Abstract
Increasing interest has been devoted in recent years to the understanding of alternative splicing in cancer. In this study, we performed a genome‐wide analysis to identify cancer‐associated splice variants in non‐small cell lung cancer. We discovered and validated novel differences in the splicing of genes known to be relevant to lung cancer biology, such as NFIB, ENAH or SPAG9. Gene enrichment analyses revealed an important contribution of alternative splicing to cancer‐related molecular functions, especially those involved in cytoskeletal dynamics. Interestingly, a substantial fraction of the altered genes found in our analysis were targets of the protein quaking (QKI), pointing to this factor as one of the most relevant regulators of alternative splicing in non‐small cell lung cancer. We also found that ESYT2, one of the QKI targets, is involved in cytoskeletal organization. ESYT2‐short variant inhibition in lung cancer cells resulted in a cortical distribution of actin whereas inhibition of the long variant caused an increase of endocytosis, suggesting that the cancer‐associated splicing pattern of ESYT2 has a profound impact in the biology of cancer cells. Finally, we show that low nuclear QKI expression in non‐small cell lung cancer is an independent prognostic factor for disease‐free survival (HR = 2.47; 95% CI = 1.11–5.46, P = 0.026). In conclusion, we identified several splicing variants with functional relevance in lung cancer largely regulated by the splicing factor QKI, a tumor suppressor associated with prognosis in lung cancer.
Keywords: Alternative splicing, Non‐small cell lung cancer, ESYT2, QKI
Highlights
We have generated an extensive list of differential splicing events in lung cancer.
The splicing factor QKI is a major regulator of alternative splicing in lung cancer.
The splicing of ESYT2, a QKI target, regulates actin and endocytosis dynamics.
QKI protein downregulation is an independent factor for poor prognosis in lung cancer.
Abbreviations
- ESYT2‐L
ESYT2 long splice variant
- ESYT2‐S
ESYT2 short splice variant
- QKI
protein quaking
- NSCLC
non‐small cell lung cancer
1. Introduction
Alternative splicing is a regulatory mechanism of gene expression with an essential role in cell homeostasis (Chen et al., 2014; Fu and Ares, 2014; Xie, 2014). Deregulation of alternative splicing affects essential biological processes and can lead to several disease conditions, including cancer (Gamazon and Stranger, 2014; Padgett, 2012). In recent years, the relevance of alternative splicing in tumor development and progression has been widely recognized (Biamonti et al., 2014; Chen and Weiss, 2015; Oltean and Bates, 2014). Alterations in splicing are frequently caused by abnormal expression or mutations in splicing regulatory factors. The best known example is the involvement of mutations in splicing factor 3b, subunit 1 (SF3B1) in the pathogenesis of myelodysplastic syndromes and chronic lymphocytic leukemia (Dolatshad et al., 2015; Wan and Wu, 2013).
Cancer‐associated alternative splicing changes are linked to the acquisition of malignant potential (Oltean and Bates, 2014). Interestingly, a relatively small number of splice factors strongly influence the alternative splicing pattern of many cancer‐related genes, suggesting that tumor progression is associated with a coordinated splicing program controlled by these few splice regulators (Oltean and Bates, 2014). In the case of lung cancer, the most lethal cancer worldwide, several studies have demonstrated the biological impact of alternative splice variants (Pio and Montuenga, 2009). The relevance of splicing in lung cancer has also been evidenced by the identification of aberrant RNA transcripts associated with somatic mutations in RNA binding motif protein 10 (RBM10) and U2 small nuclear RNA auxiliary factor 1 (U2AF1) (Cancer Genome Atlas Research Network, 2014; Imielinski et al., 2012). Serine‐arginine rich splicing factor 1 (SRSF1) has also been shown to act as a proto‐oncogene in lung tumors (Karni et al., 2007). More recently, Zong et al. have identified quaking (QKI) as a novel tumor suppressor downregulated in lung cancer and associated with poor prognosis (Zong et al., 2014). Other splicing factors, such as poly(rC) binding protein 4 (PCBP4), RBM4, RBM5, RBM6 and RBM10 are also downregulated in lung cancer and may act as tumor suppressors (Bechara et al., 2013; Pio et al., 2004; Wang et al., 2014; Xie, 2014). However, the relative contribution of any of these potential oncogenic or tumor suppressor factors to the lung cancer‐associated splicing profile is still unknown. The identification of the master regulators that control alternative splicing in lung cancer would be important to understand the functional relevance of lung cancer‐associated splice variants and to facilitate the development of novel anticancer therapeutics and biomarkers.
We recently developed an algorithm for the analysis of genome‐wide splicing changes using data from exon‐junction arrays (de Miguel et al., 2014). We have now applied this technique to perform an unbiased large‐scale characterization of splicing events in non‐small cell lung cancer (NSCLC) using a pairwise comparison with matched non‐tumor lung tissues from the same patients. NSCLC, which includes adenocarcinoma, squamous cell carcinoma and large‐cell carcinoma histosubtypes, represents approximately 85% of all lung cancer cases (Gridelli et al., 2015). We report here an extensive and highly reliable list of differentially expressed splicing events in primary NSCLC which includes known lung cancer‐associated splicing variants, as well as novel genes with no previous evidence of differential alternative splicing in lung cancer. Moreover, our analysis has allowed us to better delineate the implication of splicing regulators in this malignancy. Interestingly, a remarkable fraction of the differential splicing events were identified as targets of QKI, strongly suggesting that this RNA binding protein is a major regulator of the differential alternative splicing program linked to lung cancer. Moreover, we demonstrate that QKI regulates the splicing of genes involved in cytoskeletal dynamics, and that low QKI protein expression in primary lung tumors is an independent prognostic factor associated with worse survival.
2. Materials and methods
2.1. Biological material
Primary lung tumors were obtained from patients with lung cancer treated with surgery at the Clínica Universidad de Navarra (Pamplona, Spain). Inclusion criteria were: complete resection of the primary tumor, absence of cancer within the previous five years, and no treatment with chemo or radiotherapy prior to surgery. Non‐tumor lung specimens were sampled at a distance from the tumor to guarantee that they were free from cancerous cells. Clinicopathological characteristics of the patients are summarized in Table S1. For RNA or protein extraction, tissue samples were immediately frozen in liquid nitrogen and stored at −80 °C. Samples were included in the study if more than 70% of their cells were tumor cells. RNA extraction was performed as previously described (Valles et al., 2012). Protein extraction was carried out as previously described (Valles et al., 2012), using RIPA buffer (10 mM Tris–Cl pH 7.4, 0.5 M NaCl, 1% sodium deoxycholate, 0.1% SDS, 1% Triton X‐100) with a complete protease inhibitor cocktail (Roche). For immunocytochemistry, resected primary lung tumors were fixed in buffered formalin and embedded in paraffin. Lung tumors were classified according to the WHO 2004 classification and the International System for Staging Lung Cancer (Mountain, 1997; Travis et al., 2004). The study protocol was approved by the Institutional Ethical Committee (Institutional Review Board#015‐2014) and all patients gave written informed consent.
Lung cancer cell lines were obtained from the American Type Culture Collection (ATCC), the German Collection of Microorganisms and Cell Cultures (DSMZ), the European Collection of Cell Cultures (ECACC), or the Korean Cell Line Bank (KCLB). Cell lines were authenticated by analysis of their genetic alterations. Cells were grown in RPMI supplemented with 2 mmol/L glutamine, 10% Fetalclone (Thermo), 100 U/mL of penicillin and 100 mg/mL of streptomycin (Invitrogen).
2.2. Microarray hybridization and analysis
Samples from 21 primary lung tumors (12 squamous cell carcinomas, 8 adenocarcinomas and 1 large cell carcinoma) and matched non‐tumor lung tissues from the same patients were labeled and hybridized in Affymetrix Human GeneSplice arrays (obtained through a Technology Access program). Labeling and hybridization were performed by the Genomics Core Facility of the Center for Applied Medical Research (CIMA), following manufacturer's instructions. The ExonPointer algorithm, which combines data from exon and junction microarray probes, was used to identify annotated cassette exons (de Miguel et al., 2014).
2.3. Validation of splicing events
Validation of splicing events was carried out by endpoint PCR using primers located in the flanking exons of the spliced cassettes (Figure S1). RNA was retro‐transcribed using the PrimeScript RT reagent kit (Takara). PCR was performed using PCR Master Mix (Promega) and the following program: 94 °C, 2 min; 30 cycles at 94 °C, 30 s; 57 °C, 30 s; 72 °C, 30 s; and 72 °C, 10 min. Primers used are shown in Table S2. The PCR products were loaded in 2% agarose gels and separated for 40–60 min at 100–120 V. Bands were visualized in a UV light transilluminator (BioRad), and pictures of the gels were saved with the software Quantity One v4.5 (BioRad). A densitometric analysis of the bands was performed using Fiji software (Schindelin et al., 2012). The relative level of each band was calculated as a percentage of the total intensity of the two bands, and represented in a bar chart using red and blue colors for the long and short isoforms, respectively.
2.4. Down‐regulation and expression analyses
Cells were transfected with a 30 nM solution of a non‐targeting scrambled or a specific siRNA using lipofectamine (Invitrogen), as described by the manufacturer. Cells were incubated for 48 h after transfection. Total RNA was extracted using the NucleoSpin RNA kit (Macherey–Nagel) and retrotranscribed. The efficiency of the siRNA knockdown was determined by real‐time PCR using SYBR Green PCR Master Mix (Applied Biosystems). GAPDH was used as housekeeping gene for cell lines and IPO8 for clinical samples (Nguewa et al., 2008). Sequences of siRNAs and primers are detailed in Table S3.
2.5. Immunofluorescence analysis
Cells were cultured and transfected with ESYT2 siRNAs as described above. After 48 h of transfection, cells were plated onto 8 well slides (Nunc Lab‐Teck) pre‐coated with 50 μg/mL of type I rat tail collagen (BD Biosciences). For F‐actin and tubulin experiments, cells were subsequently permeabilized with 0.02% Triton X‐100 in PBS and stained overnight at 4 °C with an anti‐α‐tubulin monoclonal antibody (clone YL1/2; 1:400; Santa Cruz Biotechnology). Afterward, samples were incubated for 1 h at room temperature with a secondary antibody conjugated to Alexa Fluor 488 (1:400; Invitrogen) and rhodamine‐labeled phalloidin (1:75; Sigma–Aldrich). For transferrin experiments, cells were incubated with transferrin conjugated to Alexa Fluor 594 (50 μg/mL, Invitrogen), rinsed with PBS and further incubated with RPMI medium, supplemented with 10% Fetalclone for 30 min at 37 °C. Cell cultures were rinsed with PBS and fixed in 4% paraformaldehyde at 37 °C for 15 min. Images were captured with a 63X Plan‐Apochromat objective (N.A. 1.40; oil) of a laser‐scanning confocal microscope (LSM 510 META; Carl Zeiss). Images and Z sections were acquired using Aim4 software (Carl Zeiss) and processed with Fiji software and Volocity (Perkin Elmer). To quantify F‐actin and transferrin levels, the region of interest (ROI) in each cell was selected by the threshold function and divided into two different regions. In the case of F‐actin, one sector comprised the first 5 microns from the cell edge (cortical) and a second sector contained the rest of the cell (cytoplasmic). For transferrin, one unique region was defined containing the first 5 microns from the nuclear perimeter. Fluorescence intensity (arbitrary units) was measured in each sector using Fiji software. At least 30 cells for F‐actin and 15–20 cells for transferrin were analyzed per experiment.
2.6. Western blotting
Protein concentration was determined by the Pierce BCA Protein Assay kit (Thermo Scientific). Proteins were denatured in SDS sample buffer (Bio‐Rad) at 95 °C for 5 min, separated by SDS‐PAGE on NuPAGE Novex 4–12% Bis‐Tris gels (Invitrogen) and transferred to nitrocellulose membranes (0.45 μm pore size; Bio‐Rad). Membranes were blocked in 5% non‐fat milk for 2 h and incubated overnight at 4 °C with the primary antibody against QKI (HPA019123; 1:5000; Sigma Aldrich) or β‐actin (clone AC‐15; 1:10000; Sigma Aldrich). Secondary antibodies were applied (anti‐rabbit IgG NA934 or anti‐mouse IgG NA931; 1:2000; GE Healthcare), and chemiluminescent detection was performed using Lumi‐Light PLUS (Roche).
2.7. Immunohistochemistry analysis
An immunohistochemical assay was performed to evaluate QKI expression on a microarray of formalin‐fixed paraffin‐embedded tissues. Endogenous peroxidase activity was quenched with 3% H2O2 for 10 min. Antigen retrieval was carried out by heating slides in a Lab Vision PT module for 20 min with Tris–EDTA buffer (pH 9) at 95 °C. Afterward, tissues were incubated in a humidity chamber with the anti‐QKI polyclonal antibody (1:500) overnight at 4 °C. After applying the Envision + System‐HRP (Dako) for 30 min, immunostaining was developed by incubation with Liquid DAB + Substrate Chromogen System (Dako) under microscopic control. Negative controls were performed by omission of the primary antibody. Staining was evaluated by two observers independently (E.M.T. and M.J.P.) blinded to the clinical features and outcomes of patients. An H‐score was calculated as previously described (Pajares et al., 2012). The specificity of the antibody was evaluated by Western blotting and immunocytochemistry of cell lines expressing different levels of QKI (Figure S2).
2.8. Statistical analyses
The statistical analysis for the selection of significant alternative splicing events using the ExonPointer algorithm has been previously detailed (de Miguel et al., 2014). For enrichment analyses, the DAVID Functional Annotation Clustering application was used to create clusters, in accordance with annotations from several databases (Huang da et al., 2009). The DAVID Functional Annotation tool was run to obtain P values for enriched annotation terms. Enrichment analyses of networks and functions were performed with Ingenuity Pathway Analysis (IPA, Ingenuity Systems). Whole genome databases were used as references for the analyses. Correlations were assessed using the Pearson correlation test. The list of genes related to “mRNA splicing” were retrieved from UniProt (UniProt, 2015) and their expression data from the Cancer Cell Line Encyclopedia (Barretina et al., 2012). Gene set enrichment analysis of QKI targets was performed with the Wilcoxon rank‐sum test. Differences in splice variant expression in cell lines, F‐actin distribution and transferrin distribution were assessed by the Student's t‐test. QKI mRNA expression, protein expression and immunohistochemical H‐scores were analyzed as non‐normally distributed data using the Mann–Whitney U test, the Kruskal–Wallis H test or the Wilcoxon matched‐pairs signed‐ranks. Survival curves were generated by the Kaplan–Meier method using the median H‐score as the cut‐off, and differences were analyzed with the log rank test. Multivariate analyses were performed with the Cox proportional hazards model. Statistically significant variables from the univariate Cox analysis were entered into the multivariate analysis. The proportional hazards assumption was also examined. Normality of all the datasets was evaluated with the Shapiro–Wilk test. All statistical tests were two‐sided, and were considered statistically significant at P < 0.05. Tests were performed using STATA/IC version 12.1.
3. Results
3.1. Identification of differential splicing events in non‐small cell lung cancer
A genome‐wide evaluation of differential alternative cassette events in NSCLC was performed using exon‐junction arrays and the ExonPointer algorithm in 21 lung primary tumors (8 adenocarcinomas, 12 squamous cell carcinomas and 1 large cell carcinoma) and their corresponding non‐malignant lung tissues. We obtained a ranked list of splicing events differentially expressed in lung cancer tissues. Table 1 shows the top 20 ranked genes selected by ExonPointer. The complete list of events is shown in Table S4. We were able to validate, by RT‐PCR, 14 of the top 20 events (Table 1, Figure 1A and Figure S3). Twelve of the validated events showed exon retention in tumors (SCEL, ENAH, NUMB, ESYT2, EHBP1, DOCK9, FN1, RAC1, KIF13A, CYP4F3, GOLGA4 and NFIB), while two of them showed exon skipping (PRMT2 and CEACAM1). In some cases, in addition to splicing changes, there were differences in expression (e.g. NUMB, NFIB and PRMT2). No differences in the splicing patterns were observed between lung adenocarcinomas and squamous cell carcinomas (data not shown). In six of the genes (FLI1, COL12A1, WNK1, CD34, FBXO18 and SLC12A4), we did not find any significant change in splicing (data not shown). Additional validations were performed in other positions of the list (Figure 1B). As showed in Table S5, consistent with findings in the literature and indicative of the validity of our analysis, many of the alternative splicing events ranked by ExonPointer had been previously found in different types of tumors (Danan‐Gotthold et al., 2015), including lung adenocarcinoma (8 genes), head and neck squamous cell carcinoma (2 genes), breast carcinoma (2 genes), colon adenocarcinoma (9 genes) and kidney renal clear cell carcinoma (4 genes).
Table 1.
List of the top 20 ranked events identified by ExonPointer as differentially spliced in non‐small cell lung cancer.
| Rank | Gene | P value | Exon | Ensembl transcript ID | Event | Validated |
|---|---|---|---|---|---|---|
| 1 | SCEL | 5.00E‐183 | 6 | 202 | Inclusion | Yes |
| 2 | ENAH | 6.82E‐168 | 12 | 006 | Inclusion | Yes |
| 3 | NUMB | 4.44E‐151 | 11 | 003 | Inclusion | Yes |
| 4 | FLI1 | 1.01E‐128 | 3 | 002 | Inclusion | No |
| 5 | ESYT2 | 2.44E‐127 | 16 | 004 | Inclusion | Yes |
| 6 | EHBP1 | 2.59E‐117 | 17 | 001 | Inclusion | Yes |
| 7 | DOCK9 | 1.75E‐107 | 38 | 001 | Inclusion | Yes |
| 8 | FN1 | 8.76E‐105 | 25 | 001 | Inclusion | Yes |
| 9 | RAC1 | 4.15E‐103 | 4 | 002 | Inclusion | Yes |
| 10 | COL12A1 | 2.57E‐97 | 8 | 001 | Inclusion | No |
| 11 | PRMT2 | 5.36E‐96 | 2 | 001 | Exclusion | Yes |
| 12 | KIF13A | 6.26E‐94 | 38 | 001 | Inclusion | Yes |
| 13 | CYP4F3 | 1.08E‐90 | 3 | 201 | Exclusion | Yes |
| 14 | WNK1 | 1.48E‐88 | 12 | 001 | Inclusion | No |
| 15 | CD34 | 7.24E‐88 | 4 | 201 | Exclusion | No |
| 16 | CEACAM1 | 5.15E‐83 | 7 | 001 | Exclusion | Yes |
| 17 | GOLGA4 | 5.54E‐83 | 23 | 001 | Inclusion | Yes |
| 18 | FBXO18 | 1.83E‐79 | 14 | 201 | Exclusion | No |
| 19 | NFIB | 3.57E‐77 | 9 | 002 | Inclusion | Yes |
| 20 | SLC12A4 | 3.05E‐74 | 5 | 001 | Exclusion | No |
Figure 1.

Analysis of the alternative splicing events identified by ExonPointer in NSCLC. A. Differential alternative splicing was confirmed by PCR in 14 out of the top 20 genes selected by ExonPointer. The relative percentage of each isoform was measured by densitometry in 10 randomly selected clinical cases. Percentages are shown as mean ± SEM. In the case of CEACAM1, the difference was not statistically significant. N: Non‐tumor tissue, T: Tumor tissue. B. PCR validation of differential alternative splicing events in genes ranked by ExonPointer at positions: 97th (SPAG9), 98th (LIMCH1), 99th (KIAA1217), 155th (ERBB2IP) and 233th (ITGB4). C. DAVID Functional Annotation Chart for genes within the first 250 events selected by ExonPointer based on annotations from several databases. Categories are ordered by P value. The number of genes included in each category is also showed. GO: Gene Ontology, BP: Biological Process, MF: Molecular Function, CC: Cellular Component, SP: SwissProt, IP: InterPro, UP: UniProt. *P < 0.05; **P < 0.01; ***P < 0.001.
We next performed a gene cluster analysis with the first 250 events classified by ExonPointer (which corresponded to 166 genes). Ingenuity function enrichment analysis selected “Cancer” and “Tumor Morphology” as the first and the fifth enriched ontologies within the category “Diseases and Disorders” (Table 2). Notably, 140 out of the 166 genes (84%) had annotations related to cancer. Moreover, the first five ontologies within the category “Molecular and Cellular Functions” were highly related to cancer development: “Cellular Growth and Proliferation”, “Cell Cycle”, “Cellular Movement”, “Cellular Assembly and Organization” and “Cellular Function and Maintenance”. Regarding the canonical pathways, “Signaling by Rho Family GTPases” and “RhoA Signaling” were significantly enriched. DAVID functional analysis revealed a strong enrichment in annotations associated with cellular assembly among the top 20 clusters: “Cell Adhesion”, “Actin Binding”, “Cytoskeleton Organization” or “Actin Cytoskeleton Organization” (Figure 1C). These analyses suggest an important contribution of alternative splicing to cancer‐related molecular functions, especially those involved in cell adhesion and mobility.
Table 2.
Ingenuity function enrichment analysis of the top 250 events identified by ExonPointer as differentially spliced in non‐small cell lung cancer.
| Diseases and disorders | P value rank | No. genes | |
|---|---|---|---|
| Cancer | 1.50 e‐07 | 7.81e‐03 | 140 |
| Organismal injury and abnormalities | 1.50 e‐07 | 7.81e‐03 | 95 |
| Reproductive system disease | 1.50 e‐07 | 7.81e‐03 | 85 |
| Inflammatory response | 3.91 e‐05 | 7.81e‐03 | 20 |
| Tumor morphology | 6.07 e‐05 | 7.81e‐03 | 24 |
| Molecular and cellular functions | P value rank | No. genes | |
| Cellular growth and proliferation | 1.87 e‐06 | 7.81e‐03 | 69 |
| Cell cycle | 7.01 e‐06 | 7.81e‐03 | 32 |
| Cellular movement | 7.59 e‐06 | 7.81e‐03 | 47 |
| Cellular assembly and organization | 1.22 e‐05 | 7.81e‐03 | 45 |
| Cellular function and maintenance | 1.22 e‐05 | 7.81e‐03 | 55 |
| Canonical pathways | P value | No. genes a | |
| Signaling by Rho Family GTPases | 9.60 e‐05 | 9/234 | |
| RhoA signaling | 3.96 e‐04 | 6/122 | |
| IL‐3 signaling | 2.32 e‐03 | 4/71 | |
| Netrin signaling | 3.48 e‐03 | 3/39 | |
| Inhibition of matrix metalloproteases | 9.60 e‐05 | 3/39 | |
Number of genes in the top 250/Number of genes included in the pathway by IPA.
3.2. QKI is a key regulator of splicing in NSCLC
In order to identify the factor/s responsible for the splicing changes found in primary lung tumors, we reviewed the literature in search for the reported targets of splicing factors associated with lung cancer: SRSF1 (de Miguel et al., 2014), SRSF2 (Gout et al., 2012), SRSF6 (Jensen et al., 2014), RBFOX1/2 (Danan‐Gotthold et al., 2015), RBM4 (Wang et al., 2014), RBM10 (Cancer Genome Atlas Research Network, 2014), U2AF1 (Brooks et al., 2014; Imielinski et al., 2012) and QKI (Zong et al., 2014). We compared the lists of genes obtained from these studies with the list of 166 genes corresponding to the top 250 events of our analysis, and found that 21.1% of them (35 out of 166) were targets of the factor quaking (QKI). The order of the remaining splicing factors according to the number of common genes was as follows: SRSF1, RBFOX1/2, RBM10, U2AF1, SRSF2, RBM4 and SRSF6 (Figure 2A and Table S6). Furthermore, we found a remarkable and statistically significant enrichment of QKI validated targets in the top positions of the complete list of events (P < 0.001; Figure 2B). Notably, 7 out of the 14 top 20 validated events had been previously described as splicing targets for QKI (Zong et al., 2014): NUMB, ESYT2, EHBP1, FN1, PRMT2, KIF13A and GOLGA4. Moreover, in agreement with previous studies (Zong et al., 2014), we found a significant downregulation of QKI mRNA expression in our cohort of primary NSCLC tissues as compared to the adjacent non‐malignant lung tissue (P < 0.001; Figure S4A). All these results highlight the importance of QKI in the regulation of alternative splicing in NSCLC.
Figure 2.
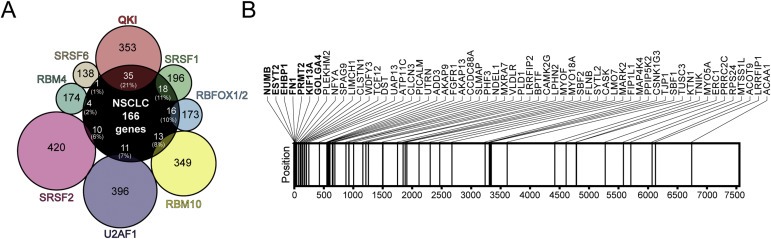
QKI is a key regulator of splicing in NSCLC. A. Venn diagram of the targets of lung cancer‐related splicing factors common to 166 genes corresponding to top 250 differentially‐spiced events identified by ExonPointer. The list of targets was obtained from the literature: QKI (Zong et al., 2014), SRSF1 (de Miguel et al., 2014), RBFOX1/2 (Zhang et al., 2008), RBM10 (Wang et al., 2013b), U2AF1 (Shirai et al., 2015), SRSF2 (Zhang et al., 2015), RBM4 (Wang et al., 2014) and SRSF6 (Jensen et al., 2014). Approximate percentages represent the proportion of genes in the ExonPointer list that are targets for a given splicing factor. B. Schematic representation of the positions occupied by 60 QKI targets, according to Zong et al. (Zong et al., 2014), in the ranked list generated by ExonPointer. Genes among the top 20 are depicted in bold. Horizontal lines represent the position of each gene in the list. A statistically significant overrepresentation of QKI target genes was observed in the ExonPointer list (P < 0.001).
3.3. Alternative spliced targets of QKI regulate cytoskeletal organization
To support the involvement of QKI in the biology of lung cancer, we performed an enrichment analysis with genes that co‐expressed with QKI in 48 lung cancer cell lines. For this purpose, we used 220 genes that correlated with QKI expression (r > |0.5|, P < 0.001). Notably, DAVID functional analysis identified annotations related to cellular assembly among the top 20 clusters: “Cell‐substrate Junction”, “Regulation of Cell Motion”, and “Actin Cytoskeleton”, among others (Figure S5A). These functions are noticeably similar to the functions found associated with the differentially‐spliced events described above (Figure 1C). Therefore, the expression levels of QKI seem to be relevant for the modulation of alternative splicing and cytoskeletal assembly. To further evaluate this association, we selected genes identified by ExonPointer which were previously associated with cytoskeletal assembly: ENAH, ESYT2 and SPAG9. The three of them have been reported to be involved in actin dynamics and metastasis in different types of tumors (Di Modugno et al., 2012; Jean et al., 2012; Tanaka et al., 2014; Yan et al., 2016; Yang et al., 2016a). We studied, in lung cancer cell lines, the correlation between the ratio of ENAH, ESYT2 and SPAG9 splice variants and the expression of 230 genes related to “mRNA splicing”. The three splicing factors with the strongest correlation were QKI, ESRP1 and ESRP2 (Table S7). Figure S5B shows the correlation between the expression of QKI and the splicing pattern of the three genes. We next knocked down the expression of QKI, ESRP1 and ESRP2 in A549 and H2009 cells (Figure S6A) and assessed the splicing of ENAH, ESYT2 and SPAG9. The splicing of ENAH was not modified after downregulation of any of the factors (Figure S6B). Downregulation of QKI resulted in a clear reduction of ESYT2 short mRNA isoform, whereas ESRP1 and ESRP2 downregulation did not show any substantial change in ESYT2 splicing (Figure S6C). Finally, there was a switch in the splicing of SPAG9 mRNA in A549 cells after neutralization of QKI, but not in H2009 cells (Figure S6D). Based on these results, we next focused our attention on ESYT2 splicing and on its role in cytoskeletal dynamics. The two main splice isoforms of ESYT2 are characterized by the presence or absence of a cassette exon between exons 13 and 14. The inclusion of this exon results in 21 additional amino acids in the C2B domain (Figure 3A). As shown previously in Figure 1, lung tumors tend to express higher levels of the long splice variant (ESYT2‐L), whereas the short variant (ESYT2‐S) is the predominant form in non‐malignant lung tissue. ESYT2‐S was the minor form in 28 out of 48 lung cancer cell lines (Figure 3B). The predominance of the long variant isoform was observed both in adenocarcinoma and squamous cell carcinoma cell lines, although it was more evident in adenocarcinomas (ESYT2 L/S ratio: 5.41 ± 6.78 vs. 1.51 ± 2.01, respectively; P = 0.070). To test for the functional significance of each variant in cytoskeletal dynamics, we knocked down ESYT2‐L or ESYT2‐S in A549 cells using variant‐specific siRNAs (Figure 3C). When ESYT2‐L was downregulated, cells showed a cytoskeleton organization similar to control cells, in which α‐tubulin spread out radially and F‐actin stress fibers were crossing the cytoplasm. However, after ESYT2‐S downregulation, α‐tubulin lost its polarity, gaining a net conformation, and F‐actin stress fibers left the cytoplasm and took a cortical distribution next to plasma membrane (Figure 3D). On the other hand, cells in which ESYT2‐L was diminished showed a higher accumulation of clathrin vesicles next to the nucleus, while ESYT2‐S downregulation showed the opposite effect (Figure 3E). Similar results were obtained when ESYT2‐L or ESTY2‐S was selectively knockdown in H2009 cells (Figure S7). All together, these results reinforce the implication of QKI in the differential splicing patterns found in non‐small cell lung cancer and, more particularly, in the splicing regulation of genes involved in cytoskeletal dynamics.
Figure 3.

ESYT2 splice variants regulate cytoskeleton and clathrin organization. A. Representative scheme of ESYT2 splicing variants: ESYT2‐L (long) and ESYT2‐S (short). Exon 13B retention disrupts the domain C2B. B. Relative percentage of each ESYT2 splicing isoform measured by PCR in 48 lung cancer cell lines ordered by ESYT2‐S expression. C. Isoform specific inhibition of ESYT2 with siRNAs in A549 cells. Mean ± SEM from three independent experiments is shown. D. α‐tubulin and F‐actin staining in A549 cells after variant‐specific siRNA inhibition. Scale bar: 20 μm. Histograms represent F‐actin fluorescence intensity measured in arbitrary units from A‐to‐B straight line (approximately 40 μm). Bar plot represents F‐actin intensity in the cortical area (5 μm from the plasma membrane) and the cytoplasm. Mean ± SEM of 30 independent cells is shown. E. Transferrin staining in A549 cells after variant‐specific siRNA inhibition. Scale bar: 20 μm. Bar plot represents transferrin fluorescence intensity measured in arbitrary units in the peripheral area of the nucleus (5 μm from the nuclear perimeter). Mean ± SEM of 15–20 independent cells is shown. **P < 0.01; ***P < 0.001.
3.4. QKI is a prognostic factor in lung cancer
Using an in silico analysis, Zong et al. found that QKI mRNA downregulation correlated with poor overall survival in NSCLC patients (Zong et al., 2014). We now aimed to characterize the expression of QKI protein in lung primary tumors both by Western blotting and by immunocytochemistry. For Western blot analysis, seven tumor tissues and their matched adjacent non‐tumor tissues were used. Expression of QKI was significantly diminished in tumor tissues as compared to their non‐tumor counterparts (Figure 4A). Interestingly, we observed two immunoreactive bands, corresponding to QKI isoforms QKI‐5 and QKI‐6 (according to their molecular weights). More importantly, a splicing shift was observed between normal and tumor tissues. In particular, QKI‐6 was the predominant form in normal tissue, while this pattern was inverted in tumors (Figure 4A). We confirmed this splicing change at the mRNA level (Figure S4B and C). We next performed an immunocytochemistry analysis of total QKI and determined its prognostic significance using a tissue microarray containing 126 primary lung cancer tissues. In normal lung tissue, QKI positive staining was found in the epithelial cells of bronchi and bronchioles. QKI was also strongly expressed in alveolar macrophages, type I and II pneumocytes, endothelial cells and stromal cells. The staining was predominantly detected in the nucleus, although a weaker staining was also observed in the cytoplasm (Figure 4B and E). The expression of nuclear QKI was significantly lower in tumor cells than in normal lung epithelium (P < 0.001 for both bronchial and alveolar tissue; Figure 4F), as it was the case for cytoplasmic QKI (data not shown). Relationships between clinicopathological characteristics and QKI nuclear expression in tumors are shown in Table S8. A significant association was found between QKI levels and histology. Specifically, we observed a lower expression of the protein in specimens with neuroendocrine differentiation (one small cell lung cancer and four atypical carcinoids). However, there were no differences in QKI expression between adenocarcinomas and squamous cell carcinomas. No statistical associations were found with sex, age, smoking status, grade or stage. Interestingly, disease‐specific survival was significantly decreased in patients with low QKI expression (log rank test; P = 0.014; Figure 4G). In a univariate Cox regression analysis, low QKI immunoreactivity was significantly associated with poor survival (HR = 2.58; 95% CI = 1.18–5.63, P = 0.018; Table S9). A multivariate analysis, adjusted by stage, showed that QKI expression was an independent prognostic factor for survival (HR = 2.47; 95% CI = 1.11–5.46, P = 0.026; Table S10).
Figure 4.

QKI expression is downregulated in lung cancer and is associated with poor prognosis. A. QKI protein expression, as determined by Western blotting, in 7 lung tumors and matched non‐tumor lung tissues. Dot plots show the expression of total QKI (gray), QKI‐5 (red) and QKI‐6 (blue), normalized with β‐actin and measured by densitometry. Mean + IQR is shown. Histogram represents the relative expression of the QKI isoforms, measured by densitometry, in the 7 paired samples. N: Non‐tumor tissue, T: Tumor tissue B‐E. Representative QKI immunohistochemical stainings. (B) Bronchial epithelium. (C) Lung parenchyma. (D) Squamous cell carcinoma of the lung. (E) Lung adenocarcinoma. QKI expression was predominantly nuclear. Scale bar: 50 μm. F. H‐score of nuclear QKI staining in bronchioles (Bro), alveoli (Alv) and lung tumors (Tum). Median + IQR is shown. G. Kaplan–Meier survival curves. Patients were divided into low and high QKI expression using the median of the H‐score values as cut‐off. The P value of the log rank test is shown. *P < 0.05; **P < 0.01; ***P < 0.001.
4. Discussion
Major progress has been made during the past decade in understanding the functional relevance of splice‐associated variants in cancer development. In this study, we have generated an extensive and highly reliable list of differentially expressed splicing events in non‐small cell lung cancer, regardless of histological subtypes. The identification of the events was performed with ExonPointer, an algorithm previously described and validated by us that detects differences in splicing based on data from microarrays containing exon and junction probes (de Miguel et al., 2014). Large‐scale screens have been previously performed to identify differential alternative splicing events in lung cancer compared to non‐malignant lung tissue (Langer et al., 2010; Misquitta‐Ali et al., 2011; Pio et al., 2010; Xi et al., 2008). Two of these studies used both adenocarcinoma and squamous cell carcinoma patients (Langer et al., 2010; Pio et al., 2010). Of them, the study by Langer et al., using exon microarrays, identified fourteen genes that exhibited differential splicing in NSCLC (Langer et al., 2010). Our group identified four genes using an in‐house analysis system and data from newly designed arrays with exon and junction probes (Pio et al., 2010). More recently, we demonstrated the advantage of the ExonPointer algorithm, also based in microarray platforms that contain exon and junction probes, to identify differentially spliced genes (de Miguel et al., 2014).
The power and reliability of our current screening method lies in the fact that we detected differences in well‐known genes with lung‐cancer associated splicing variants, such as NUMB, FN1, RAC1 or CEACAM1, as well as in several others recently described (Danan‐Gotthold et al., 2015; Langer et al., 2010; Pio et al., 2010; Xi et al., 2008; Zong et al., 2014). We have also unveiled splicing differences in novel genes with no previous evidence of differential alternative splicing in lung cancer. This is the case of NFIB, an oncogenic transcription factor amplified in small cell lung cancer (Dooley et al., 2011), or SPAG9, a scaffold protein that structurally organizes mitogen‐activated protein kinases and has been associated with lung cancer progression and prognosis (Wang et al., 2013a). In addition, the alternative splicing of other genes identified in our analysis has been previously studied, but has not been associated with lung cancer. For instance, CYP4F3, an enzyme participating in the metabolism of lipids, has splice variants with different lipophilic substrate specificity (Christmas et al., 2001). In‐depth analyses of these and other genes found in our study will help to understand the relevance of their splice variants in lung tumor progression.
Gene enrichment analyses of the identified events also yielded informative results regarding the overall implication of alternative splicing in lung cancer biology. Several enriched molecular and cellular functions of the genes undergoing different splicing were related to tumor progression and development. The most significantly altered canonical pathway was the Rho signaling pathway, long associated with cancer progression (Jaffe and Hall, 2002; Vega and Ridley, 2008). Among other functions, the Rho‐family members are master regulators of actin cytoskeleton rearrangements (Spiering and Hodgson, 2011). In line with this observation, we found a remarkable enrichment of annotations related to actin binding, cell adhesion and cytoskeleton organization. These activities are key modulators of the different steps leading to the metastatic spreading of tumors (Bezanilla et al., 2015; Feliciano and Di Pietro, 2012; Gross, 2013; Labelle and Hynes, 2012; Yao et al., 2014). In particular, one of the top genes identified by our analysis, ESYT2, had previously been related to actin dynamics and endoplasmic reticulum‐plasma membrane interactions (Giordano et al., 2013; Jean et al., 2012). The protein encoded by ESYT2 has a trans‐membrane domain followed by a synaptotagmin‐like mitochondrial‐lipid‐binding protein and three C‐terminal C2 domains (Fernandez‐Busnadiego et al., 2015). There are two main splice variants of ESYT2 characterized by the presence or absence of exon 13B between exons 13 and 14. The inclusion of this exon introduces 21 new amino acids in the C2B domain. In our study we demonstrate that lung tumors show a reduced expression of the short splice variant (ESYT2‐S), while increasing the expression of the long one (ESYT2‐L). We also showed that ESYT2‐S inhibition was associated with a clear redistribution of actin towards the cellular cortex. This cortical distribution of actin fibers is linked to high levels of cortical actomyosin contraction, and has been related to endothelial infiltration and metastasis (Olson and Sahai, 2009; Wolf et al., 2003; Wyckoff et al., 2006). Besides, ESYT2 has been described as an endocytic adapter for the clathrin‐mediated pathway (Jean et al., 2010). In our study, ESYT2‐L downregulation caused an increase in clathrin vesicles, suggesting that endocytosis is inhibited by this isoform. ESYT2‐S downregulation had the opposite effect. Considering that clathrin‐mediated endocytosis is shutdown during mitosis (Fielding et al., 2012), it can be speculated that ESYT2 participates in the inhibition of endocytosis in highly proliferative tumor cells. Based on all these results, we conclude that the alternative splicing of genes such as ESYT2 has a profound impact in the regulation of actin and endocytosis dynamics, processes directly involved in tumor biology. In this regard, SPAG9 has also been associated with invasion in several types of tumors, and has been proposed as a prognostic factor in lung cancer (Yang et al., 2016a; Yi et al., 2013). Finally, ENAH, another gene identified as differentially spliced in lung tumors, has been described as an actin cytoskeleton regulator with invasion‐specific splice variants (Di Modugno et al., 2012; Tanaka et al., 2014), which may have prognostic impact in NSCLC (Bria et al., 2014).
An important goal in the field of alternative splicing is the identification of common mechanisms that regulate cancer‐promoting splicing events (Oltean and Bates, 2014). In fact, it is thought that a limited number of splice factors may orchestrate multiple cancer‐related processes. In search for a master regulator of splicing in lung cancer, we found that the protein QKI regulated many of the differential splicing events selected in our analysis. Notably, half of the top validated events had been previously described as targets for QKI, including the splicing of ESYT2 mRNA (Zong et al., 2014). QKI has previously been shown to coordinate splicing regulatory networks in other physiological processes (de Bruin et al., 2016; Hall et al., 2013). This splicing regulator is widely involved in RNA‐binding and alternative splicing through its recognition motif NACUAAY‐N(1‐20)‐UAAY (Galarneau and Richard, 2005; Hall et al., 2013). In recent years, QKI downregulation has been shown to promote cancer cell proliferation and invasion (Bian et al., 2012; He et al., 2015; Novikov et al., 2011; Yang et al., 2010; Zhao et al., 2014). QKI is also involved in epithelial–mesenchymal transition both by the regulation of splicing (Yang et al., 2016b) and the formation of circRNAs (Conn et al., 2015). Interestingly, a limited number of specific splicing regulators, including QKI, appeared to account for many of the splicing alteration events found in a study of more than three hundred matched tumor and normal RNA‐seq samples corresponding to eight solid cancer types (Danan‐Gotthold et al., 2015). More specifically, Zong et al. have recently identified QKI as a regulator of splicing in lung cancer (Zong et al., 2014). They also demonstrated that QKI suppresses cell proliferation, at least in part, by the control of NUMB alternative splicing and the Notch signaling pathway. Other recent studies have reported the implication of QKI in aberrant splicing in breast and ovarian cancer (Brosseau et al., 2014; Wen et al., 2015). Our data now confirms the downregulation of QKI in lung carcinomas (Danan‐Gotthold et al., 2015; Novikov et al., 2011; Zong et al., 2014), and demonstrates a significant reduction in the expression of the protein as compared to both bronchial and alveolar tissue. Our study also demonstrates that nuclear QKI is an independent prognostic factor for overall survival in patients with NSCLC.
The QKI gene encodes three major splice variants, known as QKI‐5, QKI‐6 and QKI‐7, which differ in their C‐terminal amino acid sequence (Ebersole et al., 1996). Interestingly, we have now observed that the two QKI variants found in lung tissue, QKI‐5 and QKI‐6, are differentially expressed between NSCLC and non‐tumor lung tissue, both at the mRNA and protein level. This splicing switch had already been proposed after an in silico analysis of RNA‐seq data from lung adenocarcinomas (Sebestyen et al., 2015). Although some studies have evaluated the role of QKI isoforms in neuronal and development processes (Larocque et al., 2009; Saccomanno et al., 1999; Wang et al., 2013c), to our knowledge, nothing is known about the differential role of the isoforms in the biology of lung tumors. Further studies would be required to elucidate the contribution of each isoform to the splicing changes found in lung cancer, as well as to the prognosis of the disease.
In conclusion, we have applied a highly reliable methodology for a large‐scale identification of differential alternative splicing variants in lung cancer, which has allowed us to generate a list of lung cancer associated splice events, unveiling the functional relevance of some of these splice variants in cell processes such as actin and endocytosis dynamics. Besides, our study underlines the key role of QKI in the coordinated program of alternative splicing in lung cancer. Additional analyses will be needed to explore the relevance of the newly characterized splicing changes, as well as to evaluate their potential use as therapeutic targets or biomarkers in the management of lung cancer.
Funding
This work has been supported by Foundation for Applied Medical Research (FIMA), Ministerio de Economía y Competitividad‐Instituto de Salud Carlos III‐Fondo Europeo de Desarrollo Regional (RD12/0036/0040, PI11/00618, PI13/00806 and PI14/01686), and Fundación Científica Asociación Española Contra el Cáncer (GCB14‐2170). F.J. de Miguel is supported by a predoctoral fellowship from the Instituto de Salud Carlos III (FI12/00024).
Supporting information
The following are the supplementary data related to this article:
Supplementary data
Supplementary data
Supplementary data
Acknowledgments
The authors thank Cristina Sainz, Ana Remirez and Amaya Lavin for technical assistance.
Supplementary data 1.
1.1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2016.08.001.
de Miguel Fernando J., Pajares María J., Martínez-Terroba Elena, Ajona Daniel, Morales Xabier, Sharma Ravi D., Pardo Francisco J., Rouzaut Ana, Rubio Angel, Montuenga Luis M., Pio Ruben, (2016), A large-scale analysis of alternative splicing reveals a key role of QKI in lung cancer, Molecular Oncology, 10, doi: 10.1016/j.molonc.2016.08.001.
Contributor Information
Luis M. Montuenga, Email: lmontuenga@unav.es
Ruben Pio, Email: rpio@unav.es.
References
- Barretina, J. , Caponigro, G. , Stransky, N. , Venkatesan, K. , Margolin, A.A. , Kim, S. , Wilson, C.J. , Lehar, J. , Kryukov, G.V. , Sonkin, D. , Reddy, A. , Liu, M. , Murray, L. , Berger, M.F. , Monahan, J.E. , Morais, P. , Meltzer, J. , Korejwa, A. , Jane-Valbuena, J. , Mapa, F.A. , Thibault, J. , Bric-Furlong, E. , Raman, P. , Shipway, A. , Engels, I.H. , Cheng, J. , Yu, G.K. , Yu, J. , Aspesi, P. , de Silva, M. , Jagtap, K. , Jones, M.D. , Wang, L. , Hatton, C. , Palescandolo, E. , Gupta, S. , Mahan, S. , Sougnez, C. , Onofrio, R.C. , Liefeld, T. , MacConaill, L. , Winckler, W. , Reich, M. , Li, N. , Mesirov, J.P. , Gabriel, S.B. , Getz, G. , Ardlie, K. , Chan, V. , Myer, V.E. , Weber, B.L. , Porter, J. , Warmuth, M. , Finan, P. , Harris, J.L. , Meyerson, M. , Golub, T.R. , Morrissey, M.P. , Sellers, W.R. , Schlegel, R. , Garraway, L.A. , 2012. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 483, 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara, E.G. , Sebestyen, E. , Bernardis, I. , Eyras, E. , Valcarcel, J. , 2013. RBM5, 6, and 10 differentially regulate NUMB alternative splicing to control cancer cell proliferation. Mol. Cel. 52, 720–733. [DOI] [PubMed] [Google Scholar]
- Bezanilla, M. , Gladfelter, A.S. , Kovar, D.R. , Lee, W.L. , 2015. Cytoskeletal dynamics: a view from the membrane. J. Cel. Biol. 209, 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biamonti, G. , Catillo, M. , Pignataro, D. , Montecucco, A. , Ghigna, C. , 2014. The alternative splicing side of cancer. Semin. Cel. Dev. Biol. 32, 30–36. [DOI] [PubMed] [Google Scholar]
- Bian, Y. , Wang, L. , Lu, H. , Yang, G. , Zhang, Z. , Fu, H. , Lu, X. , Wei, M. , Sun, J. , Zhao, Q. , Dong, G. , Lu, Z. , 2012. Downregulation of tumor suppressor QKI in gastric cancer and its implication in cancer prognosis. Biochem. Biophys. Res. Commun. 422, 187–193. [DOI] [PubMed] [Google Scholar]
- Bria, E. , Di Modugno, F. , Sperduti, I. , Iapicca, P. , Visca, P. , Alessandrini, G. , Antoniani, B. , Pilotto, S. , Ludovini, V. , Vannucci, J. , Bellezza, G. , Sidoni, A. , Tortora, G. , Radisky, D.C. , Crino, L. , Cognetti, F. , Facciolo, F. , Mottolese, M. , Milella, M. , Nistico, P. , 2014. Prognostic impact of alternative splicing-derived hMENA isoforms in resected, node-negative, non-small-cell lung cancer. Oncotarget. 5, 11054–11063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, A.N. , Choi, P.S. , de Waal, L. , Sharifnia, T. , Imielinski, M. , Saksena, G. , Pedamallu, C.S. , Sivachenko, A. , Rosenberg, M. , Chmielecki, J. , Lawrence, M.S. , DeLuca, D.S. , Getz, G. , Meyerson, M. , 2014. A pan-cancer analysis of transcriptome changes associated with somatic mutations in U2AF1 reveals commonly altered splicing events. PloS One. 9, e87361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosseau, J.P. , Lucier, J.F. , Nwilati, H. , Thibault, P. , Garneau, D. , Gendron, D. , Durand, M. , Couture, S. , Lapointe, E. , Prinos, P. , Klinck, R. , Perreault, J.P. , Chabot, B. , Abou-Elela, S. , 2014. Tumor microenvironment-associated modifications of alternative splicing. RNA. 20, 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network, 2014. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 511, 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn, S.J. , Pillman, K.A. , Toubia, J. , Conn, V.M. , Salmanidis, M. , Phillips, C.A. , Roslan, S. , Schreiber, A.W. , Gregory, P.A. , Goodall, G.J. , 2015. The RNA binding protein quaking regulates formation of circRNAs. Cell. 160, 1125–1134. [DOI] [PubMed] [Google Scholar]
- Chen, J. , Weiss, W.A. , 2015. Alternative splicing in cancer: implications for biology and therapy. Oncogene. 34, 1–14. [DOI] [PubMed] [Google Scholar]
- Chen, L. , Bush, S.J. , Tovar-Corona, J.M. , Castillo-Morales, A. , Urrutia, A.O. , 2014. Correcting for differential transcript coverage reveals a strong relationship between alternative splicing and organism complexity. Mol. Biol. Evol. 31, 1402–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmas, P. , Jones, J.P. , Patten, C.J. , Rock, D.A. , Zheng, Y. , Cheng, S.M. , Weber, B.M. , Carlesso, N. , Scadden, D.T. , Rettie, A.E. , Soberman, R.J. , 2001. Alternative splicing determines the function of CYP4F3 by switching substrate specificity. J. Biol. Chem. 276, 38166–38172. [DOI] [PubMed] [Google Scholar]
- Danan-Gotthold, M. , Golan-Gerstl, R. , Eisenberg, E. , Meir, K. , Karni, R. , Levanon, E.Y. , 2015. Identification of recurrent regulated alternative splicing events across human solid tumors. Nucleic Acids Res. 43, 5130–5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin, R.G. , Shiue, L. , Prins, J. , de Boer, H.C. , Singh, A. , Fagg, W.S. , van Gils, J.M. , Duijs, J.M. , Katzman, S. , Kraaijeveld, A.O. , Bohringer, S. , Leung, W.Y. , Kielbasa, S.M. , Donahue, J.P. , van der Zande, P.H. , Sijbom, R. , van Alem, C.M. , Bot, I. , van Kooten, C. , Jukema, J.W. , Van Esch, H. , Rabelink, T.J. , Kazan, H. , Biessen, E.A. , Ares, M. , van Zonneveld, A.J. , van der Veer, E.P. , 2016. Quaking promotes monocyte differentiation into pro-atherogenic macrophages by controlling pre-mRNA splicing and gene expression. Nat. Commun. 7, 10846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miguel, F.J. , Sharma, R.D. , Pajares, M.J. , Montuenga, L.M. , Rubio, A. , Pio, R. , 2014. Identification of alternative splicing events regulated by the oncogenic factor SRSF1 in lung cancer. Cancer Res. 74, 1105–1115. [DOI] [PubMed] [Google Scholar]
- Di Modugno, F. , Iapicca, P. , Boudreau, A. , Mottolese, M. , Terrenato, I. , Perracchio, L. , Carstens, R.P. , Santoni, A. , Bissell, M.J. , Nistico, P. , 2012. Splicing program of human MENA produces a previously undescribed isoform associated with invasive, mesenchymal-like breast tumors. Proc. Natl. Acad. Sci. United States America. 109, 19280–19285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolatshad, H. , Pellagatti, A. , Fernandez-Mercado, M. , Yip, B.H. , Malcovati, L. , Attwood, M. , Przychodzen, B. , Sahgal, N. , Kanapin, A.A. , Lockstone, H. , Scifo, L. , Vandenberghe, P. , Papaemmanuil, E. , Smith, C.W. , Campbell, P.J. , Ogawa, S. , Maciejewski, J.P. , Cazzola, M. , Savage, K.I. , Boultwood, J. , 2015. Disruption of SF3B1 results in deregulated expression and splicing of key genes and pathways in myelodysplastic syndrome hematopoietic stem and progenitor cells. Leukemia. 29, 1092–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley, A.L. , Winslow, M.M. , Chiang, D.Y. , Banerji, S. , Stransky, N. , Dayton, T.L. , Snyder, E.L. , Senna, S. , Whittaker, C.A. , Bronson, R.T. , Crowley, D. , Barretina, J. , Garraway, L. , Meyerson, M. , Jacks, T. , 2011. Nuclear factor I/B is an oncogene in small cell lung cancer. Genes Dev. 25, 1470–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersole, T.A. , Chen, Q. , Justice, M.J. , Artzt, K. , 1996. The quaking gene product necessary in embryogenesis and myelination combines features of RNA binding and signal transduction proteins. Nat. Genet. 12, 260–265. [DOI] [PubMed] [Google Scholar]
- Feliciano, D. , Di Pietro, S.M. , 2012. SLAC, a complex between Sla1 and Las17, regulates actin polymerization during clathrin-mediated endocytosis. Mol. Biol. Cel. 23, 4256–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Busnadiego, R. , Saheki, Y. , De Camilli, P. , 2015. Three-dimensional architecture of extended synaptotagmin-mediated endoplasmic reticulum-plasma membrane contact sites. Proc. Natl. Acad. Sci. United States America. 112, E2004–E2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding, A.B. , Willox, A.K. , Okeke, E. , Royle, S.J. , 2012. Clathrin-mediated endocytosis is inhibited during mitosis. Proc. Natl. Acad. Sci. United States America. 109, 6572–6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, X.D. , Ares, M. , 2014. Context-dependent control of alternative splicing by RNA-binding proteins. Nature Rev. Genetics. 15, 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarneau, A. , Richard, S. , 2005. Target RNA motif and target mRNAs of the Quaking STAR protein. Nat. Struct. Mol. Biol. 12, 691–698. [DOI] [PubMed] [Google Scholar]
- Gamazon, E.R. , Stranger, B.E. , 2014. Genomics of alternative splicing: evolution, development and pathophysiology. Hum. Genet. 133, 679–687. [DOI] [PubMed] [Google Scholar]
- Giordano, F. , Saheki, Y. , Idevall-Hagren, O. , Colombo, S.F. , Pirruccello, M. , Milosevic, I. , Gracheva, E.O. , Bagriantsev, S.N. , Borgese, N. , De Camilli, P. , 2013. PI(4,5)P(2)-dependent and Ca(2+)-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell. 153, 1494–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout, S. , Brambilla, E. , Boudria, A. , Drissi, R. , Lantuejoul, S. , Gazzeri, S. , Eymin, B. , 2012. Abnormal expression of the pre-mRNA splicing regulators SRSF1, SRSF2, SRPK1 and SRPK2 in non small cell lung carcinoma. PloS One. 7, e46539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridelli, C. , Rossi, A. , Carbone, D.P. , Guarize, J. , Karachaliou, N. , Mok, T. , Petrella, F. , Spaggiari, L. , Rosell, R. , 2015. Non-small-cell lung cancer. Nat. Rev. Dis. Primers. 15009, [DOI] [PubMed] [Google Scholar]
- Gross, S.R. , 2013. Actin binding proteins: their ups and downs in metastatic life. Cell Adhes. Migr. 7, 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, M.P. , Nagel, R.J. , Fagg, W.S. , Shiue, L. , Cline, M.S. , Perriman, R.J. , Donohue, J.P. , Ares, M. , 2013. Quaking and PTB control overlapping splicing regulatory networks during muscle cell differentiation. RNA. 19, 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, B. , Gao, S.Q. , Huang, L.D. , Huang, Y.H. , Zhang, Q.Y. , Zhou, M.T. , Shi, H.Q. , Song, Q.T. , Shan, Y.F. , 2015. MicroRNA-155 promotes the proliferation and invasion abilities of colon cancer cells by targeting quaking. Mol. Med. Rep. 11, 2355–2359. [DOI] [PubMed] [Google Scholar]
- Huang da, W. , Sherman, B.T. , Lempicki, R.A. , 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57. [DOI] [PubMed] [Google Scholar]
- Imielinski, M. , Berger, A.H. , Hammerman, P.S. , Hernandez, B. , Pugh, T.J. , Hodis, E. , Cho, J. , Suh, J. , Capelletti, M. , Sivachenko, A. , Sougnez, C. , Auclair, D. , Lawrence, M.S. , Stojanov, P. , Cibulskis, K. , Choi, K. , de Waal, L. , Sharifnia, T. , Brooks, A. , Greulich, H. , Banerji, S. , Zander, T. , Seidel, D. , Leenders, F. , Ansen, S. , Ludwig, C. , Engel-Riedel, W. , Stoelben, E. , Wolf, J. , Goparju, C. , Thompson, K. , Winckler, W. , Kwiatkowski, D. , Johnson, B.E. , Janne, P.A. , Miller, V.A. , Pao, W. , Travis, W.D. , Pass, H.I. , Gabriel, S.B. , Lander, E.S. , Thomas, R.K. , Garraway, L.A. , Getz, G. , Meyerson, M. , 2012. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 150, 1107–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe, A.B. , Hall, A. , 2002. Rho GTPases in transformation and metastasis. Adv. Cancer Res. 84, 57–80. [DOI] [PubMed] [Google Scholar]
- Jean, S. , Mikryukov, A. , Tremblay, M.G. , Baril, J. , Guillou, F. , Bellenfant, S. , Moss, T. , 2010. Extended-synaptotagmin-2 mediates FGF receptor endocytosis and ERK activation in vivo. Developmental Cel. 19, 426–439. [DOI] [PubMed] [Google Scholar]
- Jean, S. , Tremblay, M.G. , Herdman, C. , Guillou, F. , Moss, T. , 2012. The endocytic adapter E-Syt2 recruits the p21 GTPase activated kinase PAK1 to mediate actin dynamics and FGF signalling. Biol. Open. 1, 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, M.A. , Wilkinson, J.E. , Krainer, A.R. , 2014. Splicing factor SRSF6 promotes hyperplasia of sensitized skin. Nat. Struct. Mol. Biol. 21, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni, R. , de Stanchina, E. , Lowe, S.W. , Sinha, R. , Mu, D. , Krainer, A.R. , 2007. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat. Struct. Mol. Biol. 14, 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle, M. , Hynes, R.O. , 2012. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2, 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer, W. , Sohler, F. , Leder, G. , Beckmann, G. , Seidel, H. , Grone, J. , Hummel, M. , Sommer, A. , 2010. Exon array analysis using re-defined probe sets results in reliable identification of alternatively spliced genes in non-small cell lung cancer. BMC Genom. 11, 676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocque, D. , Fragoso, G. , Huang, J. , Mushynski, W.E. , Loignon, M. , Richard, S. , Almazan, G. , 2009. The QKI-6 and QKI-7 RNA binding proteins block proliferation and promote Schwann cell myelination. PloS One. 4, e5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misquitta-Ali, C.M. , Cheng, E. , O'Hanlon, D. , Liu, N. , McGlade, C.J. , Tsao, M.S. , Blencowe, B.J. , 2011. Global profiling and molecular characterization of alternative splicing events misregulated in lung cancer. Mol. Cell. Biol. 31, 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountain, C.F. , 1997. Revisions in the International system for staging lung Cancer. Chest. 111, 1710–1717. [DOI] [PubMed] [Google Scholar]
- Nguewa, P.A. , Agorreta, J. , Blanco, D. , Lozano, M.D. , Gomez-Roman, J. , Sanchez, B.A. , Valles, I. , Pajares, M.J. , Pio, R. , Rodriguez, M.J. , Montuenga, L.M. , Calvo, A. , 2008. Identification of importin 8 (IPO8) as the most accurate reference gene for the clinicopathological analysis of lung specimens. BMC Mol. Biol. 9, 103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikov, L. , Park, J.W. , Chen, H. , Klerman, H. , Jalloh, A.S. , Gamble, M.J. , 2011. QKI-mediated alternative splicing of the histone variant MacroH2A1 regulates cancer cell proliferation. Mol. Cell. Biol. 31, 4244–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, M.F. , Sahai, E. , 2009. The actin cytoskeleton in cancer cell motility. Clin. Exp. Metastasis. 26, 273–287. [DOI] [PubMed] [Google Scholar]
- Oltean, S. , Bates, D.O. , 2014. Hallmarks of alternative splicing in cancer. Oncogene. 33, 5311–5318. [DOI] [PubMed] [Google Scholar]
- Padgett, R.A. , 2012. New connections between splicing and human disease. Trends Genetics: TIG. 28, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajares, M.J. , Agorreta, J. , Larrayoz, M. , Vesin, A. , Ezponda, T. , Zudaire, I. , Torre, W. , Lozano, M.D. , Brambilla, E. , Brambilla, C. , Wistuba, Behrens, C. , Timsit, J.F. , Pio, R. , Field, J.K. , Montuenga, L.M. , 2012. Expression of tumor-derived vascular endothelial growth factor and its receptors is associated with outcome in early squamous cell carcinoma of the lung. J. Clin. Oncol. 30, 1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pio, R. , Blanco, D. , Pajares, M.J. , Aibar, E. , Durany, O. , Ezponda, T. , Agorreta, J. , Gomez-Roman, J. , Anton, M.A. , Rubio, A. , Lozano, M.D. , Lopez-Picazo, J.M. , Subirada, F. , Maes, T. , Montuenga, L.M. , 2010. Development of a novel splice array platform and its application in the identification of alternative splice variants in lung cancer. BMC Genom. 11, 352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pio, R. , Montuenga, L.M. , 2009. Alternative splicing in lung cancer. J. Thorac. Oncol. 4, 674–678. [DOI] [PubMed] [Google Scholar]
- Pio, R. , Zudaire, I. , Pino, I. , Castano, Z. , Zabalegui, N. , Vicent, S. , Garcia-Amigot, F. , Odero, M.D. , Lozano, M.D. , Garcia-Foncillas, J. , Calasanz, M.J. , Montuenga, L.M. , 2004. Alpha CP-4, encoded by a putative tumor suppressor gene at 3p21, but not its alternative splice variant alpha CP-4a, is underexpressed in lung cancer. Cancer Res. 64, 4171–4179. [DOI] [PubMed] [Google Scholar]
- Saccomanno, L. , Loushin, C. , Jan, E. , Punkay, E. , Artzt, K. , Goodwin, E.B. , 1999. The STAR protein QKI-6 is a translational repressor. Proc. Natl. Acad. Sci. United States America. 96, 12605–12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin, J. , Arganda-Carreras, I. , Frise, E. , Kaynig, V. , Longair, M. , Pietzsch, T. , Preibisch, S. , Rueden, C. , Saalfeld, S. , Schmid, B. , Tinevez, J.Y. , White, D.J. , Hartenstein, V. , Eliceiri, K. , Tomancak, P. , Cardona, A. , 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebestyen, E. , Zawisza, M. , Eyras, E. , 2015. Detection of recurrent alternative splicing switches in tumor samples reveals novel signatures of cancer. Nucleic Acids Res. 43, 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai, C.L. , Ley, J.N. , White, B.S. , Kim, S. , Tibbitts, J. , Shao, J. , Ndonwi, M. , Wadugu, B. , Duncavage, E.J. , Okeyo-Owuor, T. , Liu, T. , Griffith, M. , McGrath, S. , Magrini, V. , Fulton, R.S. , Fronick, C. , O'Laughlin, M. , Graubert, T.A. , Walter, M.J. , 2015. Mutant U2AF1 expression alters hematopoiesis and pre-mRNA splicing in vivo. Cancer Cell. 27, 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiering, D. , Hodgson, L. , 2011. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adhes. Migration. 5, 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, N. , Yoshida, H. , Suzuki, Y. , Harigaya, K. , 2014. Relative expression of hMena11a and hMenaINV splice isoforms is a useful biomarker in development and progression of human breast carcinoma. Int. J. Oncol. 45, 1921–1928. [DOI] [PubMed] [Google Scholar]
- Travis, W.D. , Brambilla, E. , Muller-Hermelink, H.K. , Harris, C.C. , 2004. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart IARC Press; Lyon: [Google Scholar]
- UniProt, C. , 2015. UniProt: a hub for protein information. Nucleic Acids Res. 43, D204–D212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles, I. , Pajares, M.J. , Segura, V. , Guruceaga, E. , Gomez-Roman, J. , Blanco, D. , Tamura, A. , Montuenga, L.M. , Pio, R. , 2012. Identification of novel deregulated RNA metabolism-related genes in non-small cell lung cancer. PloS One. 7, e42086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega, F.M. , Ridley, A.J. , 2008. Rho GTPases in cancer cell biology. FEBS Lett. 582, 2093–2101. [DOI] [PubMed] [Google Scholar]
- Wan, Y. , Wu, C.J. , 2013. SF3B1 mutations in chronic lymphocytic leukemia. Blood. 121, 4627–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Chen, D. , Qian, H. , Tsai, Y.S. , Shao, S. , Liu, Q. , Dominguez, D. , Wang, Z. , 2014. The splicing factor RBM4 controls apoptosis, proliferation, and migration to suppress tumor progression. Cancer Cell. 26, 374–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Dong, Q. , Miao, Y. , Fu, L. , Lin, X. , Wang, E. , 2013. Clinical significance and biological roles of SPAG9 overexpression in non-small cell lung cancer. Lung Cancer. 81, 266–272. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Gogol-Doring, A. , Hu, H. , Frohler, S. , Ma, Y. , Jens, M. , Maaskola, J. , Murakawa, Y. , Quedenau, C. , Landthaler, M. , Kalscheuer, V. , Wieczorek, D. , Wang, Y. , Hu, Y. , Chen, W. , 2013. Integrative analysis revealed the molecular mechanism underlying RBM10-mediated splicing regulation. EMBO Mol. Med. 5, 1431–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Vogel, G. , Yu, Z. , Richard, S. , 2013. The QKI-5 and QKI-6 RNA binding proteins regulate the expression of microRNA 7 in glial cells. Mol. Cell. Biol. 33, 1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, J. , Toomer, K.H. , Chen, Z. , Cai, X. , 2015. Genome-wide analysis of alternative transcripts in human breast cancer. Breast Cancer Res. Treat. 151, 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, K. , Mazo, I. , Leung, H. , Engelke, K. , von Andrian, U.H. , Deryugina, E.I. , Strongin, A.Y. , Brocker, E.B. , Friedl, P. , 2003. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J. Cel. Biol. 160, 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff, J.B. , Pinner, S.E. , Gschmeissner, S. , Condeelis, J.S. , Sahai, E. , 2006. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr. Biol. CB. 16, 1515–1523. [DOI] [PubMed] [Google Scholar]
- Xi, L. , Feber, A. , Gupta, V. , Wu, M. , Bergemann, A.D. , Landreneau, R.J. , Litle, V.R. , Pennathur, A. , Luketich, J.D. , Godfrey, T.E. , 2008. Whole genome exon arrays identify differential expression of alternatively spliced, cancer-related genes in lung cancer. Nucleic Acids Res. 36, 6535–6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, J. , 2014. Differential evolution of signal-responsive RNA elements and upstream factors that control alternative splicing. Cell Mol. Life Sci.: CMLS. 71, 4347–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Q. , Lou, G. , Qian, Y. , Qin, B. , Xu, X. , Wang, Y. , Liu, Y. , Dong, X. , 2016. SPAG9 is involved in hepatocarcinoma cell migration and invasion via modulation of ELK1 expression. OncoTargets Ther. 9, 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, G. , Fu, H. , Zhang, J. , Lu, X. , Yu, F. , Jin, L. , Bai, L. , Huang, B. , Shen, L. , Feng, Y. , Yao, L. , Lu, Z. , 2010. RNA-binding protein quaking, a critical regulator of colon epithelial differentiation and a suppressor of colon cancer. Gastroenterology. 138, 231–240. e1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. , Zhou, W. , Liu, S. , 2016. SPAG9 controls the cell motility, invasion and angiogenesis of human osteosarcoma cells. Exp. Ther. Med. 11, 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Park, J.W. , Bebee, T.W. , Warzecha, C.C. , Guo, Y. , Shang, X. , Xing, Y. , Carstens, R.P. , 2016. Determination of a comprehensive alternative splicing regulatory network and combinatorial regulation by key factors during the epithelial-to-mesenchymal transition. Mol. Cell. Biol. 36, 1704–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, Y. , Gu, X. , Liu, H. , Wu, G. , Yuan, D. , Yang, X. , Song, Y. , 2014. Metadherin regulates proliferation and metastasis via actin cytoskeletal remodelling in non-small cell lung cancer. Br. J. Cancer. 111, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, F. , Ni, W. , Liu, W. , Pan, X. , Han, X. , Yang, L. , Kong, X. , Ma, R. , Chang, R. , 2013. SPAG9 is overexpressed in human astrocytoma and promotes cell proliferation and invasion. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 34, 2849–2855. [DOI] [PubMed] [Google Scholar]
- Zhang, C. , Zhang, Z. , Castle, J. , Sun, S. , Johnson, J. , Krainer, A.R. , Zhang, M.Q. , 2008. Defining the regulatory network of the tissue-specific splicing factors Fox-1 and Fox-2. Genes Dev. 22, 2550–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Lieu, Y.K. , Ali, A.M. , Penson, A. , Reggio, K.S. , Rabadan, R. , Raza, A. , Mukherjee, S. , Manley, J.L. , 2015. Disease-associated mutation in SRSF2 misregulates splicing by altering RNA-binding affinities. Proc. Natl. Acad. Sci. United States America. 112, E4726–E4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Zhang, G. , Wei, M. , Lu, X. , Fu, H. , Feng, F. , Wang, S. , Lu, W. , Wu, N. , Lu, Z. , Yuan, J. , 2014. The tumor suppressing effects of QKI-5 in prostate cancer: a novel diagnostic and prognostic protein. Cancer Biol. Ther. 15, 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong, F.Y. , Fu, X. , Wei, W.J. , Luo, Y.G. , Heiner, M. , Cao, L.J. , Fang, Z. , Fang, R. , Lu, D. , Ji, H. , Hui, J. , 2014. The RNA-binding protein QKI suppresses cancer-associated aberrant splicing. PLoS Genet. 10, e1004289 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article:
Supplementary data
Supplementary data
Supplementary data


