Abstract
The a mating-type region of Neurospora crassa controls several major events in both the sexual and asexual phases of the fungal life cycle. This 3235-base-pair DNA segment is not homologous to the comparable genetic region of the A mating type. The unique a and A regions are bordered by nearly identical DNA sequences. The a genetic region contains at least two functional segments. One segment encodes a perithecium maturation function that is dependent on the second segment for phenotypic expression. This second a segment encodes a spliced mRNA that specifies the mt a-1 polypeptide. This polypeptide appears to be responsible for vegetative incompatibility, mating identity, and perithecium induction. The a-1 transcript is produced vegetatively and under conditions that induce sexual differentiation. The amino-terminal half of the mt a-1 polypeptide is homologous to the shorter Schizosaccharomyces pombe mat-Mc polypeptide. This homology and the properties of mt a-1 mutants suggest that the a-1 polypeptide segment that is homologous to the mat-Mc polypeptide may be primarily responsible for mating functions, while the distal segment is required for vegetative incompatibility.
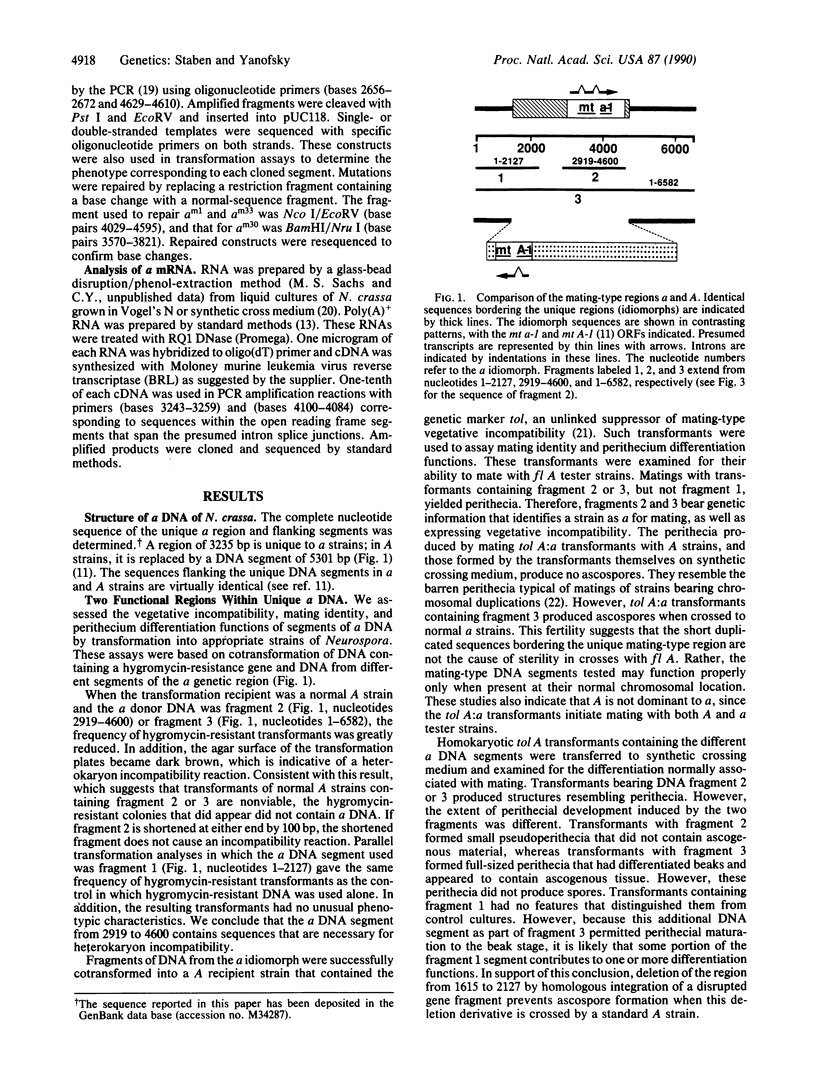
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astell C. R., Ahlstrom-Jonasson L., Smith M., Tatchell K., Nasmyth K. A., Hall B. D. The sequence of the DNAs coding for the mating-type loci of Saccharomyces cerevisiae. Cell. 1981 Nov;27(1 Pt 2):15–23. doi: 10.1016/0092-8674(81)90356-1. [DOI] [PubMed] [Google Scholar]
- Beadle G W, Coonradt V L. Heterocaryosis in Neurospora Crassa. Genetics. 1944 May;29(3):291–308. doi: 10.1093/genetics/29.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass N. L., Grotelueschen J., Metzenberg R. L. Neurospora crassa A mating-type region. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4912–4916. doi: 10.1073/pnas.87.13.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass N. L., Vollmer S. J., Staben C., Grotelueschen J., Metzenberg R. L., Yanofsky C. DNAs of the two mating-type alleles of Neurospora crassa are highly dissimilar. Science. 1988 Jul 29;241(4865):570–573. doi: 10.1126/science.2840740. [DOI] [PubMed] [Google Scholar]
- Griffiths A. J., Delange A. M. Mutations of the a Mating-Type Gene in NEUROSPORA CRASSA. Genetics. 1978 Feb;88(2):239–254. doi: 10.1093/genetics/88.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Wallace J. C. Detection of protein similarities using nucleotide sequence databases. Nucleic Acids Res. 1988 Jul 11;16(13):6191–6204. doi: 10.1093/nar/16.13.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen H. M., Admon A., Bell S. P., Tjian R. Nucleolar transcription factor hUBF contains a DNA-binding motif with homology to HMG proteins. Nature. 1990 Apr 26;344(6269):830–836. doi: 10.1038/344830a0. [DOI] [PubMed] [Google Scholar]
- Kelly M., Burke J., Smith M., Klar A., Beach D. Four mating-type genes control sexual differentiation in the fission yeast. EMBO J. 1988 May;7(5):1537–1547. doi: 10.1002/j.1460-2075.1988.tb02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermod N., O'Neill E. A., Kelly T. J., Tjian R. The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell. 1989 Aug 25;58(4):741–753. doi: 10.1016/0092-8674(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Metzenberg R. L., Glass N. L. Mating type and mating strategies in Neurospora. Bioessays. 1990 Feb;12(2):53–59. doi: 10.1002/bies.950120202. [DOI] [PubMed] [Google Scholar]
- Metzenberg R. L., Stevens J. N., Selker E. U., Morzycka-Wroblewska E. Identification and chromosomal distribution of 5S rRNA genes in Neurospora crassa. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2067–2071. doi: 10.1073/pnas.82.7.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmeyer D. A suppressor of the heterokaryon-incompatibility associated with mating type in Neurospora crassa. Can J Genet Cytol. 1970 Dec;12(4):914–926. doi: 10.1139/g70-115. [DOI] [PubMed] [Google Scholar]
- Perkins D. D., Barry E. G. The cytogenetics of Neurospora. Adv Genet. 1977;19:133–285. doi: 10.1016/s0065-2660(08)60246-1. [DOI] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Vollmer S. J., Yanofsky C. Efficient cloning of genes of Neurospora crassa. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4869–4873. doi: 10.1073/pnas.83.13.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]



