Abstract
Introduction:
Research shows that abstinence from tobacco leads to a withdrawal-related decrement in responsivity to nondrug rewards (ie, anhedonia). However, it remains unclear how anhedonia relates to other key withdrawal symptoms and withdrawal-related constructs over time. We analyzed ecological momentary assessment data to examine whether a decrement in response to rewards during a 10-day period following quitting shows a pattern of associations with other variables (ie, treatment, tobacco dependence, negative affect, and craving) that is consistent with anhedonia being a tobacco withdrawal symptom.
Methods:
As part of a randomized controlled trial of smoking cessation therapies, 1122 adults (58% female) were assigned to: placebo (n = 131), bupropion (alone or with nicotine lozenge; n = 401), or nicotine replacement therapy (NRT; lozenge, patch, both; n = 590). Participants completed 4 ecological momentary assessments per day for 10 days postquit, resulting in 22 575 assessments.
Results:
Time-varying effect modeling showed that anhedonia was significantly greater among those high in dependence relative to lower dependent smokers out to day 9 postquit. The placebo group showed elevated anhedonia immediately postquit, which fell to levels similar to the treatment groups by day 7. NRT effectively reduced anhedonia and its time-varying association with craving early in the quit attempt. The positive association between negative affect and anhedonia was moderate and stable over time for both active treatment groups.
Conclusions:
These results provide additional support that anhedonia following quitting smoking is a manifestation of the tobacco withdrawal syndrome.
Implications:
This study supported the hypothesis that diminished responsivity to nondrug rewards (ie, anhedonia) is a symptom of the tobacco withdrawal syndrome. Results showed that anhedonia: (1) was significantly associated with dependence, especially during the early postquit period when withdrawal was at its peak intensity; (2) showed significant time-varying associations with other withdrawal symptoms, especially craving; and (3) was significantly suppressed by agonist administration as was its association with craving over time.
It is vital to identify key tobacco withdrawal symptoms, given that withdrawal is central to theories of drug dependence, and is a major determinant of smoking relapse and target of smoking cessation interventions.1,2 Most cessation failures occur during early abstinence when withdrawal symptoms are at their peak3,4 and suppression of withdrawal likely accounts for much of the clinical benefit of smoking cessation pharmacotherapies.5,6 A more precise understanding of withdrawal symptoms is of theoretical and clinical interest because it may inform theories of the motivational bases of dependence and guide treatment development.
There is growing evidence that a decrement in responsivity to environmental rewards (ie, anhedonia) is a component of the tobacco withdrawal syndrome. Anhedonia reflects a combination of reward or incentive-related subdomains (eg, incentive salience, reward anticipation, interest, reward value, and costs)7,8 and is modestly associated with emotional states such as positive and negative affect.9,10 However, there is clear evidence that anhedonia is a unique construct that reflects significant motivational deficits.9,11 Our research from a large trial of treatment seeking smokers11 revealed that anhedonia conforms to the following criteria for a withdrawal symptom: (1) Cessation produced a rise in anhedonia that peaked shortly after drug deprivation, followed by a return to prequit levels (ie, a time-course consistent with withdrawal).1,12–14 (2) Agonist therapy (ie, nicotine replacement therapy [NRT])15,16 attenuated the rise in postquit anhedonia. (3) On average, anhedonia covaried with other tobacco withdrawal symptoms, reflecting the nature of a syndrome element.17 (4) Similar to other withdrawal symptoms (eg, craving and negative affect), anhedonia was associated with core indices of tobacco dependence.18 (5) Finally, postquit decrements in responsivity to environmental rewards predicted relapse, suggesting that anhedonia possesses motivational significance similar to other key withdrawal symptoms (ie, craving and negative affect).19,20
Prior human laboratory21–23 and animal research24,25 has shown that nicotine deprivation leads to a withdrawal-related decrement in reward functioning. Laboratory research with humans suggests that suspension of nicotine delivery results in diminished responding for nondrug incentive stimuli22,23,26,27 (although see Kalamboka et al.28) and decreased reports of pleasure in response to rewards21,29 (although see Snuggs and Hajek30.) Research with animals shows that nicotine deprivation following prolonged exposure diminishes instrumental responding for rewarding electrical brain stimulation (ie, anhedonia).25,31,32 Thus, the potential clinical relevance of withdrawal-related anhedonia seems clear. Withdrawal reduces the reward value of non-nicotine appetitive stimuli, thereby enhancing the relative reward or incentive value of nicotine. In addition to allowing the smoker to reexperience the direct appetitive effects of nicotine, lapsing back to smoking would reestablish the pleasurable effects of environmental rewards.11,21,33
Although some prior research supports the anhedonia-withdrawal hypothesis, further research is needed to understand how reward functioning changes during the initial course of a cessation attempt. In particular, it is unknown how variables that are theoretically and clinically related to withdrawal (eg, tobacco dependence) influence changes in the reward value of non-nicotine appetitive stimuli following quitting, as well as how other key withdrawal symptoms (eg, negative affect and craving) relate to anhedonia over time. In our previous research, we used traditional statistical models that assumed that the association between anhedonia and other variables (eg, negative affect and craving) was constant (ie, symptoms were averaged across time).11 Such an approach cannot reveal changes in the relation between anhedonia and other variables (both variant and invariant) over the course of a quit attempt. Moreover, averaging symptoms over time could positively or negatively bias the associations (eg, by overweighting the impact of trait-like influences or by underweighting the influence of episodic events that might drive reactivity in one or multiple symptom types).
In this research, we used ecological momentary assessment data34 to examine whether variables that are related to tobacco withdrawal exerted time-varying effects on anhedonia during the first 10 days following a quit attempt. Specifically, we examined the relation between anhedonia and two time-invariant predictors (baseline tobacco dependence and treatment) and two time-varying withdrawal symptoms (craving and negative affect, the two withdrawal symptoms most robustly associated with tobacco dependence and abstinence).18,35 Consistent with theory and data on the nature of withdrawal, postquit anhedonia should be elevated more among highly dependent smokers versus low dependent smokers, especially early in the deprivation period when withdrawal is at its peak. Moreover, anhedonia should be reduced (ie, reward functioning enhanced) more by a pure nicotinic agonist15,16 such as NRT than by bupropion or placebo. Any bupropion effects on postquit anhedonia, on the other hand, might be due to the dopaminergic, pleasurable-activating effects of bupropion. With regard to the time-varying relations between postquit anhedonia and both craving and negative affect, data are expected to reveal significant levels of association during the postquit period, especially early in the course of abstinence when withdrawal influences are greatest. This is because, presumably, withdrawal symptoms are driven en masse by underlying dependence processes unleashed by abstinence.17 Finally, smoking-cessation pharmacotherapies have been shown to reduce the association between two core withdrawal symptoms, craving and negative affect, in the first days after quitting.36 Therefore, to the extent that agonist administration (nicotine) reduces core, underlying withdrawal processes, it should similarly reduce the time-varying associations of anhedonia with other withdrawal symptoms.
We addressed these aims using a novel analytical approach, the time-varying effect model (TVEM).37,38 TVEM is a modeling technique that estimates within-subjects associations that unfold over time. For instance, it can evaluate the degree to which symptom co-occurrence changes during times when a disease is active versus inactive (eg, when a diabetic suspends insulin administration and symptoms emerge and cohere in magnitude and course). In TVEM, regression coefficients capturing interrelations between variables are not assumed to be constant over time. Rather, the direction and magnitude of the coefficient is estimated as a smooth function across continuous time. These functions are not assumed to be parametric (eg, linear across time). Rather, they convey the functional form that occurs naturally in the data, with the final coefficient functions reflecting a balance between model fit and parsimony. This study uses TVEM to model the time-varying associations between postquit anhedonia and well-established, clinically meaningful variables: tobacco dependence, treatment, craving, and negative affect. Given research (including with the present dataset) showing that craving, negative affect, and anhedonia reliably increase following quitting,11,12,39 the postquit symptoms observed in this study reflect, at least in part, the effects of withdrawal. Finally, we are examining these aims in a sample in which we previously examined anhedonia as a withdrawal symptom,11 because it is important to use diverse analytic strategies to further examine the anhedonia-withdrawal hypothesis.
The goal of this research was to examine whether anhedonia following a quit attempt shows a pattern of change over time, and associations with other variables, that are consistent with its being a symptom of the tobacco withdrawal syndrome. We hypothesize that elevated dependence will increase anhedonia (H1) and that cessation pharmacotherapy, particularly NRT (relative to placebo), will reduce anhedonia (H2) and that the strength of these hypothesized effects will be greatest early during the quit attempt. We also hypothesize that anhedonia will be positively correlated with craving and negative affect during the 10 days following a quit attempt, with the strength of the associations being greatest during early deprivation when withdrawal symptoms typically peak (H3). Finally, we hypothesize that treatment, particularly NRT, will diminish anhedonia’s associations with craving and negative affect, especially early in cessation (H4).
Methods
Participants
This study is a secondary analysis of a smoking cessation clinical trial.40 A total of 1504 smokers from South Central Wisconsin participated in the trial. All participants smoked at least 10 cigarettes per day for the past 6 months and were motivated to quit smoking (reported ≥7 on a single item (0–10 scale) motivation to quit smoking assessment). Exclusion criteria included a contraindication for study medication; a history of psychosis, bipolar disorder, or an eating disorder; or a consumption pattern of six or more alcoholic beverages at least 6 days a week. This study was approved by the University of Wisconsin Health Sciences Institutional Review Board. The study was registered in http://clinicaltrials.gov/ with the identification number NCT00332644.
In our analysis, we removed participants with zero observations for anhedonia or the key covariates (eg, negative affect and craving) which resulted in a final sample of 1122. The resulting sample was 58% female, 85.5% white. Participants were an average age of 45.1 years (SD = 11.0), they smoked 21.46 (SD = 9.05) cigarettes per day at baseline, and they had made 5.69 (SD = 9.21) previous quit attempts.
Procedure
Participants were recruited through media advertisements and earned media. Study candidates who passed an initial phone screen were invited to an Information Session where they provided written informed consent. Participants then attended three baseline assessments during which they underwent multiple screenings, including a medical history screening and a carbon monoxide breath test.
Treatment
Eligible participants were randomized, blocked on gender and ethnicity, to one of six treatment conditions: (1) bupropion SR (9 weeks, starting 1 week prior to the target quit day); (2) nicotine lozenge (12 weeks starting on the target quit day); (3) nicotine patch (8 weeks starting on the quit day); (4) nicotine patch + nicotine lozenge; (5) bupropion SR + nicotine lozenge; or (6) placebo. There were five placebo conditions matched to each of the active treatment conditions. All participants received six counseling sessions. For the current study, we compared the placebo condition (n = 131) with the bupropion group (alone or with nicotine lozenge; n = 401) and the NRT group (lozenge, patch, both; n = 590).
Assessments
At the screening visit, participants completed assessments on demographic and smoking variables. Tobacco dependence was assessed using one item (time-to-first-cigarette) from the Fagerström Test for Nicotine Dependence.41 Time-to-first-cigarette has been shown to relate strongly to smoking heaviness, withdrawal, and cessation failure.42–44 Time-to-first-cigarette scores were used to create two groups: those who smoked within the first 5 minutes of waking (high dependence) versus those who delayed smoking for at least 5 minutes (lower dependence).
Ecological Momentary Assessments
Participants were prompted to answer questions four times a day (morning, night, and two random prompts) via palm pilot for 10 days after their target quit day. The four daily assessments of craving and negative affect were averaged across each day. Ecological momentary assessment items were selected from validated questionnaires such as the Wisconsin Smoking Withdrawal Scale (WSWS)14 and the Positive Affect Negative Affect Scale (PANAS).45 Each ecological momentary assessment prompt asked participants to report from “disagree!!” (coded as 0) to “agree!!” (coded as 10) how they felt in the last 15 minutes on the following items: (1) six negative affect items (tense or anxious; impatient; bothered by negative moods such as anger, frustration, or irritability; irritable or easily angered; sad or depressed; and hopeless or discouraged); (2) two craving items, “Bothered by desire to smoke a cigarette” and “Urge to smoke.” A composite consisting of the mean of the items from each domain (eg, negative affect and craving) was used in the analyses.
During the evening prompt, participants reported how much pleasure they experienced that day from “no pleasure” (coded as 0) to “extreme pleasure” (coded as 10), from three domains (social, recreation, and performance/ accomplishment) that are used in standardized, well-validated anhedonia scales.46,47 Pleasure responses were reverse scored so that higher scores reflected greater anhedonia.
Statistical Analysis
The models were implemented using the TVEM SAS Macro,37 which can be applied to estimate the coefficient functions in dynamic processes measured intensively. Models were estimated using a P-spline basis, with the optimal models automatically selected on the basis of information criteria and robust standard errors to account for the nested structure of the repeated-measures data.
For Hypotheses 1 and 2, intercept-only time-varying effect models were estimated separately for high versus low dependence (H1) and treatment group (placebo, bupropion, and NRT) (H2) to capture the time-varying differences in mean anhedonia during the first 10 days after quitting across dependence and treatment groups. For Hypothesis 3, we explored the dynamic associations between anhedonia and both craving and negative affect using separate TVEMs, where anhedonia was modeled as a function of each predictor and the regression coefficient was allowed to vary flexibly as a nonparametric function of time.38,48 Finally, for Hypothesis 4, we examined how treatment influenced the time-varying associations between anhedonia and both craving and negative affect. For both craving and negative affect, separate models were estimated for the three treatment groups to capture time-varying associations within treatment conditions for the association between anhedonia and both craving and negative affect.
Finally, we conducted analyses to account for the potential influence of postquit smoking on withdrawal symptoms. During the first 10 days postquit, only 34% of participants reported no smoking; 80% reported smoking less than one cigarette per day, on average. Therefore, excluding participants who smoked at all from analysis would create an unrepresentative sample, in part because those who relapsed likely experienced the greatest withdrawal symptoms.1,13,49 To evaluate the influence of smoking on the anhedonia time course and its associations with other withdrawal symptoms, we reran the TVEMs and included smoking as a time-varying covariate. Results that included smoking as a covariate were identical to those that did not; thus, we present simplified models without smoking as a time-varying covariate.
Results
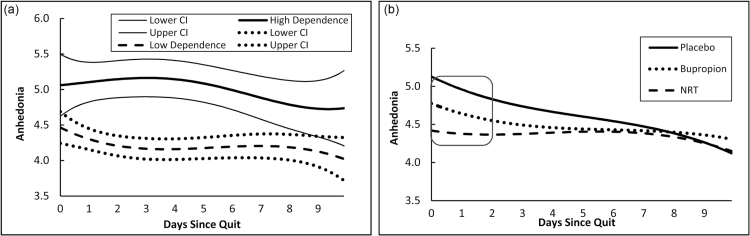
Figure 1a presents mean anhedonia over time for those high versus low in dependence. At any point in time, the level on the curve represents the estimated mean level of anhedonia for each level of dependence, with the corresponding 95% confidence interval. For both dependence groups, postquit anhedonia was significantly greater than zero at each time point the confidence intervals did not contain zero. A significant difference in mean anhedonia between the two groups occurred at any time point that the confidence intervals did not overlap (a conservative index of significance).50 As hypothesized, individuals with baseline high dependence reported significantly greater anhedonia than did less dependent individuals through approximately day 9 (see Figure 1a). Further, anhedonia remained high or increased for about 5 days post cessation among those high in dependence, but it decreased almost immediately among those low in dependence.
Figure 1.
(a) Time-varying mean anhedonia and corresponding 95% confidence interval during 10 days after cessation, by dependence level. (b) Time-varying mean anhedonia during 10 days after cessation, by treatment group. Square denotes region of time during which placebo and NRT groups had significantly different mean anhedonia.
Figure 1b presents mean anhedonia over time for each treatment group (placebo, NRT, and bupropion). As in Figure 1a, at any point in time, the level on the curve represents estimated mean anhedonia for each treatment group. The data revealed a marked elevation in anhedonia for the placebo group over the first 4–5 days post-cessation, especially relative to the NRT group, which showed an essentially flat trajectory over time. As hypothesized, anhedonia was significantly greater in the placebo group than in the NRT group during the first 2 days of the quit attempt. (Confidence intervals were not included for figures that depict the effects of treatment because they were not interpretable. Instead, a square is superimposed on the section of the trajectories where significant differences were observed.) There were no statistically significant differences in anhedonia between bupropion and placebo at any point in time.
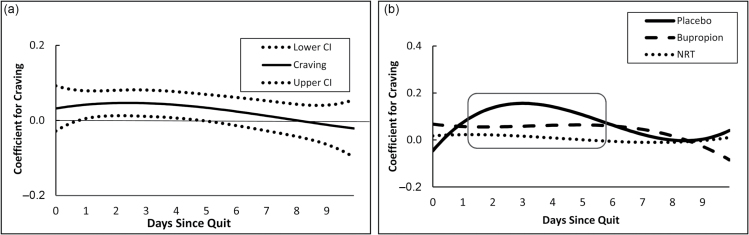
Figure 2a illustrates the time-varying association between craving and anhedonia, regardless of the level or dependence or treatment group. At any point in time, the level on a curve represents that time-specific regression coefficient indicating the association between the covariate (ie, craving) and anhedonia. Windows of time during which a confidence interval does not contain 0 indicate periods during which the relationship between craving and anhedonia is significantly greater than 0. As hypothesized, overall, there was a significant, positive time-varying association between craving and anhedonia early in the quit attempt (around days 1 through 4); the association was nonsignificant after day 4.
Figure 2.
(a) Overall time-varying effect of craving on anhedonia and corresponding 95% confidence interval during 10 days after cessation. (b) Time-varying effect of craving on anhedonia during 10 days after cessation, by treatment group. Square denotes region of time during which the association between craving and anhedonia was significantly different from zero.
Figure 2b depicts the time-varying association between craving and anhedonia for the placebo, bupropion, and NRT groups. There was a significant association between anhedonia and craving in both the placebo and bupropion groups early during the quit period (around days 2 through 4) for placebo and days 2 through 6 for bupropion, but this association declined to nonsignificance thereafter. As hypothesized, craving and anhedonia were not significantly associated for those in the NRT group at any time.
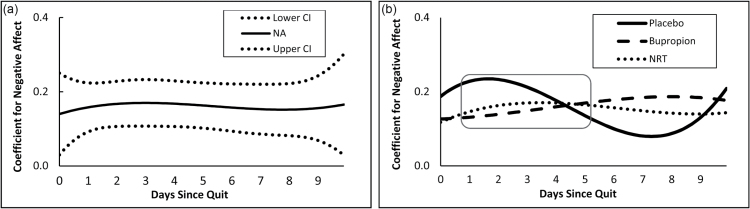
Figure 3a depicts the time-varying association between negative affect and anhedonia. There was a significant, positive association between negative affect and anhedonia starting immediately upon quitting (day 0) and lasting through day 10. Figure 3b shows that in the placebo group, the association between negative affect and anhedonia was significant and positive from day 1 through day 5, but not thereafter. For both the bupropion and NRT groups, however, the associations between negative affect and anhedonia were significant for the entire 10-day postquit period. Thus, the trajectory of the association between anhedonia and negative affect in the placebo group reflects the expected time course of symptoms driven by withdrawal; peak associations early in the post-cessation period followed by subsequent decline. This pattern is not seen in the bupropion and NRT groups, which both showed relatively flat trajectories over time. None of the three groups differed significantly from one another over the post-cessation period, contrary to the hypothesized effect.
Figure 3.
(a) Overall time-varying effect of negative affect on anhedonia and corresponding 95% confidence interval during 10 days after cessation. (b) Time-varying effect of negative affect on anhedonia during 10 days after cessation, by treatment group. Square denotes region of time during which the association between negative affect and anhedonia was significantly different from zero.
Discussion
This research supports the hypothesis that diminished responsivity to nondrug rewards (ie, anhedonia) is a symptom of the tobacco withdrawal syndrome.11,21 Consistent with long-standing characterizations of withdrawal,16,51 anhedonia was greatest among highly dependent smokers relative to those low in dependence. Although anhedonia remained elevated in the high dependence group for about 5 days postquit, the difference between the higher versus lower dependent smokers was especially apparent early in the deprivation period when withdrawal was at its peak. Moreover, postquit anhedonia was significantly reduced early in the post-cessation period by agonist-NRT administration and not by placebo or bupropion, the latter a noncompetitive nicotine agonist. As would be expected with regard to manifestations of a common underlying, emergent, disease process,17 postquit anhedonia also significantly covaried with both craving and negative affect. As hypothesized, the time-varying relation between craving and anhedonia was greatest early during the abstinence period; however, administration of a nicotinic agonist, NRT, effectively suppressed such covariation. Finally, the time-varying relation between postquit anhedonia and craving showed a prototypic rise-and-fall pattern among individuals administered placebo, which, again, is consistent with their both being influenced by withdrawal processes. Those receiving bupropion or NRT showed fairly flat trajectories over the postquit period.
The significant associations between postquit anhedonia and both craving and negative affect reveal important information about anhedonia as a withdrawal symptom as well as about the withdrawal syndrome in general. If one assumes that withdrawal significantly drives symptom expression following quitting (although other affective processes, expectancies, and cues also undoubtedly influence the expression of withdrawal symptoms), then symptoms should not only covary, but the shape of their association across time should roughly parallel individual symptom trajectories; that is, peak immediately after nicotine removal followed by gradual decline with continued deprivation.1,12 Further, when withdrawal is suppressed, either via agonist administration or a return to smoking, withdrawal symptoms and their intercorrelations should diminish. Indeed, the association between anhedonia and craving was greatest during the first few days of abstinence, when mean anhedonia and craving peaked (see Supplementary Figure 1 for mean craving time course). When NRT was administered, the correlation between anhedonia and craving became nonsignificant. Thus, we surmise that NRT reduced the severity of the withdrawal syndrome, mitigating the extent to which craving and anhedonia (both elements of withdrawal) co-occurred.
Unlike the association between anhedonia and craving, the correlation between anhedonia and negative affect was quite stable across the 10 days following quitting. This is similar to a prior finding that the time-varying association between negative affect and craving remained strong and fairly stable during the first 2 weeks postquit.36 Thus, the pattern of associations between negative affect and the other symptoms (anhedonia and craving) does not appear to mirror the trajectories of the mean levels of the individual symptoms involved (ie, postquit peak followed by gradual return to baseline; see Supplementary Figure 2 for mean negative affect time course). This conflicts with a model in which time-varying relations among symptoms are primarily driven by a single causal influence. Further, the effect of treatment yields ambiguous evidence on this issue. In support of a common withdrawal influence, participants administered placebo showed the prototypic rise-and-fall withdrawal pattern with regard to the time-varying relation between anhedonia and negative affect. However, neither bupropion nor NRT significantly suppressed this time-varying association. The lack of medication effect may simply reflect a lack of power, because the rise-and-fall pattern was present in the placebo group and not in the active medication groups (albeit, medication conditions did not differ significantly from one another). Or, it may be that mechanisms other than withdrawal influenced the associations between negative affect and other withdrawal symptoms. For instance, stress might strongly influence postquit negative affect levels.4 To the extent that a factor such as stress exerts stronger effects on negative affect than on anhedonia, it would reduce the association between the two. Thus, although withdrawal might increase negative affect and anhedonia early in the postquit period (see their association among placebo participants in Figure 3b), ongoing stressors might decouple their association. Clearly, a better understanding of the causal influences on negative affect and its relations with other withdrawal symptoms requires additional research.
This paper focused on evaluating anhedonia as a withdrawal symptom. However, it is important to use TVEM to examine the dynamic relations of other putative withdrawal symptoms. The results might have both substantive and clinical value. For instance, such results could be used to infer causal paths and construct structure, thereby complementing the results of other analytic approaches such as mediational analysis. Presumably, variables that are facets of the same construct and that share a common causal influence would reflect manipulations of that influence in terms of both their magnitude and their coherence or association over time (eg, as disease state worsens or wanes). Moreover, the coherence of symptoms over time may yield clinical insight and index sensitively the motivational impact of withdrawal. The potential influence and value of symptom coherence can be seen in the display of basic emotions such as fear; the coherence of responses across fear response systems (eg, motoric, autonomic, and cognitive) tends to reflect the magnitude of fear provocation and diagnostic status.52–54 Similarly, the challenge of quitting smoking may be a function of both the elevation of symptoms and the number of symptoms similarly elevated. Thus, TVEM, which indexes the dynamic associations between variables over time, may shed new light on the nature of withdrawal and its motivational consequences.
This research has several limitations that should be considered. First, this study comprised only individuals motivated to quit, which may reduce generalizability. Second, anhedonia was assessed via self-reports and therefore may reflect errors such as broad attitudinal factors and incorrect attributions. For instance, in addition to participants’ capacity to experience pleasure, the rate of encounters with, and interest in, environmental rewards could influence reports of pleasure in response to appetitive events. Thus, we were unable to precisely characterize the type of reward (or incentive)-related deficit. Therefore, these data should be viewed as reflecting some subset of processes associated with clinical presentations of anhedonia.7 Although basic neurobiological research might suggest that the results reflect increased reward thresholds (anhedonia), future research should address competing accounts more directly. Third, it is possible that withdrawal measures derived from different quantities of measurement occasions might differ in reliability or sensitivity (ie, craving and negative affect were averaged across four daily assessments, whereas anhedonia was assessed only once each day). Fourth, although the shapes of the profiles support our hypothesis that withdrawal influenced the relations between variables (eg, anhedonia and dependence), it is also possible that some of the postquit patterns reflect influences that existed prequit; for example, due to existing person factors or persisting contextual influences. Finally, the results of this study are based on TVEM, a novel methodologic approach. Rather than conducting specific hypothesis testing, TVEM depicts time-varying relations between variables; this limits conclusions that can be drawn from this approach. For instance, the nature of TVEM does not yield estimates of effect size that accurately capture the magnitude of effects seen in the different time-varying associations over time.
In summary, TVEM provided an innovative method for assessing whether anhedonia showed patterns of change over time, and associations with other variables, that were consistent with its being a symptom of the tobacco withdrawal syndrome. Results generally supported the hypothesis that anhedonia is a withdrawal symptom. Anhedonia: (1) was significantly associated with dependence, especially during the early postquit period when withdrawal was at its peak intensity; (2) showed significant time-varying associations with other withdrawal symptoms, especially craving; (3) was significantly suppressed by agonist administration, as was its association with craving over time, again with these effects being especially strong early in the postquit period. There was less evidence that the dynamic association between anhedonia and negative affect was strongly linked to withdrawal processes. Taken together, these results provide additional support that decrements in responsivity to environmental rewards following quitting is a manifestation of the tobacco withdrawal syndrome.11 Finally, these results suggest the potential utility of TVEM for exploring construct validity and causal relations.
Funding
JWC was funded in part by VA Merit Review Award 101CX00056 from the US Department of Veterans Affairs and by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Science Research and Development. MEP was funded in part by 1KL2RR025012. This work was also supported by grant M01 RR03186 from the General Clinical Research Centers Program of the National Center for Research Resources (NIH); by P50DA019706l from the National Institute on Drug Abuse; by grant K05CA139871 (PI: Baker) from NIH; and by grant P50 DA010075 (PI: Collins) and P50 DA039838 (PI: Collins) from the National Institute on Drug Abuse and R01 CA168676 (PI: Lanza) from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse, the National Cancer Institute, or the National Institutes of Health. Medication was provided to participants at no cost under a research agreement with GlaxoSmithKline; no part of this manuscript was written or edited by anyone employed by GlaxoSmithKline.
Declaration of Interests
None declared.
Supplementary Material
References
- 1. Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: I. Abstinence distress in lapsers and abstainers. J Abnorm Psychol. 2003;112(1):3–13. [PubMed] [Google Scholar]
- 2. Zhou X, Nonnemaker J, Sherrill B, Gilsenan AW, Coste F, West R. Attempts to quit smoking and relapse: factors associated with success or failure from the ATTEMPT cohort study. Addict Behav. 2009;34(4):365–373. [DOI] [PubMed] [Google Scholar]
- 3. Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA. 1994;271(8):589–594. [DOI] [PubMed] [Google Scholar]
- 4. McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: an electronic diary study. J Abnorm Psychol. 2006;115(3):454–466. [DOI] [PubMed] [Google Scholar]
- 5. Bolt DM, Piper ME, Theobald WE, Baker TB. Why two smoking cessation agents work better than one: role of craving suppression. J Consult Clin Psychol. 2012;80(1):54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piper ME, Federmen EB, McCarthy DE, et al. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. J Abnorm Psychol. 2008;117(1):94–105. [DOI] [PubMed] [Google Scholar]
- 7. Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35(1):68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Treadway MT, Zald DH. Parsing anhedonia: translational models of reward-processing deficits in psychopathology. Curr Dir Psychol Sci. 2013;22(3):244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Franken IH, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS). J Affect Disord. 2007;99(1-3):83–89. [DOI] [PubMed] [Google Scholar]
- 10. Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine Tob Res. 2004;6(1):39–47. [DOI] [PubMed] [Google Scholar]
- 11. Cook JW, Piper ME, Leventhal AM, Schlam TR, Fiore MC, Baker TB. Anhedonia as a component of the tobacco withdrawal syndrome. J Abnorm Psychol. 2015;124(1):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9(3):315–327. [DOI] [PubMed] [Google Scholar]
- 13. Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. J Abnorm Psychol. 2003;112(1):14–27. [PubMed] [Google Scholar]
- 14. Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7(4):354–361. [DOI] [PubMed] [Google Scholar]
- 15. Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362(24):2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siegel S. Classical conditioning, drug tolerance, and drug dependence. In: Smart RG, Glaser FB, Israel Y, Kalant R, Popham E, Schmidt W, eds. Research Advances in Alcohol and Drug Problems. 7th ed. New York: Plenum; 1983:207–246. [Google Scholar]
- 17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 18. Baker TB, Piper ME, Schlam TR, et al. Are tobacco dependence and withdrawal related amongst heavy smokers? Relevance to conceptualizations of dependence. J Abnorm Psychol. 2012;121(4):909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111(1):33–51. [DOI] [PubMed] [Google Scholar]
- 20. Swan GE, Jack LM, Javitz HS, McAfee T, McClure JB. Predictors of 12-month outcome in smokers who received bupropion sustained-release for smoking cessation. CNS Drugs. 2008;22(3):239–256. [DOI] [PubMed] [Google Scholar]
- 21. Dawkins L, Acaster S, Powell JH. The effects of smoking and abstinence on experience of happiness and sadness in response to positively valenced, negatively valenced, and neutral film clips. Addict Behav. 2007;32(2):425–431. [DOI] [PubMed] [Google Scholar]
- 22. Powell J, Dawkins L, Davis RE. Smoking, reward responsiveness, and response inhibition: tests of an incentive motivational model. Biol Psychiatry. 2002;51(2):151–163. [DOI] [PubMed] [Google Scholar]
- 23. Powell JH, Pickering AD, Dawkins L, West R, Powell JF. Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addict Behav. 2004;29(7):1407–1426. [DOI] [PubMed] [Google Scholar]
- 24. D’Souza MS, Markou A. Neural substrates of psychostimulant withdrawal-induced anhedonia. Curr Top Behav Neurosci. 2010;3:119–178. [DOI] [PubMed] [Google Scholar]
- 25. Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393(6680):76–79. [DOI] [PubMed] [Google Scholar]
- 26. al-Adawi S, Powell J. The influence of smoking on reward responsiveness and cognitive functions: a natural experiment. Addiction. 1997;92(12):1773–1782. [PubMed] [Google Scholar]
- 27. Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I–effects on incentive motivation. Psychopharmacology (Berl). 2006;189(3):355–367. [DOI] [PubMed] [Google Scholar]
- 28. Kalamboka N, Remington B, Glautier S. Nicotine withdrawal and reward responsivity in a card-sorting task. Psychopharmacology (Berl). 2009;204(1):155–163. [DOI] [PubMed] [Google Scholar]
- 29. Dawkins L, Powell J. Effects of nicotine and alcohol on affective responses to emotionally toned film clips. Psychopharmacology (Berl). 2011;216(2):197–205. [DOI] [PubMed] [Google Scholar]
- 30. Snuggs S, Hajek P. Responsiveness to reward following cessation of smoking. Psychopharmacology (Berl). 2013;225(4):869–873. [DOI] [PubMed] [Google Scholar]
- 31. Hilario MR, Turner JR, Blendy JA. Reward sensitization: effects of repeated nicotine exposure and withdrawal in mice. Neuropsychopharmacology. 2012;37(12):2661–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnson PM, Hollander JA, Kenny PJ. Decreased brain reward function during nicotine withdrawal in C57BL6 mice: evidence from intracranial self-stimulation (ICSS) studies. Pharmacol Biochem Behav. 2008;90(3):409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perkins KA, Karelitz JL. Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology (Berl). 2013;228(3):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. [DOI] [PubMed] [Google Scholar]
- 35. Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology (Berl). 2006;187(3):385–396. [DOI] [PubMed] [Google Scholar]
- 36. Lanza ST, Vasilenko SA, Liu X, Li R, Piper ME. Advancing the understanding of craving during smoking cessation attempts: a demonstration of the time-varying effect model. Nicotine Tob Res. 2014;16(Suppl 2):S127–S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li R, Dziak JD, Tan X, Huang L, Wagner AT, Yang J. TVEM (Time-Varying Effect Modeling) SAS Macro Users’ Guide (Version 3.1.0). University Park: The Methodology Center, Penn State; 2015. [Google Scholar]
- 38. Tan X, Shiyko MP, Li R, Li Y, Dierker L. A time-varying effect model for intensive longitudinal data. Psychol Methods. 2012;17(1):61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Piper ME, Schlam TR, Cook JW, et al. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology (Berl). 2011;216(4):569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Piper ME, Smith SS, Schlam TR, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66(11):1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 42. Baker TB, Piper ME, McCarthy DE, et al. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res. 2007;9(Suppl 4):S555–S570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bolt DM, Piper ME, McCarthy DE, et al. The Wisconsin Predicting Patients’ Relapse questionnaire. Nicotine Tob Res. 2009;11(5):481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84(7):791–799. [DOI] [PubMed] [Google Scholar]
- 45. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. [DOI] [PubMed] [Google Scholar]
- 46. Fawcett J, Clark DC, Scheftner WA, Gibbons RD. Assessing anhedonia in psychiatric patients. Arch Gen Psychiatry. 1983;40(1):79–84. [DOI] [PubMed] [Google Scholar]
- 47. Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167(1):99–103. [DOI] [PubMed] [Google Scholar]
- 48. Shiyko MP, Lanza ST, Tan X, Li R, Shiffman S. Using the time-varying effect model (TVEM) to examine dynamic associations between negative affect and self confidence on smoking urges: differences between successful quitters and relapsers. Prev Sci. 2012;13(3):288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hughes JR. Effects of abstinence from tobacco: etiology, animal models, epidemiology, and significance: a subjective review. Nicotine Tob Res. 2007;9(3):329–339. [DOI] [PubMed] [Google Scholar]
- 50. Schenker N, Gentleman JF. On judging the significance of differences by examining the overlap between confidence intervals. Am Statistician. 2001;55(3):182–186. [Google Scholar]
- 51. Solomon RL, Corbit JD. An opponent-process theory of motivation. II. Cigarette addiction. J Abnorm Psychol. 1973;81(2):158–171. [DOI] [PubMed] [Google Scholar]
- 52. Cacioppo JT, Uchino BN, Crites SL, et al. Relationship between facial expressiveness and sympathetic activation in emotion: a critical review, with emphasis on modeling underlying mechanisms and individual differences. J Pers Soc Psychol. 1992;62(1):110–128. [DOI] [PubMed] [Google Scholar]
- 53. Lang PJ, Levin DN, Miller GA, Kozak MJ. Fear behavior, fear imagery, and the psychophysiology of emotion: the problem of affective response integration. J Abnorm Psychol. 1983;92(3):276–306. [DOI] [PubMed] [Google Scholar]
- 54. Rosenberg EL, Ekman P. Coherence Between Expressive and Experiential Systems in Emotion. What the Face Reveals: Basic and Applied Studies of Spontaneous Expression Using the Facial Action Coding System (FACS). New York, NY: Oxford University Press; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.