swedberg MD, Goldberg SR, Katz JL. Discriminative stimulus effects of intravenous l-nicotine and nicotine analogs or metabolites in squirrel monkeys. Psychopharmacology (Berl). 1989;99(2):208–212. [DOI] [PubMed] [Google Scholar]
- 19. Clemens KJ, Caillé S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int J Neuropsychopharmacol. 2009;12(10):1355–1366. [DOI] [PubMed] [Google Scholar]
- 20. Terry AV, Jr, Hernandez CM, Hohnadel EJ, Bouchard KP, Buccafusco JJ. Cotinine, a neuroactive metabolite of nicotine: potential for treating disorders of impaired cognition. CNS Drug Rev. 2005;11(3):229–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Terry AV, Jr, Buccafusco JJ, Schade RF, et al. The nicotine metabolite, cotinine, attenuates glutamate (NMDA) antagonist-related effects on the performance of the five choice serial reaction time task (5C-SRTT) in rats. Biochem Pharmacol. 2012;83(7):941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Le Foll B, Goldberg SR. Effects of nicotine in experimental animals and humans: an update on addictive properties. Handb Exp Pharmacol. 2009;192:335–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008;325(1):302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Damaj MI, Fei-Yin M, Dukat M, Glassco W, Glennon RA, Martin BR. Antinociceptive responses to nicotinic acetylcholine receptor ligands after systemic and intrathecal administration in mice. J Pharmacol Exp Ther. 1998;284(3):1058–1065. [PubMed] [Google Scholar]
- 25. Rosecrans JA. Nicotine as a discriminative stimulus to behavior: its characterization and relevance to smoking behavior. NIDA Res Monogr. 1979;23:58–69. [PubMed] [Google Scholar]