Abstract
1. Background
Bevacizumab combination therapy is among the most frequently used treatments in recurrent glioblastoma and patients who achieve response to bevacizumab have improved survival as well as quality of life. Accordingly, the aim of this study was to identify predictive biomarkers for bevacizumab response in recurrent glioblastoma patients.
2. Methods
The study included a total of 82 recurrent glioblastoma patients treated with bevacizumab combination therapy whom were both response and biomarker evaluable. Gene expression of tumor tissue was analyzed by using a customized NanoString platform covering 800 genes. Candidate gene predictors associated with response were analyzed by multivariate logistic and Cox regression analysis.
3. Results
Two genes were independently associated with response: Low expression of angiotensinogen (2‐fold decrease in AGT; OR = 2.44; 95% CI: 1.45–4.17; P = 0.0009) and high expression of a HLA class II gene (2‐fold increase in HLA‐DQA1; OR = 1.22; 95% CI: 1.01–1.47; P = 0.04). These two genes were included in a model that is able predict response to bevacizumab combination therapy in clinical practice. When stratified for a validated prognostic index, the predictive model for response was significantly associated with improved overall survival.
4. Conclusion
Two genes (low angiotensinogen and high HLA‐class II expression) were predictive for bevacizumab response and were included in a predictive model for response. This model can be used in clinical practice to identify patients who will benefit from bevacizumab combination therapy.
Keywords: Predictive model, Angiotensin, Vascular normalization, Immune activation, Anti-angiogenic treatment, Glioblastoma, Antigen presentation
Highlights
Recurrent glioblastoma patients treated with bevacizumab combination therapy were included in the study.
Gene expression profiling of tumor tissue was performed by a customized NanoString platform covering 800 genes.
Expression of angiotensinogen and HLA class II genes were identified as independent predictors for bevacizumab response.
The two genes were included in a predictive model for response.
The predictive model, if validated, can be used to identify patients benefitting from bevacizumab combination therapy.
Abbreviations
- VEGF
vascular endothelial growth factor A
- C-index
concordance index
- AGT
angiotensinogen
- HLA-DQA1
human leukocyte antigen complex class II DQ alpha 1
- IDH1
isocitrate dehydrogenase 1
1. Introduction
Glioblastoma is the most common primary malignant brain tumor in adults. Despite aggressive standard treatment, including maximal surgical resection and post‐operative radiochemotherapy with temozolomide concomitantly and as maintenance, newly diagnosed patients have a median overall survival (OS) of less than 15 months (Stupp et al., 2005). At tumor recurrence no standard treatment is available and most known options have limited clinical effect.
Glioblastoma is characterized by increased angiogenesis and abnormal network of blood vessels. Anti‐angiogenic agents inhibiting vascular endothelial growth factor A (VEGF) have been shown to normalize the tumor vasculature and improve blood flow, emphasizing the potential value of combining anti‐angiogenic therapy with drugs targeting the tumor (Batchelor et al., 2013; Lu‐Emerson et al., 2015). However, recent results from the first randomized phase III trial investigating chemotherapy with or without the VEGF‐antibody bevacizumab did not demonstrate any difference in OS when considering the whole group of recurrent glioblastoma patients (Wick et al., 2015). Still, approximately 30% of patients achieve durable bevacizumab response and this group of patients has demonstrated improved survival as well as quality of life (Henriksson et al., 2011; Huang et al., 2016; Moller et al., 2012). This underscores the importance of identifying patients who will benefit from bevacizumab combination therapy. To date, no validated predictive tumor markers of a durable bevacizumab response have been identified. By analyzing gene expression profiles of glioblastoma patient tumors, the aim of this study was to identify predictive factors for bevacizumab response in recurrent glioblastoma patients.
2. Patients and methods
2.1. Patients
All patients with pathologically confirmed glioblastoma (WHO grade IV) who were treated at recurrence with bevacizumab plus irinotecan between May 2005 and December 2011 at Rigshospitalet were assessed for eligibility. During this period, bevacizumab (10 mg/kg) and irinotecan (125 mg/m2), administered every two weeks, could be prescribed to all recurrent glioblastoma patients in WHO performance status 0–2 according to a published treatment protocol (Poulsen et al., 2009). Alternatively, both agents were combined with cetuximab in a phase 2 trial (Hasselbalch et al., 2010). Bevacizumab monotherapy was not administered at our center. Eligibility criteria for this study were response evaluability and biomarker assessable tissue from the time of glioblastoma diagnosis. The criteria are specified in Section 2.2–2.4 and a REMARK diagram is shown in Supplementary Figure S1. The study was conducted in accordance with the Helsinki Declaration and was approved by the Danish Ethical Committee (H‐2‐2012‐069).
2.2. Clinical follow‐up
According to the treatment protocol, patients had to have measurable progressive disease by contrast‐enhanced MRI after standard therapy and be at least 4 weeks from prior chemotherapy and 3 months from completion of radiation therapy. For patients who had undergone relapse surgery a post‐surgical MRI was performed prior to treatment initiation. Clinical follow‐up was performed every 4‐weeks and MRI every 8 weeks. Treatment response was evaluated based on the RANO criteria (Wen et al., 2010). Patients were categorized according to their best response; patients who achieved complete response (CR) or partial response (PR) were classified as responders, while patients with stable disease (SD) or progressive disease (PD) were classified as non‐responders. Patients not evaluable by MRI at first response evaluation (week 8) due to early toxicity, progression or death were classified as non‐evaluable and excluded.
2.3. Sample acquisition and RNA preparation
A total of 90 archived formalin‐fixed, paraffin‐embedded tissue samples from time of initial glioblastoma diagnosis were collected and freshly cut sections (5 microns) were sent to HistogeneX, Belgium, and stored at 2–8 °C. Tissue review was conducted by a pathologist blinded to identifiers and clinical outcome, and areas containing representative tumor cells were marked on hematoxylin and eosin‐stained slides. Five samples with insufficient tumor tissue area for RNA analysis were excluded. Tumors were microdissected to enrich tumor cell RNA in the gene expression analyses. RNA was extracted using the High Pure RNA Paraffin Isolation kit (Roche, Ca. No. 03 270 289 001) and RNA extracts were stored at −80 °C.
2.4. Gene expression data generation
The platform consisted of 800 genes selected by Genentech using a custom code set for the NanoString gene expression platform (NanoString Technologies, Seattle, WA) (Geiss et al., 2008). Genes were selected from the literature to allow glioblastoma molecular subtype classification according to Phillips' classifier (Phillips et al., 2006) and to cover genes regulating angiogenesis, immune system and other glioblastoma‐related cancer hallmarks. Analyses were performed using the software R version 3.1 (R Development Core Team, Vienna, Austria, http://www.R‐project.org). Raw counts for 85 tumor samples were log2 transformed and normalized to 8 housekeeping genes recommended by Genentech and previously used on the AvaGlio dataset (Sandmann et al., 2015). The normalization procedure is described in Supplementary Method 1. Based on the distribution of normalized counts, 3 outlier samples were identified and removed from further analysis, leaving 82 evaluable samples. Subtype labels were assigned to tumor samples by Genentech blinded to clinical outcome using the 31 gene classifier previously trained on the AVAglio dataset (Sandmann et al., 2015).
2.5. Immunohistochemistry
Immunohistochemical analysis was conducted on 5 micron thick formalin‐fixed, paraffin‐embedded tissue sections. Following deparaffinization and protease treatment immunostaining was performed using the OptiView DAB IHC v4 Protocol (v1.00.0108) and the BenchMark ULTRA IHC staining Module (Ventana Medical System, Tucson, AZ, USA). The primary antibodies used were anti‐HLA‐DQA1 (dilution 1:150, Abcam, EPR7300), anti‐HLA‐DR (dilution 1:2000, DAKO, TAL 1B5), anti‐AGT (dilution 1:1500, LS Bioscience, LS‐B6575), anti‐CD31 (ready‐to‐use, Ventana Medical System, JC70), anti‐collagen‐IV (dilution 1:20, DAKO, CIV 22) and anti‐SMA (ready‐to‐use, DAKO, 1A4).
2.6. Statistical analysis
Survival probabilities (PFS and OS) were estimated with the Kaplan–Meier method. Welch's test was performed to identify differentially expressed genes between groups and significant genes with a fold‐change >1.5 were considered. Treatment response was estimated by employing logistic regression (modelling the probability of response) and the results presented by odds ratios with 95% confidence intervals (95% CI) and the area under the receiver operating characteristic curve. The Cox proportional hazards model was used for modelling survival endpoints and results are presented as hazard ratios (HR) with 95% CI. Continues covariates were log transformed (log base 2) for analysis. Assessment of the model assumptions was done using Hosmer–Lemeshow test and martingale residuals. Factors associated with response with P‐values below 0.20 in univariate analysis were considered for multivariate analysis. Penalized maximum likelihood estimation was utilized for multivariate analysis and concordance indices (C‐index) was calculated as a measure of discrimination (Harrell, Jr. et al., 1996). Five‐fold cross‐validation was applied to the analysis of response in order to assess the estimated model. P‐values < 0.05 were considered significant. Calculations were performed using SPSS (v19.0, IBM Corp., Armonk, NY), R version 3.1 and SAS (v9.3, SAS institute, Cary, NC).
3. Results
3.1. Patient characteristics
Of the 158 patients registered as receiving bevacizumab combination therapy at the time of relapse, 82 patients were response and biomarker evaluable (REMARK diagram, Supplementary Figure S1). Patient characteristics and clinical outcomes for the 82 patients are shown in Table 1. Response was observed in 29 patients (35%) of whom 22 (76%) achieved response at first treatment evaluation. After progression on bevacizumab combination treatment, 13 patients underwent surgical resection and 10 patients received various types of experimental treatments. Two patients were alive at the end of follow‐up and all had progressed (median‐follow‐up: 8.3 months, range: 2–69 months).
Table 1.
Patient characteristics.
| Total (n = 82) | |
|---|---|
| Gender, n (%) | |
| Male | 51 (62) |
| Female | 31 (38) |
| Age, years (range) | |
| Median | 56 (23–71) |
| WHO performance status, n (%) | |
| 0 | 34 (42) |
| 1 | 37 (45) |
| 2 | 11 (13) |
| Prior lines of chemotherapy, n (%) | |
| 1 | 73 (89) |
| 2 | 9 (11) |
| Multifocal disease, n (%) | |
| Yes | 21 (26) |
| No | 61 (74) |
| Corticosteroid use, n (%)a | |
| Yes | 61 (74) |
| No | 21 (26) |
| Neurocognitive deficit, n (%) | |
| Yes | 43 (52) |
| No | 39 (48) |
| Bevacizumab combination therapy, n (%) | |
| Irinotecan | 67 (82) |
| Irinotecan and cetuximab | 15 (18) |
| Response, n (%) | |
| Response (CR + PR) | 29 (35) |
| Stable disease | 42 (51) |
| Progressive disease | 11 (14) |
| Median progression‐free survival, months | 5.3 |
| Responders | 10.9 |
| Non‐responders | 3.9 |
| Median overall survival, months | 8.2 |
| Responders | 13.8 |
| Non‐responders | 7.5 |
AbbreviationsCR, complete response; PR, partial response.
Prednisolone >10 mg.
3.2. Prognostic factors
Univariate analysis was performed to test if previously identified prognostic factors, shown in Table 1, were associated with response and to test if the cetuximab combined treatment had an impact on response. None of these factors were associated with response. The gene expression profiles of glioblastomas treated with and without cetuximab were comparable and only 3 genes were significantly differentially expressed between these two groups (IFI27, IFIT3 up‐regulated and POSTN down‐regulated in the cetuximab group). None of these genes were associated with response. In addition, we tested a recently established and validated prognostic index for recurrent glioblastoma patients treated with bevacizumab and irinotecan (Urup et al., 2016). This index consists of 8 prognostic groups according to all possible combinations of the presence or absence of 3 independent prognostic factors: corticosteroid use (≥10 mg Prednisolone), neurocognitive deficit (≥minor) and multifocal disease. When applied to the current study cohort, the index was by univariate analysis significantly associated with PFS (P = 0.01) and OS (P = 0.005) but it was not associated with response (P = 0.45).
3.3. Molecular subtypes
Out of 82 samples, 27 were classified as proneural and 32 as the mesenchymal subtype. As illustrated in Supplementary Figure S2, the remaining 23 samples, categorized as proliferative or unclassified subtype, separated poorly from the proneural and mesenchymal subtypes. Consequently, it was decided to analyze only the two robust subtypes as dichotomized variables: Proneural vs. non‐proneural and mesenchymal vs. non‐mesenchymal. By univariate analysis, shown in Supplementary Table S1, the two subtypes showed no association with response. Furthermore, no association with PFS or OS was observed in univariate analysis, nor when stratified for the prognostic index described above.
3.4. Identification of biomarkers associated with bevacizumab response
As shown in Figure 1, after pre‐processing data, three steps were utilized to identify differentially expressed genes associated with treatment response. First, samples were divided into three groups according to best response: Response (CR + PR), stable disease (SD) and progressive disease (PD). To identify candidate genes differentially expressed between the two most extreme groups (response and PD) and to address unequal variance and unequal sample sizes of the groups, a Welch's t‐test was performed (Step 2). Out of 792 genes, 9 genes were found significantly differentially expressed with a median fold change >1.5. Among the 9 genes shown in Supplementary Table S2, two genes were significantly up‐regulated (BEST3 and RTN1) and one was down‐regulated (ERBB2) in the proneural subtype compared to the mesenchymal subtype. The 9 genes were screened for association with response (CR + PR) versus non‐response (SD + PD) by univariate analysis (Step 3). As shown in Supplementary Table S3, 5 genes were found associated with response (P < 0.20) and these were tested by multivariate analysis (Step 4, Supplementary Table S4). This analysis presented angiotensinogen (AGT) and a HLA class II gene (human leukocyte antigen complex class II DQ alpha 1, HLA‐DQA1) as being the most interesting markers associated with response.
Figure 1.
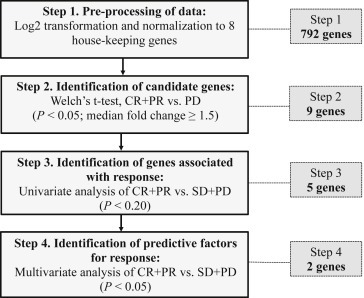
Flowchart for identification of differentially expressed genes associated with bevacizumab response. The number of genes shown in the right dotted box denotes the number of genes identified according to analytical steps.
3.5. Predictors for response
Table 2 summarizes the final multivariate model for response. Indeed, low gene expression of angiotensinogen (2‐fold decrease: OR = 2.44; 95% CI: 1.45–4.17; P = 0.0009) and high expression of HLA class II (DQA1) (2‐fold increase: OR = 1.22; 95% CI: 1.01–1.47; P = 0.04) were significantly associated with an increased likelihood of response. None of the remaining gene candidates were significantly associated with response when added to the model. The final model for response had a high C‐index of 0.78.
Table 2.
Multivariate analysis of response, PFS and OS.
| Gene expression | Response OR (95% CI) | PFSa HR (95% CI) | OSa HR (95% CI) |
|---|---|---|---|
| Angiotensinogen (2‐fold decrease) | 2.44 (1.45–4.17)P = 0.0009 | 0.75 (0.59–0.94)P = 0.01 | 0.70 (0.54–0.94)P = 0.005 |
| HLA‐class II (DQA1) (2‐fold increase) | 1.22 (1.01–1.47)P = 0.04 | 0.96 (0.88–1.04)P = 0.31 | 0.95 (0.87–1.04)P = 0.27 |
| C‐index | 0.78 | 0.67 | 0.68 |
Note: The prognostic index was not associated with response (P = 0.45).; Abbreviations: HLA‐class II (DQA1), human leukocyte antigen complex class II DQ alpha 1, HLA‐DQA1.
Stratified for a prognostic index consisting of three independent prognostic factors: Corticosteroid use, neurocognitive deficit and multifocal disease.
3.6. Association of predictors with PFS and OS
The two genes predictive for response were analyzed for association with PFS and OS. By univariate analysis, low gene expression of angiotensinogen was significantly associated with prolonged PFS (P = 0.01) and OS (P < 0.01), and high expression of HLA class II (DQA1) was significantly associated with prolonged OS (P = 0.03) but was not associated with PFS (P = 0.16). By multivariate analysis stratified for the prognostic index (Table 2), low expression of angiotensinogen was independently associated with prolonged PFS (2‐fold decrease: HR = 0.75; 95% CI: 0.59–0.94; P = 0.01) and OS (2‐fold decrease: HR = 0.70; 95% CI: 0.54–0.94; P = 0.005), while HLA class II (DQA1) expression did not significantly influence PFS or OS. The C‐indices for the PFS and OS model were 0.67 and 0.68, respectively.
3.7. Clinical predictive model for response
In order to develop a model which in clinical practice can be used to predict bevacizumab response, the multivariate model for response was used to determine a cut point for angiotensinogen and HLA class II (DQA1) gene expression. Due to limitations and difficulties in response assessment, we prioritized a high specificity in preference to a high sensitivity in order to increase the likelihood of identifying patients not responding and not benefitting from bevacizumab treatment. Accordingly, a model able to predict bevacizumab response with a sensitivity of 66% at a specificity of 80% was established. In Figure 2, the linear curve is the gene expression threshold for angiotensinogen and HLA class II (DQA1) separating responders from patients not responding, illustrating that the gene expression threshold for each gene increases as a function of the other. In clinical practice this means that a patient with a relatively high expression of angiotensinogen (e.g. 900) is predicted to achieve response only if HLA class II (DQA1) is also relatively high (e.g. 1500), while another patient with the same expression of angiotensinogen but a lower expression of HLA class II (DQA1) will not respond to bevacizumab. The cross validation procedure confirmed the estimated model for response, both covariates were significant in all cases and the C‐index was 0.75 for the test component.
Figure 2.
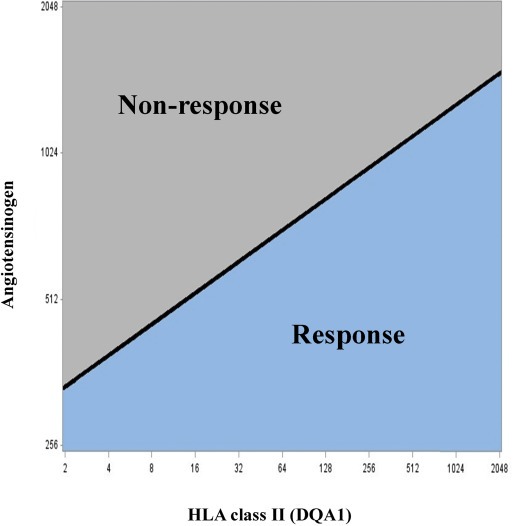
Predictive model for response to bevacizumab. The linear curve is the threshold for angiotensinogen and human leukocyte antigen complex class II DQ alpha 1 (DCA1) gene expression, separating responders from non‐responders with a sensitivity of 66% and a specificity of 80%. X‐ and Y‐axis represent gene expression count data for the two genes normalized to reference genes.
When stratified according to the prognostic index, patients who according to the predictive model were predicted to respond had a borderline significantly longer PFS (P = 0.06) and significantly longer OS (P < 0.01) compared to patients predicted not to respond. This association with OS remained significant when patients progressing at the first response evaluation were excluded from the analysis, indicating that the association of the model with OS is not due to including early progressors.
3.8. Immunohistochemistry
To examine the protein expression intensity and localization of angiotensinogen and HLA class‐II proteins in glioblastoma, immunohistochemical analysis was performed on 10 tumor samples. These were the 5 showing the highest and the 5 showing the lowest gene expression levels of angiotensinogen on the NanoString platform. Staining for HLA‐class II (DQA1) and HLA‐DR as a control resulted in a similar granular cytoplasmic staining of macrophages and microglia located perivascular, around necrosis and diffusely in the stroma to a varying degree. There were no obvious differences in amount and location of HLA expressing cells across the samples with differing angiotensinogen expression (Supplementary Figure S3).
As shown in Figure 3, angiotensinogen demonstrated a more diffuse staining in both reactive astrocytes, macrophages, microglia, glial tumor cells, endothelial cells and the extracellular matrix. The cellular staining was either cytoplasmic, nuclear or both. The intensity was varying between samples and intratumoral heterogeneity was most pronounced between malignant proliferating vessels and tumor cells. In glioblastomas with low gene expression, the staining intensity in tumor cells was mostly cytoplasmic and lower compared to tumor cells of glioblastomas with high angiotensinogen gene expression, which had a more pronounced staining in both cytoplasm and nucleus. The proliferating vessels in low angiotensinogen expressing glioblastomas seemed less compact, less fibrotic, and consisted of a mixture of positive and negative endothelial cells. In contrast, vessels in high angiotensinogen expressing tumors were more compact, fibrotic, proliferative and had smaller lumina.
Figure 3.
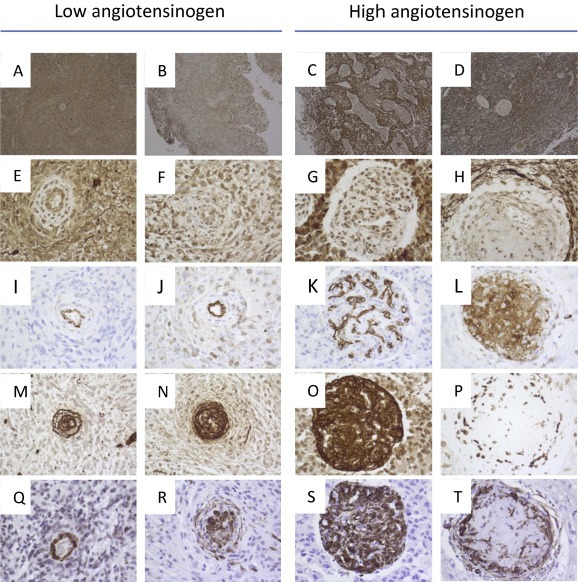
Immunohistochemistry of glioblastomas with low and high angiotensinogen expression. Overviews (×50) of angiotensinogen stains are shown for two low (A–B) and two high (C–D) angiotensinogen gene expressing tumors. Tumor blood vessels (×400) of corresponding angiotensinogen stains are shown below for low (E–F) and high (G–H) angiotensinogen expression. Serial sections of corresponding samples were stained for CD31 (I–L), Collagen IV (M–P) and smooth muscle actin (Q–T) and blood vessels (×400) are shown below for tumors with low (I‐J, M−N, Q‐R) and high (K‐L, OP, ST) angiotensinogen gene expression.
To characterize the observed differences of the vasculature in more detail, we stained for endothelial cells (CD31), Collagen IV and smooth muscle actin (SMA). As shown in Figure 3, low angiotensinogen gene expression was associated with a more normal CD31 stain of endothelial cells of vessels (I–J), Collagen IV (M−N) and SMA (Q–R) were located around the vascular lumen. These more normal vessels were also present in tumors expressing high angiotensinogen. However, in the tumors with high angiotensinogen expression the vasculature was more abnormal. Some of these highly abnormal vessels appeared highly viable with CD31 located on endothelial luminal cells (Figure 3K), hyperplastic with greater SMA (Figure 3O) and with more compact Collagen IV (Figure 3S) immunostaining. Other vessels were characterized by diffuse CD31 staining (Figure 3L), reduced Collagen IV (Figure 3P) and fragmented SMA (Figure 3T). Both of these vascular phenotypes were present in the tumors with high angiotensinogen expression and the tumors also expressed the more normal variant described in the low angiotensinogen expressing tumors.
Taken together, the vasculature varied within and between the tumors. However, the vasculature of the tumors with high angiotensinogen expression showed a greater variability of the vascular phenotype, had smaller vessel lumina and appeared more hyperplastic and more proliferative compared to tumors expressing low angiotensinogen.
4. Discussion
In this retrospective study of 82 recurrent glioblastoma patients treated with bevacizumab combination therapy, gene expression profiles of tumor tissue from the initial glioblastoma diagnosis were analyzed with the aim of identifying predictive factors for bevacizumab response. By analyzing candidate genes differentially expressed between responders and patients with early progressive disease, the expression of two genes were found independently associated with a favorable response to bevacizumab therapy: These were low gene expression of angiotensinogen (AGT) and high gene expression of HLA class II (HLA‐DQA1). Both were included in a clinically relevant model that can predict whether a patient is likely or not to respond to bevacizumab combination therapy.
In support of our findings, angiotensinogen has previously been found overexpressed in tumors of metastatic colorectal cancer patients not responding to bevacizumab combination therapy (Martin et al., 2014). In addition, it has been shown that angiotensinogen and all components of the renin‐angiotensin system, including the main effector peptide angiotensin‐II, are expressed in glioblastomas (Juillerat‐Jeanneret et al., 2004).
The renin‐angiotensin system appears to exert dual effects on the vasculature, as angiotensinogen has demonstrated anti‐angiogenic signaling (Celerier et al., 2002), while angiotensin‐II has been observed to induce angiogenesis (Arrieta et al., 2008; Paul et al., 2006). Here we found that increasing angiotensinogen expression was associated with a higher level of vascular proliferation, suggesting an angiotensin‐II dominating effect on the vasculature. Furthermore, high expression levels of angiotensinogen was associated with a more abnormal vessel architecture, characterized by excessive vascular remodeling and greater numbers blood vessels with reduced vessel lumina. These findings are also in line with angiotensin‐II signaling which stimulates vascular remodeling (Lacolley et al., 2012). Of note, angiotensinogen gene expression was not correlated to VEGF gene expression. Accordingly, we hypothesize that locally produced angiotensinogen and angiotensin‐II induce an abnormal and poorly perfused tumor vasculature which cannot sufficiently be normalized by bevacizumab therapy.
Angiotensin‐II inhibition has demonstrated a steroid‐sparring and anti‐edema effect in glioblastoma patients (Carpentier et al., 2012). In addition, preclinical and retrospective studies suggest that combination of angiotensin‐II inhibition and anti‐angiogenic therapy at least has an additive effect (Keizman et al., 2011; McKay et al., 2015; Stylianopoulos and Jain, 2013). Consequently, we are retrospectively investigating the efficacy and safety of this combination treatment in recurrent glioblastoma patients. This and other clinical studies, including an ongoing phase III trial with angiotensin‐II inhibition in combination with standard therapy (NCT01805453), will provide information on whether angiotensin‐II inhibition should be administered to glioblastoma patients.
HLA‐class II receptors are expressed on antigen presenting cells and by immunohistochemistry analysis expression was observed on microglia and macrophages. A possible explanation for the association of high HLA‐class II gene expression and bevacizumab response is that HLA class II is up‐regulated on local antigen presenting cells, which in turn directly activates and maintains a cytotoxic anti‐tumor immune response. In such a scenario, bevacizumab treatment might induce an active immune response which is otherwise often reported to be skewed towards an immunosuppressive profile in glioblastoma (Nduom et al., 2015). Indeed, accumulating data indicate that anti‐angiogenic agents activate anti‐tumor immune cells and upon normalization of the vasculature increase the number of these tumor infiltrating immune cells (Huang et al., 2013). Accordingly, HLA‐class II expression may reflect an existing anti‐tumor immune profile, which in concert with bevacizumab‐induced immune activation may explain the association of HLA‐class II with a beneficial effect of bevacizumab. Several clinical trials are currently evaluating combinatorial regimens of bevacizumab with different types of immunomodulating agents for glioblastoma patients (Reardon et al., 2015).
The molecular subtypes in our cohort had no impact on response, PFS or OS. Whether the proneural subtype (IDH1 wildtype) is a predictive factor for improved survival in bevacizumab treated glioblastoma patients, as suggested in the AvaGlio dataset (Sandmann et al., 2015), remains to be validated in a randomized trial. However, as subtype assignment has been shown to change following treatment and as a consequence of intratumoral heterogeneity, a clinically relevant subtype classification for recurrent glioblastoma has yet to be established (Patel et al., 2014; Phillips et al., 2006).
In summary, we identified low gene expression of angiotensinogen and high expression of a HLA‐class II gene (HLA‐DQA1) as independent predictors of bevacizumab response. Both genes are according to the literature involved in response and resistance mechanisms to anti‐angiogenic combination therapies and we are currently testing these hypotheses pre‐clinically as well as clinically. Based on the two identified genes we established a model which in clinical practice has the potential to predict bevacizumab response in recurrent glioblastoma patients. If validated, this model will contribute to identifying patients who will or will not benefit from bevacizumab combination therapy.
Funding
The study was supported by Rigshospitalet, the Danish Cancer Society (R90‐A6057), Doctor Sophus Carl Emil Friis and Wife Olga Friis' Grant and I.M. Daehnfeldt Foundation. Ole Winther and Lars Rønn Olsen were supported by a grant from Novo Nordisk Foundation. An unrestricted grant was provided from Roche. Genentech funded the generation of gene expression data. Roche and Genentech were not involved in interpretation of the results or presentation of the data of this study.
Conflict of interests
The authors have no conflict of interests to declare.
Supporting information
The following are the supplementary data related to this article:
Supplementary data
Supplementary data
Acknowledgments
The authors would like to thank Carlos Bais and Thomas Sandmann, Genentech, for generating gene expression data and for providing subtype classification.
Supplementary data 1.
1.1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2016.05.005.
Urup Thomas, Michaelsen Signe Regner, Olsen Lars Rønn, Toft Anders, Christensen Ib Jarle, Grunnet Kirsten, Winther Ole, Broholm Helle, Kosteljanetz Michael, Issazadeh-Navikas Shohreh, Poulsen Hans Skovgaard, Lassen Ulrik, (2016), Angiotensinogen and HLA class II predict bevacizumab response in recurrent glioblastoma patients, Molecular Oncology, 10, doi: 10.1016/j.molonc.2016.05.005.
References
- Arrieta, O. , Pineda-Olvera, B. , Guevara-Salazar, P. , Hernandez-Pedro, N. , Morales-Espinosa, D. , Ceron-Lizarraga, T.L. , Gonzalez-De la Rosa, C.H. , Rembao, D. , Segura-Pacheco, B. , Sotelo, J. , 2008. Expression of AT1 and AT2 angiotensin receptors in astrocytomas is associated with poor prognosis. Br. J. Cancer. 99, 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor, T.T. , Gerstner, E.R. , Emblem, K.E. , Duda, D.G. , Kalpathy-Cramer, J. , Snuderl, M. , Ancukiewicz, M. , Polaskova, P. , Pinho, M.C. , Jennings, D. , Plotkin, S.R. , Chi, A.S. , Eichler, A.F. , Dietrich, J. , Hochberg, F.H. , Lu-Emerson, C. , Iafrate, A.J. , Ivy, S.P. , Rosen, B.R. , Loeffler, J.S. , Wen, P.Y. , Sorensen, A.G. , Jain, R.K. , 2013. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc. Natl. Acad. Sci. U. S. A.. 110, 19059–19064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier, A.F. , Ferrari, D. , Bailon, O. , Ursu, R. , Banissi, C. , Dubessy, A.L. , Belin, C. , Levy, C. , 2012. Steroid-sparing effects of angiotensin-II inhibitors in glioblastoma patients. Eur. J. Neurol.. 19, 1337–1342. [DOI] [PubMed] [Google Scholar]
- Celerier, J. , Cruz, A. , Lamande, N. , Gasc, J.M. , Corvol, P. , 2002. Angiotensinogen and its cleaved derivatives inhibit angiogenesis. Hypertension. 39, 224–228. [DOI] [PubMed] [Google Scholar]
- Geiss, G.K. , Bumgarner, R.E. , Birditt, B. , Dahl, T. , Dowidar, N. , Dunaway, D.L. , Fell, H.P. , Ferree, S. , George, R.D. , Grogan, T. , James, J.J. , Maysuria, M. , Mitton, J.D. , Oliveri, P. , Osborn, J.L. , Peng, T. , Ratcliffe, A.L. , Webster, P.J. , Davidson, E.H. , Hood, L. , Dimitrov, K. , 2008. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat. Biotechnol.. 26, 317–325. [DOI] [PubMed] [Google Scholar]
- Harrell, F.E. , Lee, K.L. , Mark, D.B. , 1996. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med.. 15, 361–387. [DOI] [PubMed] [Google Scholar]
- Hasselbalch, B. , Lassen, U. , Hansen, S. , Holmberg, M. , Sorensen, M. , Kosteljanetz, M. , Broholm, H. , Stockhausen, M.T. , Poulsen, H.S. , 2010. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a phase II trial. Neuro. Oncol.. 12, 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson, R. , Asklund, T. , Poulsen, H.S. , 2011. Impact of therapy on quality of life, neurocognitive function and their correlates in glioblastoma multiforme: a review. J. Neurooncol.. 104, 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, R.Y. , Rahman, R. , Ballman, K.V. , Felten, S.J. , Anderson, S.K. , Ellingson, B.M. , Nayak, L. , Lee, E.Q. , Abrey, L.E. , Galanis, E. , Reardon, D.A. , Pope, W.B. , Cloughesy, T.F. , Wen, P.Y. , 2016 Feb. The impact of T2/FLAIR evaluation per RANO criteria on response assessment of recurrent glioblastoma patients treated with bevacizumab. Clin. Cancer Res.. 22, (3) 1575–1581. PMID: 26490307 [DOI] [PubMed] [Google Scholar]
- Huang, Y. , Goel, S. , Duda, D.G. , Fukumura, D. , Jain, R.K. , 2013. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res.. 73, 2943–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juillerat-Jeanneret, L. , Celerier, J. , Chapuis, B.C. , Nguyen, G. , Wostl, W. , Maerki, H.P. , Janzer, R.C. , Corvol, P. , Gasc, J.M. , 2004. Renin and angiotensinogen expression and functions in growth and apoptosis of human glioblastoma. Br. J. Cancer. 90, 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keizman, D. , Huang, P. , Eisenberger, M.A. , Pili, R. , Kim, J.J. , Antonarakis, E.S. , Hammers, H. , Carducci, M.A. , 2011. Angiotensin system inhibitors and outcome of sunitinib treatment in patients with metastatic renal cell carcinoma: a retrospective examination. Eur. J. Cancer. 47, 1955–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacolley, P. , Regnault, V. , Nicoletti, A. , Li, Z. , Michel, J.B. , 2012. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc. Res.. 95, 194–204. [DOI] [PubMed] [Google Scholar]
- Lu-Emerson, C. , Duda, D.G. , Emblem, K.E. , Taylor, J.W. , Gerstner, E.R. , Loeffler, J.S. , Batchelor, T.T. , Jain, R.K. , 2015. Lessons from anti-vascular endothelial growth factor and anti-vascular endothelial growth factor receptor trials in patients with glioblastoma. J. Clin. Oncol.. 33, 1197–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, P. , Noonan, S. , Mullen, M.P. , Scaife, C. , Tosetto, M. , Nolan, B. , Wynne, K. , Hyland, J. , Sheahan, K. , Elia, G. , O'Donoghue, D. , Fennelly, D. , O'Sullivan, J. , 2014. Predicting response to vascular endothelial growth factor inhibitor and chemotherapy in metastatic colorectal cancer. BMC Cancer. 14, 887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay, R.R. , Rodriguez, G.E. , Lin, X. , Kaymakcalan, M.D. , Hamnvik, O.P. , Sabbisetti, V.S. , Bhatt, R.S. , Simantov, R. , Choueiri, T.K. , 2015. Angiotensin system inhibitors and survival outcomes in patients with metastatic renal cell carcinoma. Clin. Cancer Res.. 21, 2471–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller, S. , Grunnet, K. , Hansen, S. , Schultz, H. , Holmberg, M. , Sorensen, M. , Poulsen, H.S. , Lassen, U. , 2012. A phase II trial with bevacizumab and irinotecan for patients with primary brain tumors and progression after standard therapy. Acta Oncol.. 51, 797–804. [DOI] [PubMed] [Google Scholar]
- Nduom, E.K. , Weller, M. , Heimberger, A.B. , 2015. Immunosuppressive mechanisms in glioblastoma. Neuro. Oncol.. 17, (Suppl. 7) vii9–vii14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, A.P. , Tirosh, I. , Trombetta, J.J. , Shalek, A.K. , Gillespie, S.M. , Wakimoto, H. , Cahill, D.P. , Nahed, B.V. , Curry, W.T. , Martuza, R.L. , Louis, D.N. , Rozenblatt-Rosen, O. , Suva, M.L. , Regev, A. , Bernstein, B.E. , 2014. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 344, 1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, M. , Poyan, M.A. , Kreutz, R. , 2006. Physiology of local renin-angiotensin systems. Physiol. Rev.. 86, 747–803. [DOI] [PubMed] [Google Scholar]
- Phillips, H.S. , Kharbanda, S. , Chen, R. , Forrest, W.F. , Soriano, R.H. , Wu, T.D. , Misra, A. , Nigro, J.M. , Colman, H. , Soroceanu, L. , Williams, P.M. , Modrusan, Z. , Feuerstein, B.G. , Aldape, K. , 2006. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 9, 157–173. [DOI] [PubMed] [Google Scholar]
- Poulsen, H.S. , Grunnet, K. , Sorensen, M. , Olsen, P. , Hasselbalch, B. , Nelausen, K. , Kosteljanetz, M. , Lassen, U. , 2009. Bevacizumab plus irinotecan in the treatment patients with progressive recurrent malignant brain tumours. Acta Oncol.. 48, 52–58. [DOI] [PubMed] [Google Scholar]
- Reardon, D.A. , Gilbert, M.R. , Wick, W. , Liau, L. , 2015. Immunotherapy for neuro-oncology: the critical rationale for combinatorial therapy. Neuro. Oncol.. 17, (Suppl. 7) vii32–vii40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmann, T. , Bourgon, R. , Garcia, J. , Li, C. , Cloughesy, T. , Chinot, O.L. , Wick, W. , Nishikawa, R. , Mason, W. , Henriksson, R. , Saran, F. , Lai, A. , Moore, N. , Kharbanda, S. , Peale, F. , Hegde, P. , Abrey, L.E. , Phillips, H.S. , Bais, C. , 2015. Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: retrospective analysis of the AVAglio trial. J. Clin. Oncol.. 33, 2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp, R. , Mason, W.P. , van den Bent, M.J. , Weller, M. , Fisher, B. , Taphoorn, M.J. , Belanger, K. , Brandes, A.A. , Marosi, C. , Bogdahn, U. , Curschmann, J. , Janzer, R.C. , Ludwin, S.K. , Gorlia, T. , Allgeier, A. , Lacombe, D. , Cairncross, J.G. , Eisenhauer, E. , Mirimanoff, R.O. , 2005. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med.. 352, 987–996. [DOI] [PubMed] [Google Scholar]
- Stylianopoulos, T. , Jain, R.K. , 2013. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc. Natl. Acad. Sci. U. S. A.. 110, 18632–18637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urup, T. , Dahlrot, R.H. , Grunnet, K. , Christensen, I.J. , Michaelsen, S.R. , Toft, A. , Larsen, V.A. , Broholm, H. , Kosteljanetz, M. , Hansen, S. , Poulsen, H.S. , Lassen, U. , 2016. Development and validation of a prognostic model for recurrent glioblastoma patients treated with bevacizumab and irinotecan. Acta Oncol.. 1–5. [DOI] [PubMed] [Google Scholar]
- Wen, P.Y. , Macdonald, D.R. , Reardon, D.A. , Cloughesy, T.F. , Sorensen, A.G. , Galanis, E. , Degroot, J. , Wick, W. , Gilbert, M.R. , Lassman, A.B. , Tsien, C. , Mikkelsen, T. , Wong, E.T. , Chamberlain, M.C. , Stupp, R. , Lamborn, K.R. , Vogelbaum, M.A. , van den Bent, M.J. , Chang, S.M. , 2010. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol.. 28, 1963–1972. [DOI] [PubMed] [Google Scholar]
- Wick, W. , Brandes, A.A. , Gorlia, T. , Bendszus, M. , Sahm, F. , Taal, W. , Taphoorn, M. , 2015. Phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 trial. Neuro Oncol.. 12, (7) [Abstract] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article:
Supplementary data
Supplementary data


