Abstract
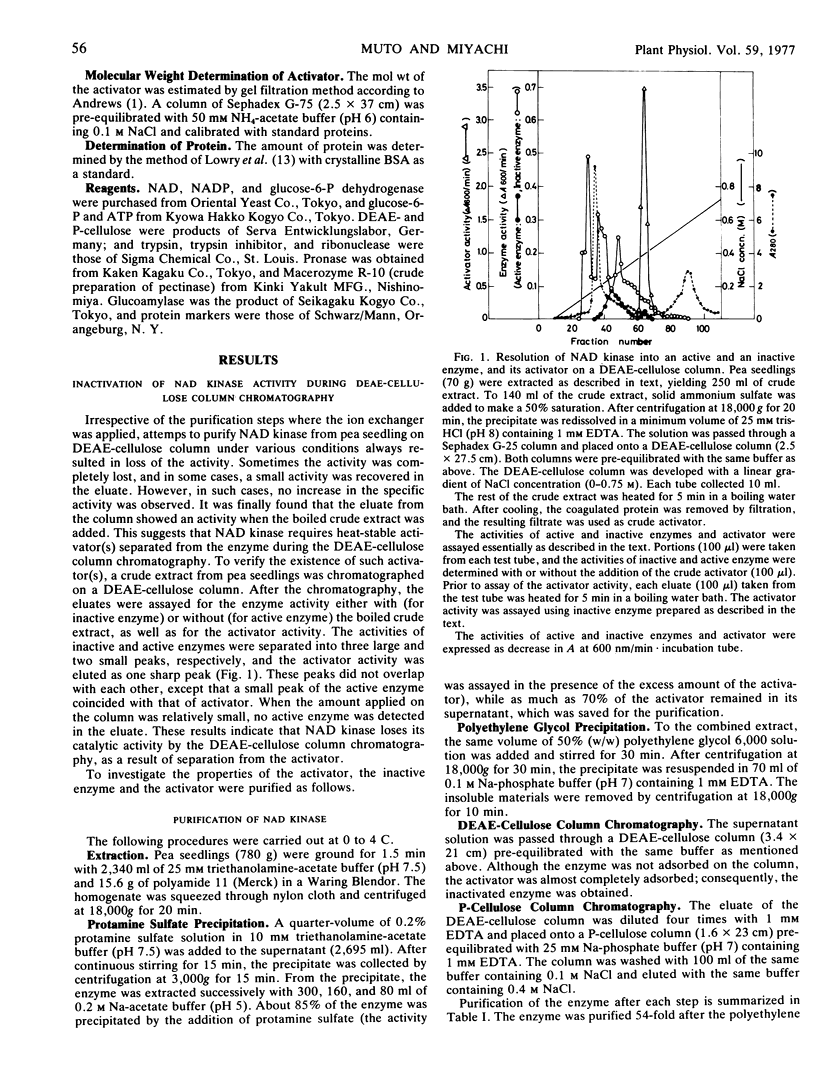
Purification of pea (Pisum sativum) seedling NAD kinase by DEAE-cellulose column chromatography resulted in loss of activity, due to dissociation of an activator from the enzyme. The purified enzyme preparation, which was almost completely inactive, regained the activity when the activator was added back.
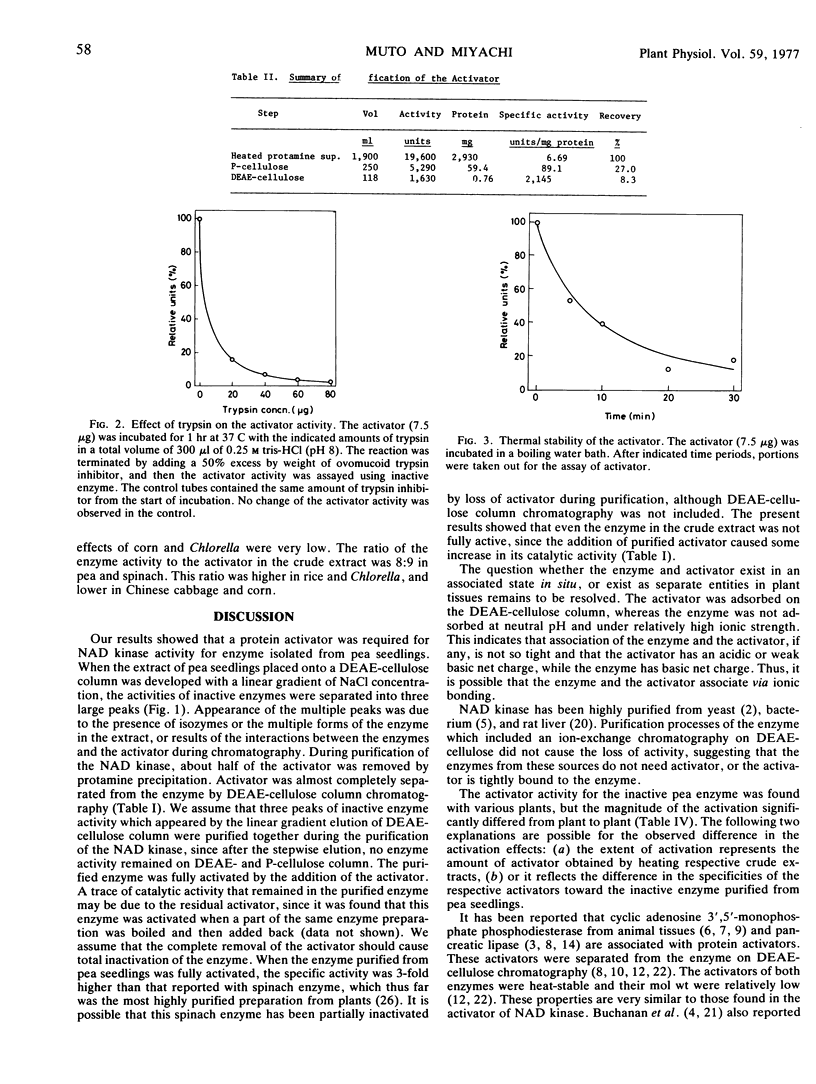
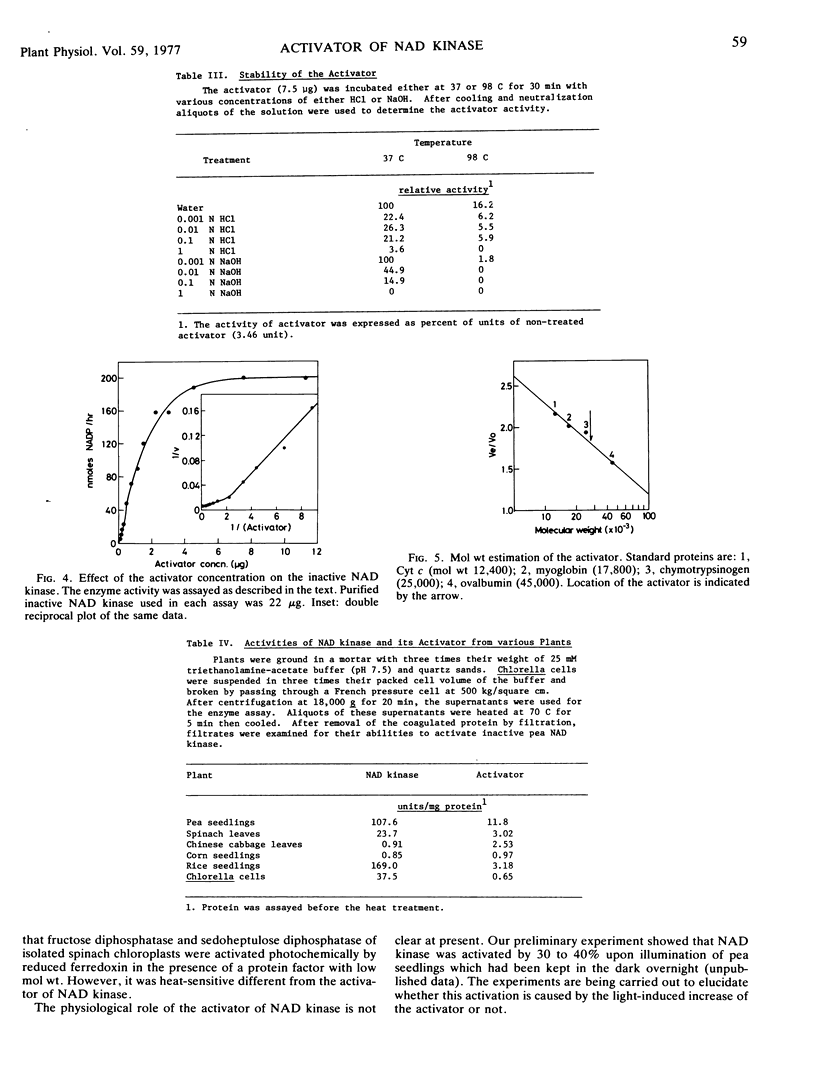
The activator was purified 320-fold by ion exchange chromatographies. The activator was susceptible to proteolytic enzymes, but not to ribonuclease, glucoamylase or pectinase, indicating that it is of a protein nature. This protein was relatively stable in boiling water, but susceptible to acid or alkali, especially under high temperatures. Restoration of catalytic activity of inactive enzyme was proportional to amounts of the activator added. Gel filtration indicated that molecular weight of the activator was 28,000.
The activator was found in extracts from various plants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps D. K. The NAD kinases of Saccharomyces cerevisiae. Eur J Biochem. 1970 Apr;13(2):223–230. doi: 10.1111/j.1432-1033.1970.tb00921.x. [DOI] [PubMed] [Google Scholar]
- BASKYS B., KLEIN E., LEVER W. F. LIPASES OF BLOOD AND TISSUES. III. PURIFICATION AND PROPERTIES OF PANCREATIC LIPASE. Arch Biochem Biophys. 1963 Aug;102:201–209. doi: 10.1016/0003-9861(63)90171-1. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Demonstration of an activator. Biochem Biophys Res Commun. 1970 Feb 6;38(3):533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Evidence for and properties of a protein activator. J Biol Chem. 1971 May 10;246(9):2859–2869. [PubMed] [Google Scholar]
- Chung A. E. Nicotinamide adenine dinucleotide kinase from Azotobacter vinelandii. I. Purification and properties of the enzyme. J Biol Chem. 1967 Mar 25;242(6):1182–1186. [PubMed] [Google Scholar]
- Erlanson C., Borgström B. Purification and further characterization of co-lipase from porcine pancreas. Biochim Biophys Acta. 1972 Jul 21;271(2):400–412. doi: 10.1016/0005-2795(72)90215-2. [DOI] [PubMed] [Google Scholar]
- Goren E. N., Rosen O. M. The effect of nucleotides and a nondialyzable factor on the hydrolysis of cyclic AMP by a cyclic nucleotide phosphodiesterase from beef heart. Arch Biochem Biophys. 1971 Feb;142(2):720–723. doi: 10.1016/0003-9861(71)90540-6. [DOI] [PubMed] [Google Scholar]
- Kimura H., Mukai M., Kitamura T. Purification and properties of a protein activator of human pancreatic lipase. J Biochem. 1974 Dec;76(6):1287–1292. doi: 10.1093/oxfordjournals.jbchem.a130682. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin Y. M., Liu Y. P., Cheung W. Y. Cyclic 3':5'-nucleotide phosphodiesterase. Purification, characterization, and active form of the protein activator from bovine brain. J Biol Chem. 1974 Aug 10;249(15):4943–4954. [PubMed] [Google Scholar]
- Maylié M. F., Charles M., Gache C., Desnuelle P. Isolation and partial identification of a pancreatic colipase. Biochim Biophys Acta. 1971 Jan 19;229(1):286–289. doi: 10.1016/0005-2795(71)90347-3. [DOI] [PubMed] [Google Scholar]
- OH-HAMA T., MIYACHI S. Effects of illumination and oxygen supply upon the levels of pyridine nucleotides in Chlorella cells. Biochim Biophys Acta. 1959 Jul;34:202–210. doi: 10.1016/0006-3002(59)90248-3. [DOI] [PubMed] [Google Scholar]
- Ogren W. L., Krogmann D. W. Studies on pyridine nucleotides in photosynthetic tissue. Concentrations, interconversions, and distribution. J Biol Chem. 1965 Dec;240(12):4603–4608. [PubMed] [Google Scholar]
- Oka H., Field J. B. Inhibition of rat liver nicotinamide adenine dinucleotide kinase by reduced nicotinamide adenine dinucleotide phosphate. J Biol Chem. 1968 Feb 25;243(4):815–819. [PubMed] [Google Scholar]
- Schürmann P., Buchanan B. B. Role of ferredoxin in the activation of sedoheptulose diphosphatase in isolated chloroplasts. Biochim Biophys Acta. 1975 Jan 31;376(1):189–192. doi: 10.1016/0005-2728(75)90217-0. [DOI] [PubMed] [Google Scholar]
- Teo T. S., Wang J. H. Mechanism of activation of a cyclic adenosine 3':5'-monophosphate phosphodiesterase from bovine heart by calcium ions. Identification of the protein activator as a Ca2+ binding protein. J Biol Chem. 1973 Sep 10;248(17):5950–5955. [PubMed] [Google Scholar]
- Tezuka T., Yamamoto Y. Photoregulation of Nicotinamide Adenine Dinucleotide Kinase Activity in Cell-free Extracts. Plant Physiol. 1972 Oct;50(4):458–462. doi: 10.1104/pp.50.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG T. P., KAPLAN N. O. Kinases for the synthesis of coenzyme A and triphosphopyridine nucleotide. J Biol Chem. 1954 Jan;206(1):311–325. [PubMed] [Google Scholar]
- Yamamoto Y. NAD Kinase in Higher Plants. Plant Physiol. 1966 Mar;41(3):523–528. doi: 10.1104/pp.41.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]


