Abstract
Hypoxia promotes an aggressive tumor phenotype with increased genomic instability, partially due to downregulation of DNA repair pathways. However, genome stability is also surveilled by cell cycle checkpoints. An important issue is therefore whether hypoxia also can influence the DNA damage‐induced cell cycle checkpoints. Here, we show that hypoxia (24 h 0.2% O2) alters the expression of several G2 checkpoint regulators, as examined by microarray gene expression analysis and immunoblotting of U2OS cells. While some of the changes reflected hypoxia‐induced inhibition of cell cycle progression, the levels of several G2 checkpoint regulators, in particular Cyclin B, were reduced in G2 phase cells after hypoxic exposure, as shown by flow cytometric barcoding analysis of individual cells. These effects were accompanied by decreased phosphorylation of a Cyclin dependent kinase (CDK) target in G2 phase cells after hypoxia, suggesting decreased CDK activity. Furthermore, cells pre‐exposed to hypoxia showed increased G2 checkpoint arrest upon treatment with ionizing radiation. Similar results were found following other hypoxic conditions (∼0.03% O2 20 h and 0.2% O2 72 h). These results demonstrate that the DNA damage‐induced G2 checkpoint can be altered as a consequence of hypoxia, and we propose that such alterations may influence the genome stability of hypoxic tumors.
Keywords: Hypoxia, G2 checkpoint, DNA damage, Ionizing radiation, Genome stability
1. Introduction
Tumor hypoxia (low oxygen concentration) can induce genomic instability resulting in more aggressive tumors (Bristow and Hill, 2008). Recent work has shown that hypoxia can suppress the homologous recombination (HR) DNA damage repair pathway through hypoxia‐induced downregulation of the repair proteins RAD51 and BRCA2 (Bindra et al., 2004; Meng et al., 2005; Bindra and Glazer, 2007; Chan et al., 2008; Marotta et al., 2011). This effect was reported both in vitro and in vivo and was most pronounced following severe hypoxia/anoxia or a prolonged hypoxia treatment of 72 h (Meng et al., 2005; Marotta et al., 2011). Such suppression of DNA damage repair has been proposed as a major mechanism leading to hypoxia‐induced genomic instability (Bristow and Hill, 2008; Luoto et al., 2013). However, genome stability is also maintained by cell cycle checkpoints, and an important issue is therefore whether hypoxia can alter the efficacy of DNA damage‐induced cell cycle checkpoints. As HR repair occurs predominantly in S and G2 phases (Rothkamm et al., 2003; Saleh‐Gohari and Helleday, 2004), the G2 checkpoint may be particularly important in preventing division of cells with defective HR repair following prolonged hypoxia. The impact of hypoxia on the G2 checkpoint is, however, poorly understood, although G2 checkpoint activation has been reported after reoxygenation‐induced DNA damage following severe hypoxia (Freiberg et al., 2006).
A key regulator of the G2 to M transition is the Cyclin B/CDK1 mitosis promoting kinase complex (Lew and Kornbluth, 1996; Ohi and Gould, 1999; Lindqvist et al., 2009). In response to DNA damage, G2 checkpoint arrest is activated through suppression of Cyclin B/CDK1 activity. The checkpoint kinase Chk1 is activated in an ATM/ATR dependent manner and directly phosphorylates Cdc25 phosphatases, leading to their suppression and thereby decreased capability to remove the inhibitory phosphorylation on the Tyr15 residue on CDK1 (Sanchez et al., 1997; Bartek and Lukas, 2003). In addition, Cyclin B/CDK1 activity is regulated by inhibitory phosphorylation of CDK1 Tyr15 by Wee1, Cyclin B transcription and protein stability, binding of the inhibitor p21, and nuclear exclusion of the kinase complex (Takizawa and Morgan, 2000; Lindqvist et al., 2009). Following repair of DNA damage, the G2 checkpoint is terminated and cells can enter mitosis (van Vugt et al., 2004). This recovery from G2 checkpoint arrest requires the Plk1 and Aurora A kinases (van Vugt et al., 2004; Macurek et al., 2008). Aurora A phosphorylates the Thr210 residue of Plk1, leading to Plk1‐mediated suppression of Wee1, activation of Cdc25 and subsequent mitotic entry (Macurek et al., 2008; Lens et al., 2010).
Although the G2 checkpoint promotes DNA repair, human cells often terminate the checkpoint prematurely and enter mitosis with low amounts of residual DNA damage (Syljuåsen et al., 2006; Deckbar et al., 2007; Lobrich and Jeggo, 2007; Tkacz‐Stachowska et al., 2011). Particularly, a process of checkpoint adaptation has been reported, where protein expression and/or cell signaling may change as a consequence of responding to the DNA damage insult, resulting in premature checkpoint termination (Syljuåsen, 2007). Given that hypoxia promotes major changes of cellular transcription and translation (Wouters and Koritzinsky, 2008), and that severe hypoxia can activate DNA damage signaling (Hammond et al., 2003; Hasvold et al., 2013), we reasoned that hypoxia would likely lead to alterations of the G2 checkpoint.
In this study we have investigated the impact of hypoxia on the G2 checkpoint. We show that mild and severe levels of hypoxia (0.2% and ∼0.03% O2) can cause altered protein levels of key G2 checkpoint regulators in individual G2 phase cells, and cause an increased ionizing radiation (IR)‐induced G2 checkpoint. Thus, in addition to the previously reported downregulation of DNA repair pathways, changes in G2 checkpoint activation may also influence genome stability after hypoxic exposure.
2. Materials and methods
2.1. Cell lines, hypoxia and IR treatment
Human U2OS osteosarcoma, HeLa cervical carcinoma cells and NCI–H460 lung cancer cells (ATCC) were cultured in DMEM (Dulbecco's modified Eagle's) medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS) and 1% Penicillin/Streptomycin (P/S) at 37 °C in a humidified atmosphere with 5% CO2. The cell lines were identity tested by STR profiling as described previously (Hasvold et al., 2013). Roscovitine (#9885, Cell Signaling) was used at 25–50 μM. Nocodazole (Sigma) was used at 0.04 μg/ml. Hypoxia treatments were carried out in an Invivo2 200 Hypoxic workstation (Ruskinn) as described previously (Hasvold et al., 2013). Oxygen concentration setpoints were 0.0% (referred to as ∼0.03%, as the recorded O2 level in gas phase was 0.00–0.03%) and 0.2% (recorded O2 level in gas phase 0.16–0.24%). Irradiation of cells was performed with an X‐ray generator (Faxitron CP160, 160 kV, 6.3 mA) at a dose‐rate of 1 Gy/min.
2.2. Microarray gene expression analysis
Gene expression profiling of cell lines was performed using the Illumina bead arrays human WG‐6v3 (Illumina Inc., San Diego, CA) with 48803 transcripts as described previously (Lando et al., 2009). Total RNA was isolated using RNeasy MiniKit (Qiagen) from cells cultured at normoxia (21% O2), or after 24 h incubation in the hypoxia chamber at 0.2% O2, as described previously (Halle et al., 2012). An aliquot of 500 ng was amplified using the Illumina® TotalPrep RNA amplification kit (Ambion Inc., Austin, TX), and cRNA was synthesized, labeled, and hybridized to the arrays. The hybridized arrays were stained with streptavidin‐Cy3 (AmershamTM, PA43001, Buckinghampshire, UK) and scanned with an Illumina beadarray reader. Signal extraction, quality control, and quantile normalization were performed using software provided by Illumina. The data have been deposited in the gene expression omnibus (GEO) database (GSE70530).
2.3. Flow cytometry
For analysis of protein expression in different cell cycle phases, cells were fixated with either 70% ethanol (Cyclin A, Aurora A and Plk1) or first 1% formaldehyde (1 h on ice) followed by 70% ethanol (Cyclin B1, p21 and Chk2) and stained with rabbit anti‐phospho‐Histone H3(Ser10) (06‐570, Millipore) and mouse anti‐Cyclin B1 (sc‐245, Santa Cruz), anti‐Chk2 (DCS 270.1 (Sørensen et al., 2003)) or anti‐Plk1 (37‐7000, Invitrogen/Life Technologies), alternatively mouse anti‐phospho‐Histone H3(Ser10) (05‐806, Millipore) and rabbit anti‐Cyclin A (sc‐751, Santa Cruz), anti‐Aurora A (ab1287, Abcam) or anti‐p21 (sc‐756, Santa Cruz). Mouse anti‐phospho‐Histone H2AX(Ser139) (05‐636, Millipore) and rabbit anti‐phospho‐BRCA2(Ser3291) (AB9986, Millipore) were used for analysis of DNA damage and CDK activity, respectively. Antibody staining was performed as described previously (Tkacz‐Stachowska et al., 2011). Barcoding (Krutzik and Nolan, 2006; Tkacz‐Stachowska et al., 2011) was used to eliminate variation in antibody staining between individual samples. For barcoding, four individually fixed samples were stained with different concentrations (0.125, 0.031, 0.0062, and 0.00078 ng/μl) of Pacific Blue succinimidyl ester (Invitrogen) for 30 min in the dark at room temperature, and subsequently mixed into one tube. The mixed cells were then stained with antibodies and the DNA‐stain FxCycle Far Red (Invitrogen). For each barcoding experiment an aliquot of the mixed cells was analyzed without any antibodies to check the need for compensation of the Pacific Blue signal, and an aliquot was stained with secondary antibodies only (no primary antibodies) to correct for background staining (see Figure S1). When repeating individual experiments, the concentrations of Pacific Blue were alternated between the treatment conditions to avoid any potential influence on the results by the Pacific Blue staining. For analysis of G2 checkpoint activation, cells were stained with rabbit anti‐phospho‐Histone H3(Ser10) (06‐570, Millipore) and the DNA stain Hoechst 33258 (1.5 μg/ml) (Sigma–Aldrich). Flow cytometry analysis was performed on a LSRII flow cytometer (BD Biosciences) using Diva software. Error bars represent standard errors of mean from at least 3 independent experiments.
2.4. Immunoblotting
Cells were lysed in SDS boiling buffer (2% SDS, 10 mM Tris–HCl pH 7.5, 100 μM Na3VO4), and immunoblotting was performed as described previously (Hasvold et al., 2013). The following antibodies were used for blotting: Anti‐CDK1 (#9112) from Cell Signaling, anti‐Cyclin B1 (sc‐245), anti‐Cyclin A (sc‐751), anti‐p21 (sc‐756), anti‐MCM7 (sc‐65469) and anti‐Cdc25A (sc‐7389) from Santa Cruz, anti‐HIF1α (610958) from BD Transduction Laboratories, anti‐H4 (05‐858) and anti‐BRCA2 (OP‐95) from Upstate (Millipore), anti‐Plk1 (Zy‐1700) from Zymed, anti‐Aurora A (ab1287) from Abcam, anti‐γ‐Tubulin (T6557) from Sigma–Aldrich, and anti‐Chk1 (DCS310) and anti‐Chk2 (DCS 270.1) (Sørensen et al., 2003).
3. Results
3.1. Hypoxia‐induced changes in gene expression of G2 checkpoint regulators
To explore whether hypoxia leads to altered expression of G2 checkpoint regulators, we first analyzed gene expression by microarray analysis in U2OS osteosarcoma cells incubated at 0.2% O2 for 24 h. U2OS cells were used for this analysis because they have functional, well characterized S and G2 DNA damage checkpoints (Sørensen et al., 2003; Syljuåsen et al., 2006). A collection of 31 G2 checkpoint regulators was selected from published studies (Table 1). These were grouped into positive and negative regulators of the G2 checkpoint and the ratio of gene expression for hypoxic versus normoxic cells was obtained. Hypoxia caused a 20–50% reduction in mRNA levels of several tested G2 checkpoint regulators, while others were less affected or even slightly upregulated (Figure 1A and B). While this change in mRNA expression was modest compared to the most downregulated genes in our dataset (Table S1), a decrease in protein levels of a panel of the G2 checkpoint regulators for which we had available antibodies was also detected by immunoblotting (Figure 1C). Protein levels of Plk1, Cdc25A, Chk2, Chk1, Cyclin A, Cyclin B, and Aurora A were all markedly decreased after 24 h of hypoxia (Figure 1C).
Table 1.
Positive and negative regulators with reference to published study.
| Gene name | Protein name | G2 checkpoint regulation | Ref. |
|---|---|---|---|
| ATM | ATM | Positive | Beamish et al.(1996) |
| ATR | ATR | Positive | Cliby et al. (1998) |
| AURKA | AURORA A | Negative | Macurek et al. (2008) |
| BRCA1 | BRCA1 | Positive | Xu et al. (1999) |
| BRCA2 | BRCA2 | Positive | Cotta‐Ramusino et al.(2011); Menzel et al. (2011) |
| C12ORF32 | RHINO | Positive | Cotta‐Ramusino et al. (2011) |
| C13ORF34 | BORA | Negative | Macurek et al. (2008) |
| CCNA2 | CYCLIN A2 | Negative | Walker et al. (1995); Furuno et al. (1999) |
| CCNB1 | CYCLIN B1 | Negative | Kao et al. (1997) |
| CCNB2 | CYCLIN B2 | Negative | Gimenez‐Abian et al. (2002); Wu et al. (2010) |
| CDC2 | CDK1 | Negative | Kao et al. (1997) |
| CDC25A | CDC25A | Negative | Zhao et al. (2002) |
| CDC25B | CDC25B | Negative | van Vugt et al. (2004) |
| CDC25C | CDC25C | Negative | Sanchez et al. (1997) |
| CDKN1A | p21 | Positive | Bunz et al. (1998) |
| CHEK1 | Chk1 | Positive | Sanchez et al. (1997); Liu et al. (2000) |
| CHEK2 | Chk2 | Positive | Hirao et al. (2000) |
| CLSPN | Claspin | Positive | Bassermann et al. (2008) |
| HUS1 | HUS1 | Positive | Roos‐Mattjus et al. (2002); Cotta‐Ramusino et al. (2011) |
| MYT1 | MYT1 | Positive | Wang et al. (2004) |
| NEK11 | NEK11 | Positive | Melixetian et al. (2009) |
| PALB2 | PALB2 | Positive | Cotta‐Ramusino et al. (2011); Menzel et al. (2011) |
| PLK1 | PLK1 | Negative | van Vugt et al. (2004) |
| PPP2R4 | PP2A | Positive | Yan et al. (2010) |
| RAD17 | RAD17 | Positive | Bao et al. (2001) |
| RAD9A | RAD9A | Positive | Roos‐Mattjus et al. (2002); Cotta‐Ramusino et al. (2011) |
| RAD9B | RAD9B | Positive | Dufault et al. (2003) |
| RBBP8 | CTIP | Positive | Kousholt et al. (2012) |
| TOPBP1 | TOPBP1 | Positive | Yamane et al. (2003) |
| TP53 | TP53 | Positive | Bunz et al. (1998) |
| WEE1 | WEE1 | Positive | Wang et al. (2001) |
Figure 1.
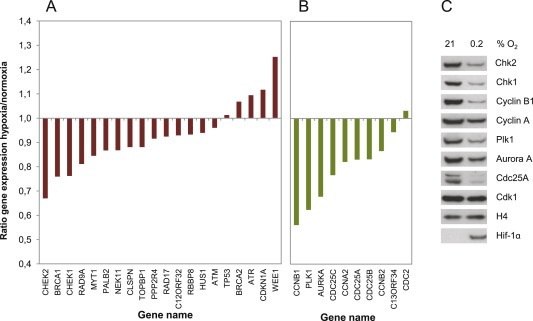
Hypoxia‐induced alterations in mRNA and protein expression of G2 checkpoint regulators. A. Gene expression of positive G2 checkpoint regulators in U2OS cells. The ratio of mRNA expression in cells treated with hypoxia (0.2% O2, 24 h) relative to mRNA expression in cells cultured at normoxia (21% O2) is shown. Data were obtained from genome wide microarray analysis. The positive G2 checkpoint regulators were found from published studies as described in Table 1. B. Gene expression of negative G2 checkpoint regulators similar as in A. C. Immunoblot analysis of protein extracts from U2OS cells exposed to hypoxia or normoxia for 24 h. The samples are from the same experiment as the microarray results shown in A and B. HIF1α was shown to confirm hypoxia. H4 was used as loading control.
3.2. Analysis of protein levels in individual G2 phase cells (after 0.2% O2, 24 h)
Hypoxia treated cells accumulated in G1, with a lower fraction of cells in S and G2 phase, compared to the normoxic cells (Figure 2A). As expression of many G2 checkpoint regulators is cell cycle phase dependent, changes in mRNA and protein levels in pooled cell populations (Figure 1) could potentially be a consequence of hypoxia‐mediated suppression of cell cycle progression. We therefore measured protein levels in G2 phase cells by multiparameter flow cytometry for a smaller panel (Cyclin A & B, Plk1, Aurora A, Chk2 and p21) of the G2 checkpoint regulators. In these experiments cells were incubated for 24 h at 0.2% O2 followed by 0–6 h at 21% O2, and compared to cells cultured at normoxia (21% O2). Barcoding with Pacific Blue (Krutzik and Nolan, 2006) was included to minimize sample‐to‐sample variation (Figure 2B and S1). By this method, we observed a marked reduction in Cyclin B levels in G2 phase cells after hypoxia, which persisted at least until 6 h after reoxygenation (Figure 2B and C). Minor reductions were observed in levels of Plk1 and Aurora A, whereas Chk2 levels were not affected by the hypoxic exposure itself, and only slightly decreased after reoxygenation. However, no changes were observed in the G2 levels of Cyclin A, and the observed increase in p21 was minor (Figure 2C). Thus, although the whole‐population based microarray and immunoblotting analysis showed altered expression of all of these proteins after exposure to 0.2% O2 (Figure 1), only levels of Cyclin B, and to some extent Plk1 and Aurora A, were reduced in individual G2 phase cells.
Figure 2.
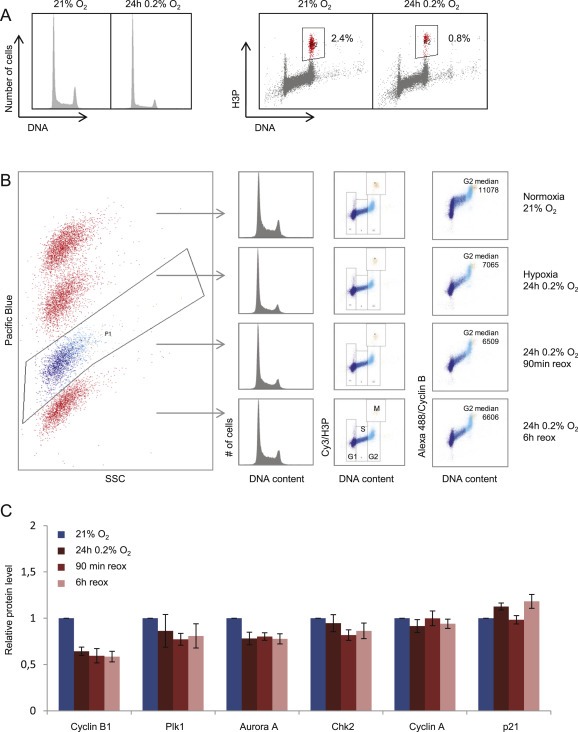
Protein levels of G2 checkpoint regulators in individual G2 cells following hypoxia. A. Cell cycle profiles of U2OS cells after hypoxia treatment as in Figure 1 (24 h 0.2% O2). Flow cytometric analysis was performed after staining with anti phospho‐H3Ser10 (H3P) to mark mitotic cells, and the DNA stain Hoechst. Numbers indicate fraction of mitotic cells. B. Flow cytometric barcoding analysis for accurate measurement of protein levels in G2 phase cells. U2OS cells treated with four different conditions, as indicated in the right column, were labeled with different concentrations of Pacific Blue and combined into a single sample. The single sample of cells was then stained with antibodies to Cyclin B and phospho‐H3 and with the DNA‐stain FxCycle Far Red, and analyzed by flow cytometry. Gating of the Pacific Blue‐SSC plot (left) was used to separate the four original samples. The G1, S, G2 and M cell cycle phase populations were gated from the scatter plot of phospho‐Histone H3(Ser10) (H3P) versus DNA content, and the median signal for Cyclin B levels in each cell cycle phase could thus be obtained. C.Median values of G2 phase levels of the indicated proteins obtained as in B, after subtraction of background values obtained as in Figure S1. U2OS cells were grown at 21% O2, or exposed to 24 h hypoxia at 0.2% O2, or first exposed to 0.2% O2 for 24 h followed by subsequent incubation at 21% O2 for 90 min (90 min reox) or 6 h (6 h reox). Average results from at least 3 independent experiments are shown. Error bars indicate SEM.
3.3. Altered G2/M phenotypes after hypoxia (0.2% O2, 24 h)
Our initial microarray and immunoblotting results suggested that both positive and negative G2 checkpoint regulators may be affected by hypoxia (Figure 1). It was therefore not clear whether the overall balance between these regulators would be shifted, potentially resulting in altered G2/M phenotypes. We therefore next examined if CDK activity in G2 phase cells and G2 checkpoint activation in response to DNA damage were altered by hypoxia.
To measure CDK activity in G2 phase cells we used a similar flow cytometry barcoding approach as above, to assess CDK‐dependent phosphorylation of BRCA2 on Ser3291 (Esashi et al., 2005). The method was validated by addition of the Wee1 inhibitor MK1775 and the CDK‐inhibitor Roscovitine to increase and decrease the CDK activity, respectively (Figure S2). Interestingly, we observed a decrease in Ser3291 phosphorylated BRCA2 in G2 cells after exposure to hypoxia (24 h 0.2% O2) compared to normoxic U2OS cells (Figure 3A). Of note, the BRCA2 protein was not down‐regulated by hypoxia at this condition (Figure 3B), which is in agreement with our own microarray data (Figure 1) and with previous studies showing downregulation of DNA repair proteins only after anoxia or longer hypoxic incubations (Meng et al., 2005; Marotta et al., 2011). The measurements of CDK activity by this method after incubation at 0.2% O2 for 24 h were thus not disturbed by changes in BRCA2 expression levels. We conclude that hypoxia (24 h 0.2% O2) leads to reduced phosphorylation of a CDK target in G2 phase cells, indicating decreased CDK activity.
Figure 3.
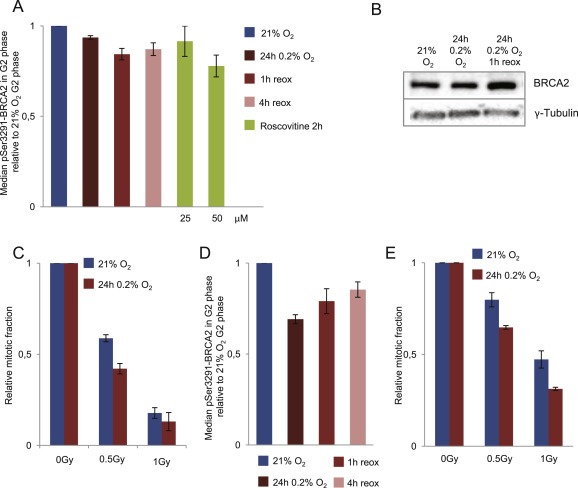
Hypoxia‐induced changes in CDK activity and G2 checkpoint activation. A. CDK activity in G2 phase cells as measured by phosphorylation of BRCA2‐Ser3291. U2OS cells were grown at 21% O2, or incubated at 0.2% O2 for 24 h and harvested inside the hypoxia chamber and at 1 and 4 h after reoxygenation, or treated with Roscovitine for 2 h at 21% O2. Flow cytometry barcoding analysis of phospho‐BRCA2‐Ser3291 was performed as in Figure 2 and S2. B. Immunoblot analysis of U2OS cells treated as in A with antibodies to total BRCA2 and γ‐tubulin (loading control). C. G2 checkpoint activation after IR (0.2%O2 24 h). Flow cytometric analysis of G2 checkpoint arrest after X‐ray irradiation (0, 0.5, 1 Gy) of normoxic U2OS cells (21%O2) or U2OS cells exposed to 24 h of hypoxia at 0.2%O2 and irradiated 15 min after reoxygenation. Nocodazole was added to all samples 1 h after IR, and the samples were harvested 5 h later. The relative mitotic fraction was determined as the fraction of phospho‐H3 positive cells in irradiated samples divided by the fraction of phospho‐H3 positive cells in non‐irradiated samples. Average values from 3 independent experiments are shown. Error bars indicate SEM. D. Phosphorylation of BRCA2‐Ser3291 in H460 cells treated with hypoxia and analyzed as in A. E. G2 checkpoint activation in H460 cells treated with hypoxia and IR and analyzed as in C.
To investigate whether G2 checkpoint activation also was affected, we examined the G2 checkpoint after IR in cells pre‐exposed to hypoxia (24 h 0.2% O2) compared to normoxic cells. Because IR induces less DNA damage when performed in hypoxic conditions (Brown, 1999), we allowed the hypoxic cells to reoxygenate at 21% O2 for 15 min before the IR treatment. In this way the IR‐induced DNA damage was similar for the normoxic and hypoxia‐exposed cells, as measured by the DNA damage marker γH2AX (Figure S3A). The microtubule inhibitor Nocodazole was added at 1 h after IR to trap mitotic cells that escaped the G2 checkpoint until cell harvest 5 h later. The results showed a more pronounced G2 arrest in cells that had been exposed to hypoxia (Figure 3C), confirming that hypoxia indeed can cause altered G2 arrest. Similar effects on BRCA2 phosphorylation and G2 checkpoint activation were observed in H460 lung cancer cells (Figure 3D and E), accompanied by decreased Cyclin B levels after hypoxic exposure (Figure S4A). Altogether, these results suggest that hypoxia can change the balance between positive and negative G2 checkpoint regulators, resulting in altered G2/M phenotypes.
3.4. Responses to severe and prolonged hypoxia (∼0.03% O2, 20 h and 0.2% O2, 72 h)
Hypoxia in tumors can be characterized as mild (0.2%–2% O2), leading to altered transcriptional and translational responses, or severe (<0.1% O2), often triggering replication stress and activation of DNA damage signaling in addition to altered transcription/translation (Hammond et al., 2014). Furthermore, the duration of hypoxia can vary. Reoxygenation can for instance occur as a consequence of cell death during radiation therapy, or due to fluctuations in blood flow (Bussink et al., 2000). As mentioned above, downregulation of HR repair was previously reported after severe levels of hypoxia and prolonged mild hypoxia (Meng et al., 2005; Pires et al., 2010). To address whether the G2 checkpoint was altered after similar hypoxic conditions, we examined the G2 checkpoint after exposure to ∼0.03% O2 for 20 h (severe hypoxia) or 0.2% O2 for 72 h (prolonged mild hypoxia). Indeed, the IR‐induced G2 checkpoint was also increased after these hypoxic incubations (Figure 4A and B). Furthermore, Cyclin B and Plk1 levels were clearly reduced in G2 phase cells, and remained low at least until 6 h after reoxygenation (Figure 4C and D). As was the case after 24 h 0.2% O2, no changes were observed in the Cyclin A levels, whereas only some minor changes were observed for Aurora A and Chk2. Upregulation of p21 was mainly seen after severe hypoxia, consistent with activation of a replication stress response in these cells (Figure 4C and D), and may contribute to further decrease the CDK activity.
Figure 4.
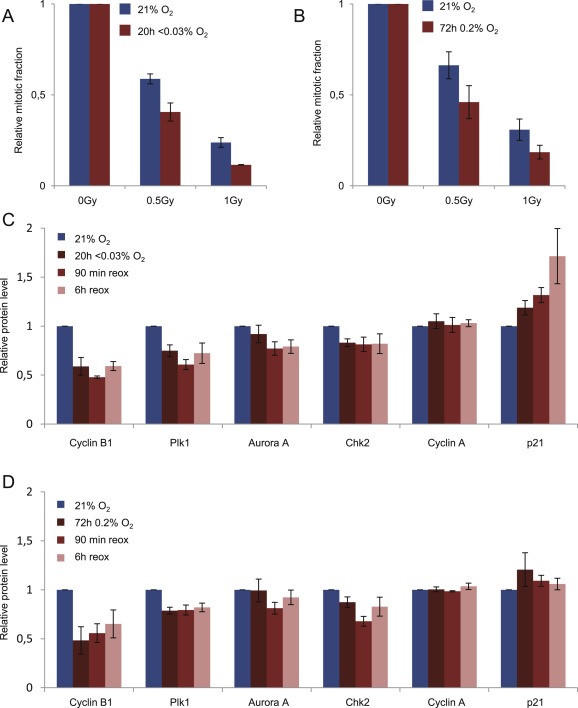
IR‐induced G2 checkpoint and expression of G2 checkpoint regulators in U2OS cells after severe hypoxia (∼0.03% O2 20 h) and prolonged mild hypoxia (0.2%O2, 72 h). A. Similar G2 checkpoint measurement after IR as in Figure 3C following incubation at severe hypoxia (∼0.03% O2 20 h). B. Similar as in A following incubation at prolonged mild hypoxia (0.2% O2 72 h). C. Similar flow cytometric barcoding analysis of protein levels in G2 phase cells as in Figure 2C following incubation at severe hypoxia (∼0.03% O2 20 h). D. Similar as in C following incubation at 0.2% O2, 72 h.
Thus, among the G2 checkpoint regulators we have analyzed by flow cytometry after hypoxia treatment of U2OS cells, in particular Cyclin B and to some extent Plk1 were the most downregulated. To address whether similar changes were found in an additional cell line, we also have performed analysis of HeLa cervical cancer cells. Consistent with our results in U2OS cells, though with even more pronounced effects, we observed downregulation of Cyclin B, Plk1 and Aurora A in G2 phase cells after incubation of HeLa cells at severe hypoxia (Figure S4B). However, in HeLa cells some of the other factors were also downregulated in G2 phase, such as Cyclin A, whereas after mild hypoxia (72 h 0.2% O2) these cells overall displayed less change in the levels of G2 checkpoint regulators than that observed in U2OS cells (data not shown). Therefore, different cell lines may show slightly different hypoxia‐induced alterations of G2 checkpoint regulators.
4. Discussion
The aim of this study was to investigate whether hypoxia can lead to an altered DNA damage G2 checkpoint. For the first time we have used a flow cytometric barcoding approach to accurately compare protein levels in G2 phase cells after hypoxic and normoxic incubation. We demonstrate that in particular Cyclin B, and to some extent Plk1 and Aurora A, were downregulated in G2 phase cells after exposure to hypoxia. However, other proteins, such as Cyclin A, showed no difference in G2 phase levels even though immunoblotting and microarray analysis of cell lysates from non‐synchronized pooled cell populations indicated downregulation. Thus, our results highlight the need for careful examination of expression levels in each cell cycle phase, in order to evaluate whether protein expression is truly altered after a treatment that also has an impact on cell cycle progression, such as hypoxia.
As hypoxia may affect both positive and negative G2 checkpoint regulators, it was not obvious in what direction hypoxia would be expected to alter the G2 checkpoint. However, we consistently observed reduced phosphorylation of a CDK target in G2 phase cells after hypoxic incubation, and increased IR‐induced G2 checkpoint arrest. Our results thus demonstrate that hypoxia can lead to altered G2 checkpoint regulation. These effects were not likely a consequence of additional DNA damage caused by reoxygenation. We found no increase in γH2AX after reoxygenation following exposure to 0.2% O2 for 24 h (Figure S3B), and the incidence of micronuclei was not increased after incubation at ∼0.03% O2 for 20 h or 0.2% O2 for 72 h (Figure S3C). Moreover, our hypoxic conditions did not reduce clonogenic survival (Hasvold et al., 2013). In contrast, a previous report demonstrated DNA damage induction, G2 arrest and strongly reduced clonogenic survival after reoxygenation following hypoxia (Freiberg et al., 2006). Most likely a more severe hypoxic/anoxic condition was used in this study. They used glass while we used plastic dishes, and plastic will bind and release small amounts of oxygen (Chapman et al., 1970), thereby preventing anoxic conditions in our experiments. Furthermore, by calculating the numbers of mitotic cells after IR and hypoxia relative to the numbers after exposure to hypoxia alone, we avoided influence from any hypoxia/reoxygenation‐related cell cycle effects on our measurements of the IR‐induced checkpoint (Figure 3C and E).
The enhanced G2 checkpoint in the hypoxia treated cells presumably provides more time for DNA damage repair before cell division. However, as hypoxia also can affect DNA repair pathways (Meng et al., 2005; Pires et al., 2010), an interesting issue is whether hypoxia might alter the stringency of the G2 checkpoint. Potentially, the G2 checkpoint could be less stringent after hypoxia, contributing to increased genomic instability. Particularly, one could imagine that the DNA damage checkpoints may be less stringent after severe hypoxia, due to a process of “adapation” to hypoxia‐induced replication stress. To briefly examine this issue, we have analyzed G2 checkpoint stringency by live cell imaging of the DNA damage marker 53BP1 in G2 phase after severe hypoxia, and by counting of the micronuclei formed during the first division after IR after mild and severe hypoxia (data not shown). The results suggest that neither mild nor severe hypoxia causes major changes in G2 checkpoint stringency. Thus, based on our analysis, the enhanced G2 checkpoint seems sufficient to maintain checkpoint stringency in U2OS cells at a normal level after hypoxia.
Our analysis of G2 phase cells by flow cytometry included a small panel of G2 checkpoint regulators for which we found well functioning antibodies. Among the factors examined, Cyclin B, Plk1 and to some extent Aurora A were the most downregulated proteins in G2 phase after hypoxic incubation. The mechanisms of hypoxia‐induced alterations of G2 checkpoint regulators remain to be elucidated, but may potentially involve HIF1‐α mediated regulation of gene expression, or regulation of translation via the mTOR or UPR pathways (Wouters and Koritzinsky, 2008). Of note, additional G2 checkpoint regulators would likely show altered expression in G2 phase cells if examined by similar flow cytometry analysis. For instance, Chk1 and Cdc25A were reported to be downregulated by hypoxia (Hammer et al., 2007; de Oliveira et al., 2009), as we also show in Figure 1, although protein expression levels were only measured in lysates from pooled cell populations in these studies.
In conclusion, we have shown that hypoxia can lead to altered expression of key G2 checkpoint regulators associated with an increased G2 checkpoint in response to IR and decreased phosphorylation of a CDK target in G2 phase. We propose that an enhanced G2 checkpoint may allow extra time for repair and thereby contribute to maintaining genome stability in some hypoxic tumors. Thus, hypoxia‐induced genomic instability may be influenced by hypoxia‐induced changes in G2 checkpoint regulation, in addition to the previously reported suppression of DNA damage repair pathways (Luoto et al., 2013) and hypoxia‐induced replication stress (Olcina et al., 2010).
Conflict of interest
The authors declare no conflict of interest.
Supporting information
The following are the supplementary data related to this article:
Supplementary data
Figure S1
Figure S2
Figure S3
Figure S4
Acknowledgments
This work was supported by The Norwegian Cancer Society, Simon Fougner Hartmann Foundation and South‐Eastern Norway Health Authority.
Supplementary data 1.
1.1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2015.12.015.
Hasvold Grete, Lund-Andersen Christin, Lando Malin, Patzke Sebastian, Hauge Sissel, Suo ZhenHe, Lyng Heidi, Syljuåsen Randi G., (2016), Hypoxia‐induced alterations of G2 checkpoint regulators, Molecular Oncology, 10, doi: 10.1016/j.molonc.2015.12.015.
References
- Bao, S. , Tibbetts, R.S. , Brumbaugh, K.M. , Fang, Y. , Richardson, D.A. , Ali, A. , Chen, S.M. , Abraham, R.T. , Wang, X.F. , 2001. ATR/ATM-mediated phosphorylation of human Rad17 is required for genotoxic stress responses. Nature. 411, 969–974. [DOI] [PubMed] [Google Scholar]
- Bartek, J. , Lukas, J. , 2003. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 3, 421–429. [DOI] [PubMed] [Google Scholar]
- Bassermann, F. , Frescas, D. , Guardavaccaro, D. , Busino, L. , Peschiaroli, A. , Pagano, M. , 2008. The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell. 134, 256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamish, H. , Williams, R. , Chen, P. , Lavin, M.F. , 1996. Defect in multiple cell cycle checkpoints in ataxia-telangiectasia postirradiation. J. Biol. Chem. 271, 20486–20493. [DOI] [PubMed] [Google Scholar]
- Bindra, R.S. , Glazer, P.M. , 2007. Repression of RAD51 gene expression by E2F4/p130 complexes in hypoxia. Oncogene. 26, 2048–2057. [DOI] [PubMed] [Google Scholar]
- Bindra, R.S. , Schaffer, P.J. , Meng, A. , Woo, J. , Maseide, K. , Roth, M.E. , Lizardi, P. , Hedley, D.W. , Bristow, R.G. , Glazer, P.M. , 2004. Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol. Cell Biol. 24, 8504–8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow, R.G. , Hill, R.P. , 2008. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer. 8, 180–192. [DOI] [PubMed] [Google Scholar]
- Brown, J.M. , 1999. The hypoxic cell: a target for selective cancer therapy–eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 59, 5863–5870. [PubMed] [Google Scholar]
- Bunz, F. , Dutriaux, A. , Lengauer, C. , Waldman, T. , Zhou, S. , Brown, J.P. , Sedivy, J.M. , Kinzler, K.W. , Vogelstein, B. , 1998. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 282, 1497–1501. [DOI] [PubMed] [Google Scholar]
- Bussink, J. , Kaanders, J.H. , Rijken, P.F. , Raleigh, J.A. , Van der Kogel, A.J. , 2000. Changes in blood perfusion and hypoxia after irradiation of a human squamous cell carcinoma xenograft tumor line. Radiat. Res. 153, 398–404. [DOI] [PubMed] [Google Scholar]
- Chan, N. , Koritzinsky, M. , Zhao, H. , Bindra, R. , Glazer, P.M. , Powell, S. , Belmaaza, A. , Wouters, B. , Bristow, R.G. , 2008. Chronic hypoxia decreases synthesis of homologous recombination proteins to offset chemoresistance and radioresistance. Cancer Res. 68, 605–614. [DOI] [PubMed] [Google Scholar]
- Chapman, J.D. , Sturrock, J. , Boag, J.W. , Crookall, J.O. , 1970. Factors affecting the oxygen tension around cells growing in plastic Petri dishes. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 17, 305–328. [DOI] [PubMed] [Google Scholar]
- Cliby, W.A. , Roberts, C.J. , Cimprich, K.A. , Stringer, C.M. , Lamb, J.R. , Schreiber, S.L. , Friend, S.H. , 1998. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. Embo J. 17, 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotta-Ramusino, C. , McDonald, E.R. , Hurov, K. , Sowa, M.E. , Harper, J.W. , Elledge, S.J. , 2011. A DNA damage response screen identifies RHINO, a 9-1-1 and TopBP1 interacting protein required for ATR signaling. Science. 332, 1313–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira, P.E. , Zhang, L. , Wang, Z. , Lazo, J.S. , 2009. Hypoxia-mediated regulation of Cdc25A phosphatase by p21 and miR-21. Cell Cycle. 8, 3157–3164. [DOI] [PubMed] [Google Scholar]
- Deckbar, D. , Birraux, J. , Krempler, A. , Tchouandong, L. , Beucher, A. , Walker, S. , Stiff, T. , Jeggo, P. , Lobrich, M. , 2007. Chromosome breakage after G2 checkpoint release. J. Cell Biol. 176, 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufault, V.M. , Oestreich, A.J. , Vroman, B.T. , Karnitz, L.M. , 2003. Identification and characterization of RAD9B, a paralog of the RAD9 checkpoint gene. Genomics. 82, 644–651. [DOI] [PubMed] [Google Scholar]
- Esashi, F. , Christ, N. , Gannon, J. , Liu, Y. , Hunt, T. , Jasin, M. , West, S.C. , 2005. CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature. 434, 598–604. [DOI] [PubMed] [Google Scholar]
- Freiberg, R.A. , Hammond, E.M. , Dorie, M.J. , Welford, S.M. , Giaccia, A.J. , 2006. DNA damage during reoxygenation elicits a Chk2-dependent checkpoint response. Mol. Cell Biol. 26, 1598–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuno, N. , den Elzen, N. , Pines, J. , 1999. Human cyclin A is required for mitosis until mid prophase. J. Cell Biol. 147, 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Abian, J.F. , Weingartner, M. , Binarova, P. , Clarke, D.J. , Anthony, R.G. , Calderini, O. , Heberle-Bors, E. , Moreno Diaz de la Espina, S. , Bogre, L. , De la Torre, C. , 2002. A topoisomerase II-dependent checkpoint in G2-phase plant cells can be bypassed by ectopic expression of mitotic cyclin B2. Cell Cycle. 1, 187–192. [PubMed] [Google Scholar]
- Halle, C. , Andersen, E. , Lando, M. , Aarnes, E.K. , Hasvold, G. , Holden, M. , Syljuåsen, R.G. , Sundfor, K. , Kristensen, G.B. , Holm, R. , Malinen, E. , Lyng, H. , 2012. Hypoxia-induced gene expression in chemoradioresistant cervical cancer revealed by dynamic contrast-enhanced MRI. Cancer Res. 72, 5285–5295. [DOI] [PubMed] [Google Scholar]
- Hammer, S. , To, K.K. , Yoo, Y.G. , Koshiji, M. , Huang, L.E. , 2007. Hypoxic suppression of the cell cycle gene CDC25A in tumor cells. Cell Cycle. 6, 1919–1926. [DOI] [PubMed] [Google Scholar]
- Hammond, E.M. , Asselin, M.C. , Forster, D. , O'Connor, J.P. , Senra, J.M. , Williams, K.J. , 2014. The meaning, measurement and modification of hypoxia in the laboratory and the clinic. Clin. Oncol. (R Coll. Radiol.). 26, 277–288. [DOI] [PubMed] [Google Scholar]
- Hammond, E.M. , Dorie, M.J. , Giaccia, A.J. , 2003. ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J. Biol. Chem. 278, 12207–12213. [DOI] [PubMed] [Google Scholar]
- Hasvold, G. , Nähse-Kumpf, V. , Tkacz-Stachowska, K. , Rofstad, E.K. , Syljuåsen, R.G. , 2013. The efficacy of CHK1 inhibitors is not altered by hypoxia, but is enhanced after reoxygenation. Mol. Cancer Ther. 12, 705–716. [DOI] [PubMed] [Google Scholar]
- Hirao, A. , Kong, Y.Y. , Matsuoka, S. , Wakeham, A. , Ruland, J. , Yoshida, H. , Liu, D. , Elledge, S.J. , Mak, T.W. , 2000. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 287, 1824–1827. [DOI] [PubMed] [Google Scholar]
- Kao, G.D. , McKenna, W.G. , Maity, A. , Blank, K. , Muschel, R.J. , 1997. Cyclin B1 availability is a rate-limiting component of the radiation-induced G2 delay in HeLa cells. Cancer Res. 57, 753–758. [PubMed] [Google Scholar]
- Kousholt, A.N. , Fugger, K. , Hoffmann, S. , Larsen, B.D. , Menzel, T. , Sartori, A.A. , Sorensen, C.S. , 2012. CtIP-dependent DNA resection is required for DNA damage checkpoint maintenance but not initiation. J. Cell Biol. 197, 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutzik, P.O. , Nolan, G.P. , 2006. Fluorescent cell barcoding in flow cytometry allows high-throughput drug screening and signaling profiling. Nat. Methods. 3, 361–368. [DOI] [PubMed] [Google Scholar]
- Lando, M. , Holden, M. , Bergersen, L.C. , Svendsrud, D.H. , Stokke, T. , Sundfor, K. , Glad, I.K. , Kristensen, G.B. , Lyng, H. , 2009. Gene dosage, expression, and ontology analysis identifies driver genes in the carcinogenesis and chemoradioresistance of cervical cancer. PLoS Genet. 5, e1000719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens, S.M. , Voest, E.E. , Medema, R.H. , 2010. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat. Rev. Cancer. 10, 825–841. [DOI] [PubMed] [Google Scholar]
- Lew, D.J. , Kornbluth, S. , 1996. Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr. Opin. Cell Biol. 8, 795–804. [DOI] [PubMed] [Google Scholar]
- Lindqvist, A. , Rodriguez-Bravo, V. , Medema, R.H. , 2009. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J. Cell Biol. 185, 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q. , Guntuku, S. , Cui, X.S. , Matsuoka, S. , Cortez, D. , Tamai, K. , Luo, G. , Carattini-Rivera, S. , DeMayo, F. , Bradley, A. , Donehower, L.A. , Elledge, S.J. , 2000. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 14, 1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Lobrich, M. , Jeggo, P.A. , 2007. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat. Rev. Cancer. 7, 861–869. [DOI] [PubMed] [Google Scholar]
- Luoto, K.R. , Kumareswaran, R. , Bristow, R.G. , 2013. Tumor hypoxia as a driving force in genetic instability. Genome Integr. 4, 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macurek, L. , Lindqvist, A. , Lim, D. , Lampson, M.A. , Klompmaker, R. , Freire, R. , Clouin, C. , Taylor, S.S. , Yaffe, M.B. , Medema, R.H. , 2008. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 455, 119–123. [DOI] [PubMed] [Google Scholar]
- Marotta, D. , Karar, J. , Jenkins, W.T. , Kumanova, M. , Jenkins, K.W. , Tobias, J.W. , Baldwin, D. , Hatzigeorgiou, A. , Alexiou, P. , Evans, S.M. , Alarcon, R. , Maity, A. , Koch, C. , Koumenis, C. , 2011. In vivo profiling of hypoxic gene expression in gliomas using the hypoxia marker EF5 and laser-capture microdissection. Cancer Res. 71, 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melixetian, M. , Klein, D.K. , Sorensen, C.S. , Helin, K. , 2009. NEK11 regulates CDC25A degradation and the IR-induced G2/M checkpoint. Nat. Cell Biol. 11, 1247–1253. [DOI] [PubMed] [Google Scholar]
- Meng, A.X. , Jalali, F. , Cuddihy, A. , Chan, N. , Bindra, R.S. , Glazer, P.M. , Bristow, R.G. , 2005. Hypoxia down-regulates DNA double strand break repair gene expression in prostate cancer cells. Radiother. Oncol. 76, 168–176. [DOI] [PubMed] [Google Scholar]
- Menzel, T. , Nähse-Kumpf, V. , Kousholt, A.N. , Klein, D.K. , Lund-Andersen, C. , Lees, M. , Johansen, J.V. , Syljuåsen, R.G. , Sørensen, C.S. , 2011. A genetic screen identifies BRCA2 and PALB2 as key regulators of G2 checkpoint maintenance. EMBO Rep. 12, 705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi, R. , Gould, K.L. , 1999. Regulating the onset of mitosis. Curr. Opin. Cell Biol. 11, 267–273. [DOI] [PubMed] [Google Scholar]
- Olcina, M. , Lecane, P.S. , Hammond, E.M. , 2010. Targeting hypoxic cells through the DNA damage response. Clin. Cancer Res. 16, 5624–5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires, I.M. , Bencokova, Z. , McGurk, C. , Hammond, E.M. , 2010. Exposure to acute hypoxia induces a transient DNA damage response which includes Chk1 and TLK1. Cell Cycle. 9, 2502–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos-Mattjus, P. , Vroman, B.T. , Burtelow, M.A. , Rauen, M. , Eapen, A.K. , Karnitz, L.M. , 2002. Genotoxin-induced Rad9-Hus1-Rad1 (9-1-1) chromatin association is an early checkpoint signaling event. J. Biol. Chem. 277, 43809–43812. [DOI] [PubMed] [Google Scholar]
- Rothkamm, K. , Kruger, I. , Thompson, L.H. , Lobrich, M. , 2003. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol. Cell Biol. 23, 5706–5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh-Gohari, N. , Helleday, T. , 2004. Conservative homologous recombination preferentially repairs DNA double-strand breaks in the S phase of the cell cycle in human cells. Nucleic Acids Res. 32, 3683–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez, Y. , Wong, C. , Thoma, R.S. , Richman, R. , Wu, Z. , Piwnica-Worms, H. , Elledge, S.J. , 1997. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 277, 1497–1501. [DOI] [PubMed] [Google Scholar]
- Syljuåsen, R.G. , 2007. Checkpoint adaptation in human cells. Oncogene. 26, 5833–5839. [DOI] [PubMed] [Google Scholar]
- Syljuåsen, R.G. , Jensen, S. , Bartek, J. , Lukas, J. , 2006. Adaptation to the ionizing radiation-induced G2 checkpoint occurs in human cells and depends on checkpoint kinase 1 and Polo-like kinase 1 kinases. Cancer Res. 66, 10253–10257. [DOI] [PubMed] [Google Scholar]
- Sørensen, C.S. , Syljuåsen, R.G. , Falck, J. , Schroeder, T. , Ronnstrand, L. , Khanna, K.K. , Zhou, B.B. , Bartek, J. , Lukas, J. , 2003. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell. 3, 247–258. [DOI] [PubMed] [Google Scholar]
- Takizawa, C.G. , Morgan, D.O. , 2000. Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C. Curr. Opin. Cell Biol. 12, 658–665. [DOI] [PubMed] [Google Scholar]
- Tkacz-Stachowska, K. , Lund-Andersen, C. , Velissarou, A. , Myklebust, J.H. , Stokke, T. , Syljuåsen, R.G. , 2011. The amount of DNA damage needed to activate the radiation-induced G2 checkpoint varies between single cells. Radiother. Oncol. 101, 24–27. [DOI] [PubMed] [Google Scholar]
- van Vugt, M.A. , Bras, A. , Medema, R.H. , 2004. Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol. Cell. 15, 799–811. [DOI] [PubMed] [Google Scholar]
- Walker, D.H. , Adami, G.R. , Dold, K.M. , Babiss, L.E. , 1995. Misregulated expression of the cyclin dependent kinase 2 protein in human fibroblasts is accompanied by the inability to maintain a G2 arrest following DNA damage. Cell Growth Differ. 6, 1053–1061. [PubMed] [Google Scholar]
- Wang, Y. , Decker, S.J. , Sebolt-Leopold, J. , 2004. Knockdown of Chk1, Wee1 and Myt1 by RNA interference abrogates G2 checkpoint and induces apoptosis. Cancer Biol. Ther. 3, 305–313. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Li, J. , Booher, R.N. , Kraker, A. , Lawrence, T. , Leopold, W.R. , Sun, Y. , 2001. Radiosensitization of p53 mutant cells by PD0166285, a novel G(2) checkpoint abrogator. Cancer Res. 61, 8211–8217. [PubMed] [Google Scholar]
- Wouters, B.G. , Koritzinsky, M. , 2008. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat. Rev. Cancer. 8, 851–864. [DOI] [PubMed] [Google Scholar]
- Wu, T. , Zhang, X. , Huang, X. , Yang, Y. , Hua, X. , 2010. Regulation of cyclin B2 expression and cell cycle G2/m transition by menin. J. Biol. Chem. 285, 18291–18300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X. , Weaver, Z. , Linke, S.P. , Li, C. , Gotay, J. , Wang, X.W. , Harris, C.C. , Ried, T. , Deng, C.X. , 1999. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol. Cell. 3, 389–395. [DOI] [PubMed] [Google Scholar]
- Yamane, K. , Chen, J. , Kinsella, T.J. , 2003. Both DNA topoisomerase II-binding protein 1 and BRCA1 regulate the G2-M cell cycle checkpoint. Cancer Res. 63, 3049–3053. [PubMed] [Google Scholar]
- Yan, Y. , Cao, P.T. , Greer, P.M. , Nagengast, E.S. , Kolb, R.H. , Mumby, M.C. , Cowan, K.H. , 2010. Protein phosphatase 2A has an essential role in the activation of gamma-irradiation-induced G2/M checkpoint response. Oncogene. 29, 4317–4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. , Watkins, J.L. , Piwnica-Worms, H. , 2002. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc. Natl. Acad. Sci. U S A. 99, 14795–14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article:
Supplementary data
Figure S1
Figure S2
Figure S3
Figure S4


