Abstract
The tumor suppressor 15‐hydroxyprostaglandin dehydrogenase (15‐PGDH) is the key enzyme in prostaglandin E2 catabolism and is down‐regulated in colorectal cancer (CRC) tissue. Canonical Wnt signaling is frequently elevated in colon cancers and has been shown to down‐regulate 15‐PGDH expression. Therefore, we have in the current study investigated if the non‐canonical ligand WNT5A relates to increased expression of 15‐PGDH in colon cancer cells. In the same cohort of patients, we demonstrated a parallel and significant loss of 15‐PGDH and WNT5A protein expression in CRC tissues compared with matched normal colon tissues. Furthermore, patients with low 15‐PGDH/WNT5A expression in their tumors showed reduced survival compared with patients with high 15‐PGDH/WNT5A expression. To investigate if WNT5A signaling directly affects 15‐PGDH expression, we performed in vitro analyses of colon cancer cells (HT‐29 and Caco‐2). Both cell lines, when treated with recombinant WNT5A (rWNT5A) or Foxy‐5, a WNT5A‐mimicking peptide, responded by increasing their expression of 15‐PGDH mRNA and protein. Our investigations showed that rWNT5A and Foxy‐5 induced this increased expression of 15‐PGDH through reduced β‐catenin signaling as well as increased JNK/AP‐1 signaling in colon cancer cells. WNT5A signaling also induced increased 15‐PGDH expression in a breast cancer cell line both in vitro and in vivo. In agreement, WNT5A signaling also increased the expression of the differentiation markers sucrose‐isomaltase and mucin‐2 in colon cancer cells. Our results show that WNT5A signaling regulates 15‐PGDH expression, thus uncovering a novel mechanism by which WNT5A acts as a tumor suppressor and suggests that increased 15‐PGDH expression could be used as an indicator of a positive response to Foxy‐5 in patients treated with this WNT5A agonist.
Keywords: 15‐PGDH, WNT‐5A, JNK, β‐Catenin, Colon cancer
Highlights
Low 15‐PGDH/WNT5A expression in CRC tissue correlates with reduced patient survival.
A parallel loss of 15‐PGDH and WNT5A protein was observed in CRC tissues.
WNT5A signaling induces 15‐PGDH expression at the transcriptional level in colon cancer cells.
WNT5A signaling induced 15‐PGDH expression in breast cancer cells both in vitro and in vivo.
The expression of 15‐PGDH is regulated via β‐catenin as well as JNK/AP‐1 signaling.
Abbreviations
- rWNT‐5a
recombinant WNT‐5A
- 15‐PGDH
15‐hydroxyprostaglandin dehydrogenase
- JNK
c‐Jun N‐terminal kinase
1. Introduction
Every year, more than one million new cases of colorectal cancer (CRC) are diagnosed worldwide (Tenesa and Dunlop, 2009). CRC is the third most common cancer in both men and women and the third most frequent cause of cancer‐related deaths (Siegel et al., 2014). Etiological factors include the consumption of large quantities of alcohol and red meat, diets low in fiber and high in fat, and a lack of physical activity (Huxley et al., 2009). Inflammation, particularly protracted or chronic inflammation, including inflammatory bowel disease (IBD), is commonly associated with CRC (Triantafillidis et al., 2009). The development of CRC involves the accumulation of genetic and epigenetic alterations that cause the transformation of normal colonic epithelium into colon adenocarcinoma (Grady and Markowitz, 2002). These changes lead to the activation of various oncogenic pathways and the inactivation of tumor suppressor pathways (Markowitz and Bertagnolli, 2009). The up‐regulation of cyclooxygenase‐2 (COX‐2), which occurs in the majority of colorectal tumors, plays a crucial role in colon cancer development (Sinicrope and Gill, 2004). The deregulation of COX‐2 expression leads to increases in the levels of inflammatory lipids, including prostaglandins, particularly prostaglandin E2 (PGE2) (Brown and DuBois, 2005). COX‐2‐derived PGE2 is known to induce proliferation, neovascularization, cell death inhibition and motility of tumor cells (Turini and DuBois, 2002) and more recently to expand the number of colon cancer stem cells (Wang et al., 2015). 15‐hydroxyprostaglandin dehydrogenase (15‐PGDH), an important enzyme responsible for the degradation of PGE2 (Ensor and Tai, 1995), has been shown to act as a colorectal tumor suppressor (Backlund et al., 2005; Yan et al., 2004). The loss of 15‐PGDH is associated with CRC (Backlund et al., 2005), and its decreased expression has also been implicated in other cancers, including lung cancer, bladder cancer, pancreatic cancer and gastric cancer (Pham et al., 2010; Song et al., 2011; Tai et al., 2007; Tseng‐Rogenski et al., 2010). Similarly, in the majority of breast cancer subtypes, the expression of 15‐PGDH is reduced or lost, although it has been suggested that 15‐PGDH might serve as a marker for the rare apocrine molecular subtype of breast cancer (Celis et al., 2008) possibly related to the finding that 15‐PGDH tumor expression in a small sub‐population of breast cancer patients is associated with a poor prognosis (Lehtinen et al., 2012).
Canonical Wnt/β‐catenin pathway signaling is one of the crucial signaling pathways controlling the proliferation, differentiation and morphogenesis of cells during development, and several mutations in this pathway have been positively linked to CRC (White et al., 2012). In addition to canonical Wnt signaling, there also exists a non‐canonical arm of Wnt signaling, and one of the most extensively studied non‐canonical ligands is WNT5A (Kikuchi et al., 2012). We previously showed that WNT5A significantly reduces the migration of colon and breast cancer cells (Dejmek et al., 2005a; Jonsson and Andersson, 2001). Immunohistochemical data from both colon and breast cancer patients demonstrated that high WNT5A expression is a good prognostic marker (Dejmek et al., 2005, 2005). Similar findings have also been presented for other cancers, including prostate cancer, lymphoma, thyroid carcinoma and neuroblastoma (Blanc et al., 2005; Kremenevskaja et al., 2005; Liang et al., 2003; Syed Khaja et al., 2011). Foxy‐5, a formylated hexapeptide derived from WNT5A, has been shown to mimic the inhibitory effect of WNT5A on cancer cell migration and tumor metastasis (Säfholm et al., 2006, 2008), making it an attractive molecule for future anti‐metastatic cancer therapy. Foxy‐5 has been tested in a completed phase‐I clinical trial of patients with breast, colon and prostate cancer (www.clinicalTrials.gov; NCT02020291).
Several studies have shown that the expression of 15‐PGDH is associated with the suppression of colon carcinogenesis and invasiveness (Choi et al., 2014; Li et al., 2008; Myung et al., 2006). This has resulted in attempts to re‐express 15‐PGDH in this type of cancer as a strategic area of therapeutic research. In this context, canonical Wnt/β‐catenin signaling has been shown to suppress 15‐PGDH expression in colorectal cancer cells (Smartt et al., 2012b). Based on demonstrations in different studies that non‐canonical WNT5A signaling has the ability to oppose canonical Wnt/β‐catenin stabilization in colon cancer cells (Cheng et al., 2014; Topol et al., 2003), we decided to investigate whether the WNT5A ligand has the ability to regulate the expression of 15‐PGDH, thus mechanistically linking these two tumor suppressors.
The present results reveal for the first time that WNT5A signaling, induced by either recombinant WNT5A or the WNT5A‐mimicking peptide Foxy‐5, can positively regulate the expression of 15‐PGDH mRNA and protein in colon cancer cells. These data reveal a novel mechanism by which WNT5A acts as a tumor suppressor and indicate that increased 15‐PGDH expression might serve as a marker of a positive response to Foxy‐5 in patients treated with this WNT5A agonist.
2. Materials and methods
2.1. Antibodies and reagents
The antibodies used were as follows: rabbit polyclonal against 15‐PGDH (dilution 1:5000 for western blotting and dilution 1:500 for immunohistochemistry; Novus Biologicals, Cambridge, UK), goat polyclonal against WNT5A (dilution 1:200 for western blotting and immunohistochemistry; R&D Systems, Inc. Minneapolis, MN, USA), mouse monoclonal against phospho‐JNK (p‐JNK dilution 1:1000) or JNK (dilution 1:1000; Santa Cruz Biotechnology Inc. CA, USA). The p‐JNK antibody is raised against an epitope from JNK1, however it recognizes both JNK1 and JNK2 and therefore we use JNK when referring to our results on this kinase. Rabbit polyclonal against sucrase‐isomaltase (SI) (dilution 1:1000; Sigma Life Science, St. Louis, MO, USA), mouse monoclonal against β‐catenin (dilution 1:500 for immunofluorescence and 1:1000 for western blotting; BD Transduction Laboratories, Franklin Lakes, NJ, USA), Rabbit monoclonal against non‐phospho active β‐catenin (Ser33/37/Thr41, D13A1; dilution 1:1000), rabbit monoclonal against phospho‐c‐JUN/AP‐1 or c‐JUN/AP‐1 (both diluted 1:1000; Cell Signaling Technology, Danvers, MA, USA). Rabbit monoclonal against c‐Myc (dilution 1:1000; abcam, Cambridge, MA, USA). Rabbit polyclonal against phospho‐β‐catenin (Thr 41/45) (dilution 1:500; Genway, San Diego, CA), mouse monoclonal against β‐actin (dilution 1:1000; Sigma–Aldrich, St. Louis, USA). The recombinant WNT5A was from R&D Systems. Foxy‐5 was obtained from Bachem (Bubendorf, Switzerland). XAV‐939 (S1180) and CHIR‐99021 (CT99021) were from Selleckchem (Houston, TX, USA). The secondary antibodies used for western blotting were peroxidase‐linked goat anti‐rabbit, goat anti‐mouse and rabbit anti‐goat (dilution 1:5000; Dako, Glustrup, Denmark).
2.2. Tissue micro array (TMA) details
We utilized TMA from patients who underwent surgery for at Malmö University Hospital (Malmö, Sweden) during a selected time period in 1990 and who were included in a retrospective study (Magnusson et al., 2010; Salim et al., 2013). Clinico‐pathological details for the patients are provided in Suppl. Table 1. The Ethical Committee at Lund University approved this study (no. LU 52‐99 and 367/2005).
2.3. Immunohistochemistry
Immunohistochemical staining was performed as previously described (Magnusson et al., 2010). All immunohistochemical procedures were performed using a Dako automatic slide stainer (Dako) according to the manufacturer's instructions. All the stained slides were scored independently and in a blinded manner by RE, a senior specialist in clinical pathology, and LM (MD). The slides were scored for the percentage of cells showing immunoreactivity, which was scored as 0 = negative, 1 = weak, 2 = intermediate, and 3 = strong.
2.4. Cell lines
All three cancer cells lines were procured from ATCC. HT‐29 colon cancer cells (ATCC HTB‐38™ has a mutant carboxy‐truncated APC gene but a wild‐type KRAS gene) was grown in McCoy's 5A medium with glutamine, Caco‐2 colon cancer cells (ATCC HTB‐37™, has a mutated APC gene but a wild‐type KRAS gene) and MDA‐MB‐468 (ATCC HTB‐132, breast cancer) were grown in DMEM and all media was supplemented with 10% fetal bovine serum and 100 μg/ml penicillin/streptomycin, and were maintained at 37 °C in a humidified atmosphere containing 5% CO2. The cells were routinely screened for the absence of mycoplasma contamination.
2.5. Western blotting
Whole cell lysates were prepared from cells stimulated or not with either rWNT5A (400 ng/ml) or Foxy‐5 (100 μM) for 24, 48, or 72 h. After treatment, the cells were washed twice with ice‐cold PBS and either lysed in buffer A (Salim et al., 2013) or in a buffer included in a subcellular protein fractionation kit for cultured cells (Thermo scientific Rockford, IL, USA). The resulting lysates from buffer A were homogenized by 10 passages through a syringe and then centrifuged at 10,000 × g for 10 min. Laemmle buffer (4×) was added the each of these samples and those obtained from the subcellular protein fractionation kit. The samples were adjusted to contain equal amounts of protein and boiled before being loaded onto 10% SDS polyacrylamide gels and analyzed by electrophoresis (SDS‐PAGE). After separation, the proteins were transferred to a PVDF membrane (Bio‐Rad, Hercules, CA, USA). The membranes were blocked for 1 h with 3% BSA/PBS at room temperature followed by incubation with a primary antibody overnight at 4 °C. The membranes were washed extensively and incubated with the corresponding secondary antibody for 1 h at RT. The membranes were washed and incubated with Immobilon™ Western Chemiluminescent HRP Substrate (Immobilon™ Western, Merck Millipore, Billerica, MA, USA), and the proteins were detected using the Bio‐Rad ChemiDoc XRS + system. The densitometric analyses were conducted using Image Lab 3.0 software.
2.6. Real Time‐qPCR
The cells were incubated with or without the stimulants in the presence or absence of the inhibitors for the specified time periods. The cells were washed with PBS and then immediately frozen at −80 °C. Total RNA from the different cell samples was isolated using Qiagen RNeasy Plus Mini Kits (Qiagen GmbH, Hilden, Germany). The cDNA synthesis was performed using RevertAid H Minus M‐MuLV reverse transcriptase (ThermoFisher Scientific, USA). The following primers were used: CCND1 (cyclin D1, Hs00765553_m1), HPGD (15‐PGDH, HS00168359_m1), SI (SI, HS00356112_m1), MUC2 (mucin‐2, HS00159374_m1), and HPRT1 (HS99999909_m1). Amplifications were performed in an Mx3005P system (Agilent Technologies, Inc., CA, USA). The reactions were normalized to the housekeeping gene HPRT1 and analyzed with MxPro qPCR software (Agilent Technologies, Santa Clara, CA, USA).
2.7. Luciferase assays
A Dual‐Luciferase Reporter Assay System (Promega, Madison, WI, USA) was used. 15‐PGDH promoter plasmids (Greenland et al., 2000) (a gift from Professor Birgit Gellersen, University of Hamburg, Germany) at a final concentration of 1 μg/ml together with a control Renilla luciferase reporter plasmid of 50 ng/ml were used for the transfections. The DNA plasmids were allowed to form complexes with PolyFect Transfection Reagent (Qiagen) (ratio 4:1) in Opti‐MEM I Reduced Serum Medium (Gibco/Thermo Fisher Scientific, Waltham, MA, USA) and the cells were treated with the DNA‐PolyFect mixture at 37 °C for 24 h. The medium was changed to a serum‐containing medium and the cells were allowed to recover for 24 h. Thereafter, the cells were incubated for 2 h with a serum‐free medium before treatment with recombinant WNT5A (400 ng/ml) or Foxy‐5 (100 μM) for 24 h. The cells were washed twice with PBS and lysed by the addition of the Passive Lysis Buffer from the Dual‐Luciferase Reporter Assay System. The lysed samples were cleared by centrifugation at 1000 × g for 5 min according to the manufacturer's instructions. Firefly and the control Renilla luminescence were measured on a MiniLumat LB 9506 luminometer (Berthold Technologies GmbH, Dusseldorf, Germany) according to the protocol and the ratio was calculated. Triplicate samples were prepared in every set of experiments.
2.8. MDA‐MB‐468 cell xenografts
The generation of MDA‐MB‐468 breast cancer cells stably expressing a WNT5A vector or an empty control vector were previously described by Prasad et al. (2013). Four‐ to five‐week‐old female athymic nude mice were purchased from Harlan Laboratories Inc. (Boxmeer, Netherlands). The cells (2 × 106 cells in 100 μl of serum‐free medium) were inoculated subcutaneously into both flanks of the mice (10 mice/group). The animals were sacrificed 24 days after they had been inoculated and the tumors were removed, measured and weighed. The primary tumors were dissected, fixed in 4% paraformaldehyde and embedded in paraffin. Sections (4 μm) were cut and subsequently stained with the 15‐PGDH‐specific antibody. The stained tissue sections were scanned with the Scanscope CS System (Aperio, Bristol, UK) and analyzed with the Aperio Image Scope software from where we obtained the percentage of strong positive cells (Nsr%). These animal experiments were performed at Pharmatest Services Ltd (Turku, Finland) under the ethical permission no. 3257/04.10.07/2014.
2.9. Statistical analysis
Graph‐pad Prism software 5.0 (San Diego, CA, USA) was used for the statistical analyses. The differences between groups of data were considered statistically significant if P ≤ 0.05 by the two‐tailed Student's t‐test. All means were calculated based on data from at least three different experiments. SPSS version 19.0 (SPSS, IBM, Armonk, NY, USA) was used for the statistical analyses of all the immunostaining data. Univariate survival analyses were performed by the Kaplan–Meier analysis with a log rank test to determine the risk of death.
3. Results
3.1. Low 15‐PGDH and WNT5A expression in colorectal carcinoma
It was previously demonstrated that normal colon epithelium expresses a large amount of 15‐PGDH and that there is a loss of this enzyme in colon cancers (Yan et al., 2004). In the current study, our initial experiments used a tissue micro array (TMA) containing human colorectal carcinoma tissues and matched normal tissues. These paired samples were stained with a 15‐PGDH‐specific antibody. In all normal colon samples we observed a high level of 15‐PGDH expression (Figure 1A). However, a lower level of 15‐PGDH staining was observed in the matched CRC tissues (Figure 1A). An intensity plot revealed that the level of 15‐PGDH expression in the colorectal tumor tissues was significantly less than in there matched normal controls (Figure 1A). These results confirm that our TMA is representative and valid because they yielded similar results to those previously published by Yan et al., 2004.
Figure 1.
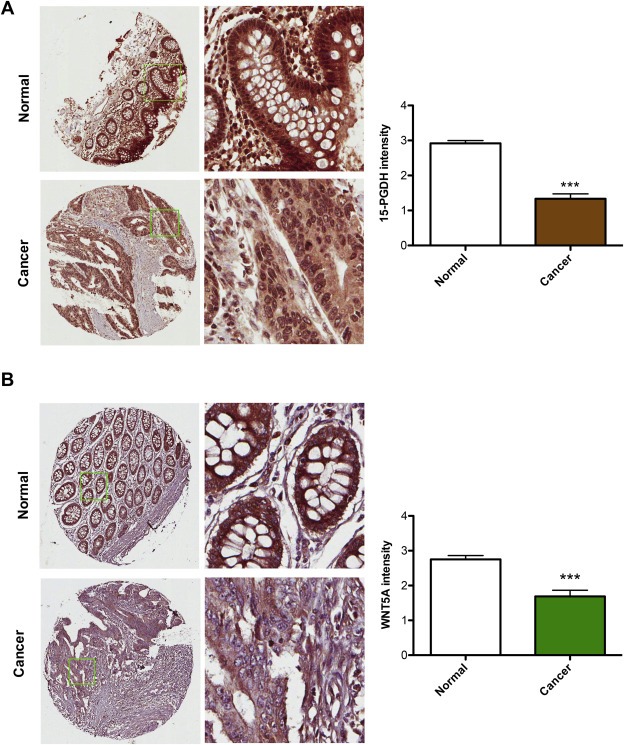
Expression of 15‐PGDH and WNT5A in colorectal carcinomas and in matched normal control tissue. A, The images shown are representative immunohistochemical stainings of 15‐PGDH protein expression in colorectal carcinoma tissue and matched normal controls. The intensity of 15‐PGDH protein staining was scored and the results from this scoring are presented in the graph. B, The images shown are representative immunohistochemical stainings of WNT5A protein expression in colorectal carcinoma tissue and matched normal controls. The intensity of WNT5A protein staining was scored and the results from this scoring are presented in the graph. The results are presented as mean ± standard error of the mean (SEM); ***P < 0.001.
The TMA was then stained for the expression of WNT5A, and we found that WNT5A expression, similar to that of 15‐PGDH, was significantly decreased in CRC tissues compared with their matched normal controls (Figure 1B). Overall, the analyses of 15‐PGDH and WNT5A expression in the same TMA tissues demonstrated that both proteins are expressed at high levels in normal colorectal mucosa and that they are both significantly down‐regulated in CRC patients. These findings suggest that the loss of expression of these proteins may play a role in the progression of a tumor. These findings also allowed us to speculate that a relationship might exist between the two proteins, whereby the WNT5A ligand might trigger an intracellular signaling pathway that regulates and maintains 15‐PGDH expression in normal colorectal mucosa. If WNT5A is lost in CRC tissue, a down‐regulation of 15‐PGDH expression might occur.
3.2. 15‐PGDH and WNT5A expression in primary tumor tissue reveals similar survival trends for colorectal carcinoma patients
Similar to 15‐PGDH, WNT5A has been shown to act as a tumor suppressor in colon, prostate and breast cancer (Dejmek et al., 2005, 2005, 2001). WNT5A is expressed in normal colon epithelium, where its promotor is unmethylated, which is in contrast to CRC, where WNT5A is frequently down‐regulated owing to tumor specific methylation (Ying et al., 2008). Similar to 15‐PGDH (Backlund et al., 2005; Yan et al., 2004), WNT5A has been demonstrated to be a good prognostic marker in patients with colon, prostate and breast cancer (Dejmek et al., 2005a; Jonsson et al., 2002). Therefore, we next investigated the expression of the 15‐PGDH and WNT5A proteins in the same cohort of CRC patients to enable direct correlation of the expression of these proteins with overall survival.
The immunohistochemical staining of 15‐PGDH was performed in a TMA containing 84 tissues from CRC patients. The clinico‐pathological details of the patients are provided in Supplementary Table S1. Of the 84 patients, 74 (88%) showed down‐regulation, whereas 10 (12%) maintained high 15‐PGDH expression in their primary tumors. Representative tumor samples expressing high and low levels of 15‐PGDH are shown in Figure 2A. Of the 84 samples analyzed for 15‐PGDH, 66 were also analyzed for the expression of WNT5A. Representative tumor samples with low and high levels of WNT5A are shown in Figure 2C. We found that approximately 39% (26/66) of the primary tumor samples from these CRC patients showed no or reduced expression of WNT5A. Based on these finding we found a positive correlation between 15‐PGDH and WNT5A protein expressions using the Pearson correlation test (r = 0.3 with a statistical significance of 0.027).
Figure 2.
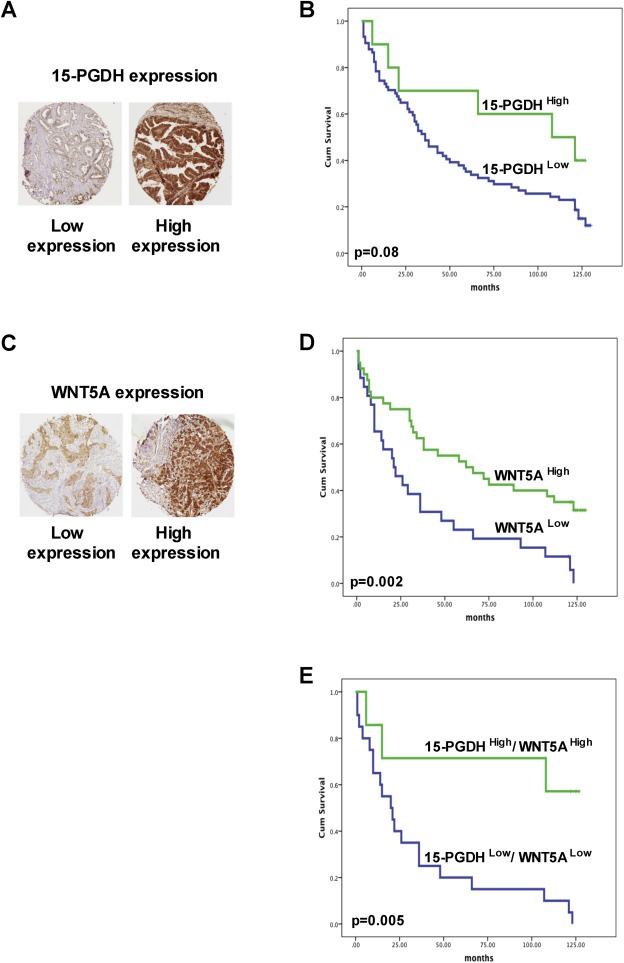
Kaplan–Meier survival curves of colorectal cancer patients with different levels of 15‐PGDH and WNT5A protein in their tumor tissue. A and C, Representative immunohistochemical images of low and high levels of 15‐PGDH and WNT5A expression in the tumor tissue from colon cancer patients. B, Kaplan–Meier survival curves of colorectal cancer patients with low or high levels of 15‐PGDH expression. D, Kaplan–Meier survival curves of colorectal cancer patients with low or high levels of WNT5A expression. E, Kaplan–Meier survival curves of colorectal cancer patients with low or high expression levels of both 15‐PGDH and WNT5A. Cumulative survival is shown in months, and the differences between the groups were assessed using log‐rank testing.
Kaplan–Meier survival analysis revealed that the patients expressing high levels of 15‐PGDH (Figure 2B, 15‐PGDHHigh) had a better cumulative survival than the patients expressing low levels of 15‐PGDH (Figure 2B, 15‐PGDHLow). Kaplan–Meier survival analysis revealed that the WNT5A negative group (WNT5ALow; Figure 2D) had worse survival than the WNT5A positive group (WNT5AHigh; Figure 2D). All of the 26 WNT5ALow samples also revealed a loss of 15‐PGDH protein expression, suggesting that the loss of WNT5A protein expression in CRC might be related to the simultaneous loss of 15‐PGDH protein expression. Furthermore, CRC patients showing 15‐PGDHLow/WNT5ALow expression in their primary tumors showed reduced overall survival compared with patients showing 15‐PGDHHigh/WNT5AHigh expression in their primary tumors (Figure 2E).
3.3. WNT5A and the WNT5A mimicking peptide Foxy‐5 both up‐regulate 15‐PGDH expression in colon cancer cells
To pursue the above findings we next assessed whether WNT5A signaling can re‐constitute the expression of 15‐PGDH in colon cancer cells (HT‐29 and Caco‐2). First, we investigated endogenous WNT5A expression in HT‐29 and Caco‐2 by western blotting. Our results showed that both cell lines were negative for endogenous WNT5A (Suppl. Figure 1A), making them perfect model cells with which to test whether WNT5A signaling can be initiated by treating the cells with either recombinant WNT5A (rWNT5A) or Foxy‐5. Both these molecules trigger non‐canonical WNT5A signaling as demonstrated by their abilities to induce a robust Ca2+ signal, an essential activation marker of this pathway, in both HT‐29 and Caco‐2 colon cancer cells (Suppl. Figure 2). We then treated HT‐29 cells with rWNT5A at a concentration of 400 ng/ml for different periods of time (24, 48 or 72 h), after which the cells were lysed and 15‐PGDH expression was analyzed by western blotting. The treated HT‐29 cells showed significant increases in the expression of 15‐PGDH at all the time points analyzed (Figure 3A). A similar increase in 15‐PGDH was detected when HT‐29 cells were exposed to the WNT5A‐mimicking peptide Foxy‐5 (100 μM) for 24, 48 or 72 h. To investigate whether this WNT5A signaling‐induced expression of 15‐PGDH is observed at the transcript level, we performed Real Time‐qPCR in HT‐29 cells after treatment with either rWNT5A or Foxy‐5 for 6, 9, 12, 18, 24, 48 or 72 h (Figure 3C,D). Our results showed that cells treated with either rWNT5A or Foxy‐5 for the indicated periods of time responded with an increase in their 15‐PGDH mRNA levels. We found a robust increase in 15‐PGDH mRNA already after 9 h of stimulation with rWNT5A (Figure 3C) whereas the most robust increase in 15‐PGDH mRNA was seen after 48 h of stimulation with Foxy‐5 (Figure 3D). However, we observe a clear and statistically significant increase of 15‐PGDH mRNA after 24 h stimulation with both WNT5A and Foxy‐5. These data were complemented with an additional Ct graph showing that basal 15‐PGDH mRNA (gene name HPGD) is detected after 26 cycles similarly to the mRNA of the housekeeping gene HPRT1 (Suppl. Figure 1B). We also treated Caco‐2 colon cancer cells with rWNT5A or Foxy‐5 for 24 h. Western blot analysis revealed a significant up‐regulation of 15‐PGDH protein expression also in this cell line after rWNT5A or Foxy‐5 treatment (Figure 3E). Our results suggest that the activation of WNT5A signaling triggers an increase in 15‐PGDH protein expression initiated at the transcription level.
Figure 3.
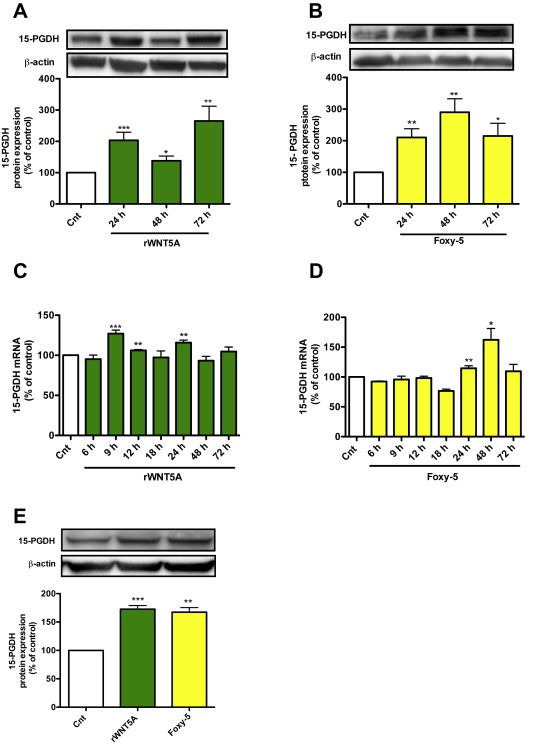
The effect of rWNT5A and Foxy‐5 on the expression of 15‐PGDH in colon cancer cells. A, Western blot analyses and subsequent densitometric evaluations of 15‐PGDH protein expression in HT‐29 colon cancer cells not stimulated (Cnt) or stimulated with rWNT5A (400 ng/ml) for 24, 48 or 72 h. B, Western blot analyses and subsequent densitometric evaluations of 15‐PGDH protein expression in HT‐29 colon cancer cells not stimulated (Cnt) or stimulated with Foxy‐5 (100 μM) for 24, 48 or 72 h. The images shown in A, and B, are representative western blots of 15‐PGDH and re‐probed blots for β‐actin to ensure equal loading. The diagrams outline the results of the densitometric analyses expressed as 15‐PGDH/β‐actin ratio. C and D, QPCR analyses of 15‐PGDH mRNA levels in HT‐29 cells not stimulated (Cnt) or stimulated with C, 400 ng/ml rWNT5A or D, 100 μM Foxy‐5 for 6, 9, 12, 18, 24, 48 or 72 h. E, Analyses of 15‐PGDH protein expression in Caco‐2 colon cancer cells not stimulated (Cnt) or stimulated with either 400 ng/ml rWNT5A or 100 μM Foxy‐5 for 24 h. The images shown are a representative western blot of 15‐PGDH and a re‐probed blot for β‐actin to ensure equal loading. The diagram outlines the results of the densitometric analyses expressed as 15‐PGDH/β‐actin ratio. The data are calculated as percentage of untreated control cells and given as mean ± standard error of mean (SEM) of at least five separate experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
3.4. WNT5A/Foxy‐5 signaling regulates 15‐PGDH expression
To substantiate our findings, we analyzed the c‐Jun N‐terminal kinase (JNK), a well‐studied target of WNT5A signaling (Nomachi et al., 2008; Wang et al., 2013), to determine whether it might mediate the effect of WNT5A signaling on 15‐PGDH expression. We first stimulated HT‐29 cells with Foxy‐5 (100 μM) for different periods of time (0.5, 1, 2 and 24 h). Cell lysates were prepared from un‐stimulated and stimulated cells, and the activation of JNK was visualized by western blotting using an anti‐phospho JNK antibody raised against the epitope corresponding to a short amino acid sequence containing phosphorylated Thr 183 and Tyr 185 of JNK1 of human origin. We performed a ratio between p‐JNK and total JNK and observed a 1.5‐fold increase in the phosphorylation of JNK within 30 min and a maximal increase after 1 h of Foxy‐5 treatment, an increase that was maintained even after 24 h (Figure 4A). In the next set of experiments, we investigated whether the WNT5A signaling‐induced activation of JNK is involved in mediating 15‐PGDH expression. The basis for these experiments was the facts that the 15‐PGDH promoter contains several AP‐1 transcription factor binding sites and that JNK is a regulator of AP‐1 (Greenland et al., 2000). Accordingly, we transfected HT‐29 cells with a luciferase construct (1024 bp) that contained a 15‐PGDH promoter with 3 AP‐1 sites and then treated the cells with 10 μM JNK inhibitor1 (JNKI1, L‐form) for 30 min before finally stimulating them with either rWNT5A or Foxy‐5 for 24 h. Our results showed that both rWNT5A and Foxy‐5 induced 15‐PGDH promoter activity and that this activation was inhibited by the JNK inhibitor (Figure 4B). To validate these results we also investigated the effects of rWNT5A and Foxy‐5, in the absence or presence of the JNK inhibitor, on 15‐PGDH mRNA expression. We found that both rWNT5A and Foxy‐5 caused a statistically significant increase in 15‐PGDH mRNA levels that were inhibited by the JNK inhibitor (Figure 4C). Furthermore, we also checked if and how rWNT5A and Foxy‐5 affected AP‐1 activity, a well‐know target for p‐JNK activity and a transcription factor for the 15‐PGDH promoter. Indeed, we found that both rWNT5A and Foxy‐5 induced increased phosphorylation/activation of the transcription factor AP‐1 in the nuclear fraction of the cells (Figure 4D).
Figure 4.
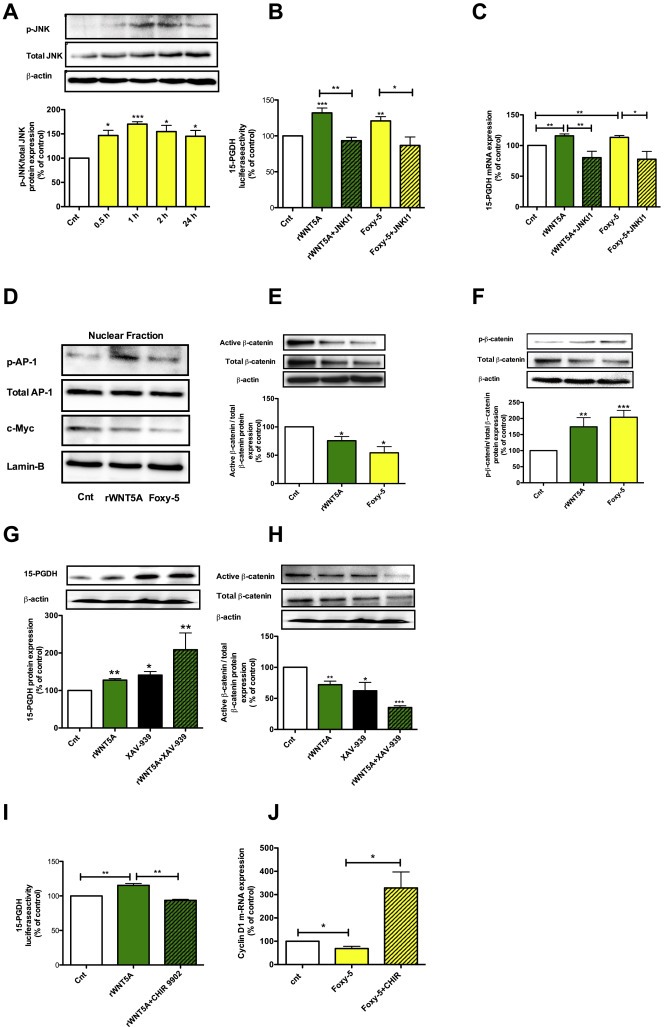
WNT5A signaling modulates 15‐PGDH expression via different mechanisms A, Western blot analyses and densitometric evaluations of phosphorylated JNK (p‐JNK) expression in HT‐29 colon cancer cells not stimulated (Cnt) or stimulated with 100 μM Foxy‐5 for 0.5, 1, 2, or 24 h. The images shown are a representative western blot of p‐JNK and re‐probed blots for total JNK and β‐actin to ensure equal loading. The diagram outlines the results of the densitometric analyses expressed as p‐JNK/total JNK ratio. B, Luciferase activity in HT‐29 cells transfected with a 15‐PGDH promoter construct for 24 h, incubated for 30 min in the absence or presence of the JNK inhibitor1 (JNKI1, 10 μM) and, finally, either not stimulated (Cnt) or stimulated with rWNT5A (400 ng/ml) or Foxy‐5 (100 μM) for 24 h in the absence or presence of the JNKI1. C, 15‐PGDH mRNA expression in HT‐29 cells incubated for 30 min in the absence or presence of JNKI1 (10 μM) and then either not stimulated (Cnt) or stimulated with either 400 ng/ml rWNT5A or 100 μM Foxy‐5 for 24 h in the absence or presence of JNKI1 (10 μM). D, Western blot analyses of nuclear fractions from HT‐29 cells not stimulated (Cnt) or stimulated with either 400 ng/ml rWNT5A or 100 μM Foxy‐5 for 24 h. The images shown are western blots of phosphorylated AP‐1 (p‐AP‐1; re‐probed for total AP‐1) and c‐Myc (re‐probed for Lamin‐B), the re‐probing were performed to ensure equal loading. E and F, Western blot analyses and subsequent densitometric evaluations of active β‐catenin and phosphorylated β‐catenin protein expressions in HT‐29 cells not stimulated (Cnt) or stimulated with rWNT5A (400 ng/ml) or Foxy‐5 (100 μM) for 24 h. The images shown are representative western blots that were re‐probed for total β‐catenin and finally also for β‐actin to ensure equal loading. The diagrams in E and F outline the results of the densitometric analyses. G, Western blot analyses and subsequent densitometric evaluations of 15‐PGDH protein expression in HT‐29 cells not stimulated (Cnt) or stimulated with 400 ng/ml rWNT5A for 24 h in the absence or presence of the XAV‐939 (10 μM) and H, the same blots analyzed in G were also re‐probed for active β‐catenin and total β‐catenin. The images shown in G and H are representative western blots and all blots were re‐probed for β‐actin to ensure equal loading. I, Luciferase activity in HT‐29 cells transfected with a 15‐PGDH promoter construct for 24 h, incubated for 30 min in the absence or presence of the β‐catenin activator CHIR‐99021 (1.25 μM) and, finally, either not stimulated (Cnt) or stimulated with rWNT5A (400 ng/ml) for 24 h in the absence or presence of CHIR‐99021. J, Cyclin D1 mRNA expression in HT‐29 cells not stimulated (Cnt) or stimulated with 100 μM Foxy‐5 in the absence or presence of the β‐catenin activator CHIR‐99021 (1.25 μM) for 48 h. The data are normalized against untreated controls, and the results are presented as mean ± standard error of the mean (SEM) of at least five separate experiments; *P < 0.05, **P < 0.01, ***P < 0.001.
As previously discussed, canonical Wnt/β‐catenin signaling can suppress the expression of 15‐PGDH, thus making it possible that WNT5A signaling can influence 15‐PGDH protein expression by not only increasing 15‐PGDH promoter activity but also by counteracting canonical Wnt/β‐catenin signaling in colon cancer cells. In support of this dual mechanism, we found that both rWNT5A and Foxy‐5 reduced the non‐phosphorylated active form of β‐catenin (Figure 4E), induced phosphorylation of β‐catenin and reduced the total level of β‐catenin in both HT‐29 (Figure 4F) and Caco‐2 cells (Suppl. Figure 3A,B). The increased degradation of β‐catenin induced by WNT5A signaling was not compensated for by a simultaneous recruitment of β‐catenin from the membrane pool of β‐catenin as revealed by confocal immunofluorescence images of β‐catenin expression from the focal plane of the plasma membrane in both HT‐29 and Caco‐2 cells (Suppl. Figure 3C,D). To further investigate if inhibition of Wnt/β‐catenin signaling contributes to 15‐PGDH expression we used a small molecule inhibitor of Wnt/β‐catenin signaling (XAV‐939) that function by increasing the stability of Axin which results in increased β‐catenin degradation. We found that XAV‐939 increased 15‐PGDH protein expression to a similar level as rWNT5A (Figure 4G) and that these effects occurred in parallel to similar reductions in the levels of non‐phosphorylated active β‐catenin (Figure 4H). We found that when XAV‐939 and rWNT5A were combined, the increase in 15‐PGDH protein expression (Figure 4G) and the reductions in non‐phosphorylated active β‐catenin as well as total β‐catenin were even more pronounced (Figure 4H). To further validate the interaction between canonical Wnt signaling and non‐canonical Wnt signaling, we used the GSK‐3β inhibitor (CHIR‐99021), a documented activator of canonical Wnt/β‐catenin signaling (Chen et al., 2014). We first transfected HT‐29 cells with the 15‐PGDH promoter construct and then treated the cells in the absence or presence of CHIR‐99021 (1.25 μM) for 30 min, after which we finally stimulated the cells with rWNT5A for 24 h. Our results demonstrated that the rWNT5A‐induced activation of the 15‐PGDH promoter activity was impaired by CHIR‐99021, indicating that the effect of WNT5A signaling on 15‐PGDH expression is counteracted by CHIR‐99021‐induced excessive canonical β‐catenin signaling in colon cancer cells (Figure 4I). Control experiments were performed to confirm that non‐canonical WNT5A signaling caused a functional inhibition of β‐catenin signaling. Both rWNT5A and Foxy‐5 caused significant reductions in the level of the β‐catenin signaling targets cyclin D1 (Figure 4J and Suppl. Figure 3E,F) and c‐Myc (Figure 4D). The action of CHIR‐99021 was functionally confirmed by its ability to significantly increase the expression of cyclin D1 even in the presence of Foxy‐5 (Figure 4J). Taken together, these findings support our notion that WNT5A signaling has the capacity to reduce canonical β‐catenin signaling in colon cancer cells.
3.5. WNT5A/Foxy‐5 signaling induces 15‐PGDH protein expression in breast cancer
WNT5A acts as a tumor suppressor in breast cancer as well as in colon cancer. To determine whether the observed effect of WNT5A signaling on 15‐PGDH expression in colon cancer cells is unique or if it occurs also in other cancer cell types, we also investigated its effect on 15‐PGDH expression in breast cancer cells. We treated MDA‐MB‐468 breast cancer cells (endogenously negative for WNT5A expression) with either rWNT5A or Foxy‐5 for 24 h and then determined the effect on 15‐PGDH expression by western blotting (Figure 5A). We observed a significant up‐regulation of 15‐PGDH expression following treatment with either rWNT5A or Foxy‐5 (Figure 5A). To ascertain whether such WNT5A‐mediated regulation of 15‐PGDH occurs in vivo, we injected WNT5A transfected MDA‐MB‐468 cells [468(WNT5A)] subcutaneously into the flanks of mice. For a control, MDA‐MB‐468 cells transfected with the empty vector [468(EV)] were used. After three weeks, the animals were sacrificed, and the primary tumors were harvested. The tumor growth was significantly reduced in the WNT5A transfected cells [468(WNT5A)] when compared to empty vector transfected control cells [468(EV)] as demonstrated by reductions in both tumor volume (Figure 5B) and tumor weight (Figure 5C). Tumor sections were immunostained with an anti‐15‐PGDH antibody. We observed a 2‐fold increase in the staining intensity of 15‐PGDH in tumors that originated from 468(WNT5A) cells compared with the 468(EV) cells (Figure 5D). Overall, our results demonstrated that WNT5A signaling can induce the expression of 15‐PGDH in both colon cancer cells and breast cancer cells in vitro and in vivo.
Figure 5.
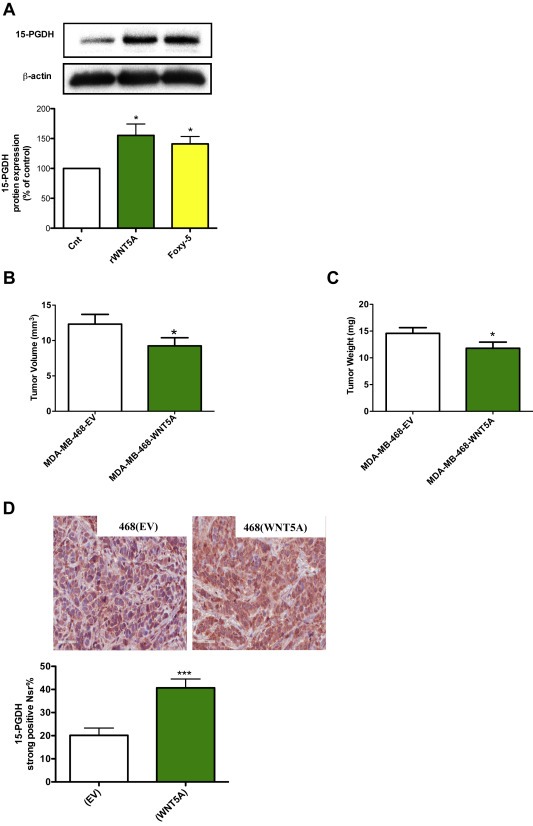
The effect of rWNT5A and Foxy‐5 on the expression of the 15‐PGDH protein in breast cancer cells. A, Western blot analyses and subsequent densitometric evaluations of 15‐PGDH protein expression in MDA‐MB‐468 breast cancer cells not stimulated (Cnt) or stimulated with either rWNT5A (400 ng/ml) or Foxy‐5 (100 μM) for 24 h. The images shown are a representative western blot of 15‐PGDH that was re‐probed for β‐actin to ensure equal loading. The diagram outlines the results of the densitometric analyses and the results are presented as a percentage of untreated controls. The results are presented as mean ± standard error of the mean (SEM) of at least four separate experiments; *P < 0.05. B and C, Effect of WNT5A on tumor growth. Volumes and weights of tumors derived from MDA‐MB‐468 cells either transfected with an empty vector (EV) or a WNT5A expressing vector (WNT5A). Volumes and weights were determined at day 24 when the experiments were terminated. D, Representative images from in vivo experiments showing the expression of 15‐PGDH in tumors derived from MDA‐MB‐468 cells transfected with either an empty vector (EV; left panel) or a WNT5A expressing vector (WNT5A; right panel) at day 24. The accumulated 15‐PGDH data are presented as percentage of cells with strong 15‐PGDH expression (Nsr). The animal results are given as means ± standard error of the mean (SEM) for 18 tumors derived from 468(EV) cells and 18 tumors derived from 468(WNT5A) cells; *P < 0.05, ***P < 0.001.
3.6. WNT5A/Foxy‐5 signaling induces the differentiation of colon cancer cells
Differentiation is an essential cellular process of normal physiology. The proliferative nature of cancer cells is related to their poor differentiation capacity. WNT5A signaling has been shown to inhibit the proliferation of colon cancer cells (Cheng et al., 2014); however, their ability to induce the differentiation of colon cancer cells is unclear. To investigate this possibility, we studied how increased WNT5A signaling in colon cancer cells affects their differentiation by measuring the levels of two key terminal differentiation markers, SI and mucin‐2 (Taupin and Podolsky, 1999). In the initial experiment, we stimulated HT‐29 cells with rWNT5A for 24–72 h, after which we analyzed the expression of SI by western blotting with a specific SI antibody. A significant up‐regulation of the protein level of SI was observed at all time points (24, 48 and 72 h; Figure 6A). These findings were validated in Caco‐2 colon cancer cells, in which we found a similar significant increase in SI expression when they were treated with either rWNT5A or Foxy‐5 for 24 h (Figure 6B). We also analyzed the WNT5A signaling axis‐mediated increase in SI mRNA in HT‐29. We stimulated HT‐29 cells for 48 h with Foxy‐5 in the absence or presence of the JNK inhibitor (JNKl1) or CHIR‐99021. As expected, based on the above data, Foxy‐5 significantly increased the level of SI mRNA, and this increase was neutralized in the presence of JNKl1, suggesting that WNT5A regulates the expression of SI (Figure 6C) via the same signaling pathway that regulates the expression of 15‐PGDH. Similarly, the activation of canonical Wnt signaling by CHIR‐99021 reduced the Foxy‐5‐induced mRNA level of SI. In addition to SI, we analyzed how increased WNT5A signaling affected the transcription levels of the differentiation marker Mucin‐2. We observed significantly increased levels of Mucin‐2 mRNA after stimulation with either rWNT5A or Foxy‐5 (Figure 6D). Similar to the SI response, the Foxy‐5‐induced Mucin‐2 mRNA increase was significantly inhibited by JNKl1, and when canonical Wnt/β‐catenin signaling was activated by CHIR‐99021, the effect of Foxy‐5 on Mucin‐2 expression was reduced.
Figure 6.
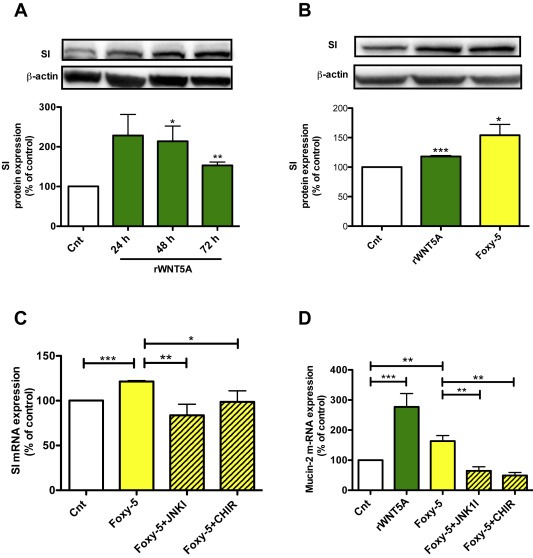
WNT5A signaling induces differentiation of colon cancer cells. A, Western blot analyses and subsequent densitometric evaluations of sucrase‐isomaltase (SI) expression in HT‐29 colon cancer cells not stimulated (Cnt) or stimulated with 400 ng/ml rWNT5A for 24, 48, or 72 h. The images shown are a representative western blot of SI that was re‐probed for β‐actin to ensure equal loading. The diagram outlines the results of the densitometric analyses. B, Western blot analyses and subsequent densitometric evaluations of SI expression in Caco‐2 colon cancer cells not stimulated or stimulated with either rWNT5A (400 ng/ml) or Foxy‐5 (100 μM) for 24 h. The images shown are a representative western blot of SI that was re‐probed for β‐actin to ensure equal loading. The diagram outlines the results of the densitometric analyses. C and D, QPCR analyses of SI mRNA and mucin‐2 mRNA levels in HT‐29 colon cancer cells incubated for 30 min in the absence or presence of JNKl1 (10 μM) or the β‐catenin activator CHIR‐99021 (1.25 μM) and either not stimulated (Cnt) or stimulated with rWNT5A (400 ng/ml) or Foxy‐5 (100 μM) for C, 48 h and D, 72 h. The accumulated data are calculated as the percentage of untreated controls and the results are presented as mean ± standard error of the mean (SEM) of at least four separate experiments; *P < 0.05, **P < 0.01, ***P < 0.001.
4. Discussion
The re‐expression of tumor suppressor proteins, to elicit their functional effects, is an attractive therapeutic strategy in the treatment of cancer patients. The non‐canonical ligand WNT5A is thought to exert its tumor suppressor activities in several cancer types, including breast, colon and prostate cancer, primarily by impairing tumor cell migration and invasion (Dejmek et al., 2005a; Jonsson and Andersson, 2001; Syed Khaja et al., 2011). These findings are strongly supported by the observation that the loss or reduced expression of WNT5A in tumor cells in these types of cancer is strongly correlated with a more rapid progression of the disease (Dejmek et al., 2005a; Nomachi et al., 2008; Sand‐Dejmek et al., 2013; Syed Khaja et al., 2011). The reconstitution of WNT5A signaling in patients with these types of cancer is therefore an attractive treatment strategy. To make such a treatment alternative possible, our laboratory has developed a WNT5A‐mimicking hexapeptide Foxy‐5 (Säfholm et al., 2006). This peptide has also been shown to have a strong anti‐metastatic effect in vivo (Säfholm et al., 2008). This peptide has been tested in a recently completed phase 1 clinical trial in patients with breast, colon and prostate cancer. Similar to the non‐canonical ligand WNT5A, the intracellular enzyme 15‐PGDH has been characterized as a tumor suppressor protein in colon and breast cancer, where it catalyzes the oxidation of the 15(S) hydroxyl group of prostaglandin to produce the inactive 15‐keto‐metabolite. In colon and breast tumor cells, the expression of this protein is often lost. Interestingly, the transfection and re‐expression of 15‐PGDH is a potent in vivo suppressor of colon carcinogenesis (Myung et al., 2006). However, at present there is no molecule that can be used as a treatment alternative in vivo to induce the re‐expression of 15‐PGDH in tumor cells lacking expression of this enzyme.
In the present study we demonstrated that in the same cohort, colorectal tumors expressed less 15‐PGDH and WNT5A than matched normal tissue from the same patient. These findings are compatible with the suggested tumor suppressor function of these proteins. Preliminary findings from those whole tumor sections that contained normal, polyp, adenoma and cancer tissues in the same section suggest that reduced expression of both these proteins can be detected at the adenoma stage (Suppl. Figure 4). Further analyses of these data disclosed that around 40% of the patients analyzed for WNT5A expression showed low levels of expression of the WNT5A protein and that the tumor samples from all of these patients were also negative for 15‐PGDH expression. These results revealed a statistically positive correlation between 15‐PGDH and WNT5A protein expressions. We therefore suggest that the WNT5A ligand might regulate the expression of the intracellular enzyme 15‐PGDH and that the low levels of WNT5A expression in these cancers might cause a subsequent decrease in 15‐PGDH.
To ascertain whether WNT5A signaling actually regulates 15‐PGDH expression in colon cancer cells, we treated two different colon cancer cell lines, HT‐29 and Caco‐2, which have low endogenous levels of WNT5A and 15‐PGDH, with either rWnt5a or the WNT5A‐mimicking hexapeptide Foxy‐5. These treatments induced up‐regulation of 15‐PGDH expression at the protein level. Furthermore, this effect of WNT5A signaling on 15‐PGDH expression was initiated at the transcription level as indicated by the effect of WNT5A signaling on 15‐PGDH mRNA levels and on luciferase promoter activity. The concept that WNT5A signaling induces the expression of 15‐PGDH was supported by the data demonstrating that WNT5A signaling regulates 15‐PGDH expression via the activation of JNK, a documented WNT5A downstream signal (Nomachi et al., 2008). This activation led to activation of AP‐1 a transcription factor known to bind to and regulate the promoter for 15‐PGDH. Overall, our results show that restoring WNT5A signaling and tumor suppressor function also restores the expression of the tumor suppressor 15‐PGDH in colon cancer cells, thereby, for the first time, linking these two tumor suppressors. The above data were further validated in vitro and in vivo in breast cancer cells and the results obtained from these experiments strengthen our conclusion that WNT5A signaling can positively regulate 15‐PGDH expression. Importantly, our finding that Foxy‐5 is as effective as rWNT5A in regulating the expression of 15‐PGDH suggests that this drug candidate can also be used to restore the expression of the tumor suppressor 15‐PGDH.
The fact that WNT5A signaling reduced the non‐phosphorylated active form of β‐catenin and in parallel increased the level of phosphorylated β‐catenin in colon cancer cells are interesting from the perspective that Wnt/β‐catenin signaling can suppress 15‐PGDH expression in colon cancer (Smartt et al., 2012a). These observations were further investigated using a specific inhibitor of Wnt/β‐catenin signaling (XAV‐939). This inhibitor reduced the non‐phosphorylated active form of β‐catenin and in parallel increased the expression of 15‐PGDH both to the same extent as WNT5A signaling. Furthermore, an activator of canonical β‐catenin signaling (CHIR‐99021) counteracted the effect of WNT5A signaling on 15‐PGDH expression. These data show that non‐canonical WNT5A signaling in colon cancer cells have an opposite effect than canonical β‐catenin signaling on 15‐PGDH expression, due to its ability to inhibit β‐catenin signaling.
At present there is no validated biomarker that can be used to evaluate a treatment response in patients treated with the drug candidate Foxy‐5. Based on the finding that Foxy‐5 induces increase 15‐PGDH expression in tumor cells similar to that induced by rWNT5A, it should be possible to use intracellular 15‐PGDH as an immunohistochemical marker in tumor biopsies from patients treated with Foxy‐5.
In summary, our data reveal a novel interaction between the two tumor suppressors WNT5A and 15‐PGDH in which the WNT5A ligand induces the expression of the intracellular 15‐PGDH enzyme in colon and breast cancer cells via at least two different mechanisms. A practical consequence of this finding is that the level of 15‐PGDH in tumor tissue and possibly also the levels of its substrate and degradation products in blood can be used as biomarker in future clinical studies of the WNT5A‐mimicking peptide Foxy‐5.
Conflict of interest
T.A. is a shareholder of and part‐time Chief Scientific Officer of WntResearch AB. This does not alter the authors' adherence to all the policies on sharing data and materials as the guidelines for the Molecular Oncology.
Supporting information
The following are the supplementary data related to this article:
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgments
The authors thank Lena Axelsson, Maria Juhas, Gunilla Jönsson, Elise Nilsson and Dr Qing Liu for excellent technical assistance. The 15‐PGDH promoter plasmid was a kind gift from Professor Birgit Gellersen, University of Hamburg, Germany.
The study was supported by grants to T.A. and A.S. from Malmö University Hospital Cancer Foundation, Skåne University Hospital Research Foundations, the Swedish Cancer Foundation, the Swedish Research Council, Gunnar Nilsson's Cancer Foundation, and by Governmental Funding of Clinical Research within the national health services. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary data 1.
1.1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2016.07.011.
Mehdawi Lubna M., Prasad Chandra Prakash, Ehrnström Roy, Andersson Tommy, Sjölander Anita, (2016), Non-canonical WNT5A signaling up-regulates the expression of the tumor suppressor 15-PGDH and induces differentiation of colon cancer cells, Molecular Oncology, 10, doi: 10.1016/j.molonc.2016.07.011.
References
- Backlund, M.G. , Mann, J.R. , Holla, V.R. , 2005. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J. Biol. Chem. 280, 3217–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc, E. , Roux, G.L. , Benard, J. , 2005. Low expression of Wnt-5a gene is associated with high-risk neuroblastoma. Oncogene. 24, 1277–1283. [DOI] [PubMed] [Google Scholar]
- Brown, J.R. , DuBois, R.N. , 2005. COX-2: a molecular target for colorectal cancer prevention. J. Clin. Oncol. 23, 2840–2855. [DOI] [PubMed] [Google Scholar]
- Celis, J.E. , Gromov, P. , Cabezon, T. , 2008. 15-Prostaglandin dehydrogenase expression alone or in combination with ACSM1 defines a subgroup of the apocrine molecular subtype of breast carcinoma. Mol. Cell. Prot. MCP. 7, 1795–1809. [DOI] [PubMed] [Google Scholar]
- Chen, E.Y. , DeRan, M.T. , Ignatius, M.S. , 2014. Glycogen synthase kinase 3 inhibitors induce the canonical WNT/beta-catenin pathway to suppress growth and self-renewal in embryonal rhabdomyosarcoma. Proc. Natl. Acad. Sci. U. S. A. 111, 5349–5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, R. , Sun, B. , Liu, Z. , 2014. Wnt5a suppresses colon cancer by inhibiting cell proliferation and epithelial-mesenchymal transition. J. Cell. Physiol. 229, (12) 1908–1917. [DOI] [PubMed] [Google Scholar]
- Choi, S.H. , Kim, B.G. , Robinson, J. , 2014. Synthetic triterpenoid induces 15-PGDH expression and suppresses inflammation-driven colon carcinogenesis. J. Clin. Invest. 124, 2472–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejmek, J. , Dejmek, A. , Safholm, A. , 2005. Wnt-5a protein expression in primary dukes B colon cancers identifies a subgroup of patients with good prognosis. Cancer Res. 65, 9142–9146. [DOI] [PubMed] [Google Scholar]
- Dejmek, J. , Leandersson, K. , Manjer, J. , 2005. Expression and signaling activity of Wnt-5a/discoidin domain receptor-1 and Syk plays distinct but decisive roles in breast cancer patient survival. Clin. Cancer Res. 11, 520–528. [PubMed] [Google Scholar]
- Ensor, C.M. , Tai, H.H. , 1995. 15-Hydroxyprostaglandin dehydrogenase. J. Lipid Med. Cell Signal. 12, 313–319. [DOI] [PubMed] [Google Scholar]
- Grady, W.M. , Markowitz, S.D. , 2002. Genetic and epigenetic alterations in colon cancer. Annu. Rev. Genomics Hum. Genet. 3, 101–128. [DOI] [PubMed] [Google Scholar]
- Greenland, K.J. , Jantke, I. , Jenatschke, S. , 2000. The human NAD+-dependent 15-hydroxyprostaglandin dehydrogenase gene promoter is controlled by Ets and activating protein-1 transcription factors and progesterone. Endocrinology. 141, 581–597. [DOI] [PubMed] [Google Scholar]
- Huxley, R.R. , Ansary-Moghaddam, A. , Clifton, P. , 2009. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int. J. Cancer. 125, 171–180. [DOI] [PubMed] [Google Scholar]
- Jonsson, M. , Andersson, T. , 2001. Repression of Wnt-5a impairs DDR1 phosphorylation and modifies adhesion and migration of mammary cells. J. Cell Sci. 114, 2043–2053. [DOI] [PubMed] [Google Scholar]
- Jonsson, M. , Dejmek, J. , Bendahl, P.O. , 2002. Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res. 62, 409–416. [PubMed] [Google Scholar]
- Kikuchi, A. , Yamamoto, H. , Sato, A. , 2012. Wnt5a: its signalling, functions and implication in diseases. Acta Physiol. 204, 17–33. [DOI] [PubMed] [Google Scholar]
- Kremenevskaja, N. , von Wasielewski, R. , Rao, A.S. , 2005. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene. 24, 2144–2154. [DOI] [PubMed] [Google Scholar]
- Lehtinen, L. , Vainio, P. , Wikman, H. , 2012. 15-Hydroxyprostaglandin dehydrogenase associates with poor prognosis in breast cancer, induces epithelial-mesenchymal transition, and promotes cell migration in cultured breast cancer cells. J. Pathol. 226, 674–686. [DOI] [PubMed] [Google Scholar]
- Li, M. , Xie, J. , Cheng, L. , 2008. Suppression of invasive properties of colorectal carcinoma SW480 cells by 15-hydroxyprostaglandin dehydrogenase gene. Cancer Invest. 26, 905–912. [DOI] [PubMed] [Google Scholar]
- Liang, H. , Chen, Q. , Coles, A.H. , 2003. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell. 4, (5) 349–360. [DOI] [PubMed] [Google Scholar]
- Magnusson, C. , Mezhybovska, M. , Lörinc, E. , 2010. Low expression of CysLT1R and high expression of CysLT2R mediate good prognosis in colorectal cancer. Eur. J. Cancer. 46, 826–835. [DOI] [PubMed] [Google Scholar]
- Markowitz, S.D. , Bertagnolli, M.M. , 2009. Molecular origins of cancer: molecular basis of colorectal cancer. N. Engl. J. Med. 361, 2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung, S.J. , Rerko, R.M. , Yan, M. , 2006. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 103, 12098–12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomachi, A. , Nishita, M. , Inaba, D. , 2008. Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal kinase via actin-binding protein filamin A. J. Biol. Chem. 283, 27973–27981. [DOI] [PubMed] [Google Scholar]
- Pham, H. , Chen, M. , Li, A. , 2010. Loss of 15-hydroxyprostaglandin dehydrogenase increases prostaglandin E2 in pancreatic tumors. Pancreas. 39, 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad, C.P. , Chaurasiya, S.K. , Axelsson, L. , 2013. WNT-5A triggers Cdc42 activation leading to an ERK1/2 dependent decrease in MMP9 activity and invasive migration of breast cancer cells. Mol. Oncol. 7, 870–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Säfholm, A. , Leandersson, K. , Dejmek, J. , 2006. A formylated hexapeptide ligand mimics the ability of Wnt-5a to impair migration of human breast epithelial cells. J. Biol. Chem. 281, 2740–2749. [DOI] [PubMed] [Google Scholar]
- Säfholm, A. , Tuomela, J. , Rosenkvist, J. , 2008. The Wnt-5a-derived hexapeptide Foxy-5 inhibits breast cancer metastasis in vivo by targeting cell motility. Clin. Cancer Res. 14, 6556–6563. [DOI] [PubMed] [Google Scholar]
- Salim, T. , Sjölander, A. , Sand-Dejmek, J. , 2013. Nuclear expression of glycogen synthase kinase-3beta and lack of membranous beta-catenin is correlated with poor survival in colon cancer. Int. J. Cancer. 133, 807–815. [DOI] [PubMed] [Google Scholar]
- Sand-Dejmek, J. , Ehrnström, R. , Berglund, P. , 2013. The prognostic significance of Wnt-5a expression in primary breast cancer is extended to premenopausal women. PLoS One. 8, e70890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, R. , Desantis, C. , Jemal, A. , 2014. Colorectal cancer statistics, 2014. CA – Cancer J. Clin. 64, 104–117. [DOI] [PubMed] [Google Scholar]
- Sinicrope, F.A. , Gill, S. , 2004. Role of cyclooxygenase-2 in colorectal cancer. Cancer Met. Rev. 23, 63–75. [DOI] [PubMed] [Google Scholar]
- Smartt, H.J. , Greenhough, A. , Ordonez-Moran, P. , 2012. beta-catenin negatively regulates expression of the prostaglandin transporter PGT in the normal intestinal epithelium and colorectal tumour cells: a role in the chemopreventive efficacy of aspirin?. Br. J. Cancer. 107, 1514–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smartt, H.J. , Greenhough, A. , Ordonez-Moran, P. , 2012. Beta-catenin represses expression of the tumour suppressor 15-prostaglandin dehydrogenase in the normal intestinal epithelium and colorectal tumour cells. Gut. 61, 1306–1314. [DOI] [PubMed] [Google Scholar]
- Song, H.J. , Myung, S.J. , Kim, I.W. , 2011. 15-Hydroxyprostaglandin dehydrogenase is downregulated and exhibits tumor suppressor activity in gastric cancer. Cancer Invest. 29, 257–265. [DOI] [PubMed] [Google Scholar]
- Syed Khaja, A.S. , Helczynski, L. , Edsjo, A. , 2011. Elevated level of Wnt5a protein in localized prostate cancer tissue is associated with better outcome. PLoS One. 6, e26539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai, H.H. , Tong, M. , Ding, Y. , 2007. 15-Hydroxyprostaglandin dehydrogenase (15-PGDH) and lung cancer. Prost. Lipid Med. 83, 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin, D. , Podolsky, D.K. , 1999. Mitogen-activated protein kinase activation regulates intestinal epithelial differentiation. Gastroenterology. 116, 1072–1080. [DOI] [PubMed] [Google Scholar]
- Tenesa, A. , Dunlop, M.G. , 2009. New insights into the aetiology of colorectal cancer from genome-wide association studies. Nat. Rev. Genet. 10, 353–358. [DOI] [PubMed] [Google Scholar]
- Topol, L. , Jiang, X. , Choi, H. , 2003. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J. Cell Biol. 162, 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafillidis, J.K. , Nasioulas, G. , Kosmidis, P.A. , 2009. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. Int. J. Cancer Res. Treat. 29, 2727–2737. [PubMed] [Google Scholar]
- Tseng-Rogenski, S. , Gee, J. , Ignatoski, K.W. , 2010. Loss of 15-hydroxyprostaglandin dehydrogenase expression contributes to bladder cancer progression. Am. J. Pathol. 176, 1462–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turini, M.E. , DuBois, R.N. , 2002. Cyclooxygenase-2: a therapeutic target. Annu. Rev. Med. 53, 35–57. [DOI] [PubMed] [Google Scholar]
- Wang, C. , Zhao, Y. , Su, Y. , 2013. C-Jun N-terminal kinase (JNK) mediates Wnt5a-induced cell motility dependent or independent of RhoA pathway in human dental papilla cells. PLoS One. 8, e69440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Fu, L. , Sun, H. , 2015. Prostaglandin E promotes colorectal cancer stem cell expansion and metastasis in mice. Gastroenterology. 149, (7) 1884–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, B.D. , Chien, A.J. , Dawson, D.W. , 2012. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology. 142, 219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, M. , Rerko, R.M. , Platzer, P. , 2004. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proc. Natl. Acad. Sci. U. S. A. 101, 17468–17473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying, J. , Li, H. , Yu, J. , 2008. WNT5A exhibits tumor-suppressive activity through antagonizing the Wnt/beta-catenin signaling, and is frequently methylated in colorectal cancer. Clin. Cancer Res. 14, 55–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article:
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data


