Abstract
Background: Signaling via the Insulin‐like Growth Factor type 1 Receptor (IGF1R) plays a crucial role in cancer development. In breast cancer (BC), IGF1R and estrogen receptor expression are correlated. In this current study we explored the hypothesis that postmenopausal hormone receptor positive (HR+ve) BC patients with high IGF1R tumor expression still have estrogen driven IGF1R stimulated tumor growth when treated with tamoxifen, resulting in detrimental clinical outcome compared to patients treated with exemestane. Additionally, we assessed the added value of metformin as this drug may lower IGF1R stimulation.
Methods: Of 2,446 Dutch TEAM patients, randomized to either exemestane for 5 years or sequential treatment (tamoxifen for 2–3 years followed by exemestane for another 3–2 years) tumor tissue microarray sections were immunohistochemically stained for IGF1R. Overall Survival (OS), Breast Cancer specific Survival (BCSS) and Relapse‐Free Survival (RFS) were assessed in patient subgroups with low and high IGF1R expression, and in patients with or without metformin use.
Results: High IGF1R tumor expression was significantly associated with exemestane therapy for RFS (Hazard Ratio (HR) 0.74, 95% Confidence Interval (CI) 0.58–0.95, p = 0.02). In addition, the combination of metformin with exemestane resulted in improved efficacy, yielding a 5‐yrs RFS of 95% (HR 0.32, 95% CI 0.10–1.00, p = 0.02, compared to sequential treatment). No relation was observed in tumors with low IGF‐1R expression.
Conclusion: This study suggests IGF1R as a potential biomarker of improved clinical outcome in HR+ve BC patients treated with exemestane. Adding metformin to exemestane treatment may add to this effect.
Keywords: Breast cancer, Clinical outcome, IGF1 receptor, Hormone receptor, Endocrine treatment, Metformin
Highlights
Breast tumor tissue sections from Dutch TEAM patients were immunohistochemically stained for IGF1R status.
Part of this cohort was using metformin because of Diabetes type 2.
Breast cancer patients on exemestane harboring high IGF1R tumor expression had significantly less breast cancer relapse.
The addition of metformin to exemestane treatment resulted in improved efficacy.
Dual blockade of insulin‐ and estrogen‐related growth pathway may improve outcome in selective breast cancer patients.
1. Introduction
Breast cancer (BC) is still the most frequent cause of cancer related death in women in developed countries, and marks one of the leading health problems worldwide (Ferlay et al., 2010; Jemal et al., 2011; Parkin et al., 2002). Over the past decades, a substantial reduction in BC related mortality has been observed, mostly due to mayor advances in (neo‐)adjuvant systemic treatment (Berry et al., 2005; Kim et al., 2005; Pritchard, 2005; Tria, 2013; Viale et al., 2008). Decisions regarding optimal treatment of breast cancer patients are largely based on prognostic and predictive markers. However, the various currently used classical markers do not provide optimal risk stratification, hampering further personalization of therapy.
Estrogen receptor (ER) expression is present in approximately 65–75% of all postmenopausal breast cancers (Hammond et al., 2010). Anti‐estrogens, such as tamoxifen, are known to inhibit cell proliferation and disease progression by competitive blocking of estrogen binding to the ER, whereas aromatase inhibitors (AIs) act by blocking the estrogen biosynthesis via aromatase inhibition in postmenopausal women, thus lowering the already low postmenopausal estrogen levels.
It is known that signaling via the Insulin‐like Growth Factor type 1 Receptor (IGF1R) plays a crucial role in the development of many cancers, including BC, by influencing cellular proliferation, cell survival, invasion and metastatic behavior (Hartog et al., 2007; Pollak, 2008). It has been shown that IGF1R expression is correlated with the expression of the ER (Happerfield et al., 1997; Winder et al., 2014). IGF1R has been shown to be up‐regulated in tamoxifen‐resistant BC, which retained the tamoxifen antagonism of classical ER genomic function (Massarweh et al., 2008). Subsequently, a study performed by Song et al. has shown that 17β‐Estradiol, although to a lesser extent than IGF1, can activate a linear pathway involving the activation of IGF1R, which subsequently leads to a boost of the mitogen‐activated protein kinase (MAPK) (Song et al., 2007; Richards et al., 1996; Song et al., 2004). Patients treated with an AI could lose this additional tumor growth‐stimulating pathway due to complete blockage of estrogen production, independent of IGF1 stimulation.
Another drug that may be of interest in relation to the IGF1R is metformin, which has long been known for lowering plasma insulin and insulin growth factor levels by increasing insulin sensitivity (Giugliano et al., 1993). Several observational studies have suggested that metformin may be beneficial in BC treatment (Jiralerspong et al., 2009; Kiderlen et al., 2013). It could be postulated that an additional effect of metformin treatment in BC patients with high IGF1R expression could be observed, by means of lowering direct IGF1R stimulation.
Therefore, in the current analyses we explored the hypothesis that postmenopausal hormone‐receptor positive (HR+ve) early BC patients with high IGF1R tumor expression treated with tamoxifen still have estrogen driven IGF1R stimulated tumor growth, resulting in detrimental clinical outcome compared to patients treated with the AI exemestane. In addition, the combined effect of endocrine therapy with metformin use on clinical outcome in both IGF1R positive and IGF1R negative HR+ve postmenopausal BC patients was explored.
2. Material and methods
2.1. Patients and tumors
For this study, intra‐operative breast tumor samples of Dutch patients participating in the Tamoxifen and Exemestane Adjuvant Multicenter trial (TEAM) (n = 2,764) were used. All patients signed an informed consent form prior to enrollment in the TEAM study. Local ethics approval was received and the study was conducted in accordance with the Declaration of Helsinki.
The TEAM study is a randomized, open‐label, phase III trial, conducted in postmenopausal women with early stage ER and/or progesterone receptor‐positive BC, who were eligible for adjuvant endocrine treatment. Patients were randomly assigned to receive either exemestane 25 mg once daily for 5 years or tamoxifen 20 mg once daily for 2.5–3 years, followed by exemestane 25 mg once daily for 2.5–2 years (sequential regimen) (van de Velde et al., 2011). All patients were diagnosed and treated between 2001 and 2006. For this sub‐study, patients with bilateral tumors or a history of another cancer within five years prior to inclusion in the TEAM study were excluded, with an exception for patients with basal cell carcinoma of the skin and cervical intraepithelial neoplasia.
For all patients included in this study the following data was retrieved from the central TEAM database at the datacenter of the Leiden University Medical Center: age at diagnosis, histological tumor grade (classified as Grade I, II or III) and tumor type (ductal, lobular or “other”), ER and progesterone receptor status, pathological tumor and nodal stage, adjuvant treatment received, Body Mass Index (BMI), used co‐medication, date and type of loco‐regional/distant recurrence, and date and cause of death if relevant.
It should be noted that some differences were seen between the Dutch patients and the other patients in the TEAM trial. Most of these can be explained by differences in the number of patients with missing data. However, patients from the Netherlands presented with more advanced tumor stages than patients from other countries, as they had higher T‐ and N‐stages (web‐table 1). This probably also explains the difference in survival between the countries (web‐table 2).
2.2. Immunohistochemistry
All tumor samples were handled in a coded fashion, according to national ethical guidelines (“Code for Proper Secondary Use of Human Tissue”, Dutch Federation of Medical Scientific Societies). Immunohistochemical staining for IGF1R was performed on 4 μm tissue sections from FFPE tumor samples of the Dutch TEAM BC patients processed into a Tissue Micro Array (TMA, containing three 0.6 mm2 tumor tissue punches per patient) (Bartlett et al., 2011). The tissue sections were deparaffinized and rehydrated according to standard protocols (de Kruijf et al., 2010). Endogenous peroxidase activity was blocked with hydrogen peroxidase 0.3% in PBS for 20 min. Antigen retrieval was performed using a Pre Treatment (PT) module (PT link, DAKO, Denmark) in low pH buffer. Sections were incubated at room temperature over night with rabbit polyclonal antibody (1:50, diluted in 1% PBSA) directed against IGF‐I receptor β (#3027 Cell Signaling, BIOKÉ, Leiden, the Netherlands). The following day all TMA slides were washed in PBS and incubated with Envision anti‐rabbit (DAKO Cytomation K4003) for 30 min at room temperature. DAB was used for visualization of positively stained breast tumor tissue on the TMA and counterstained with haematoxylin, dehydrated and finally mounted with pertex. All slides were stained simultaneously to avoid inter‐assay variation. Placenta tissue served as positive‐ and negative‐control, the latter was obtained by omitting the primary antibody.
2.3. Evaluation of immunostaining
Microscopic quantification of positive tumor cells for the IGF1R antibody was performed in a blinded manner by two independent observers (CCE and AS). IGF1R expression was scored: 0 for no staining at all or membrane staining in <10% of the tumor cells; 1+ for a faint/barely perceptible partial membrane staining in >10% of the tumor cells; 2+ for weak to moderate complete membrane staining in >10% of the tumor cells; and 3+ for strong to complete membrane staining in >10% of the tumor cells (Figure 1). In accordance with previous studies, the highest score out of the three punches of the same tumor was used for statistical analyses (Hartog et al., 2011). If one or more punches were missing, the highest score of the remaining punch(es) was included for analyses. The Cohen's Kappa was 0.86, indicating substantial agreement between the two observers.
Figure 1.
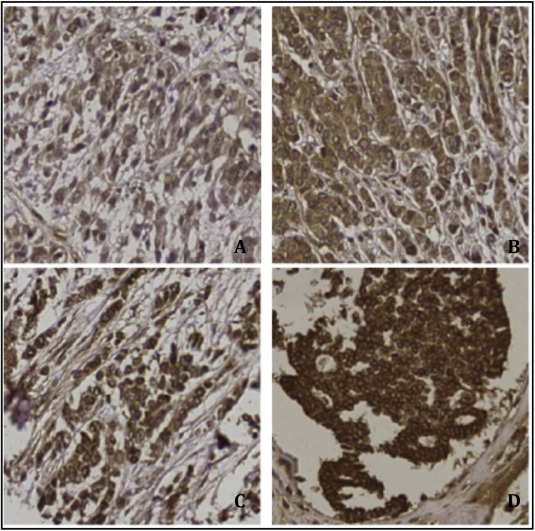
20× pictures of immunohistochemical IGF‐1R staining showing: A – no staining at all or membrane staining in <10% of the tumor cells (0). B – faint/barely perceptible partial membrane staining in >10% of the tumor cells (1+). C – weak to moderate complete membrane staining in >10% of the tumor cells (2+). D – strong to complete membrane staining in >10% of the tumor cells (3+).
2.4. Statistical analysis
Statistical analyses were performed using the statistical package SPSS (version 20.0 for Windows, IBM SPSS statistics). Hypotheses and analysis plan were drafted before the pathological data became available. Patients with missing data regarding IGF1R, due to material handling, were excluded from statistical analyses as it can be assumed that these data were “missing at random”. IGF1R scores were dichotomized: scores 0 and 1+ were considered IGF‐1R low, and scores 2+ and 3+ were considered IGF1R high (Vermeulen et al., 2012, 2013). The χ2 test was used to evaluate associations between various clinico‐pathological parameters and tumor IGF1R expression. The clinical endpoints examined were Overall Survival (OS), defined as the time from date of randomization in the TEAM‐trial until death by any reason; Breast Cancer Specific Survival (BCSS), defined as the time from date of randomization until death due to BC; and Relapse‐Free Survival (RFS), defined as the time from date of randomization until loco‐regional recurrence, contralateral BC, distant recurrence or BC death (whichever came first).
First, we assessed the relation between the two treatment regimens of the TEAM trial in patients with either high or low IGF1R expression. The Kaplan–Meier method was used to compose survival plots, and the log‐rank test was performed for comparison of OS, BCSS and RFS curves. Cox Proportional Hazard analyses were used to calculate corresponding Hazard Ratio's (HRs), using univariate analyses for OS, BCSS and RFS. Since the TEAM‐trial was randomized, no additional adjustments were made for these analyses.
Next, we assessed the relation between the type of adjuvant endocrine treatment with or without metformin use (subgroups: sequential endocrine treatment only, sequential endocrine treatment with metformin, exemestane only, and exemestane with metformin) in patients with either high or low IGF1R tumor expression using Cox Proportional Hazard Models. These analyses were additionally adjusted for clinically relevant confounders (including age at diagnosis, Body Mass Index (BMI), T‐stage, N‐stage and histological grade).
3. Results
3.1. Patient and tumor characteristics
Of the original Dutch TEAM cohort (n = 2,764), 2,446 postmenopausal, early hormone sensitive BC patients were included in the current analyses (116 patients were excluded because of history of malignancy within five years prior to inclusion, and 202 patients were excluded due to missing IGF1R‐status), as a result of sample errors. Clinico‐pathological and treatment characteristics of the selected patients are shown in Table 1. Median age at diagnosis was 64 years (range 38–91 years). Median follow‐up of patients who were alive was 5.4 years (range 0.1–8.7 years). The majority of the BCs had high IGF1R expression (n = 1,616, 66.0%). IGF1R expression was not significantly associated with any of the patient, tumor or treatment characteristics.
Table 1.
Patient characteristics.
| IGF‐1R low (n = 830) | IGF‐1R high (n = 1,616) | p‐Value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Age | |||
| <55 | 110 (13.3) | 221 (13.7) | 0.33 |
| 55–59 | 175 (21.1) | 321 (19.9) | |
| 60–64 | 145 (17.5) | 315 (19.5) | |
| 65–69 | 123 (14.8) | 277 (17.1) | |
| 70–74 | 130 (15.7) | 222 (13.7) | |
| ≥75 | 147 (17.7) | 260 (16.1) | |
| BMI | |||
| <20 | 23 (2.8) | 39 (2.4) | 0.81 |
| 20–24 | 253 (30.5) | 484 (30) | |
| 25–29 | 282 (34) | 559 (34.6) | |
| ≥30 | 176 (21.2) | 366 (22.6) | |
| Unknown | 96 (11.6) | 168 (10.4) | |
| T‐stage | |||
| T1 | 359 (43.3) | 733 (45.4) | 0.62 |
| T2 | 411 (49.5) | 781 (48.3) | |
| T3 | 35 (4.2) | 62 (3.8) | |
| T4 | 22 (2.7) | 38 (2.4) | |
| Missing | 3 (0.4) | 2 (0.1) | |
| N‐stage | |||
| N0 | 239 (28.8) | 517 (32) | 0.16 |
| N+ | 591 (71.2) | 1097 (67.9) | |
| Unknown | 0 (0) | 2 (0.1) | |
| Histological grade | |||
| Grade 1 | 130 (15.7) | 227 (14) | 0.06 |
| Grade 2 | 368 (44.3) | 717 (44.4) | |
| Grade 3 | 270 (32.5) | 586 (36.3) | |
| Unknown | 62 (7.5) | 86 (5.3) | |
| ER‐ and/or PR‐status | |||
| Negative | 3 (0.4) | 3 (0.2) | 0.33 |
| Positive | 827 (99.6) | 1613 (99.8) | |
| Most extensive surgery | |||
| No surgery | 0 (0) | 1 (0.1) | 0.77 |
| BCS | 380 (45.8) | 735 (45.5) | |
| Mastectomy | 450 (54.2) | 880 (54.5) | |
| Radiotherapy | |||
| No | 318 (38.3) | 623 (38.6) | 0.92 |
| Yes | 511 (61.6) | 990 (61.3) | |
| Unknown | 1 (0.1) | 3 (0.2) | |
| Chemotherapy | |||
| No | 592 (71.3) | 1119 (69.2) | 0.29 |
| Yes | 238 (28.7) | 497 (30.8) | |
| Randomisation | |||
| TAM → EXE | 402 (48.4) | 822 (50.9) | 0.26 |
| EXE | 428 (51.6) | 794 (49.1) | |
| Metformin user | |||
| No | 780 (94) | 1511 (93.5) | 0.65 |
| Yes | 50 (6) | 105 (6.5) | |
3.2. Stratified analyses for endocrine therapy and metformin use
After stratification of the cohort by IGF1R status, exemestane therapy was significantly associated with improved RFS in patients with high IGF1R tumor expression (HR for exemestane versus sequential therapy: 0.74, 95% Confidence Interval (CI) 0.58–0.95, p = 0.02) (Table 2 and Figure 2). In this cohort, OS and BCSS were not significantly related with either of the treatment arms, showing a HR of 0.83 (95% CI 0.66–1.04, p = 0.10) for OS, and a HR of 0.74 (95% CI 0.54–1.01, p = 0.06) for BCSS. However, it should be noted that both estimates were below one and the p‐value for BCSS approached statistical significance. In low IGF‐1R expressing tumors, no association between treatment and any of the outcomes was observed.
Table 2.
Survival.
| Patients | Overall survival | Breast cancer specific survival | Relapse‐free survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | HR | 95% CI | p‐Value | Events | HR | 95% CI | p‐Value | Events | HR | 95% CI | p‐Value | ||
| IGFR low | |||||||||||||
| TAM → EXE | 402 | 75 | Ref | 0.6 | 52 | Ref | 0.66 | 68 | Ref | 0.73 | |||
| EXE | 428 | 84 | 1.08 | (0.81–1.43) | 46 | 1.09 | (0.74–1.63) | 76 | 1.06 | (0.76–1.47) | |||
| IGFR high | |||||||||||||
| TAM → EXE | 822 | 142 | Ref | 0.1 | 92 | Ref | 0.06 | 142 | Ref | 0.02 | |||
| EXE | 794 | 118 | 0.83 | (0.66–1.04) | 67 | 0.74 | (0.54–1.01) | 105 | 0.74 | (0.58–0.95) | |||
Figure 2.
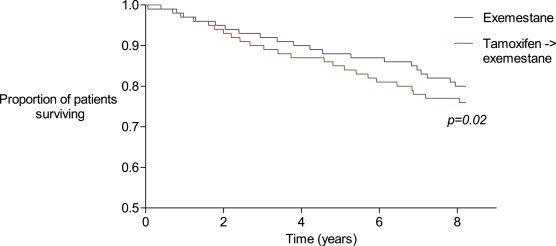
Relapse‐free survival of patients with high IGF1‐R expression.
Regarding metformin use in addition to the endocrine therapy, survival analyses showed no significant association in the patient population with low IGF1R tumor expression (Table 3). In contrast, in patients with high IGF1R expressing tumors, the combination of metformin with the endocrine treatment arm was significantly associated with RFS (HR 1.12, 95% CI 0.57–2.23 for sequential treatment with metformin, HR 0.73 95% CI 0.56–0.94 for exemestane only, and HR 0.32, 95% CI 0.10–1.00 for exemestane with metformin, p = 0.02, compared to the sequential treatment arm, Table 3). Although BCSS was not significantly associated with the combined therapies, the estimates were similar to the RFS outcomes. Ultimately, significant association was also seen for the OS (multivariable adjusted HR for OS 1.72, 95% CI 0.96–3.08 for sequential treatment with metformin, HR 0.80, 95% CI 0.62–1.03 for exemestane only, and HR 0.67, 95% CI 0.31–1.45 for exemestane with metformin, p = 0.03, compared to the sequential treatment arm, Table 3). It should be noted that in all analyses the number of events was low for the metformin users.
Table 3.
Metformin use and survival.
| Patients | Overall survival | Breast cancer specific survival | Relapse‐free survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Adjusteda | Events | Adjusteda | Events | Adjusteda | ||||||||
| HR | 95% CI | p‐Value | HR | 95% CI | p‐Value | HR | 95% CI | p‐Value | |||||
| IGFR low | |||||||||||||
| Tam only | 376 | 70 | Ref | 0.98 | 44 | Ref | 0.95 | 64 | Ref | 0.94 | |||
| Tam & metformin | 26 | 5 | 0.82 | (0.33–2.07) | 2 | 0.73 | (0.18–3.08) | 4 | 1.22 | (0.48–3.09) | |||
| Exe only | 404 | 81 | 0.99 | (0.71–1.37) | 50 | 1.04 | (0.68–1.62) | 72 | 1.06 | (0.75–1.49) | |||
| Exe & metformin | 24 | 3 | 1.07 | (0.42–2.70) | 2 | 0.8 | (0.19–3.39) | 4 | 1.28 | (0.50–3.26) | |||
| IGFR high | |||||||||||||
| Tam only | 775 | 131 | Ref | 0.03 | 87 | Ref | 0.19 | 133 | Ref | 0.02 | |||
| Tam & metformin | 47 | 11 | 1.72 | (0.96–3.08) | 5 | 1.11 | (0.44–2.77) | 9 | 1.12 | (0.57–2.23) | |||
| Exe only | 736 | 108 | 0.8 | (0.62–1.03) | 64 | 0.74 | (0.52–1.04) | 100 | 0.73 | (0.56–0.94) | |||
| Exe & metformin | 58 | 10 | 0.67 | (0.31–1.45) | 3 | 0.38 | (0.09–1.56) | 5 | 0.32 | (0.10–1.00) | |||
Adjusted for age, BMI, T‐stage, N‐stage, histological grade.
4. Discussion
This study showed a significantly improved RFS in patients with high IGF1R expression on their breast tumor surface treated with exemestane compared to sequential therapy. Additionally, our data suggested a further enhancement of the RFS when metformin was added to exemestane in these patients, although it must be noted that the number of events in patients who received metformin was low.
The findings of our analyses are interesting, and are in contrast with the main results of the TEAM‐trial, which showed no difference in OS, BCSS nor DFS for either one of the two treatment arms (van de Velde et al., 2011).
There may be several explanations for the observed benefit of exemestane in patients with high IGF1R expression. Evidence is building for a novel view that that estrogen can, next to binding and activating its classical receptor, the ER, also phosphorylate and activate the IGF1R (Song et al., 2007). In view of our results, we hypothesize that the interaction between the degree of IGF1R expression on the tumor surface and the efficacy of exemestane is mainly induced by the fact that exemestane, an aromatase inhibitor, suppresses estrogen production. Suppression of estrogen production could lead to reduced estrogen induced activation of IGF1R and thus less activation of the mitogen‐stimulating pathway. Since this ultimately leads to less proliferation of the BC cells, this can translate into a clinical benefit for the high IGF1R expressing, hormone sensitive BC patients. This hypothesis also supports our finding that patients with high IGF1R expression who were treated with tamoxifen (an ER blocker) for the first 2.5 years following local therapy did not experience clinical benefit, as the unaffected levels of circulating estrogens can still phosphorylate the IGF1R, thereby stimulating breast cancer cell growth. The fact that no clinical benefit of exemestane treatment was observed in patients with tumors harboring low IGF1R expression also supports our proposed hypothesis, as the effect of estrogen induced tumor growth promoting signaling by IGF1R is too small in these tumors.
When metformin was added to the endocrine treatment received, an additional significant benefit was seen with respect to the clinical outcome parameters OS and RFS for patients treated with exemestane and metformin, and non‐significant similar estimates were seen for BCSS in patients treated with exemestane and metformin. However, these results must be interpreted with caution, as the number of events was small in patients who were treated with metformin. However, these findings support our hypothesis concerning inhibition of the IGF‐1 pathway, as metformin induces lowering of insulin and IGF concentrations (Charles and Eschwege, 1999). Thus, we propose that metformin induced lowering of the IGF concentration leads to direct loss of IGF1R stimulation. Therefore, our hypothesis states that patients with high IGF1R expression on their tumor surface treated with both exemestane and metformin will encounter dual blockage of IGF1R activation, thus blocking both estrogen‐driven as well as insulin‐driven IGF1R activation. This study showed that dual blockage of the IGF1R results in better clinical outcome. These findings are promising, as several previous observational studies have shown benefits of metformin treatment in cancer patients (Jiralerspong et al., 2009; Lega et al., 2013). By stratifying patients according to IGF1R expression of the tumor, which is up‐regulated in roughly two‐thirds of the postmenopausal breast cancer population and thus widely applicable, it may become possible to identify a subgroup of patients who may benefit of these combined treatments, thereby further individualizing treatment and improving outcomes for particular subgroups within the heterogeneous BC population. Of course, our interesting and promising results need first to be confirmed in other large studies containing HR+ve BC patients treated with AI, such as, for example the ATAC, BIG, or IES study, all with tumor material available, or preferably in a randomized trial setting, before they can be implemented in clinical practice. To our knowledge, there are no ongoing trials that specifically assess the value of metformin added to treatment with an AI, nor are there trials that assess the benefit of AI in relation to IGF1R expression.
The main strength of this study is the fact that we clearly defined hypotheses before data collection and analyses. Biomarker substudies of clinical trials frequently “search” for significant associations between many different subgroups or biomarkers, and present the significant associations only. Although the current study was not a prospectively planned subgroup analysis, it can still be considered a major strength of this study that we only assessed the IGF1R receptor and formulated hypotheses before data collection. Secondly, to our knowledge this is the first study that assessed the relation between adjuvant endocrine therapy in relation to IGF1R expression on the tumor surface of postmenopausal HR+ve, early BC patients. Furthermore, no previous studies assessed the added benefits of metformin in relation to IGF1R expression. Another major strength of this study is the use of data from the TEAM‐trial, as this provides well‐registered data in a large number of patients.
This study, however, also has its limitations. First, there were no uniform cut‐off values for IGF1‐R expression available from previous literature. Therefore, we categorized patients by defining a moderate to strong expression in >10% of the tumor cells as high IGF‐1R expression, in line with e.g. categorization of endocrine receptor positivity. Furthermore, patients on sequential hormonal therapy received exemestane after the first 2.5 years of tamoxifen treatment. It would be desirable to compare two endocrine treatment regimens, consisting of solely exemestane and solely tamoxifen given for five consecutive years. Also, metformin use was not randomized in this trial, which makes that these analyses must be interpreted with caution, as they may be subjected to confounding by indication. However, RFS and BCSS in relation to metformin use can be considered as unintended effects of metformin, and therefore we believe that it is possible to assess this relation in this study. Furthermore, it is plausible that the patients using metformin in this study are diabetics. Therefore, it is unclear whether the results concerning the effect of metformin in this specific population can be extrapolated to the non‐diabetic population. Finally, the relatively small number of events for BCSS may be considered as a limitation, but the estimates for BCSS strongly resembled the estimates for RFS. Especially for the analyses where the additional value of metformin on clinical outcome was assessed, the small number of events was a strong limitation of this study.
In conclusion, these study results add to the ongoing discussion of the value of optimal endocrine treatment as well as metformin use in BC patients, as it appears that high IGF1R expression on the breast tumor surface is a potential biomarker of improved clinical outcome in HR+ve BC patients treated with exemestane. Combining metformin with exemestane may further add to this effect.
Funding
This study was funded by the Dutch Cancer Foundation (KWF 2007‐3968).
Supporting information
The following are the supplementary data related to this article:
Supplementary data
Supplementary data
Acknowledgments
We would like to thank Professor John MS Bartlett from the Ontario Institute of Cancer Research, Toronto, Canada, for providing us with the Dutch TEAM breast cancer research material.
Supplementary data 1.
1.1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2015.10.010.
Engels Charla C., de Glas Nienke A., Sajet Anita, Bastiaannet Esther, Smit Vincent T.H.B.M., Kuppen Peter J.K., Seynaeve Caroline, van de Velde Cornelis J.H., Liefers Gerrit Jan, (2016), The influence of insulin‐like Growth Factor‐1‐Receptor expression and endocrine treatment on clinical outcome of postmenopausal hormone receptor positive breast cancer patients: A Dutch TEAM substudy analysis, Molecular Oncology, 10, doi: 10.1016/j.molonc.2015.10.010.
References
- Bartlett, J.M. , Brookes, C.L. , Robson, T. , 2011. Estrogen receptor and progesterone receptor as predictive biomarkers of response to endocrine therapy: a prospectively powered pathology study in the tamoxifen and exemestane adjuvant multinational trial. J. Clin. Oncol. 29, 1531–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry, D.A. , Cronin, K.A. , Plevritis, S.K. , 2005. Effect of screening and adjuvant therapy on mortality from breast cancer. N. Engl. J. Med. 353, 1784–1792. [DOI] [PubMed] [Google Scholar]
- Charles, M.A. , Eschwege, E. , 1999. Prevention of type 2 diabetes: role of metformin. Drugs. 58, (Suppl. 1) 71–73. [DOI] [PubMed] [Google Scholar]
- de Kruijf, E.M. , van Nes, J.G. , Sajet, A. , 2010. The predictive value of HLA class I tumor cell expression and presence of intratumoral tregs for chemotherapy in patients with early breast cancer. Clin. Cancer Res. 16, 1272–1280. [DOI] [PubMed] [Google Scholar]
- Ferlay, J. , Shin, H.R. , Bray, F. , Forman, D. , Mathers, C. , Parkin, D.M. , 2010. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 127, 2893–2917. [DOI] [PubMed] [Google Scholar]
- Giugliano, D. , De, R.N. , Di, M.G. , 1993. Metformin improves glucose, lipid metabolism, and reduces blood pressure in hypertensive, obese women. Diabetes Care. 16, 1387–1390. [DOI] [PubMed] [Google Scholar]
- Hammond, M.E. , Hayes, D.F. , Wolff, A.C. , Mangu, P.B. , Temin, S. , 2010. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Oncol. Pract. 6, 195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happerfield, L.C. , Miles, D.W. , Barnes, D.M. , Thomsen, L.L. , Smith, P. , Hanby, A. , 1997. The localization of the insulin-like growth factor receptor 1 (IGFR-1) in benign and malignant breast tissue. J. Pathol. 183, 412–417. [DOI] [PubMed] [Google Scholar]
- Hartog, H. , Wesseling, J. , Boezen, H.M. , van der Graaf, W.T. , 2007. The insulin-like growth factor 1 receptor in cancer: old focus, new future. Eur. J. Cancer. 43, 1895–1904. [DOI] [PubMed] [Google Scholar]
- Hartog, H. , Horlings, H.M. , van, d, V. , 2011. Divergent effects of insulin-like growth factor-1 receptor expression on prognosis of estrogen receptor positive versus triple negative invasive ductal breast carcinoma. Breast Cancer Res. Treat. 129, 725–736. [DOI] [PubMed] [Google Scholar]
- Jemal, A. , Bray, F. , Center, M.M. , Ferlay, J. , Ward, E. , Forman, D. , 2011. Global cancer statistics. CA Cancer J. Clin. 61, 69–90. [DOI] [PubMed] [Google Scholar]
- Jiralerspong, S. , Palla, S.L. , Giordano, S.H. , 2009. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J. Clin. Oncol. 27, 3297–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiderlen, M. , de Glas, N.A. , Bastiaannet, E. , 2013. Diabetes in relation to breast cancer relapse and all-cause mortality in elderly breast cancer patients: a FOCUS study analysis. Ann. Oncol. 24, (12) 3011–3016. [DOI] [PubMed] [Google Scholar]
- Kim, R. , Osaki, A. , Toge, T. , 2005. Current and future roles of neoadjuvant chemotherapy in operable breast cancer. Clin. Breast Cancer. 6, 223–232. [DOI] [PubMed] [Google Scholar]
- Lega, I.C. , Austin, P.C. , Gruneir, A. , Goodwin, P.J. , Rochon, P.A. , Lipscombe, L.L. , 2013. Association between metformin therapy and mortality after breast cancer: a population-based study. Diabetes Care. 36, 3018–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarweh, S. , Osborne, C.K. , Creighton, C.J. , 2008. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 68, 826–833. [DOI] [PubMed] [Google Scholar]
- Parkin, D.M. , Bray, F. , Ferlay, J. , Pisani, P. , 2002. Global cancer statistics. CA Cancer J. Clin. 2005, (55) 74–108. [DOI] [PubMed] [Google Scholar]
- Pollak, M. , 2008. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer. 8, 915–928. [DOI] [PubMed] [Google Scholar]
- Pritchard, K.I. , 2005. Adjuvant endocrine therapies for pre-/perimenopausal women. Breast. 14, 547–554. [DOI] [PubMed] [Google Scholar]
- Richards, R.G. , DiAugustine, R.P. , Petrusz, P. , Clark, G.C. , Sebastian, J. , 1996. Estradiol stimulates tyrosine phosphorylation of the insulin-like growth factor-1 receptor and insulin receptor substrate-1 in the uterus. Proc. Natl. Acad. Sci. U. S. A. 93, 12002–12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, R.X. , Barnes, C.J. , Zhang, Z. , Bao, Y. , Kumar, R. , Santen, R.J. , 2004. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc. Natl. Acad. Sci. U. S. A. 101, 2076–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, R.X. , Zhang, Z. , Chen, Y. , Bao, Y. , Santen, R.J. , 2007. Estrogen signaling via a linear pathway involving insulin-like growth factor I receptor, matrix metalloproteinases, and epidermal growth factor receptor to activate mitogen-activated protein kinase in MCF-7 breast cancer cells. Endocrinology. 148, 4091–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tria, T.M. , 2013. Breast cancer screening update. Am. Fam. Physician. 87, 274–278. [PubMed] [Google Scholar]
- van de Velde, C.J. , Rea, D. , Seynaeve, C. , 2011. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet. 377, 321–331. [DOI] [PubMed] [Google Scholar]
- Vermeulen, J.F. , van Brussel, A.S. , van der Groep, P. , 2012. Immunophenotyping invasive breast cancer: paving the road for molecular imaging. BMC Cancer. 12, 240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen, J.F. , van der Wall, E. , Witkamp, A.J. , van Diest, P.J. , 2013. Analysis of expression of membrane-bound tumor markers in ductal carcinoma in situ of the breast: paving the way for molecular imaging. Cell Oncol. (Dordr.). 36, 333–340. [DOI] [PubMed] [Google Scholar]
- Viale, G. , Regan, M.M. , Maiorano, E. , 2008. Chemoendocrine compared with endocrine adjuvant therapies for node-negative breast cancer: predictive value of centrally reviewed expression of estrogen and progesterone receptors–International Breast Cancer Study Group. J. Clin. Oncol. 26, 1404–1410. [DOI] [PubMed] [Google Scholar]
- Winder, T. , Giamas, G. , Wilson, P.M. , 2014. Insulin-like growth factor receptor polymorphism defines clinical outcome in estrogen receptor-positive breast cancer patients treated with tamoxifen. Pharmacogenomics J. 14, 28–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article:
Supplementary data
Supplementary data


