Abstract
Introduction:
Blunted nucleus accumbens (NAc) reactivity to reward is common across drug users. One theory is that individuals abuse substances due to this reward deficit. However, whether there is a relationship between the amount an individual uses and the severity of NAc dysfunction is unclear. It also is possible that such a relationship is substance specific, as nicotine transiently increases reward system sensitivity while alcohol, another commonly used substance, does not. As smokers may use nicotine to bolster NAc reward function, we hypothesize that NAc reactivity to reward will be related to volume of cigarette use, but not volume of alcohol use.
Methods:
A functional magnetic resonance imaging incentive-processing task collected by the Human Connectome Project was assessed in a cohort of tobacco smokers who reported smoking between 5–20 cigarettes/day and a cohort of alcohol users who reported drinking 7–25 drinks/wk. Number of cigarettes/day and drinks/wk were correlated with right and left NAc reactivity to the receipt of a monetary reward relative to baseline.
Results:
Individuals who smoke greater numbers of cigarettes/day showed lower right NAc reactivity to reward (r = 0.853, p ≤ .001). Left NAc reactivity was not correlated with cigarettes/day. No association was found with drinks/wk.
Conclusions:
A negative association was found between NAc reactivity to reward and cigarettes/day, but not alcohol drinks/wk. Given nicotine’s unique ability to increase sensitivity to rewards, these findings suggest that individuals who smoke more cigarettes/day may be compensating for more dysfunctional NAc reward reactivity.
Implications:
The present study demonstrates that a relationship between NAc reactivity to nondrug reward and volume of substance use is present in nicotine but not alcohol use. While prior work has implicated dysfunctional reward processing in addictions, these findings clarify a substance-specific role that blunted reward function has in determining patterns of use among chronic users.
Introduction
Dysfunctional reward processing plays a key role in maintaining substance abuse despite aversive consequences.1–6 While addiction-related changes in reward function are often associated with heightened reactivity to drug-related rewards,7–8 substance users also demonstrate relatively blunted reward reactivity to nondrug rewards.9–11 Blunted reward reactivity to nondrug rewards in brain regions such as the nucleus accumbens (NAc) has been observed in individuals who use substances including cocaine,9 nicotine,2,5,12–13 and alcohol.11
One theory is that individuals abuse substances due to a deficit in reward reactivity to nondrug rewards.1 This may be particularly true for nicotine users, as nicotine transiently increases reward system sensitivity14–16 and may be used to ameliorate deficits in reward reactivity. However, whether there is a relationship between the extent of blunted NAc reactivity to nondrug rewards and the amount an individual uses of a substance remains unclear. Preliminary evidence has linked volume of cigarette use in adolescence with blunted NAc reactivity12 to nondrug rewards. Yet, it is unknown if the degree of blunted NAc reactivity is associated with patterns of use after chronic nicotine exposure in adults.
Further, it is possible that this relationship is specific to nicotine. While all drugs of abuse result in increase of the reward value of drug-related contextual cues, nicotine administration transiently increases reward system sensitivity to all rewarding stimuli16 while other commonly used substances such as alcohol do not.17 Exploring whether a relationship between NAc reactivity to nondrug rewards and volume of use is specific to nicotine may provide insight into motivational factors impacting severity of use. One such hypothesis is that individuals with more severely blunted NAc reactivity to nondrug rewards may smoke a greater volume of cigarettes in an attempt to normalize reward function.
To explore relationships between volume of substance use and NAc reactivity to nondrug reward in adults, we examined NAc reactivity to monetary reward during an incentive-processing task collected by the Human Conectome Project (HCP).18 Specifically, we examined the relationship between volume of tobacco use and NAc reactivity to reward in cigarette smokers. Further, to determine if the relationship between substance use and NAc reactivity to reward is specific to nicotine, we also determined whether a similar relationship exists between reward reactivity and alcohol use severity, another commonly used, legal substance. To confirm blunted NAc reactivity to reward in substance users, NAc reactivity to reward in nicotine and alcohol groups were compared to a group of matched drug naïve controls. Given nicotine’s unique ability to increase reward system sensitivity,14–16 we hypothesize that the relationship between NAc reactivity to reward and volume of use will found in nicotine and not alcohol users.
Methods
Participants
Data analyzed were obtained from the 500 subjects release of the HCP database. A detailed description of the recruitment for the HCP is provided by Van Essen et al.18 Briefly, individuals were excluded by the HCP if they reported a history of psychiatric disorder, neurological disorder, or medical disorder known to influence brain function. Inclusion/exclusion criteria were determined using a detailed questionnaire developed explicitly for the HCP followed by the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA).19
Nineteen smokers (14 female), 23 alcohol users (15 female), and 19 nondrug users (14 female) were included. All demographic characteristics are listed in Table 1. Participants were excluded if they gave positive urine sample indicating use of any illicit drugs, provided a breath sample indicating >0.05 blood alcohol content, reported current or lifetime DSM-IV disorder aside from alcohol dependence (for the alcohol group), or reported a sum of >5 current and lifetime depressive symptoms.
Table 1.
Demographics by Group
| Group | Sex | Age | Education | Cigarettes/day | Drinks/wk | ||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | ||
| Nicotine | 5M, 14F | 30.42 | 3.45 | 13.74 | 1.73 | 10.02 | 4.57 | 5.63 | 7.82 |
| Alcohol | 8M, 15F | 28.04 | 3.33 | 15.57 | 1.59 | 0 | 0 | 12.3 | 5.3 |
| Drug naïve | 5M, 14F | 29.95 | 3.39 | 14.53 | 1.74 | 0 | 0 | 0 | 0 |
Inclusion criterion for the nicotine group was a self-report of smoking at least 5 cigarettes/day in the past 7 days. Participants reported smoking an average of 10.02 cigarettes/day (SD = 4.57, range 5–20). Alcohol use was not exclusionary, and was controlled for in the analyses. However, no individuals in the nicotine group met DSM-IV criteria for alcohol dependence.
Inclusion criterion for the alcohol group was a self-report of drinking behaviors that exceed “low-risk” as defined by the National Institute on Alcohol Abuse and Alcoholism.20 The National Institute on Alcohol Abuse and Alcoholism defines low-risk drinking as no more than 7 drinks/wk or three drinks on a single day for women, and no more than 14 drinks/wk or four drinks on single day for men. These participants reported drinking an average of 12.21 drinks/wk (SD = 5.21, range 7–25). No participants in this cohort reported current nicotine use of any form and they were excluded if they reported >20 lifetime tobacco uses.
Participants in the nondrug group were matched to the nicotine group for age, sex, and race. Participants were excluded if they reported any instances of prior nicotine use, reported drinking >0 alcoholic drinks in the past week, reported any prior illicit drug use, tested positive for illicit drug use, or reported any current or lifetime symptoms of depression.
fMRI Data Acquisition
All imaging data were acquired with a 32-channel head coil on a Siemens 3T Skyra modified to achieve a maximum gradient strength of 100 mT/m.18 Whole brain echo planar imaging images were acquired with the following parameters: repetition time = 720 ms, echo time = 33.1 ms, flip angle = 52°, bandwidth = 2290 Hz/Px, in-plan field of view = 208 × 180 mm, 72 slices, 2-mm isotropic voxels, with a multi-band acceleration factor of 8.21,22 Two runs of each task were acquired, one with right-to-left and the other left-to-right phase encoding. Scanner modifications and data acquisition parameters are described in detail in Urgurbil et al.23 At the time of acquisition, all smokers were at least 1-hour abstinent.
fMRI Data Preprocessing
The “minimally preprocessed” Quarter 3 release of the HCP data was used for this study.24 These data were preprocessed by the HCP Functional pipeline v2.0 consisting of tools from FSL and FreeSurfer. Preprocessing steps included gradient unwarping, motion correction, fieldmap-based echo planar imaging distortion correction, brain-boundary-based registration of echo planar imaging to structural T1-weighted scan, nonlinear registration into MNI152 space using FNIRT, and grand-mean intensity normalization.24
Data from the HCP were further preprocessed using tools from FSL 5.0.6.25 Data were re-registered to subject specific T1-wighted scans, spatially smoothed with a 4-mm full-width half-maximum Gaussian kernel and a high-pass temporal filter with Gaussian-weighted least-squares straight-line fitting with σ = 100 s was applied.
Experimental Task Design
The incentive-processing task conducted by the HCP was adapted from Delgado and colleagues.26 In this task, participants were presented with a card of an unknown number, denoted by a “?”. They were told that potential card numbers ranged from 1 to 9, and were instructed to indicate if the number of the card was greater or less than 5 by pressing one of two buttons on the response box. Although participant responses were irrelevant, they believed they were monetarily “rewarded” or “punished” for the accuracy of their response.
Feedback on each response consisted of either (1) a green up arrow and “$1” for reward trials, (2) a red down arrow and “$-0.50” for loss trials, or (3) a grey double sided arrow and the number “5” for neutral trials. Two 2 minutes 52 second runs of the task were acquired with four 28-second active blocks interleaved with four 15-second fixation blocks. Each active block consisted of eight 3.5-second trials with presentation of the “?” for 1.5 seconds, feedback for 1.0 second, and a 1.0-second fixation cross ITI. Each active block consisted of eight trials, such that blocks included primarily reward or primarily loss trails. That is, reward blocks consisted of six reward trials pseudo randomly interleaved with two out-of-set trials (one neutral and one loss trial, two neutral trials, or two loss trials). Similarly, loss blocks consisted of six loss trials pseudo randomly interleaved with two out-of-set trials (one reward and one neutral trial, two neutral trials, or two reward trials). All participants were provided with a standardized amount of money as a result of completing the task.
fMRI Task Data Analysis
First-level analyses were conducted separately on each of the participant’s two incentive processing task runs. The first-level general linear model included a regressor corresponding to either reward or punishment feedback presentation. This regressor was convolved with the gamma hemodynamic response function. Confound regressors were included to model motion effects (x, y, z translation and rotation motion). Contrasts were conducted between baseline (fixation) and reward conditions, baseline (fixation) and punishment conditions, and punishment and reward conditions. First-level results were combined across runs. Beta weights for the right NAc and left NAc regions of interest were extracted from second level reward > baseline contrasts for each participant. NAc regions of interest were defined using the Oxford/Harvard Atlas (www.cma.mgh.harvard.edu).
Statistical Analyses
To determine if a relationship exists between volume of substance use and NAc reactivity to monetary reward, two-tailed Pearson’s correlations were performed separately within the nicotine and alcohol use groups. Correlations were performed between average activation in the right NAc and left NAc during reward blocks and cigarettes/day in the nicotine group and between the NAc activation and average drinks/wk in the alcohol use group. We used a partial correlation to control for alcohol use in the nicotine group. A one-way between-groups analysis of variance was used to examine group differences in NAc reward reactivity between the drug naïve group, nicotine group, and alcohol group.
Results
Participant Demographics
All demographics and demographic comparisons are listed in Table 1.
Nicotine Group
Seven of 19 participants reported drinking 0 drinks/wk, seven reported drinking ≤6 drinks/wk, and five reported drinking between 13–22 drinks/wk. None of these participants met DSM-IV criteria for alcohol dependence. Although the HCP does not provide exact age of smoking onset, four participants began smoking before the age of 14, 10 began smoking between the ages of 15 and 17, and five began smoking between the ages of 18 and 20. All smokers have used nicotine daily for 6 years, 13 have used for >10.
Alcohol Group
One of 23 participants in the alcohol group met criteria for DSM-IV alcohol dependence. Five participants endorsed between 1–19 lifetime tobacco uses and 18 reported smoking 0 lifetime cigarettes.
NAc Correlations and Group Differences
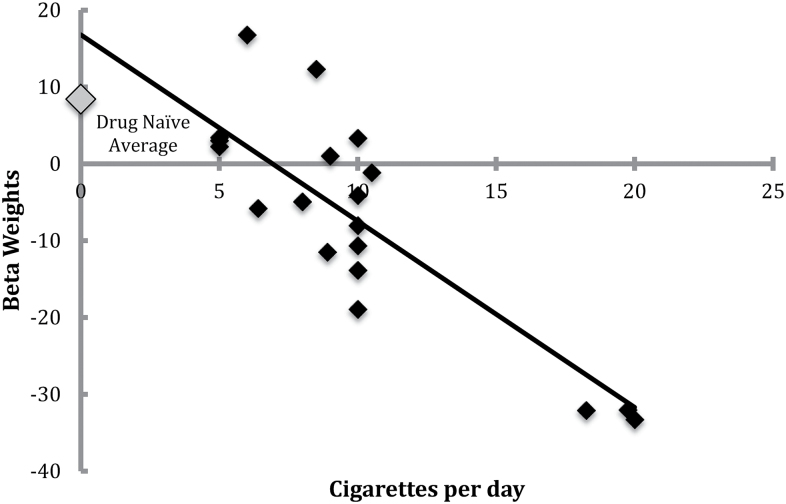
In line with our a priori hypothesis, right NAc reactivity to reward versus baseline was negatively correlated with self-report of cigarettes/day in the nicotine use group (r = −0.853, p < .001, Figure 1). When controlling for drinks/wk, the relationship between right NAc reactivity to reward versus baseline remained significant (r = −0.851, p ≤ .001).
Figure 1.
Right accumbens activation to reward in smokers.
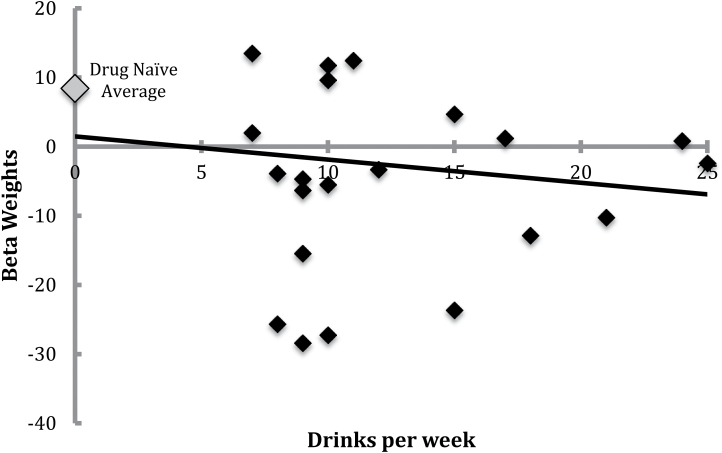
There was no relationship between right NAc reactivity to reward versus baseline and drinks/wk in the alcohol group (r = −0.070, p = .750, Figure 2).
Figure 2.
Right accumbens activation to reward in alcohol drinkers.
There were no relationships between left NAc reactivity to reward versus baseline and cigarettes/day in the nicotine group (r = −0.331, p = .166) or drinks/wk in the alcohol group (r = −0.295, p = .172).
One-way between-groups analysis of variance revealed significant differences in right NAc reactivity to reward versus baseline among groups (F(2,58) = 5.74, p = .005). Post hoc comparisons using Tukey honest significant difference test indicated that the drug naïve group (M = 8.41, SD = 14.85) had greater right NAc reactivity to monetary reward than both the nicotine group (M = −7.06, SD = 14.19) and alcohol group (M = −2.95, SD = 14.84). Post hoc tests revealed that right NAc reactivity did not differ between the nicotine (M = −7.06, SD = 14.19) and alcohol (M = −2.95, SD = 14.84) groups.
A second one-way between-groups analysis of variance revealed significant differences in left NAc activity to reward versus baseline among groups (F(2,58) = 3.42, p = .040). Post hoc comparisons using Tukey honest significant difference test indicated that the drug naïve group (M = 4.24, SD = 12.66) had greater left NAc reactivity to monetary reward than the alcohol group (M = −6.19, SD = 13.80). Left NAc reactivity did not differ between the nicotine group (M = −4.36, SD = 13.86) and the drug naïve (M = 4.24, SD = 12.66) and alcohol (M = −6.19, SD = 13.80) groups.
Discussion
It is well understood that blunted reward reactivity plays a central role in addiction.2,5,9–13 The current results clarify that role by demonstrating that while both nicotine users and alcohol users demonstrated blunted NAc reactivity to reward relative to the nondrug using group, severity of blunted right NAc reward reactivity is associated with volume of cigarette, but not volume of alcohol use. Furthermore, the laterality observed in these results is consistent with literature identifying the right NAc’s involvement in anticipation27 and receipt28 of rewards. Together, these findings suggest that although NAc reactivity to reward is comparable among individuals who use nicotine and alcohol, the relationship between NAc reactivity and volume of use is specific to nicotine use.
One explanation for the nicotine specific relationship between severity of blunted NAc reactivity and volume of use is that nicotine transiently increases sensitivity to nondrug rewards,14 whereas alcohol does not.17 While blunted reward reactivity is present in both nicotine and alcohol users, smokers may be using nicotine to normalize blunted reactivity to nondrug rewards. Moreover, smokers with greater NAc dysfunction may smoke greater volumes of nicotine to achieve that goal. Further, we found a significant association between NAc reactivity to nondrug reinforcers in chronic, adult smokers. Although the HCP data did not allow us to examine exact age of onset or length of tobacco use, there were no relationships between NAc reactivity and binned estimates of either time variable. This point is important, as others have linked volume of use to NAc function in early onset adolescent smokers12 and demonstrated that individuals with history of depression-related reward dysfunction begin smoking at an earlier age.29 While blunted reward function may increase the likelihood of early nicotine use,12,29 the current results show that blunted NAc reactivity may also play a role in determining lifetime smoking patterns after chronic nicotine use, regardless of age of onset.
The present finding that no relationship exists between NAc function and alcohol volume suggests that motivational factors other than enhancing nondrug related reward are driving alcohol use in moderate to heavy drinkers. Twenty-two of 23 participants did not meet criteria for alcohol dependence, suggesting that additional work examining individuals that meet for alcohol dependence is necessary to confirm these findings in heavier users. Furthermore, future studies should examine men and women separately given sex differences in criteria for moderate to heavy alcohol use. Although alcohol did not impact the relationship between cigarette use and NAc reactivity in our sample of smokers, alcohol and nicotine are commonly used in combination.30 We were not able to separately assess the relationship between reward function and patterns of use in individuals who use both alcohol and nicotine heavily. Conversely, findings should be replicated in a smoking cohort that does not report any alcohol use.
In addition to evaluating the relative valence of a rewarding stimulus, the NAc is involved in anticipating and responding to the relative salience of a stimulus.31–33 Valence, or magnitude of pleasurable hedonic impact, is the most commonly explored aspect of reward. However several theories of reward function suggest that reward is not a unitary construct, instead proposing that factors such as a reward salience also play an important role.31 It is likely that blunted reward reactivity is in part, due to decreased salience of nondrug related rewards. In fact, examination of the punishment versus baseline contrast demonstrated a marginally significant relationship between cigarettes/day and NAc reactivity to loss of money (r = −0.448, p = .055). While these results suggest that the relationship between NAc reactivity and volume of nicotine use is not specific to positively valenced stimuli, further investigation using a task designed to examine valence and salience is needed to clarify if blunted reward reactivity in nicotine users is related to a specific component of reward.
There are several limitations to the present study. Due to our correlational study design, we are not able to directly examine if blunted NAc reactivity causes individuals to smoke a higher volume of cigarettes or vice versa. The work of Peters and colleagues12 provides evidence to support that NAc reactivity influences smoking, as they examined adolescents before chronic nicotine exposure. However, directly testing this hypothesis should be considered in further work. As our ability to evaluate daily nicotine use was limited by the variables provided by the HCP, future studies would benefit from examining further aspects of nicotine use beyond number of cigarettes/day. Furthermore, while all tobacco users were abstinent for at least 1 hour before beginning the task we were not able to confirm the specific length of abstinence. While we can speculate that heavier smokers were more likely to smoke up until the study and during breaks, variability in length of abstinence and withdrawal severity may be related to blunted reward reactivity. In the present analyses we did not examine sex differences due to relatively limited sample size, thus we cannot rule out sex as a possible confounding factor. Finally, the interpretation of these findings is somewhat limited due to our small sample size and inability to adjust for sex differences in alcohol use severity, and should be considered preliminary.
Conclusion
The observed relationship between blunted right NAc reactivity to reward and volume of cigarette use, but not alcohol use suggests that nicotine’s unique ability to enhance nondrug reward sensitivity may play a role in severity of nicotine use. While prior work has implicated dysfunctional reward processing in addictions, these findings clarify a substance-specific role that blunted reward function has in determining patterns of use among chronic users.
Funding
This work was supported by the National Institute on Drug Abuse (grant number K01 DA029645) to ACJ.
Declaration of Interests
None declared.
Acknowledgments
Data were provided (in part) by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
References
- 1. Blum K, Braverman ER, Holder JM. Reward deficiency syndrome: a biogenic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. 2000;32(suppl 1):1–112. doi:10.1080/02791072.2000.10736099 [DOI] [PubMed] [Google Scholar]
- 2. Bühler M, Vollstädt-Klein S, Kobiella A, et al. Nicotine dependence is characterized by disorder reward processing in a network driving motivation. Biol Psychiatry. 2010;67(8):745–752. doi:10.1016/j.biopsych.2009.10.029 [DOI] [PubMed] [Google Scholar]
- 3. Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. doi:10.1176/appi.ajp.162.8.1403 [DOI] [PubMed] [Google Scholar]
- 4. Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsycho pharmacology. 2010;35(8):217–238. doi:10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Versace F, Lam CY, Engelmann JM, et al. Beyond cue reactivity: blunted brain responses to pleasant stimuli predict long-term smoking abstinence. Addict Biol. 2012;17(6):991–1000. doi:10.1111/j.1369-1600.2011.00372.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Volkow ND, Wang G, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm, the brain’s control circuit. Bioessays. 2010;32(9):748–755. doi:10.1002/bies.201000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. David SP, Munafò MR, Johansen-Berg H, et al. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58(6):488–494. doi:10.1016/j.biopsych.2005.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159(6):954–960. doi:10.1176/appi.ajp.159.6.954 [DOI] [PubMed] [Google Scholar]
- 9. Garavan H, Pankiewicz J, Bloom A, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157(11):1789–1798. doi:10.1176/appi.ajp.157.11.1789 [DOI] [PubMed] [Google Scholar]
- 10. Powell JH, Pickering AD, Dawkins L, West R, Powell JF. Cognitive psychological correlates of smoking abstinence and predictors of successful cessation. Addict Behav. 2004;29(7):1407–1426. doi:10.1016/j.addbeh.2004.06.006 [DOI] [PubMed] [Google Scholar]
- 11. Wrase J, Schlagenhauf F, Kienast T, et al. Dysfunction of reward processing correlated with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35(2):787–794. doi:10.1016/j.neuroimage.2006.11.043 [DOI] [PubMed] [Google Scholar]
- 12. Peters J, Bromberg U, Schneider S, et al. Lower ventral striatal activation during reward anticipation in adolescent smokers. Am J Psychiarty. 2011;168(5):540–549. [DOI] [PubMed] [Google Scholar]
- 13. Rose EJ, Ross TJ, Salmeron B, et al. Chronic exposure to nicotine is associated with reward-related activity in the striatum but not the midbrain. Biol Psychiatry. 2012;71(3):206–213. doi:10.1016/j.biopsych.2011.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biol Psychiatry. 2008;63(11):1061–1065. doi:10.1016/j.biopsych.2007.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I-Effects on incentive motivation. Psychopharmcology. 2006;189(3):355–367. doi:10.1007/s00213-006-0588-8 [DOI] [PubMed] [Google Scholar]
- 16. Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31(6):1203–1211. doi:10.1038/sj.npp.1300905 [DOI] [PubMed] [Google Scholar]
- 17. Schulteis G, Liu J. Brain reward deficits accompany withdrawal (hangover) from acute ethanol in rats. Alcohol. 2006:39(1):21–28. doi:10.1016/j.alcohol.2006.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Essen DC, Ugurbil K, Auerbach et al. The human connectome project: A data acquisition perspective. Neuroimage. 2012;62(4):2222–2231. doi:10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bucholz KK, Cadoret R, Cloninger CR, et al. A new, semi-structured psychiatric interview for using in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994:55(2):149–158. doi:10.15288/jsa.1994.55.149 [DOI] [PubMed] [Google Scholar]
- 20. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/ moderate-binge-drinking Accessed February 16, 2016.
- 21. Feinberg DA, Moeller S, Smith SM, et al. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS One. 2010:5(12):e15710. doi:10.1371/journal.pone.0015710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moeller S, Yacoub E, Olman CA, et al. Multiband multislice GE-EPI at 7 tesla, with 16-Fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain FMRI. Magn Reson Med. 2009:66:1144–11153. doi:10.1002/mrm.22361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ugurbil K, Xu J, Auerbach EJ, et al. Pushing spatial and temporal resolution for functional and diffusion MRI in the human connectome project. Neuroimage. 2013;80:80–104. doi:10.1016/j.neuroimage.2013.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Glasser MF, Sotiropoulos SN, Wilson JA, et al. The minimal preprocessing pipelines for the human connectome project. Neuroimage. 2013;80:105–124. doi:10.1016/j.neuroimage.2013.04.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. FMRIB Software Library. Analysis Group. Oxford, UK: FMRIB; www.fmrib.ox.ac.uk/fsl. [Google Scholar]
- 26. Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84(6):3072–3077. [DOI] [PubMed] [Google Scholar]
- 27. Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(16):RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martin-Soelch C, Szczepanik J, Nugent A, et al. Lateralization and gender differences in the dopaminergic response to unpredictable reward in the human ventral striatum. Eur J Neurosci. 2011;33(9):1706–17175. doi:10.1111/j.1460-9568.2011.07642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Janes AC, Pedrelli P, Whitton AE, et al. Reward responsiveness varies by smoking status in women with a history of major depressive disorder. Neuropsychopharmacology. 2015;40;1940–1946. doi:10.1038/npp.2015.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Batel P, Pessione F, Maître C, Rueff B. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction. 1995;90(7):977–980. doi:10.1046/j.1360-0443.1995.90797711.x [DOI] [PubMed] [Google Scholar]
- 31. Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28(3):309–369. doi:10.1016/S0165-0173(98)00019-8 [DOI] [PubMed] [Google Scholar]
- 32. Cooper JC, Knutson B. Valence and salience contribute to nucleus accumbens activation. Neuroimage. 2008;39(1):538–547. doi:10.1016/j.neuroimage.2007.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zink CF, Pagnoni G, Martin ME. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003;23(22):8092–8097. [DOI] [PMC free article] [PubMed] [Google Scholar]