Abstract
Tumor cells leave the primary tumor and enter the circulation. Once there, they are called circulating tumor cells (CTCs). A fraction of CTCs are capable of entering distant sites and persisting as disseminated tumor cells (DTCs). An even smaller fraction of DTCs are capable of progressing toward metastases. It is known that the DTC microenvironment plays an important role in sustaining their survival, regulating their growth, and conferring resistance to therapy. But we still have much to learn about the nature of these rare cell populations to predict which will progress and what exactly should cause concern for future relapse. Although recent technological advances in our ability to detect and molecularly and functionally characterize CTCs and DTCs promise to unravel this ambiguity, the timing of dissemination and the precise source of CTCs and DTCs profiled will impact the conclusions that can be made from these endeavors. In this review, we discuss the biology of CTCs and DTCs; the technologies to detect, isolate, and profile these cells; and the exceptions we must apply to our understanding of what role these cells play in the metastatic process. We conclude that a greater effort to understand the unique biology of these cells in context will positively impact our ability to use these cells to predict outcome, monitor treatment efficacy, and reveal therapeutically relevant targets to deplete these populations and ultimately prevent metastasis.
Keywords: chemoresistance, circulating tumor cells, disseminated tumor cells, dormancy, metastatic microenvironment, perivascular niche
Abbreviations
- AML
acute myeloid leukemia
- BMP
bone morphogenic protein
- CDXs
CTC‐derived explants
- CGH
comparative genome hybridization
- CTCs
circulating tumor cells
- DCIS
ductal carcinoma in situ
- DTCs
disseminated tumor cells
- ECM
extracellular matrix
- EGFR
epithelial growth factor receptor
- EMT
epithelial‐to‐mesenchymal transition
- EpCAM
epithelial cell adhesion molecule
- ERBB2
erb‐B2 receptor tyrosine kinase 2
- ER
estrogen receptor
- G‐CSF
granulocyte colony‐stimulating factor
- HER2
human epidermal growth factor receptor 2
- HSCs
hematopoietic stem cells
- ICC
immunocytochemistry
- IHC
immunohistochemistry
- ISH
in situ hybridization
- LIF
leukemia inhibitory factor
- MET
mesenchymal‐to‐epithelial transition
- MHCI
major histocompatibility complex I
- MICs
metastasis‐initiator cells
- MMPs
matrix metalloproteinases
- NKG2D
natural killer group 2D
- NSCLC
non‐small‐cell lung cancer
- PCR
polymerase chain reaction
- PVN
perivascular niche
- RNA‐ISH
RNA in situ hybridization
- RNA‐seq
RNA sequencing
- RT‐PCR
real‐time polymerase chain reaction
- SCLC
small cell lung cancer
- TGF‐β2
transforming growth factor‐β2
- TGF‐β
transforming growth factor‐β
- TSP‐1
thrombospondin‐1
1. Introduction
Perhaps the most important discovery in the last 15 years of basic metastasis research is the finding that tumor cells disseminate at the so‐called in situ stage (Braun et al., 2005; Gruber et al., 2016; Sänger et al., 2011), well before detection of the primary tumor. In retrospect, this should not have been so surprising. Five to ten percent of all cases of metastatic cancer are cancer of unknown primary (Abbruzzese et al., 1994; Klein, 2009; Massard et al., 2011; van de Wouw et al., 2002), where the metastatic lesion is detected before the primary tumor. The implications of early dissemination – that disseminated tumor cells (DTCs) a) lack many of the genomic abnormalities that characterize the primary tumor, and b) evolve in parallel with the primary tumor – have great relevance now that we are at the dawn of personalized medicine.
The question is how to instigate a paradigm shift in metastasis research so that personalized medicine is applied to DTCs and their circulating counterparts, CTCs, rather than solely to the primary tumor. How do we shift the focus from treating metastasis to preventing metastasis when we currently do not have any drugs that specifically target DTCs or CTCs? What approaches are necessary to identify actionable targets that could lead to CTC and/or DTC depletion? This is essentially the only way to test the logical assumption that successful depletion of these cell populations would result in prolonged metastasis‐free survival. And lastly, once we successfully develop these approaches, we must understand heterogeneity within and among patients so that we can determine which patients would benefit from such therapies.
Addressing these challenges not only requires bold thinking, it necessitates the development of technologies and models to study rare cell populations in context. In this review, we shine a spotlight on these harbingers of metastasis with a focus on early stages of the disease. We highlight the biology that implicates CTCs and DTCs in metastasis; the technologies and models developed to detect, profile, and study these cells; the clinical implications of current findings; and what is left to uncover.
2. Biology of CTCs
The study of CTC biology is simplified by the fact that CTCs can be isolated from blood using a standard blood draw. Several new high‐throughput technologies have been developed to isolate these cells from the blood and study them at the single‐cell level. CTCs are considered to be en route to or from disseminated sites and reflective of metastasis, and several studies have shown their diagnostic and prognostic significance.
Attempts to further develop CTC isolation, detection, and analysis techniques as clinical platforms to aid disease management are underway. The information gained from CTCs isolated over the course of disease or treatment can provide a more dynamic, therapeutically relevant, and patient‐centric means to treat cancer. We will begin with a primer on CTC biology – primarily, the characteristics that facilitate exit from primary tissue and survival through several stressful bottlenecks to reach their site of dissemination.
2.1. To leave for the unknown
Circulating tumor cells must overcome a number of physiological hurdles to disseminate. To enter the circulation, tumor cells must invade from the epithelium or tumor of origin, navigate through their local microenvironment, and traverse the endothelium (intravasation). Once in circulation, CTCs must survive shear and immunological pressures, exit from circulation (extravasation), and successfully incorporate within their new tissue (Fig. 1).
Figure 1.
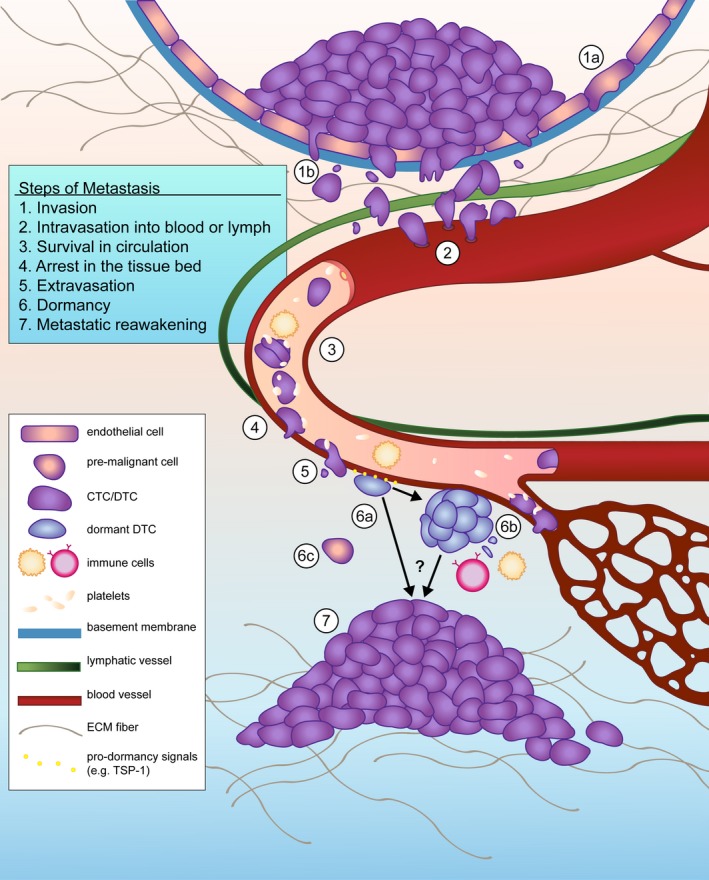
Tumor cell dissemination enables the spread of cancer from its site of origin. Local invasion through the basement membrane of a duct and ECM fibers may occur early (1a) or later (1b) in malignant progression. Upon reaching a local or intratumoral vessel conduit, DTCs intravasate into hematogenous or lymphatic vessels (2). Blood is a hostile environment and CTCs must contend with circulating immune cells, loss of cell–cell junctions, and shear stress to survive (3). Platelets offset these hurdles by providing a protective coat that promotes the formation of tumor microemboli and arrest on the luminal side of capillaries and venules (4). As DTCs extravasate (5), they encounter a foreign microenvironment containing new obstacles to survival. Some DTCs enter dormancy during this stressful transition, a process in which microenvironmental factors such as TSP‐1, BMP‐4, and BMP‐7 have been implicated. Single dormant cells that undergo cell cycle arrest have been observed in the perivascular niche (6a), where they apparently avoid immunodetection. Small DTC clusters and micrometastases may continue to divide, but are prevented from crossing a size threshold due to angiogenic limitations or immunosurveillance (6b). The contribution of early disseminators, which may or may not have ever entered a completely malignant state, is unknown (6c). The mechanisms through which dormant DTCs eventually reawaken are currently being elucidated (7) and likely include tissue‐specific factors, molecules derived from angiogenic neovessels, and other unknown effectors.
How do tumor cells emigrate from a primary site? One widely prescribed theory is that cells within solid tumors must undergo an epithelial‐to‐mesenchymal transition (EMT) in order to invade (Hanahan and Weinberg, 2011; Labelle et al., 2011; Pantel and Speicher, 2015; Yu et al., 2013). This phenotypic transition is accompanied by molecular changes that enable epithelial cells to become more invasive, motile, and capable of seeding distant sites (Joosse et al., 2015; Yang and Weinberg, 2008). Several of the physical hurdles faced by CTCs can be addressed by the potential of malignant cells (and future CTCs) to undergo EMT. For example, a cell undergoing EMT downregulates E‐cadherin, which enables detachment from neighboring epithelial cells, and upregulates matrix metalloproteinase (MMP) activity, which facilitates navigation through the local extracellular matrix (ECM) and entry to microvasculature (Lamouille et al., 2014; Lee et al., 2006).
Circulating tumor cells with mesenchymal features predict poor outcome in a number of cancers, indicating that this phenotypic shift provides an advantage in circulation and/or distant sites. In a cohort of 39 patients with metastatic breast cancer, 62% of CTC‐positive patients had CTCs that were positive for at least one of three EMT markers (Akt2, PI3K, and Twist1) multiplexed using RT‐PCR. This comprised the majority of patients that did not respond to standard chemo‐, antibody, or hormonal therapy, as opposed to just 10% in responders (Aktas et al., 2009).
The caveat with this study and others like it are the limited number of genes and markers used to define the occurrence of a phenotypic switch that involves much more than a handful of genes (Diepenbruck and Christofori, 2016). Application of gene expression microarrays in a mouse model of breast cancer metastasis showed that the EMT signature detected in CTCs is not limited to one or two transcripts or molecules (LeBleu et al., 2014), but instead involves significant changes in expression of several genes, for example, in at least 76 genes in lung cancer (Byers et al., 2013). These signatures also evolve over time with treatment. In a study where CTCs were analyzed from an individual patient using RNA in situ hybridization (RNA‐ISH), resistance to therapy was accompanied by a shift toward the mesenchymal molecular signature (Yu et al., 2013). The dominance of the mesenchymal‐like fraction in this patient's CTCs showed a dramatic decrease when another effective therapy was introduced (Yu et al., 2013). Ideally, establishing accurate molecular correlates between CTC phenotype and metastasis‐initiating potential would enable one to monitor treatment efficacy via serial liquid biopsies. Whether EMT constitutes this phenotypic signature on its own remains to be seen.
However, questions have been raised about the obligate requirement of EMT in the generation of cells capable of leaving the primary tumor (Christiansen and Rajasekaran, 2006; Tarin et al., 2005). If EMT is necessary for dissemination, then it follows that the reverse, or mesenchymal‐to‐epithelial transition (MET), is necessary for CTCs to ultimately grow within a distant tissue. This would require another overhaul of the transcriptome and proteome in what is already considered an inefficient process (i.e., dissemination). In fact, DTCs from several cancers found in the bone marrow are epithelial in nature (Braun et al., 2000; Pantel et al., 1996; Schardt et al., 2005); perhaps for these early‐stage disseminated cells, this transition never (or only partially) took place. Indeed, under varying experimental conditions, EMT‐associated transcriptional factors have both positive (Yang and Weinberg, 2008; Yang et al., 2004) and negative (Fischer et al., 2015; Ocaña et al., 2012; Tsai et al., 2012) effects on metastasis. Cancer cells destined to leave the primary tumor display only a small part of the spectrum of changes associated with EMT in development (Vanharanta and Massagué, 2013; Yang and Weinberg, 2008). Eventually, the specific influence of EMT ‘drivers’ on the release of CTCs from the primary tissue and the ability of mesenchymal‐like cells to seed and survive within distant organs will have to be elucidated.
In addition to individual CTCs, CTC clusters are also found in patient blood. CTC clusters are composed of 2 to more than 50 cells and are relatively rare compared with single cells in circulation. Larger clusters, also called tumor microemboli, have been detected in several cancers using various technologies, and the presence of these microemboli in circulation generally correlates with very poor clinical outcome in lung and breast cancer (Aceto et al., 2015; Al‐Mehdi et al., 2000; Christiansen and Rajasekaran, 2006). In the case of breast cancer, clusters appear to be oligoclonal in nature and not an artifact of cellular replication in circulation (Aceto et al., 2014). This could provide cells within a CTC cluster with a survival advantage over single cells. Indeed, cells in clusters display a stark lack of apoptosis (Hou et al., 2012), possibly due to maintaining a basal level of cell junction‐mediated survival signaling that helps them avoid anoikis (Paoli et al., 2013). Upon extravasation, autocrine signaling within a cluster may facilitate more rapid adaptation to a new environment in distant organs. CTC clusters may also contain normal cells from the primary site (e.g., stromal cells), which has major implications for the ‘seed‐and‐soil’ hypothesis (Paget, 1889). Bringing its own soil should only enhance the ability of a seed to sprout at distant sites (Aceto et al., 2015; Duda et al., 2010; Jones et al., 2013).
2.2. Platelets contribute to the survival of CTCs and clusters
Circulating tumor cells get help from other nontumor entities along the way. For instance, platelets contribute in multiple ways to CTC persistence in blood. Experiments in mouse models with no circulating platelets (e.g., NF‐E2−/− mice), nonfunctional platelets (e.g., Par4−/− mice), or a reduced number of platelets following treatment with neuraminidase have shown that lack of appropriate platelet function hampers metastasis (Camerer et al., 2004; Gasic et al., 1968; Pearlstein et al., 1980). The prometastatic effects of platelets are both physical and molecular. From a mechanical perspective, platelets rapidly coat CTCs in the bloodstream, shielding them from violent shear forces (McCarty et al., 2000; Nieswandt et al., 1999) (Fig. 1, Step 3). Tumor cells bind platelet adhesive proteins fibronectin and von Willebrand factor via integrins, promoting crosslinking and coagulation (Nierodzik et al., 1991). Tumor cell‐induced platelet aggregates result in tumor emboli, which have a greater chance of entrapment in small vessels, allowing more time for extravasation to occur. Further, platelets promote adhesion to the luminal side of the endothelia via surface molecules such as selectins (Läubli and Borsig, 2010).
Platelets also provide a defense against the immune system. For instance, platelet‐derived TGF‐β inactivates natural killer cells via downregulation of NKG2D (Kopp et al., 2009). Immune attenuation also occurs through transfer of MHC I complexes from granulated platelets to CTCs, endowing the latter with a ‘self’ identity that discourages natural killer cell‐mediated cytolytic attack (Placke et al., 2012).
Despite all of this help, CTCs persist in circulation for only 1–2.4 h according to in vivo experimental modeling and data from a small cohort of five patients (Meng et al., 2004). Most CTCs die in circulation as a result of shear stress and/or anoikis (Labelle and Hynes, 2012; Vanharanta and Massagué, 2013). Paradoxically, the half‐life of CTC clusters in circulation has been reported to be shorter than that of single CTCs, but this may be because large clusters get ‘cleared’ from the bloodstream or get stuck in capillaries more often than single CTCs (Aceto et al., 2014). Theoretically, survival signaling due to cell–cell junction‐derived signaling, reduction in shear stress experienced by individual components of the cluster, and longer dwell time in tiny capillaries should promote viability of CTC clusters versus single CTCs.
3. Isolation and characterization of CTCs
3.1. CTC isolation
Although highly aggressive tumors shed thousands of cancer cells into circulation each day (Allard et al., 2004; Nagrath et al., 2007), CTCs comprise a fairly rare population within blood. Technologies developed over the past two decades to detect CTCs are generally based on properties that either define CTCs (positive selection) or are uncharacteristic of CTCs (negative selection), as reviewed thoroughly elsewhere (Aceto et al., 2015; Joosse et al., 2015; Krebs et al., 2014; Millner et al., 2013). CTCs originating from epithelial tumors are thought by many to exclusively express epithelial cell adhesion molecule (EpCAM; a faulty assumption that is discussed below). EpCAM selection underlies the basic strategy of the CellSearch system, which has been approved by the US FDA for use in breast, colorectal, and prostate cancer. More recent technologies such as the micropost‐based CTC chip and the herringbone (HB)‐based CTC chip allow increased sensitivity due to the incorporation of additional epithelial tumor antigens such as HER2 (human epidermal growth factor receptor 2) and EGFR (epithelial growth factor receptor) (Nagrath et al., 2007; Stott et al., 2010; Yu et al., 2013).
Given the relatively rare nature of CTCs and CTC clusters, GILUPI Nanomedizin developed a technology that enables sampling of CTCs in vivo by inserting an EpCAM‐coated detection wire into the antecubital vein for 30 min. This allows the probing of a large volume of blood to isolate CTCs and CTC clusters (Saucedo‐Zeni et al., 2012). Essentially the opposite approach – diagnostic leukapheresis – has been used to sample much larger volumes of blood to overcome issues with detection that occur due to the rare nature of the CTCs (Fischer et al., 2013).
Regardless of the approach, it is important to remember that tumor classifications represent population averages; therefore, one cannot expect all CTCs to express a primary tumor marker over a threshold level. Indeed, a study using breast cancer cell lines showed that approximately 20% of cell lines expressed EpCAM at low levels. These cells instead showed a basal‐like phenotype with lower overall expression of epithelial markers and increased expression of mesenchymal markers (Punnoose et al., 2010). This datum suggests that mesenchymal‐like cells may be missed or undersampled using only EpCAM to select for CTCs (Punnoose et al., 2010). This is important for prognostic purposes because EpCAM− circulating breast tumor cells may actually constitute a Her2+/EGFR+/HPSE+/Notch1+ population with enhanced propensity to colonize brain (Zhang et al., 2013). On the other hand, EpCAM‐based selection also falsely identifies normal cells as tumor cells. Erythroid progenitor cells in the bone marrow express EpCAM transiently (Lammers et al., 2002), and this has been taken into consideration in the definition of a dormancy signature of EpCAM+ disseminated prostate tumor cells in bone marrow (Chéry et al., 2014).
Antigen‐independent methods have also been developed to isolate and study CTCs. The CTC‐iCHIP offers an improved technology by separating out blood components through hydrodynamic separation followed by immune depletion to deliver purified CTCs that have not been tagged and manipulated (Ozkumur et al., 2013). Furthermore, some technologies work optimally with CTC clusters when compared with the previously mentioned technologies that are limited to single cells or two to four cell clusters. Technologies such as the ‘cluster chip’ have been developed to take into account the need to isolate structurally sound and viable CTC clusters for downstream studies (Sarioglu et al., 2015).
3.2. Molecular characterization of CTCs
Isolation of viable CTCs enables their molecular and functional characterization. Chromosomal rearrangements or changes in CTC gene copy number can be studied using techniques such as interphase fluorescence in situ hybridization (ISH) and array‐CGH (comparative genome hybridization). Additionally, next‐generation DNA sequencing allows the study of the genome‐wide mutation spectrum in CTCs. Using a combination of array‐CGH and next‐generation sequencing, several cancer‐associated copy number changes shared with the primary tumor were described in 37 intact CTCs isolated from six patients with colorectal cancer (Heitzer et al., 2013). Comparison of the mutation spectrum among tumors, CTCs, and metastases using massive parallel sequencing in two of these patients revealed not only the expected ‘driver mutations’, but also CTC‐specific mutations initially. However, it was subsequently confirmed that these ‘CTC‐specific’ mutations were in fact present also in subclonal levels in the primary tumor and in metastases (Heitzer et al., 2013). This study serves as an example of the power of sequencing technologies available to researchers to study CTC biology in the context of systemic disease. Coupled with its ever‐reducing cost, sequencing technologies could eventually transform patient‐specific treatment over the course of systemic disease.
Whereas traditional techniques such as RNA in situ hybridization with limited pooling of targets, multiplexed quantitative PCR, and gene expression microarrays have been utilized to study CTCs (Payne et al., 2012; Smirnov et al., 2005; Xi et al., 2007; Yu et al., 2013), single‐cell expression profiling approaches are coming to the fore given the relative rarity of CTCs and the limited amount of genetic material one can isolate from them (Sandberg, 2013). Powerful techniques such as single nucleus (cell) sequencing and whole‐exome sequencing have been employed to study clonal composition among primary breast cancer cells and prostate cancer‐derived CTCs (Lohr et al., 2014; Wang et al., 2014). These studies shed light on the heterogeneity of CTCs with respect to time and response to therapy. For example, the application of RNA‐Seq technology to single prostate CTCs (77 intact CTCs from 13 patients) showed considerable heterogeneity between CTCs from a single patient, and identified noncanonical WNT signaling as a mechanism of resistance to therapies targeting androgen receptor (Miyamoto et al., 2015).
It remains to be seen if such assays will become predictive, specific, and inexpensive enough to be used routinely. Although it would require a technological leap, one can envision single‐cell proteomic approaches being applied to CTCs eventually in order to measure antigen diversity in metastatic disease or to identify targets for immunotherapy in the context of minimal residual disease.
3.3. Functional characterization of CTCs
Functional characterization of CTCs is technically challenging due to their rarity. Thus, developing standardized techniques to culture CTCs isolated from patients with different types of cancer is critical, keeping in mind that the changes CTCs undergo after isolation and passaging (particularly if immortalized) cast a shadow on subsequent results. Cell lines and organoid cultures have been established from CTCs, but with very low efficiency and success. Such endeavors have come to fruition only for a handful of cancers (e.g., Cayrefourcq et al., 2015).
Circulating tumor cell functionality has been tested in culture and in mice, such as an assay where CTCs are plated on fluorescently labeled ECM in order to measure invasiveness (Friedlander et al., 2014) or xenotransplantation of CTCs into immunodeficient mice. The tumors that grow out in these mice, called CTC‐derived explants (CDXs), could serve as vital material for the choice or design of therapeutic regimens. Xenotransplantation experiments using luminal breast cancer patient‐derived CTCs identified an EPCAMlowMEThighCD47highCD44high population proposed to be metastasis‐initiator cells (MICs) (Baccelli et al., 2013). Utilizing a CDX model of small cell lung cancer (SCLC), one group identified a CTC profile that predicted response to platinum‐based drugs and etoposides (Hodgkinson et al., 2014).
It should be noted, however, that in vivo strategies used to study CTCs lack uniformity in one important aspect – the site of injection. In the breast cancer and SCLC studies mentioned previously, CTCs were injected into the femoral medullary cavity and flanks of immunocompromised mice, respectively (Baccelli et al., 2013; Hodgkinson et al., 2014). Injection sites for CTCs are chosen often for technical reasons governed by the number of CTCs at hand, the number of assays to be performed, and in‐house expertise. However, there needs to be careful consideration about artifacts that are introduced by the choice of these injection sites, which clearly comprise very different microenvironments. For example, injecting patient‐derived CTCs into solid tissue (e.g., the flanks of mice) could provide a survival advantage by eliminating the need to a) survive in circulation, b) extravasate as an individual cell, and c) survive and outgrow from an individual cell in the absence of potentially stimulating factors from other tumor cells.
4. Clinical relevance of CTCs: does timing of detection matter?
A number of studies have sought to validate the degree to which CTCs mirror their tumor of origin and the metastases they initiate. The presence of CTCs in blood negatively correlates with progression‐free and overall survival at early stages of lung cancer, gastric cancer, melanoma, and pancreatic cancer (Ilie et al., 2014; Mimori et al., 2008; Reid et al., 2013; Rhim et al., 2014). Accumulating evidence suggests that CTCs are detectable at very early stages in multiple types of cancer, yet the discrepancy between the number of metastatic lesions and the number of detectable CTCs suggests that not all CTCs are created equal with regard to colony‐forming potential.
The metastasis‐initiating capacity of early CTCs at the neoplastic or hyperplastic stage of primary tumor development is not well characterized (Baccelli et al., 2013; Tsai et al., 2016); however, given that 5–10% of metastases come from primary tumors of unknown origin, early CTCs are clearly capable of colonizing distant sites and thus are important to the timeline of metastatic progression (van de Wouw et al., 2002). Indeed, a CTC screen conducted on healthy individuals who were, nonetheless, positive for an independent risk factor of non‐small‐cell lung cancer (NSCLC) led to the detection of CTCs in a subset of this cohort (Ilie et al., 2014). The CTC‐positive individuals all developed lung nodules within 4 years of CTC detection, prompting immediate surgical intervention.
Whether the relevance of CTCs depends on cancer type remains to be confirmed, especially in light of accumulating data that certain cancer types tend to disseminate early (discussed in greater detail in Section 3.1) (Klein, 2009). Further, early CTC detection may be confounded by normal or benign circulating epithelial cells, as is the case with inflammatory colorectal diseases (Pantel et al., 2012). The development of panels including epithelial, mesenchymal, and tissue of origin markers may help filter out normal circulating epithelial cells and avoid false positives.
Recent work also linked CTC number with primary tumor size, invasiveness (lymph node status), presence of metastasis, and clinical outcome (Ma et al., 2012; Mocellin et al., 2006; Rahbari et al., 2010; Wang et al., 2015; Zhang et al., 2012). Detecting as few as two to five CTCs per 7.5–10 mL blood predicts shorter progression‐free and overall survival across cancer types and stages (Cohen et al., 2008; Cristofanilli et al., 2004; Hiraiwa et al., 2008; Karhade et al., 2014; Rack et al., 2014; Thalgott et al., 2013). CTCs may also be useful predictors of metastatic burden. Metastatic patients are more likely to be CTC‐positive than nonmetastatic patients in the case of prostate and GI cancer (Hiraiwa et al., 2008; Thalgott et al., 2013), whereas higher CTC count predicts liver metastases from pancreatic adenocarcinoma (Tien et al., 2016). Therefore, defining CTC thresholds would be useful to predict patients at risk for recurrence. Developing means to faithfully monitor disease progression, recurrence, and mechanisms of resistance using CTCs will direct us toward successful clinical avenues for both treatment and prevention.
5. Lost in transition: CTCs and DTCs in the context of early dissemination
If the technologies to detect and characterize CTCs are to become routine in the prevention of disease, then the timing of their application needs to be carefully optimized. Mounting evidence for the parallel progression of cancer (Klein, 2009) argues that knowing the species (primary tumor or DTC) contributing to the CTC pool is important to accurately predict progression and/or response to therapy.
Tumor cells leave the primary site well before the primary tumor is clinically established. In fact, pancreatic cancer CTCs with mesenchymal and stem cell‐like phenotypes can be detected in the circulation of mouse of models even before detection of the primary tumor (Rhim et al., 2012). Additionally, some of these early DTCs seed the liver at this early stage, suggesting that early‐stage DTCs have the ability to not only leave the primary site but also successfully seed a secondary distant site (and perhaps even re‐enter the circulation). Blood‐borne dissemination is also an early event in patients with breast cancer, as DTCs have been detected in patients with preinvasive ductal carcinoma in situ (DCIS) (Gruber et al., 2016; Sänger et al., 2011), a phenomenon that has been recapitulated in detail in mouse models (Hüsemann et al., 2008).
What does early dissemination mean from the perspective of utilizing CTCs as a diagnostic and prognostic tool? There is a distinct possibility that chronologically disparate CTCs differ at the molecular level. Indeed, CTCs that disseminate early and form micrometastatic lesions undetectable by standard clinical imaging technologies may actually be comprised of a greater number of MICs than those derived later from frank lesions. But the CTC compartment may be dominated by contributions from the primary tumor, which contains several orders of magnitude more cells, at the time of diagnosis, essentially masking the metastasis‐initiating population that may be more critical to monitor. It should be noted that currently, there is no concrete evidence that CTCs detected later in disease are more (or less) capable of disseminating and forming metastases than early CTCs. Testing such a hypothesis is technically challenging due to the small number of CTCs that can be isolated from patients and animal models; however, investigating the proportion of early CTCs that are dissemination‐ and metastasis‐competent compared with late CTCs warrants exploration given that early DTCs have been shown to form metastases (Hüsemann et al., 2008).
A final, related caveat is that substantial heterogeneity exists in the techniques used to isolate and characterize CTCs. For instance, the location of sample collection varies widely from study to study. CTC count (and potentially source) varies with the blood compartment from which it is drawn, that is, right versus left side of the heart (Rhim et al., 2012) or mesenteric versus venous blood (Rahbari et al., 2012). These observational differences may underlie a natural enrichment of CTCs in somatic distribution based on circulation patterns.
6. Biology of DTCs
The vast majority of cancer‐associated mortalities are not due to the primary tumor but because of the presence of tumor emboli or metastatic lesions at distant sites, which often cause organ failure. CTCs may seed these distant sites, but even if they survive, the dynamics of outgrowth vary considerably between cells, cancer types, and individual patients. Indeed, in melanoma, breast, and prostate cancer, DTCs may enter into a quiescent state, only to ‘awaken’ after years or even decades and form deadly metastases (Chambers et al., 2002).
Long considered a ‘black box’ in the metastatic cascade, progress is being made to understand DTC biology. Evidence is accumulating that the DTC microenvironment plays a significant role in determining their signature properties: prolonged survival, reversible growth arrest, and therapeutic resistance (Ghajar, 2015).
6.1. Dormancy: the rest after the long travel
What are the pressures faced by a cell after it leaves circulation and enters a foreign tissue? The new microenvironment is thought to ‘stress’ DTCs, although whether all tissues induce equal stress is unclear. However, cancers of different types exhibit discrete patterns of metastatic colonization, driven by specific gene expression and chemokine secretion patterns (Bos et al., 2009; Kang et al., 2003; Minn et al., 2005; Tabariès et al., 2015). This is commonly referred to as ‘organo‐tropism’ or cancer cell ‘homing’ (Nguyen et al., 2009; Wan et al., 2013). DTCs face an unfamiliar milieu of growth factors, ECM, and metabolic stresses upon extravasating (Aguirre‐Ghiso, 2007; Sosa et al., 2014) regardless of the destination tissue. An exception is when the primary tumor induces tissue remodeling from afar to create a premetastatic niche (Hoshino et al., 2015; Kaplan et al., 2005; Peinado et al., 2012; Psaila and Lyden, 2009). In this case, arrival within a familiar wound‐like milieu accelerates the dynamics of metastasis. But in healthy tissues, a potential result of these pressures is that DTCs are steered into a state of dormancy, a phrase coined by Willis in the context of tumor progression as early as 1934 (Willis, 1934). Indeed, perhaps most DTCs go through a phase of dormancy of some length as part of their normal biology (Aguirre‐Ghiso, 2007; Sosa et al., 2014).
Dormancy can imply one of two states that reflect two very different underlying biologies. Dormancy can be induced in tumor cells at the cellular level, where they exit the cell cycle and undergo a G0–G1 arrest or differentiation as a means to avoid replication in the absence of appropriate growth factor and adhesion signaling (Aguirre‐Ghiso, 2007; Ghajar, 2015) (Fig. 1, Step 6a). However, dormancy can also be induced at the population level due to the presence of cell‐depleting phenomena (such as apoptosis induced by diffusion limitations and/or immunosurveillance) that counter the proliferation of DTC masses (Aguirre‐Ghiso, 2007; Folkman, 1971; Gimbrone et al., 1972; Rakhra et al., 2010) (Fig. 1, Step 6b). Theoretically, these two dormancy types are not independent of one another and may indeed reflect two separate barriers that a DTC must overcome in order to progress toward a full metastatic lesion.
6.2. DTCs and their time of arrival at distant sites
Linear progression requires that DTCs be derived from the primary tumor at or around the time of detection (Klein, 2009; Klein et al., 2008). Due to the popularity of the ‘Vogelgram (Fearon and Vogelstein, 1990)’, many assume this to be the case. If so, DTCs should contain most/all of the mutations present in the primary tumor at the time of detection, and accumulate additional mutations over time.
Several lines of evidence are at odds with this paradigm, none more so than the presence of DTCs in the bone marrow of breast cancer patients with so‐called in situ disease (Gruber et al., 2016; Hüsemann et al., 2008; Sänger et al., 2011), and the emergence of metastases in both early‐stage cancers (T1M1 or T2M1) and in patients with cancer of unknown primary (Abbruzzese et al., 1994; Engel et al., 2003; Klein, 2009; van de Wouw et al., 2002). Thus, temporally speaking, early DTCs are exposed to selection pressures different from those experienced by the primary tumor, which influences their biology in a unique manner.
Several studies comparing DTC genotypes with that of primary tumor show that early DTCs are actually less evolved than the primary tumor. For example, in the case of breast cancer, bone marrow DTCs display fewer genetic abnormalities (chromosomal aberrations, subchromosomal allelic losses, and gene amplification) than cells from primary tumors as assayed by CGH; in fact, at this resolution, only half of bone marrow DTCs display abnormal karyograms, as opposed to 100% of cells from the primary tumor (Schardt et al., 2005; Schmidt‐Kittler et al., 2003). In the case of esophageal cancers, comparison of chromosomal rearrangements revealed significant heterogeneity between primary tumor cells and DTCs, and even between DTCs from different sites. These results argue for parallel evolution under potentially site‐specific selection pressures (Stoecklein et al., 2008).
The implications of parallel evolution are twofold: First, the therapeutic based on properties of the primary tumor (e.g., targeting certain receptors that have genetic amplifications) might be effective only against a subset DTCs. And second, DTCs occupy microenvironments totally distinct from that found in the primary tumor. The role that these ‘foreign’ microenvironments play in the biology of DTCs is thus a necessary consideration.
6.3. DTCs: a product of their (micro)environment
There are three characteristic properties of dormant DTCs – prolonged survival in a foreign microenvironment, reversible growth arrest at these sites, and resistance to targeted and cytotoxic treatment (Ghajar, 2015) (Fig. 2). DTCs from carcinomas originate from epithelia, and nearly all epithelial cells depend on adhesion to basement membrane in order to survive (Boudreau et al., 1995). As DTCs leave the primary tissue through either a hematogenous or a lymphatic route, the first basement membrane they come across at a distant site laminates the vascular endothelium (Fig. 1, Steps 4–5). Therefore, it is not surprising that dormant DTCs are found in close association with vascular basement membrane in various experimental models of breast cancer, lung cancer, and melanoma (Chambers et al., 2002; Ghajar et al., 2013; Kienast et al., 2010; Price et al., 2016) (Fig. 1, Step 6). For instance, in brain, fate mapping individual lung and melanoma DTCs via intravital microscopy revealed that adhesion to the abluminal surface of vasculature was a prerequisite for survival and ultimately progression to metastasis (Kienast et al., 2010). This microenvironment, called the perivascular niche (PVN), plays a dynamic role in normal tissue development and differentiation (Butler et al., 2010; Rafii et al., 2016). Stem cells from several different organs localize to the PVN, where they are growth‐regulated and maintained in a stem‐like state by endothelial‐derived/angiocrine factors (Butler et al., 2010; Christov et al., 2007; Goldman and Chen, 2011; Kiel et al., 2005; Xiao et al., 2013). Clear parallels can be drawn between stem cell maintenance and induction and maintenance of DTC quiescence by this niche.
Figure 2.
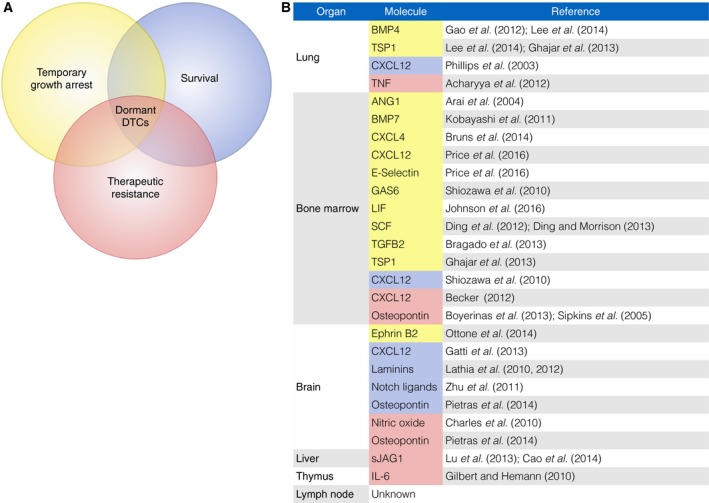
Characterization of dormant disseminated tumor cells. (A) Dormant tumor cells have three primary characteristics that distinguish them from other tumor cells and contribute to their metastasis‐initiating potential: the ability to survive in foreign and often hostile environments for a decade or more, temporary and reversible growth arrest, and resistance to therapies. (B) Dormant DTC characteristics are driven by tissue‐specific microenvironmental effectors. Molecules demonstrated to contribute to the DTC dormancy program, a significant fraction of which are derived from microvascular endothelium, are presented here by tissue type and by the dormant property endowed. Color coding matches that of the Venn diagram in panel A.
While there is currently no conclusive evidence that the niche occupied by DTCs and stem cells is one and the same, there is evidence that these niches overlap (Ghajar, 2015). First, the size of the primary tumor correlates with the number of detectable CTCs, but not DTCs in bone marrow, suggesting that niches available for engraftment are limited at least in bone marrow (Braun et al., 2005; Klein, 2013; Zhang et al., 2003). Mouse model experiments demonstrate that human prostate cancer cells compete with hematopoietic stem cells (HSCs) for HSC niche occupancy and that dissemination can be compromised by reducing the niche size (Shiozawa et al., 2011). Once prostate DTCs occupy a HSC niche, they take on a cancer stem cell‐like phenotype (Shiozawa et al., 2016). This is in line with evidence that stem cell niches can induce so‐called terminally differentiated daughter cells to become stem‐like (Calabrese et al., 2007; Rompolas et al., 2013; Scadden, 2014; Tata et al., 2013). Second, mobilization of HSCs from bone marrow by the application of G‐CSF also leads to the eviction of DTCs (and a concordant spike in CTC number) in breast cancer and multiple myeloma patients, suggesting that there is substantial overlap in the physical, physiological, and biological control of these cells (Fischer et al., 2013). Finally, recent evidence suggests that DTCs modify stem cell niches in distant tissues, indicating that the properties of DTCs are, unsurprisingly, the result of dynamic interactions between DTCs and their niche. A detailed study of disseminated acute myeloid leukemia (AML) stem cells revealed that the arrival of AML stem cells in the bone marrow disrupts sympathetic nerves, destroying periarteriolar nestin+ HSC niche cells and ultimately depleting HSC niches (Hanoun et al., 2014). Interestingly, the leukemic bone marrow also shows an expansion of endothelial cells via proliferation (Hanoun et al., 2014), suggesting that aberrant perivascular AML stem cell niches might also expand to allow a greater number of DTCs to engraft, although this has not been tested.
Several known and hypothesized factors that confer DTCs with key properties have been discussed elsewhere, and it is interesting to note that the dormancy‐associated effect of many of these molecules is tissue specific (Ghajar, 2015) (Fig. 2). This likely speaks to the tissue specificity of the PVN. But one component common to every PVN is microvascular endothelium, and in the case of breast cancer, growth‐arrested DTCs are intimately associated with brain, lung, and bone marrow vasculature (Ghajar et al., 2013). This phenomenon is observed in patients (Price et al., 2016) and has been re‐created in 3D culture models (Ghajar et al., 2013). Endothelial‐derived thrombospondin‐1 (TSP‐1) is a pivotal player in the induction of tumor dormancy in lung and bone marrow. Bone morphogenetic protein (BMP)‐4 has been hypothesized to play a key role in inducing dormancy in the lung, although it is potentially relevant to note that autocrine BMP‐4 signaling results in TSP‐1 expression by lung endothelium (Gao et al., 2012; Lee et al., 2014). On the other hand, dormancy induction in bone marrow has been attributed to the action of stromal BMP‐7, transforming growth factor‐β2 (TGF‐β2), and leukemia inhibitory factor (LIF) in prostate cancer, head‐and‐neck squamous cell carcinoma, and breast cancer, respectively (Bragado et al., 2013; Johnson et al., 2016; Kobayashi et al., 2011). Similarly, factors that play a role in therapeutic resistance of DTCs in different niches may not completely overlap. Other known and hypothesized factors identified in different tissues are referenced in Fig. 2.
Eradicating the reservoir of minimal residual disease requires total elimination of DTC burden; thus, the final property of dormant DTCs – therapeutic resistance – is a particularly key hurdle to overcome. Many assume that cell cycle status is the reason that dormant DTCs resist chemotherapy, but there is currently no clear answer as to why DTCs are chemoresistant. A careful look at their biology and microenvironment provides some clues that perhaps cell cycle status is not the only answer.
The heterogeneity between DTCs and the primary tumor (a combination of early dissemination, site‐specific selection pressure, and parallel evolution) results in a substantial fraction of DTCs that do not express molecular targets derived from the primary tumor. For example, EGFR mutations did not correlate in more than 75% of paired primary tumors and metastases in a NSCLC cohort in a failed adjuvant clinical trial where EGFR was targeted using gefitinib (Goss et al., 2010; Gow et al., 2009). Additionally, pathways active in dormant DTCs such as the p38 pathway might supersede oncogenic pathways, making these cells resistant to targeted therapies (Adam et al., 2009; Polzer and Klein, 2013). Finally, the niche of dormant DTCs may also play a role in chemoresistance (Ghajar, 2015). Disseminated colorectal cancer cells expressing the putative cancer stem cell marker CD133 are enriched on the microvasculature of human liver and are sustained in the face of 5‐fluorouracil and oxaliplatin by cleaved Notch ligand jagged‐1 derived from liver endothelium (Lu et al., 2013). The soluble form of angiocrine Jagged‐1 in liver has also been implicated in doxorubicin resistance of lymphoma cells (Cao et al., 2014). The induction of certain cancer stem cell‐like properties may also underlie resistance mechanisms conferred by the PVN. The brain's PVN induces a stem cell‐like phenotype in proneural glioblastoma cells, resulting in expression of ABC transporter G family member 2 (ABCG2), known to actively efflux chemotherapeutics (Charles et al., 2010; Fletcher et al., 2010). Additionally, the DTC microenvironment itself may respond to chemotherapy and release factors that enable DTC survival (Acharyya et al., 2012; Sun et al., 2012). To understand the chemoresistance of these cells, efforts that take into account the timing of dissemination, the influence of the microenvironment on DTC survival, and response of the DTC microenvironment to treatment are needed.
7. Isolation and molecular and functional characterization of DTCs
Several tools, techniques, and platforms available for detection and characterization of DTCs have been reviewed exhaustively (e.g., Magbanua et al., 2015). In this section, we will provide a general overview of techniques and interesting findings from a subset of these studies.
7.1. DTC isolation
The molecular and functional characterization of DTCs is made difficult because their isolation invariably requires invasive procedures and positive selection. Detection of single DTCs in a noninvasive manner even with the use of high‐resolution imaging technologies is currently not possible in the clinic. Thus, current strategies rely on isolating DTCs from accessible tissue that lacks common epithelial tumor markers – primarily lymph node and bone marrow biopsy. Whole lymph node isolation and bone marrow biopsy samples are routinely performed in the clinic, and cytokeratins are currently used as standard markers for the detection of epithelial tumor cells in organs of mesenchymal origin. Additional markers such as proliferation‐associated Ki67 (Pantel et al., 1993), stem cell markers (CD44high CD24low) (Balic et al., 2006), and HER2 (Schmidt‐Kittler et al., 2003) are also used to characterize DTCs. As new markers are identified and verified, they are increasingly being applied in multi‐marker fashion that is higher throughput than ICC/IHC alone. For example, using a system that detects transcripts of 38 genes (nCounter™ system), it was found that three patients with metastatic breast cancer had detectable levels of ERBB2 expression in their bone marrow despite their corresponding tumors being HER2/ERBB2 negative (Siddappa et al., 2013). This has clinical relevance as the Her2 gene is a common target for systemic therapy and these patients did not receive trastuzumab. Subsets of DTCs that express breast cancer stem cell markers (e.g., CD44+ CD24−/low) (Bartkowiak et al., 2010) have also been identified, although it is unclear whether this subset has greater metastasis‐initiating capacity than others.
7.2. Molecular characterization of DTCs
Single‐cell DNA sequencing technologies are increasingly being utilized to study DTCs. These allow the detection of small, novel, and copy‐neutral genetic changes. Comparing primary tumor cells and DTCs from two patients with breast cancer in this fashion revealed that although DTCs often carry the major chromosomal aberrations found in the primary tumor (whole arm gains/losses), they often carry unique genetic changes as well, further evidence of dynamic and divergent evolution (Møller et al., 2013). Whole transcriptome profiling using oligonucleotide microarray analysis revealed that the transcriptome signature of some prostate cancer bone marrow DTCs from patients with advanced disease clustered closely with that of DTCs from patients with no evidence of disease (Chéry et al., 2014), perhaps indicative of the bone marrow microenvironment's dominance over DTC gene expression. Efforts to correlate genetic and expression profiles in single DTCs (Gužvić et al., 2014) could potentially generate a specific expression signature to predict the metastasis‐initiating capacity of DTCs in different cancers.
7.3. Functional characterization of DTCs
Functional characterization (culture‐based and in vivo tumor/metastases‐forming assays) of DTCs is hampered mainly by the limited number of DTCs that can be isolated from patients. DTCs have been used to establish cell lines to address this problem. Although genetic changes may be initially maintained, the biology of DTC lines cannot be expected to fully recapitulate in situ DTC biology due to the lack of the full complement of microenvironmental factors. Nevertheless, important conclusions have been made from DTC lines. Analysis of DTCs from a number of different sites that either commonly support metastasis or are rarely affected by metastasis challenged the notion that tumor cells ‘home’ to specific tissues. Solitary MDA‐MB‐435 CL16 breast cancer cells isolated from metastasis‐free organs such as bone and spleen were shown to be just as tumorigenic and metastatic as cells isolated from preferred organs of metastasis such as lungs and lymph nodes (Suzuki et al., 2006), suggesting the need for a permissive microenvironment. DTC lines also provide further evidence of parallel progression. The proliferation rate of breast cancer cell lines established from M0 DTCs (i.e., patients without detectable metastatic disease) does not correlate with the proliferative potential of cells from the corresponding primary tumor (Gangnus et al., 2004). CGH analysis showed that the genomic profiles of these DTCs and their matched primary were very different, suggesting early dissemination and independent evolution of DTCs (Gangnus et al., 2004). Efforts are underway to test whether the dormancy phenotype can be established in cell lines by recapitulating the niche compartment that regulates DTC dormancy in each tissue. For breast cancer, this involves establishing microvascular niche cultures because dormant DTCs are often found in close association with endothelia – a phenomenon observed both in patients and in mouse models (Ghajar et al., 2013; Price et al., 2016). DTC biology, especially the influence of microenvironment on quiescence and re‐emergence, can thus be studied in tractable models that do not necessarily depend on establishment of cell lines derived from isolated DTCs.
8. Clinical relevance of DTCs: the persistent face of cancer
Disseminated tumor cells detected before and after adjuvant therapy, radiotherapy, or surgical resection of the primary tumor have been described as independent risk factors for relapse and death (Becker et al., 2007; Berg et al., 2007; Gruber et al., 2014; Hartkopf et al., 2013; Janni et al., 2011; Lilleby et al., 2013; Mathiesen et al., 2012; Morgan et al., 2009; Naume et al., 2014; Tjensvoll et al., 2012). Yet, despite DTCs predicting adverse outcomes, the majority of DTC‐positive patients (specifically, 2/3 for breast cancer) do not develop metastases (Braun et al., 2005). This suggests that the ability to take up residence in bone marrow is not sufficient to initiate metastases.
Factors that may help explain this outcome include heterogeneity in the timeline of dissemination, influence of the microenvironment, and largely unknown effects of adjuvant therapy on DTC phenotype. There may be an as yet unknown trigger of DTC re‐emergence that occurs in the right space and time in patients that develop metastases that does not occur in others. In culture and in zebrafish, neovascular tips ‘awaken’ dormant breast cancer cells (Ghajar et al., 2013) by depositing many of the factors known to cause aggressive growth of many cancers in the premetastatic niche (Psaila and Lyden, 2009). These data suggest that physiological stresses that induce neoangiogenesis, such as systemic inflammation or surgery, could cause dormant cells to re‐enter the cell cycle. Along these lines, it was shown in a retrospective study that early breast cancer relapse incidents in the 9‐ to 18‐month period after surgery are reduced approximately fivefold in patients taking preoperative nonsteroidal anti‐inflammatory drugs (Retsky et al., 2013), suggesting that systemic inflammation is a indeed a trigger. Additionally, there are a number of case studies involving surgery conducted on breast cancer survivors years after treatment that caused noncanonical metastatic outgrowth at the surgical site, such as in the vicinity of dental implants and the site of tracheotomy (Dib et al., 2007; Rotolo et al., 2013).
Overall, very little progress has been made on therapeutic strategies to address DTCs. The heterogeneity between the primary tumor and DTCs, and between DTCs at different sites, are doubtlessly contributing factors. Additionally, it has never been proven that DTCs are the source of future metastasis/metastases (Goss and Chambers, 2010; Meng et al., 2004; Uhr and Pantel, 2011). Ironically, the only proof is an effective therapy. Here, it is important to note that small trials targeting DTCs post‐treatment have been successful. Second‐line therapies such as bisphosphonates (Banys et al., 2013; Kasimir‐Bauer et al., 2016) or docetaxel (Naume et al., 2014) have led to increased survival for patients with breast cancer. The latter trial was particularly promising as the risk of recurrence for docetaxel‐treated patients effectively treated to eliminate DTCs was reduced to the level of patients who were initially DTC negative (Naume et al., 2014). These clinical successes in targeting DTCs are promising, but leave room to identify more specific strategies to eliminate DTCs with first‐line therapy. Another unexplored approach to disease management could involve prolonged induction of dormancy to make DTCs, dormant or otherwise, incapable of progressing from a growth‐arrested state (Ghajar, 2015).
Thus, deeper understanding of the factors that induce and sustain dormancy may open avenues to a) inhibit factors that trigger activation of dormant DTCs or b) sustain quiescence indefinitely (Ghajar, 2015). Analysis of the dormant niche via mass spectrometry, metabolomics, etc., may identify proteins and metabolites that induce dormancy and/or activation. Development of tissue‐specific niche models that recapitulate the cellular and molecular composition of native DTC niches are pivotal to better understand dynamic changes in niche composition under different pressures such as chemotherapy. Such efforts may reveal novel factors or signaling pathways involved in conferring and/or sustaining dormant properties. In this endeavor, the ideal therapeutic would be a prophylactic therapy administered as soon as the primary tumor is detected in order to keep the DTCs dormant. This strategy has been elaborated on elsewhere along with a careful consideration of clinical trial design (Ghajar, 2015).
9. Concluding remarks
In this review, we examined our current understanding of CTC and DTC biology and profiled technologies available to detect and molecularly characterize these cells. Additionally, we discussed the clinical relevance of CTCs and DTCs and posed several important questions and considerations – from technical caveats and challenges in analyzing DTCs and CTCs, to the need to address the current discrepancy in the timing of dissemination as a clinically relevant factor. Regardless, studying these cells through the use of more refined detection, isolation, and characterization technologies, and carefully designed animal models, has the potential to make CTCs and DTCs important diagnostic and prognostic biomarkers in disease management. Ultimately, however, our aim should be to either eliminate or permanently incapacitate these cells so that they are no longer able to progress toward lethal metastases. So how do we progress from a lack of therapies that specifically target CTC and DTCs to making metastasis prevention by targeting the relevant population(s) a reality?
As is evident from Fig. 2, we still have much to learn about the dormant niche. What sustains the survival of DTCs from different cancers within different tissues, how do tissue microenvironments steer DTCs into a quiescent state, and how do these controls go awry to foster DTC re‐emergence? The hope is to identify universal mechanisms that will allow us to target DTCs throughout the body, but in the absence of a ubiquitous control mechanism, we will need tissue‐specific approaches that can be applied to patients with cancers prone to metastasize to restricted sites (e.g., pancreatic cancer to the liver) or cancer subtypes with a defined risk of metastasis to a particular site (e.g., ER‐positive breast cancer to the bone).
Once we realize the above, we still have to answer the most relevant question: Whom do we treat? In patients with breast cancer, it is well established that DTC‐positive bone marrow aspirates reflect a greatly enhanced risk of metastasis. But still, only one‐third of this population will progress, meaning that applying DTC‐targeted therapies to all marrow‐positive breast cancer patients would result in overtreatment. This is not acceptable. Profiling DTCs or CTCs derived from DTCs at the molecular level could reveal indicators of metastasis‐initiating potential, allowing us to stratify DTC‐positive patients further. Then, the goal would be to treat only this subset of patients and eliminate metastasis‐initiating cells so that they perish of causes unrelated to cancer.
Ultimately, it is important to remember the old adage, ‘an ounce of prevention is worth a pound of cure.’ Applying this to CTCs and DTCs to prevent metastasis has the potential to prolong the lives of many cancer survivors. The time is ripe to commit to this approach.
Acknowledgements
Work in the Laboratory for the Study of Metastatic Microenvironments (LSM2) is funded by an Era of Hope Scholar Award from the Department of Defense Breast Cancer Research Program (W81XWH‐15‐1‐0201), the National Institutes of Health (U54 CA193461‐01), the Cuyamaca Foundation, the Pink Gene Foundation, the Prostate Cancer Foundation, and new development funds provided by Fred Hutchinson Cancer Research Center.
Contributor Information
Arko Dasgupta, Email: adasgupt@fredhutch.org.
Cyrus M. Ghajar, Email: cghajar@fredhutch.org
References
- Abbruzzese JL, Abbruzzese MC, Hess KR, Raber MN, Lenzi R and Frost P (1994) Unknown primary carcinoma: natural history and prognostic factors in 657 consecutive patients. J Clin Oncol 12, 1272–1280. [DOI] [PubMed] [Google Scholar]
- Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H et al (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aceto N, Toner M, Maheswaran S and Haber DA (2015) En route to metastasis: circulating tumor cell clusters and epithelial‐to‐mesenchymal transition. Trends Cancer 1, 44–52. [DOI] [PubMed] [Google Scholar]
- Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova‐Todorova K, Leversha M, Hogg N, Seshan VE et al (2012) A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 150, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam AP, George A, Schewe D, Bragado P, Iglesias BV, Ranganathan AC, Kourtidis A, Conklin DS and Aguirre‐Ghiso JA (2009) Computational identification of a p38SAPK‐regulated transcription factor network required for tumor cell quiescence. Cancer Res 69, 5664–5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre‐Ghiso JA (2007) Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer 7, 834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R and Kasimir‐Bauer S (2009) Stem cell and epithelial‐mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res 11, R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AGJ, Uhr JW and Terstappen LWMM (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10, 6897–6904. [DOI] [PubMed] [Google Scholar]
- Al‐Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A and Muschel RJ (2000) Intravascular origin of metastasis from the proliferation of endothelium‐attached tumor cells: a new model for metastasis. Nat Med 6, 100–102. [DOI] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY and Suda T (2004) Tie2/angiopoietin‐1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118, 149–161. [DOI] [PubMed] [Google Scholar]
- Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bäuerle T, Wallwiener M et al (2013) Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol 31, 539–544. [DOI] [PubMed] [Google Scholar]
- Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, Datar RH and Cote RJ (2006) Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res 12, 5615–5621. [DOI] [PubMed] [Google Scholar]
- Banys M, Solomayer E‐F, Gebauer G, Janni W, Krawczyk N, Lueck H‐J, Becker S, Huober J, Kraemer B, Wackwitz B et al (2013) Influence of zoledronic acid on disseminated tumor cells in bone marrow and survival: results of a prospective clinical trial. BMC Cancer 13, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkowiak K, Effenberger KE, Harder S, Andreas A, Buck F, Peter‐Katalinic J, Pantel K and Brandt BH (2010) Discovery of a novel unfolded protein response phenotype of cancer stem/progenitor cells from the bone marrow of breast cancer patients. J Proteome Res 9, 3158–3168. [DOI] [PubMed] [Google Scholar]
- Becker PS (2012) Dependence of acute myeloid leukemia on adhesion within the bone marrow microenvironment. ScientificWorldJournal 2012, 856467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Solomayer E, Becker‐Pergola G, Wallwiener D and Fehm T (2007) Primary systemic therapy does not eradicate disseminated tumor cells in breast cancer patients. Breast Cancer Res Treat 106, 239–243. [DOI] [PubMed] [Google Scholar]
- Berg A, Berner A, Lilleby W, Bruland ØS, Fosså SD, Nesland JM and Kvalheim G (2007) Impact of disseminated tumor cells in bone marrow at diagnosis in patients with nonmetastatic prostate cancer treated by definitive radiotherapy. Int J Cancer 120, 1603–1609. [DOI] [PubMed] [Google Scholar]
- Bos PD, Zhang XH‐F, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA et al (2009) Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z and Bissell MJ (1995) Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 267, 891–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyerinas B, Zafrir M, Yesilkanal AE, Price TT, Hyjek EM and Sipkins DA (2013) Adhesion to osteopontin in the bone marrow niche regulates lymphoblastic leukemia cell dormancy. Blood 121, 4821–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragado P, Estrada Y, Parikh F, Krause S, Capobianco C, Farina HG, Schewe DM and Aguirre‐Ghiso JA (2013) TGF‐β2 dictates disseminated tumour cell fate in target organs through TGF‐β‐RIII and p38α/β signalling. Nat Cell Biol 15, 1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S, Pantel K, Müller P, Janni W, Hepp F, Kentenich CR, Gastroph S, Wischnik A, Dimpfl T, Kindermann G et al (2000) Cytokeratin‐positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med 342, 525–533. [DOI] [PubMed] [Google Scholar]
- Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G et al (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 353, 793–802. [DOI] [PubMed] [Google Scholar]
- Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y, Scheiermann C, Schiff L, Poncz M, Bergman A et al (2014) Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med 20, 1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JM, Kobayashi H and Rafii S (2010) Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer 10, 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M, Shen L, Fan Y, Giri U, Tumula PK et al (2013) An epithelial‐mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res 19, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M et al (2007) A perivascular niche for brain tumor stem cells. Cancer Cell 11, 69–82. [DOI] [PubMed] [Google Scholar]
- Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R and Coughlin SR (2004) Platelets, protease‐activated receptors, and fibrinogen in hematogenous metastasis. Blood 104, 397–401. [DOI] [PubMed] [Google Scholar]
- Cao Z, Ding B‐S, Guo P, Lee SB, Butler JM, Casey SC, Simons M, Tam W, Felsher DW, Shido K et al (2014) Angiocrine factors deployed by tumor vascular niche induce B cell lymphoma invasiveness and chemoresistance. Cancer Cell 25, 350–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrefourcq L, Mazard T, Joosse S, Solassol J, Ramos J, Assenat E, Schumacher U, Costes V, Maudelonde T, Pantel K et al (2015) Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res 75, 892–901. [DOI] [PubMed] [Google Scholar]
- Chambers AF, Groom AC and MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2, 563–572. [DOI] [PubMed] [Google Scholar]
- Charles N, Ozawa T, Squatrito M, Bleau A‐M, Brennan CW, Hambardzumyan D and Holland EC (2010) Perivascular nitric oxide activates notch signaling and promotes stem‐like character in PDGF‐induced glioma cells. Cell Stem Cell 6, 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chéry L, Lam H‐M, Coleman I, Lakely B, Coleman R, Larson S, Aguirre‐Ghiso JA, Xia J, Gulati R, Nelson PS et al (2014) Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget 5, 9939–9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen JJ and Rajasekaran AK (2006) Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res 66, 8319–8326. [DOI] [PubMed] [Google Scholar]
- Christov C, Chrétien F, Abou‐Khalil R, Bassez G, Vallet G, Authier F‐J, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B et al (2007) Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell 18, 1397–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, Punt CJA, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC et al (2008) Relationship of circulating tumor cells to tumor response, progression‐free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 26, 3213–3221. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LWMM et al (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351, 781–791. [DOI] [PubMed] [Google Scholar]
- Dib LL, Soares AL, Sandoval RL and Nannmark U (2007) Breast metastasis around dental implants: a case report. Clin Implant Dent Relat Res 9, 112–115. [DOI] [PubMed] [Google Scholar]
- Diepenbruck M and Christofori G (2016) Epithelial–mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol 43, 7–13. [DOI] [PubMed] [Google Scholar]
- Ding L and Morrison SJ (2013) Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G and Morrison SJ (2012) Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda DG, Duyverman AMMJ, Kohno M, Snuderl M, Steller EJA, Fukumura D and Jain RK (2010) Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci USA 107, 21677–21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Eckel R, Kerr J, Schmidt M, Fürstenberger G, Richter R, Sauer H, Senn H‐J and Hölzel D (2003) The process of metastasisation for breast cancer. Eur J Cancer 39, 1794–1806. [DOI] [PubMed] [Google Scholar]
- Fearon ER and Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61, 759–767. [DOI] [PubMed] [Google Scholar]
- Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong STC, Choi H, El Rayes T, Ryu S, Troeger J et al (2015) Epithelial‐to‐mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527, 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JC, Niederacher D, Topp SA, Honisch E, Schumacher S, Schmitz N, Zacarias Föhrding L, Vay C, Hoffmann I, Kasprowicz NS et al (2013) Diagnostic leukapheresis enables reliable detection of circulating tumor cells of nonmetastatic cancer patients. Proc Natl Acad Sci USA 110, 16580–16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JI, Haber M, Henderson MJ and Norris MD (2010) ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer 10, 147–156. [DOI] [PubMed] [Google Scholar]
- Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285, 1182–1186. [DOI] [PubMed] [Google Scholar]
- Friedlander TW, Ngo VT, Dong H, Premasekharan G, Weinberg V, Doty S, Zhao Q, Gilbert EG, Ryan CJ, Chen W‐T et al (2014) Detection and characterization of invasive circulating tumor cells derived from men with metastatic castration‐resistant prostate cancer. Int J Cancer 134, 2284–2293. [DOI] [PubMed] [Google Scholar]
- Gangnus R, Langer S, Breit E, Pantel K and Speicher MR (2004) Genomic profiling of viable and proliferative micrometastatic cells from early‐stage breast cancer patients. Clin Cancer Res 10, 3457–3464. [DOI] [PubMed] [Google Scholar]
- Gao H, Chakraborty G, Lee‐Lim AP, Mo Q, Decker M, Vonica A, Shen R, Brogi E, Brivanlou AH and Giancotti FG (2012) The BMP inhibitor coco reactivates breast cancer cells at lung metastatic sites. Cell 150, 764–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic GJ, Gasic TB and Stewart CC (1968) Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci USA 61, 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti M, Pattarozzi A, Bajetto A, Würth R, Daga A, Fiaschi P, Zona G, Florio T and Barbieri F (2013) Inhibition of CXCL12/CXCR4 autocrine/paracrine loop reduces viability of human glioblastoma stem‐like cells affecting self‐renewal activity. Toxicology 314, 209–220. [DOI] [PubMed] [Google Scholar]
- Ghajar CM (2015) Metastasis prevention by targeting the dormant niche. Nat Rev Cancer 15, 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DYR et al (2013) The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 15, 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LA and Hemann MT (2010) DNA damage‐mediated induction of a chemoresistant niche. Cell 143, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone MA, Leapman SB, Cotran RS and Folkman J (1972) Tumor dormancy in vivo by prevention of neovascularization. J Exp Med 136, 261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA and Chen Z (2011) Perivascular instruction of cell genesis and fate in the adult brain. Nat Neurosci 14, 1382–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss PE and Chambers AF (2010) Does tumour dormancy offer a therapeutic target? Nat Rev Cancer 10, 871–877. [DOI] [PubMed] [Google Scholar]
- Goss GD, Lorimer I, Tsao MS, O'Callaghan CJ, Ding K, Masters GA, Roberts P, Jett JR, Edelman MJ and Shepherd FA (2010) A phase III randomized, double‐blind, placebo‐controlled trial of the epidermal growth factor receptor inhibitor gefitinb in completely resected stage IB‐IIIA non‐small cell lung cancer (NSCLC): NCIC CTG BR.19. ASCO Meet. Abstr. 28, LBA7005.
- Gow C‐H, Chang Y‐L, Hsu Y‐C, Tsai M‐F, Wu C‐T, Yu C‐J, Yang C‐H, Lee Y‐C, Yang P‐C and Shih J‐Y (2009) Comparison of epidermal growth factor receptor mutations between primary and corresponding metastatic tumors in tyrosine kinase inhibitor‐naive non‐small‐cell lung cancer. Ann Oncol 20, 696–702. [DOI] [PubMed] [Google Scholar]
- Gruber I, Fehm T, Taran FA, Wallwiener M, Hahn M, Wallwiener D, Krawzyck N, Hoffmann J and Hartkopf AD (2014) Disseminated tumor cells as a monitoring tool for adjuvant therapy in patients with primary breast cancer. Breast Cancer Res Treat 144, 353–360. [DOI] [PubMed] [Google Scholar]
- Gruber IV, Hartkopf AD, Hahn M, Taran F‐A, Staebler A, Wallwiener D, Brucker SY, Hanke J and Fehm T (2016) Relationship between hematogenous tumor cell dissemination and cellular immunity in DCIS patients. Anticancer Res 36, 2345–2351. [PubMed] [Google Scholar]
- Gužvić M, Braun B, Ganzer R, Burger M, Nerlich M, Winkler S, Werner‐Klein M, Czyż ZT, Polzer B and Klein CA (2014) Combined genome and transcriptome analysis of single disseminated cancer cells from bone marrow of prostate cancer patients reveals unexpected transcriptomes. Cancer Res 74, 7383–7394. [DOI] [PubMed] [Google Scholar]
- Hanahan D and Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Hanoun M, Zhang D, Mizoguchi T, Pinho S, Pierce H, Kunisaki Y, Lacombe J, Armstrong SA, Dührsen U and Frenette PS (2014) Acute myelogenous leukemia‐induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell 15, 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartkopf AD, Taran F‐A, Wallwiener M, Hagenbeck C, Melcher C, Krawczyk N, Hahn M, Wallwiener D and Fehm T (2013) The presence and prognostic impact of apoptotic and nonapoptotic disseminated tumor cells in the bone marrow of primary breast cancer patients after neoadjuvant chemotherapy. Breast Cancer Res 15, R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzer E, Auer M, Gasch C, Pichler M, Ulz P, Hoffmann EM, Lax S, Waldispuehl‐Geigl J, Mauermann O, Lackner C et al (2013) Complex tumor genomes inferred from single circulating tumor cells by array‐CGH and next‐generation sequencing. Cancer Res 73, 2965–2975. [DOI] [PubMed] [Google Scholar]
- Hiraiwa K, Takeuchi H, Hasegawa H, Saikawa Y, Suda K, Ando T, Kumagai K, Irino T, Yoshikawa T, Matsuda S et al (2008) Clinical significance of circulating tumor cells in blood from patients with gastrointestinal cancers. Ann Surg Oncol 15, 3092–3100. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CL, Morrow CJ, Li Y, Metcalf RL, Rothwell DG, Trapani F, Polanski R, Burt DJ, Simpson KL, Morris K et al (2014) Tumorigenicity and genetic profiling of circulating tumor cells in small‐cell lung cancer. Nat Med 20, 897–903. [DOI] [PubMed] [Google Scholar]
- Hoshino A, Costa‐Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S et al (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J‐M, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, Priest LJC, Greystoke A, Zhou C, Morris K et al (2012) Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small‐cell lung cancer. J Clin Oncol 30, 525–532. [DOI] [PubMed] [Google Scholar]
- Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmüller G et al (2008) Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68. [DOI] [PubMed] [Google Scholar]
- Ilie M, Hofman V, Long‐Mira E, Selva E, Vignaud J‐M, Padovani B, Mouroux J, Marquette C‐H and Hofman P (2014) “Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS ONE 9, e111597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janni W, Vogl FD, Wiedswang G, Synnestvedt M, Fehm T, Jückstock J, Borgen E, Rack B, Braun S, Sommer H et al (2011) Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse – a European pooled analysis. Clin Cancer Res 17, 2967–2976. [DOI] [PubMed] [Google Scholar]
- Johnson RW, Finger EC, Olcina MM, Vilalta M, Aguilera T, Miao Y, Merkel AR, Johnson JR, Sterling JA, Wu JY et al (2016) Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat Cell Biol 18, 1078–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ML, Siddiqui J, Pienta KJ and Getzenberg RH (2013) Circulating fibroblast‐like cells in men with metastatic prostate cancer. Prostate 73, 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosse SA, Gorges TM and Pantel K (2015) Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol Med 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón‐Cardo C, Guise TA and Massagué J (2003) A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA et al (2005) VEGFR1‐positive haematopoietic bone marrow progenitors initiate the pre‐metastatic niche. Nature 438, 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karhade M, Hall C, Mishra P, Anderson A, Kuerer H, Bedrosian I, Krishnamurthy S and Lucci A (2014) Circulating tumor cells in non‐metastatic triple‐negative breast cancer. Breast Cancer Res Treat 147, 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasimir‐Bauer S, Bittner A‐K, König L, Reiter K, Keller T, Kimmig R and Hoffmann O (2016) Does primary neoadjuvant systemic therapy eradicate minimal residual disease? Analysis of disseminated and circulating tumor cells before and after therapy. Breast Cancer Res 18, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C and Morrison SJ (2005) SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121. [DOI] [PubMed] [Google Scholar]
- Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WEF, Goldbrunner R, Herms J and Winkler F (2010) Real‐time imaging reveals the single steps of brain metastasis formation. Nat Med 16, 116–122. [DOI] [PubMed] [Google Scholar]
- Klein CA (2009) Parallel progression of primary tumours and metastases. Nat Rev Cancer 9, 302–312. [DOI] [PubMed] [Google Scholar]
- Klein CA (2013) Selection and adaptation during metastatic cancer progression. Nature 501, 365–372. [DOI] [PubMed] [Google Scholar]
- Klein CA, Kuhn TS, Podsypanina K, Ansieau S, Hüsemann Y, Mani SA, Bernards R, Weinberg RA, Chambers AF, Groom AC et al (2008) Cancer. The metastasis cascade. Science 321, 1785–1787. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C et al (2011) Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem‐like cells in bone. J Exp Med 208, 2641–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp H‐G, Placke T and Salih HR (2009) Platelet‐derived transforming growth factor‐beta down‐regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res 69, 7775–7783. [DOI] [PubMed] [Google Scholar]
- Krebs MG, Metcalf RL, Carter L, Brady G, Blackhall FH and Dive C (2014) Molecular analysis of circulating tumour cells‐biology and biomarkers. Nat Rev Clin Oncol 11, 129–144. [DOI] [PubMed] [Google Scholar]
- Labelle M, Begum S and Hynes RO (2011) Direct signaling between platelets and cancer cells induces an epithelial‐mesenchymal‐like transition and promotes metastasis. Cancer Cell 20, 576–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle M and Hynes RO (2012) The initial hours of metastasis: the importance of cooperative host‐tumor cell interactions during hematogenous dissemination. Cancer Discov 2, 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers R, Giesert C, Grünebach F, Marxer A, Vogel W and Bühring H‐J (2002) Monoclonal antibody 9C4 recognizes epithelial cellular adhesion molecule, a cell surface antigen expressed in early steps of erythropoiesis. Exp Hematol 30, 537–545. [DOI] [PubMed] [Google Scholar]
- Lamouille S, Xu J and Derynck R (2014) Molecular mechanisms of epithelial‐mesenchymal transition. Nat Rev Mol Cell Biol 15, 178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, MacSwords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB et al (2010) Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 6, 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia JD, Li M, Hall PE, Gallagher J, Hale JS, Wu Q, Venere M, Levy E, Rani MRS, Huang P et al (2012) Laminin alpha 2 enables glioblastoma stem cell growth. Ann Neurol 72, 766–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läubli H and Borsig L (2010) Selectins promote tumor metastasis. Semin Cancer Biol 20, 169–177. [DOI] [PubMed] [Google Scholar]
- LeBleu VS, O'Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, de Carvalho FM, Damascena A, Domingos Chinen LT, Rocha RM et al (2014) PGC‐1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 16, 992–1003, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R and Thompson EW (2006) The epithelial‐mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 172, 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J‐H, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, Wagers AJ, Tseng Y‐H, Ryeom S, Kim CF et al (2014) Lung stem cell differentiation in mice directed by endothelial cells via a BMP4‐NFATc1‐thrombospondin‐1 axis. Cell 156, 440–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilleby W, Stensvold A, Mills IG and Nesland JM (2013) Disseminated tumor cells and their prognostic significance in nonmetastatic prostate cancer patients. Int J Cancer 133, 149–155. [DOI] [PubMed] [Google Scholar]
- Lohr JG, Adalsteinsson VA, Cibulskis K, Choudhury AD, Rosenberg M, Cruz‐Gordillo P, Francis JM, Zhang C‐Z, Shalek AK, Satija R et al (2014) Whole‐exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat Biotechnol 32, 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Ye X, Fan F, Xia L, Bhattacharya R, Bellister S, Tozzi F, Sceusi E, Zhou Y, Tachibana I et al (2013) Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged‐1. Cancer Cell 23, 171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X‐L, Xiao Z‐L, Liu L, Liu X‐X, Nie W, Li P, Chen N‐Y and Wei Y‐Q (2012) Meta‐analysis of circulating tumor cells as a prognostic marker in lung cancer. Asian Pac J Cancer Prev 13, 1137–1144. [DOI] [PubMed] [Google Scholar]
- Magbanua MJM, Das R, Polavarapu P and Park JW (2015) Approaches to isolation and molecular characterization of disseminated tumor cells. Oncotarget 6, 30715–30729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massard C, Loriot Y and Fizazi K (2011) Carcinomas of an unknown primary origin—diagnosis and treatment. Nat Rev Clin Oncol 8, 701–710. [DOI] [PubMed] [Google Scholar]
- Mathiesen RR, Borgen E, Renolen A, Løkkevik E, Nesland JM, Anker G, Ostenstad B, Lundgren S, Risberg T, Mjaaland I et al (2012) Persistence of disseminated tumor cells after neoadjuvant treatment for locally advanced breast cancer predicts poor survival. Breast Cancer Res 14, R117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty OJ, Mousa SA, Bray PF and Konstantopoulos K (2000) Immobilized platelets support human colon carcinoma cell tethering, rolling, and firm adhesion under dynamic flow conditions. Blood 96, 1789–1797. [PubMed] [Google Scholar]
- Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF, Beitsch PD, Leitch M, Hoover S, Euhus D et al (2004) Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res 10, 8152–8162. [DOI] [PubMed] [Google Scholar]
- Millner LM, Linder MW and Valdes R (2013) Circulating tumor cells: a review of present methods and the need to identify heterogeneous phenotypes. Ann Clin Lab Sci 43, 295–304. [PMC free article] [PubMed] [Google Scholar]
- Mimori K, Fukagawa T, Kosaka Y, Kita Y, Ishikawa K, Etoh T, Iinuma H, Sasako M and Mori M (2008) Hematogenous metastasis in gastric cancer requires isolated tumor cells and expression of vascular endothelial growth factor receptor‐1. Clin Cancer Res 14, 2609–2616. [DOI] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL and Massagué J (2005) Genes that mediate breast cancer metastasis to lung. Nature 436, 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto DT, Zheng Y, Wittner BS, Lee RJ, Zhu H, Broderick KT, Desai R, Fox DB, Brannigan BW, Trautwein J et al (2015) RNA‐Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science 349, 1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocellin S, Hoon D, Ambrosi A, Nitti D and Rossi CR (2006) The prognostic value of circulating tumor cells in patients with melanoma: a systematic review and meta‐analysis. Clin Cancer Res 12, 4605–4613. [DOI] [PubMed] [Google Scholar]
- Møller EK, Kumar P, Voet T, Peterson A, Van Loo P, Mathiesen RR, Fjelldal R, Grundstad J, Borgen E, Baumbusch LO et al (2013) Next‐generation sequencing of disseminated tumor cells. Front Oncol 3, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TM, Lange PH, Porter MP, Lin DW, Ellis WJ, Gallaher IS and Vessella RL (2009) Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res 15, 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A et al (2007) Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450, 1235–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naume B, Synnestvedt M, Falk RS, Wiedswang G, Weyde K, Risberg T, Kersten C, Mjaaland I, Vindi L, Sommer HH et al (2014) Clinical outcome with correlation to disseminated tumor cell (DTC) status after DTC‐guided secondary adjuvant treatment with docetaxel in early breast cancer. J Clin Oncol 32, 3848–3857. [DOI] [PubMed] [Google Scholar]
- Nguyen DX, Bos PD and Massagué J (2009) Metastasis: from dissemination to organ‐specific colonization. Nat Rev Cancer 9, 274–284. [DOI] [PubMed] [Google Scholar]
- Nierodzik ML, Plotkin A, Kajumo F and Karpatkin S (1991) Thrombin stimulates tumor‐platelet adhesion in vitro and metastasis in vivo . J Clin Invest 87, 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieswandt B, Hafner M, Echtenacher B and Männel DN (1999) Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 59, 1295–1300. [PubMed] [Google Scholar]
- Ocaña OH, Córcoles R, Fabra A, Moreno‐Bueno G, Acloque H, Vega S, Barrallo‐Gimeno A, Cano A and Nieto MA (2012) Metastatic colonization requires the repression of the epithelial‐mesenchymal transition inducer Prrx1. Cancer Cell 22, 709–724. [DOI] [PubMed] [Google Scholar]
- Ottone C, Krusche B, Whitby A, Clements M, Quadrato G, Pitulescu ME, Adams RH and Parrinello S (2014) Direct cell‐cell contact with the vascular niche maintains quiescent neural stem cells. Nat Cell Biol 16, 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkumur E, Shah AM, Ciciliano JC, Emmink BL, Miyamoto DT, Brachtel E, Yu M, Chen P, Morgan B, Trautwein J et al (2013) Inertial focusing for tumor antigen‐dependent and ‐independent sorting of rare circulating tumor cells. Sci Transl Med 5, 179ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget S (1889) The distribution of secondary growths in cancer of the breast. Lancet 133, 571–573. [PubMed] [Google Scholar]
- Pantel K, Denève E, Nocca D, Coffy A, Vendrell J‐P, Maudelonde T, Riethdorf S and Alix‐Panabières C (2012) Circulating epithelial cells in patients with benign colon diseases. Clin Chem 58, 936–940. [DOI] [PubMed] [Google Scholar]
- Pantel K, Izbicki J, Passlick B, Angstwurm M, Häussinger K, Thetter O and Riethmüller G (1996) Frequency and prognostic significance of isolated tumour cells in bone marrow of patients with non‐small‐cell lung cancer without overt metastases. Lancet 347, 649–653. [DOI] [PubMed] [Google Scholar]
- Pantel K, Schlimok G, Braun S, Kutter D, Lindemann F, Schaller G, Funke I, Izbicki JR and Riethmüller G (1993) Differential expression of proliferation‐associated molecules in individual micrometastatic carcinoma cells. J Natl Cancer Inst 85, 1419–1424. [DOI] [PubMed] [Google Scholar]
- Pantel K and Speicher MR (2015) The biology of circulating tumor cells. Oncogene 35, 1216–1224. [DOI] [PubMed] [Google Scholar]
- Paoli P, Giannoni E and Chiarugi P (2013) Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta 1833, 3481–3498. [DOI] [PubMed] [Google Scholar]
- Payne RE, Wang F, Su N, Krell J, Zebrowski A, Yagüe E, Ma X‐J, Luo Y and Coombes RC (2012) Viable circulating tumour cell detection using multiplex RNA in situ hybridisation predicts progression‐free survival in metastatic breast cancer patients. Br J Cancer 106, 1790–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein E, Salk PL, Yogeeswaran G and Karpatkin S (1980) Correlation between spontaneous metastatic potential, platelet‐aggregating activity of cell surface extracts, and cell surface sialylation in 10 metastatic‐variant derivatives of a rat renal sarcoma cell line. Proc Natl Acad Sci USA 77, 4336–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa‐Silva B, Moreno‐Bueno G, Hergueta‐Redondo M, Williams C, Garcia‐Santos G, Ghajar C et al (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro‐metastatic phenotype through MET. Nat Med 18, 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Burdick MD, Lutz M, Belperio JA, Keane MP and Strieter RM (2003) The stromal derived factor‐1/CXCL12‐CXC chemokine receptor 4 biological axis in non‐small cell lung cancer metastases. Am J Respir Crit Care Med 167, 1676–1686. [DOI] [PubMed] [Google Scholar]
- Pietras A, Katz AM, Ekström EJ, Wee B, Halliday JJ, Pitter KL, Werbeck JL, Amankulor NM, Huse JT and Holland EC (2014) Osteopontin‐CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell 14, 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placke T, Örgel M, Schaller M, Jung G, Rammensee H‐G, Kopp H‐G and Salih HR (2012) Platelet‐derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res 72, 440–448. [DOI] [PubMed] [Google Scholar]
- Polzer B and Klein CA (2013) Metastasis awakening: the challenges of targeting minimal residual cancer. Nat Med 19, 274–275. [DOI] [PubMed] [Google Scholar]
- Price TT, Burness ML, Sivan A, Warner MJ, Cheng R, Lee CH, Olivere L, Comatas K, Magnani J, Kim Lyerly H et al (2016) Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci Transl Med 8, 340ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaila B and Lyden D (2009) The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9, 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnoose EA, Atwal SK, Spoerke JM, Savage H, Pandita A, Yeh R‐F, Pirzkall A, Fine BM, Amler LC, Chen DS et al (2010) Molecular biomarker analyses using circulating tumor cells. PLoS ONE 5, e12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack B, Schindlbeck C, Jückstock J, Andergassen U, Hepp P, Zwingers T, Friedl TWP, Lorenz R, Tesch H, Fasching PA et al (2014) Circulating tumor cells predict survival in early average‐to‐high risk breast cancer patients. J Natl Cancer Inst 106, dju066. doi: 10.1093/jnci/dju066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii S, Butler JM and Ding B‐S (2016) Angiocrine functions of organ‐specific endothelial cells. Nature 529, 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbari NN, Aigner M, Thorlund K, Mollberg N, Motschall E, Jensen K, Diener MK, Büchler MW, Koch M and Weitz J (2010) Meta‐analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology 138, 1714–1726. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Bork U, Kircher A, Nimitz T, Schölch S, Kahlert C, Schmidt T, Steinert G, Ulrich AB, Reissfelder C et al (2012) Compartmental differences of circulating tumor cells in colorectal cancer. Ann Surg Oncol 19, 2195–2202. [DOI] [PubMed] [Google Scholar]
- Rakhra K, Bachireddy P, Zabuawala T, Zeiser R, Xu L, Kopelman A, Fan AC, Yang Q, Braunstein L, Crosby E et al (2010) CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell 18, 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AL, Millward M, Pearce R, Lee M, Frank MH, Ireland A, Monshizadeh L, Rai T, Heenan P, Medic S et al (2013) Markers of circulating tumour cells in the peripheral blood of patients with melanoma correlate with disease recurrence and progression. Br J Dermatol 168, 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retsky M, Demicheli R, Hrushesky WJM, Forget P, De Kock M, Gukas I, Rogers RA, Baum M, Sukhatme V and Vaidya JS (2013) Reduction of breast cancer relapses with perioperative non‐steroidal anti‐inflammatory drugs: new findings and a review. Curr Med Chem 20, 4163–4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH et al (2012) EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim AD, Thege FI, Santana SM, Lannin TB, Saha TN, Tsai S, Maggs LR, Kochman ML, Ginsberg GG, Lieb JG et al (2014) Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology 146, 647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR and Greco V (2013) Spatial organization within a niche as a determinant of stem‐cell fate. Nature 502, 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotolo N, Dominioni L, De Monte L, Conti V, La Rosa S and Imperatori A (2013) Metastasis at a tracheostomy site as the presenting sign of late recurrent breast cancer. Head Neck 35, E359–E362. [DOI] [PubMed] [Google Scholar]
- Sandberg R (2013) Entering the era of single‐cell transcriptomics in biology and medicine. Nat Methods 11, 22–24. [DOI] [PubMed] [Google Scholar]
- Sänger N, Effenberger KE, Riethdorf S, Van Haasteren V, Gauwerky J, Wiegratz I, Strebhardt K, Kaufmann M and Pantel K (2011) Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ . Int J Cancer 129, 2522–2526. [DOI] [PubMed] [Google Scholar]
- Sarioglu AF, Aceto N, Kojic N, Donaldson MC, Zeinali M, Hamza B, Engstrom A, Zhu H, Sundaresan TK, Miyamoto DT et al (2015) A microfluidic device for label‐free, physical capture of circulating tumor cell clusters. Nat Methods 12, 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucedo‐Zeni N, Mewes S, Niestroj R, Gasiorowski L, Murawa D, Nowaczyk P, Tomasi T, Weber E, Dworacki G, Morgenthaler NG et al (2012) A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int J Oncol 41, 1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden DT (2014) Nice neighborhood: emerging concepts of the stem cell niche. Cell 157, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardt JA, Meyer M, Hartmann CH, Schubert F, Schmidt‐Kittler O, Fuhrmann C, Polzer B, Petronio M, Eils R and Klein CA (2005) Genomic analysis of single cytokeratin‐positive cells from bone marrow reveals early mutational events in breast cancer. Cancer Cell 8, 227–239. [DOI] [PubMed] [Google Scholar]
- Schmidt‐Kittler O, Ragg T, Daskalakis A, Granzow M, Ahr A, Blankenstein TJF, Kaufmann M, Diebold J, Arnholdt H, Muller P et al (2003) From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci USA 100, 7737–7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Berry JE, Eber MR, Jung Y, Yumoto K, Cackowski FC, Yoon HJ, Parsana P, Mehra R, Wang J et al (2016) The marrow niche controls the cancer stem cell phenotype of disseminated prostate cancer. Oncotarget 7, 41217–41232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM et al (2011) Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest 121, 1298–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Pedersen EA, Patel LR, Ziegler AM, Havens AM, Jung Y, Wang J, Zalucha S, Loberg RD, Pienta KJ et al (2010) GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia 12, 116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddappa CM, Watson MA, Pillai SG, Trinkaus K, Fleming T and Aft R (2013) Detection of disseminated tumor cells in the bone marrow of breast cancer patients using multiplex gene expression measurements identifies new therapeutic targets in patients at high risk for the development of metastatic disease. Breast Cancer Res Treat 137, 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipkins DA, Wei X, Wu JW, Runnels JM, Côté D, Means TK, Luster AD, Scadden DT and Lin CP (2005) In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 435, 969–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov DA, Zweitzig DR, Foulk BW, Miller MC, Doyle GV, Pienta KJ, Meropol NJ, Weiner LM, Cohen SJ, Moreno JG et al (2005) Global gene expression profiling of circulating tumor cells. Cancer Res 65, 4993–4997. [DOI] [PubMed] [Google Scholar]
- Sosa MS, Bragado P and Aguirre‐Ghiso JA (2014) Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 14, 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoecklein NH, Hosch SB, Bezler M, Stern F, Hartmann CH, Vay C, Siegmund A, Scheunemann P, Schurr P, Knoefel WT et al (2008) Direct genetic analysis of single disseminated cancer cells for prediction of outcome and therapy selection in esophageal cancer. Cancer Cell 13, 441–453. [DOI] [PubMed] [Google Scholar]
- Stott SL, Hsu C‐H, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK et al (2010) Isolation of circulating tumor cells using a microvortex‐generating herringbone‐chip. Proc Natl Acad Sci USA 107, 18392–18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, True L and Nelson PS (2012) Treatment‐induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med 18, 1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Mose ES, Montel V and Tarin D (2006) Dormant cancer cells retrieved from metastasis‐free organs regain tumorigenic and metastatic potency. Am J Pathol 169, 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabariès S, Ouellet V, Hsu BE, Annis MG, Rose AAN, Meunier L, Carmona E, Tam CE, Mes‐Masson A‐M and Siegel PM (2015) Granulocytic immune infiltrates are essential for the efficient formation of breast cancer liver metastases. Breast Cancer Res 17, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarin D, Thompson EW and Newgreen DF (2005) The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res 65, 5996–6000; discussion 6000–6001. [DOI] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo‐Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A et al (2013) Dedifferentiation of committed epithelial cells into stem cells in vivo . Nature 503, 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalgott M, Rack B, Maurer T, Souvatzoglou M, Eiber M, Kreß V, Heck MM, Andergassen U, Nawroth R, Gschwend JE et al (2013) Detection of circulating tumor cells in different stages of prostate cancer. J Cancer Res Clin Oncol 139, 755–763. [DOI] [PubMed] [Google Scholar]
- Tien YW, Kuo H‐C, Ho B‐I, Chang M‐C, Chang Y‐T, Cheng M‐F, Chen H‐L, Liang T‐Y, Wang C‐F, Huang C‐Y et al (2016) A high circulating tumor cell count in portal vein predicts liver metastasis from periampullary or pancreatic cancer: a high portal venous CTC count predicts liver metastases. Medicine (Baltimore) 95, e3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjensvoll K, Oltedal S, Heikkilä R, Kvaløy JT, Gilje B, Reuben JM, Smaaland R and Nordgård O (2012) Persistent tumor cells in bone marrow of non‐metastatic breast cancer patients after primary surgery are associated with inferior outcome. BMC Cancer 12, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai W‐S, Chen J‐S, Shao H‐J, Wu J‐C, Lai J‐M, Lu S‐H, Hung T‐F, Chiu Y‐C, You J‐F, Hsieh P‐S et al (2016) Circulating tumor cell count correlates with colorectal neoplasm progression and is a prognostic marker for distant metastasis in non‐metastatic patients. Sci Rep 6, 24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JH, Donaher JL, Murphy DA, Chau S and Yang J (2012) Spatiotemporal regulation of epithelial‐mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 22, 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhr JW and Pantel K (2011) Controversies in clinical cancer dormancy. Proc Natl Acad Sci USA 108, 12396–12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanharanta S and Massagué J (2013) Origins of metastatic traits. Cancer Cell 24, 410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Pantel K and Kang Y (2013) Tumor metastasis: moving new biological insights into the clinic. Nat Med 19, 1450–1464. [DOI] [PubMed] [Google Scholar]
- Wang Y, Waters J, Leung ML, Unruh A, Roh W, Shi X, Chen K, Scheet P, Vattathil S, Liang H et al (2014) Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature 512, 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H‐Y, Wei J, Zou Z‐Y, Qian X‐P and Liu B‐R (2015) Circulating tumour cells predict survival in gastric cancer patients: a meta‐analysis. Contemp Oncol (Pozn) 19, 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis RA (1934) The Spread of Tumours in the Human Body. J. & A. Churchill, London, p. 321. [Google Scholar]
- van de Wouw AJ, Janssen‐Heijnen MLG, Coebergh JWW and Hillen HFP (2002) Epidemiology of unknown primary tumours; incidence and population‐based survival of 1285 patients in Southeast Netherlands, 1984–1992. Eur J Cancer 38, 409–413. [DOI] [PubMed] [Google Scholar]
- Xi L, Nicastri DG, El‐Hefnawy T, Hughes SJ, Luketich JD and Godfrey TE (2007) Optimal markers for real‐time quantitative reverse transcription PCR detection of circulating tumor cells from melanoma, breast, colon, esophageal, head and neck, and lung cancers. Clin Chem 53, 1206–1215. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Woo W‐M, Nagao K, Li W, Terunuma A, Mukouyama Y, Oro AE, Vogel JC and Brownell I (2013) Perivascular hair follicle stem cells associate with a venule annulus. J Invest Dermatol 133, 2324–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and Weinberg RA (2004) Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939. [DOI] [PubMed] [Google Scholar]
- Yang J and Weinberg RA (2008) Epithelial‐mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 14, 818–829. [DOI] [PubMed] [Google Scholar]
- Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM et al (2013) Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong W‐G, Ross J, Haug J, Johnson T, Feng JQ et al (2003) Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ridgway LD, Wetzel MD, Ngo J, Yin W, Kumar D, Goodman JC, Groves MD and Marchetti D (2013) The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci Transl Med 5, 180ra48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Riethdorf S, Wu G, Wang T, Yang K, Peng G, Liu J and Pantel K (2012) Meta‐analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res 18, 5701–5710. [DOI] [PubMed] [Google Scholar]
- Zhu TS, Costello MA, Talsma CE, Flack CG, Crowley JG, Hamm LL, He X, Hervey‐Jumper SL, Heth JA, Muraszko KM et al (2011) Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self‐renewal of cancer stem‐like cells. Cancer Res 71, 6061–6072. [DOI] [PMC free article] [PubMed] [Google Scholar]


