ABSTRACT
Background
Sjögren’s syndrome is a chronic systemic disease, characterized by lymphocytic infiltration and destruction mainly of the salivary and lacrimal glands, resulting in xerostomia and xeropthalmia. Sjögren’s syndrome patients have a 44-fold excess risk for the development of non-Hodgkin’s lymphoma particularly mucosa-associated lymphoid tissue (MALT) lymphoma, prevalently affecting the major salivary glands. In this report, a rare case of MALT lymphoma of minor salivary glands in a patient with Sjögren’s syndrome is described. A review of the published cases of MALT lymphoma located in the minor salivary glands of patients with Sjögren’s syndrome is provided.
Methods
In a 64-year-old female patient previously diagnosed with Sjögren’s syndrome, an asymptomatic soft tissue mass at the palate was noticed, exhibiting rapid enlargement within one month. With a main differential diagnosis of salivary gland neoplasm or lymphoproliferative lesion, a partial biopsy was performed accompanied by proper immunohistochemical analysis.
Results
A final diagnosis of MALT lymphoma was rendered and the patient was referred for further multidisciplinary evaluation. Gastric endoscopy and biopsy revealed a Helicobacter pylori-negative gastric MALT lymphoma, while spleen involvement and bone marrow infiltration were also identified. Patient was classified as having stage IV disseminated disease and a standard chemotherapy protocol was administered; the treatment was well tolerated and resulted in complete remission.
Conclusions
This case emphasizes the need for close monitoring of patients with Sjögren’s syndrome by oral medicine specialists, which, besides ensuring proper management of xerostomia and its sequelae, may also lead to early recognition of lymphoma development.
Keywords: MALT lymphoma, minor salivary glands, Sjögren’s syndrome
INTRODUCTION
Sjögren’s syndrome (SS) is a chronic systemic autoimmune disorder, characterized by lymphocytic infiltration of the exocrine glands, mainly the salivary and lacrimal glands, as well as by systemic symptoms and extraglandular manifestations. It may present as a solitary entity (primary SS) or it may occur in conjunction with another autoimmune condition, such as rheumatoid arthritis, systemic lupus erythematosus or scleroderma, in which case it is termed secondary SS [1]. SS has a strong female predisposition (female to male ratio of 9:1), usually beginning during the 4th or 5th decade of life. Patients with SS suffer from classic sicca symptoms of dry eyes and dry mouth, associated with objective evidence of keratoconjunctivitis sicca and xerostomia, respectively. The most significant complication of SS is the development of lymphoproliferative malignancy, which occurs in about 5% of SS patients [2].
Malignant lymphoma, particularly mucosa-associated lymphoid tissue (MALT) lymphoma, is a relatively frequent complication of SS with an incidence ranging between 5 - 10% and a median time from SS to lymphoma diagnosis of 7.5 years [3-6]. Potential explanations for this incidence variation include differences in the criteria used for the diagnosis of SS and the variable duration of follow-up. The risk of lymphoma development in SS patients has been assessed to be 44 times higher compared to the normal population [4]. According to a more recent study, this high risk is probably valid only for selected patient populations with severe disease, while a lower danger appraisal of 16-fold is probably more representative of a low-risk SS subpopulation [7]. According to a meta-analysis of 20 studies, SS exhibits a higher standardized incidence rate (SIR 18.8%) of non-Hodgkin’s lymphoma development compared to other immune systemic diseases, e.g. systemic lupus erythematosus (SIR 7.5%) and rheumatoid arthritis (SIR 3.3%) [8].
The purpose of the present report is to describe a rare case of MALT lymphoma of the palatal minor salivary glands, which was detected during routine oral examination of a patient previously diagnosed with secondary Sjögren’s syndrome. Additionally, a summary of the published cases of MALT lymphoma affecting the minor salivary glands of Sjögren’s syndrome patients as well as the risk factors for lymphoma development in Sjögren’s syndrome patients, are also discussed.
CASE DESCRIPTION AND RESULTS
A 64-year-old female patient was referred by her prosthodontist for evaluation of dry mouth and oral burning sensation to the Department of Oral Medicine and Pathology, School of Dentistry, National and Kapodistrian University of Athens, Greece.
The patient reported a 15-year history of secondary SS associated with rheumatoid arthritis, managed by her rheumatologist with hydroxychloroquine and pilocarpine intermittently for several years. Medical history was also significant for haemorrhagic spastic colitis and iron deficiency anaemia, which were managed with appropriate medication.
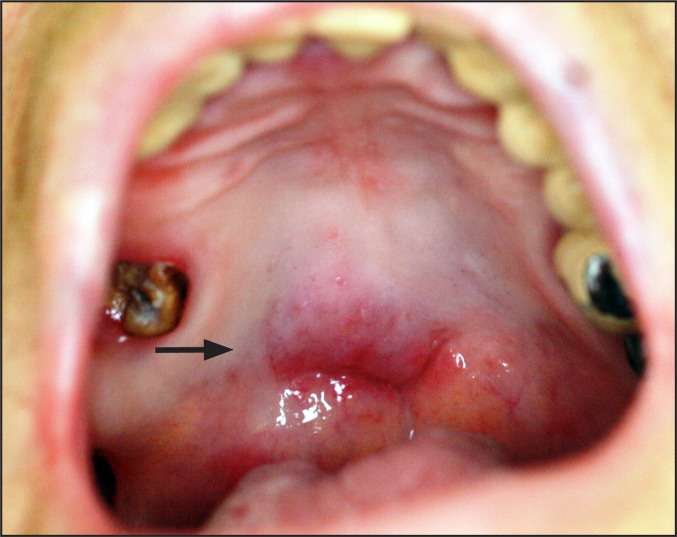
Clinical examination revealed signs of xerostomia (including lack of saliva secretion during palpation of major salivary glands), erythematous candidiasis (confirmed by a positive cytologic smear) and angular cheilitis. Appropriate systemic (per os fluconazole 100 mg once daily for 15 days) and topical antifungal (miconazole 2% oral gel 4 times daily diffusely on the oral mucosa, and miconazole 2%/hydrocortisone 1% cream 4 times daily on the comissures for 15 days) medication, along with antixerostomic products (topical gel and mouthwash), were prescribed and the patient was placed on regular follow-up. During follow-up, an asymptomatic submucosal mass of soft tissue consistency was noticed at the junction of the hard and soft palate crossing the midline (Figure 1). The lesion, which was not present at the initial visit, exhibited rapid enlargement (reaching approximately 1 cm in diameter) within one month of observation. With a main differential diagnosis of a salivary gland neoplasm or a lymphoproliferative lesion, a partial biopsy was performed.
Figure 1.
Asymptomatic mass noticed at the junction of hard and soft palate (arrow).
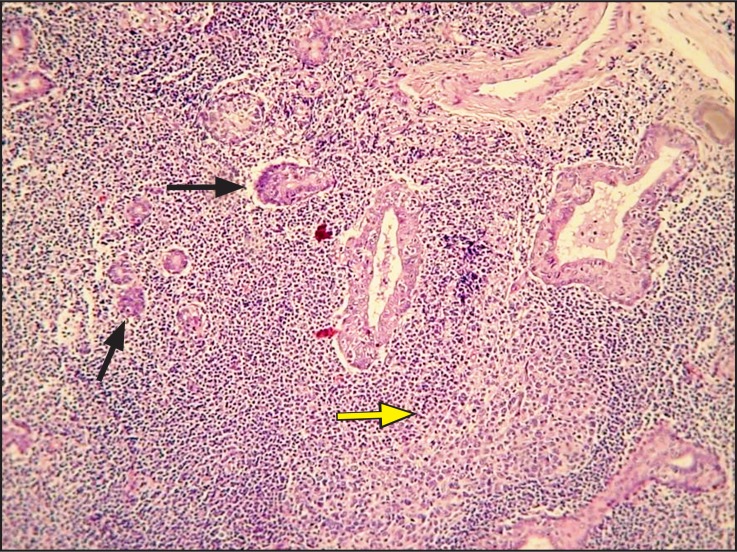
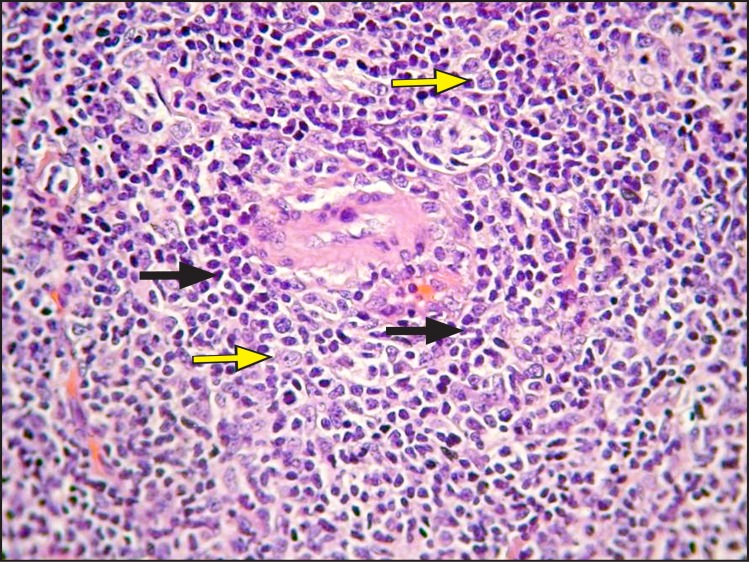
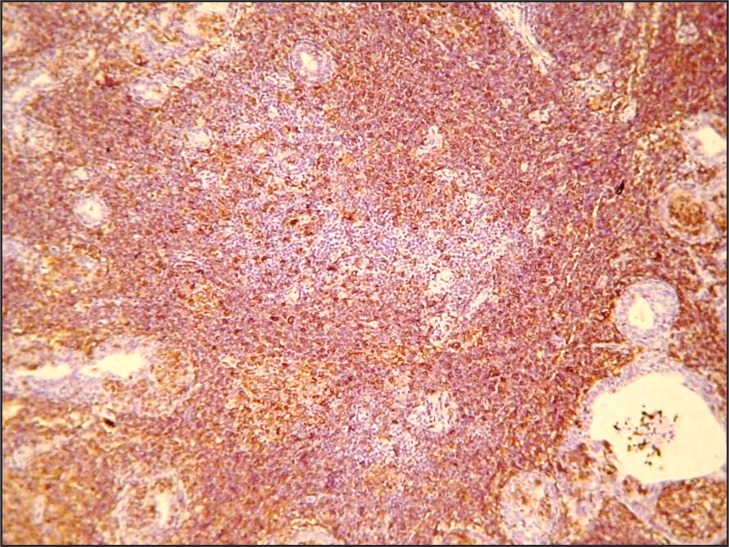
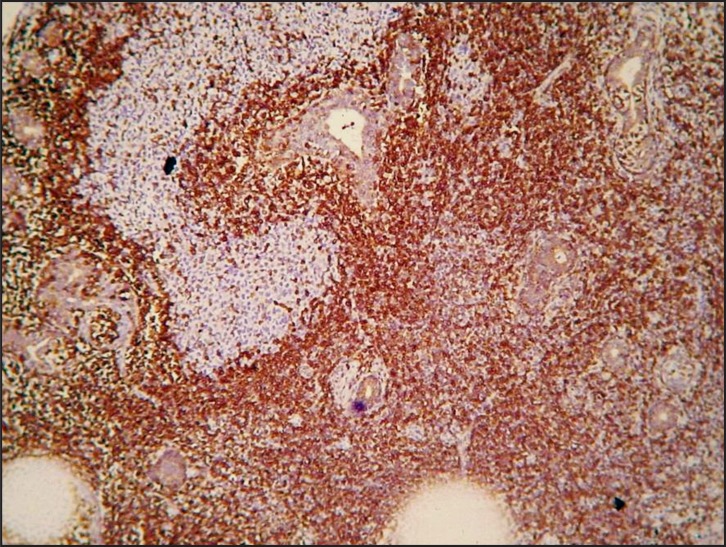
Histopathologically, a dense and diffuse infiltration of minor salivary glands by small sized centrocyte-like neoplastic lymphoid cells with formation of abundant lymphoepithelial lesions was observed. A few scattered blast cells were also noted (Figure 2 and 3). Immunohistochemistry revealed positivity of the neoplastic cells for CD20, CD43 and BCL-2; individual cells were also positive for DBA44 and MUM1. In contrast, stains for BCL-6, CD10, cyclin D1, CD5 and CD3 were negative, while Ki-67 positivity was observed in about 8 - 10% of the neoplastic cells (Figure 4 and 5).
Figure 2.
Dense, diffuse infiltration of a minor salivary gland by a monotonous population of lymphoid cells, destroying the normal architecture.
An organized lymphoid follicle (arrows) and lymhoepithelial islands (yellow arrow) are also observed (haematoxylin and eosin stain, original magnification x100).
Figure 3.
Infiltration by numerous centrocyte-like cells (yellow arrows) is observed; scattered blast cells are also noted (arrows) (haematoxylin and eosin stain, original magnification x400).
Figure 4.
Neoplastic cells exhibiting positivity for anti-CD20 (original magnification x100).
Figure 5.
Strong positivity for anti-BCL2 (original magnification x100).
A final diagnosis of an extranodal NHL, MALT-type was rendered and the patient was referred for further multidisciplinary evaluation, disease staging and management to a specialized Hematology-Oncology Center. Gastric endoscopy and biopsy revealed a Helicobacter pylori-negative gastric MALT lymphoma, while further imaging showed spleen involvement. Bone marrow infiltration by neoplastic lymphocytes (at approximately 10%) was identified. Neutropenia, lymphocytopenia, anaemia, low IgM and IgA, as well as elevated β2-microglobulin, beta 1 globulin and LDH levels were also observed.
The patient was classified as having a stage IV disseminated disease and a standard treatment chemotherapy protocol, including rituximab and fludarabine, was recommended. Although the patient initially refused treatment (for approximately one year), no significant disease progression was noticed during this time after a complete re-evaluation of lymphoma with computed tomography scan and bone marrow biopsy. Eventually, the patient complied with the suggested treatment; no major complications developed and sustained complete remission of the lymphoma, including resolution of the palatal mass, was achieved; no signs of recurrence were noticed during the subsequent 2 years follow-up period.
DISCUSSION
Malignant lymphoma development is a major and potentially lethal complication in patients with SS. The standardized mortality ratio in SS patients with lymphoma is 3.25 compared to 1.08 in SS patients without lymphoma [9]. Many clinical and biological parameters have been related to lymphoma development in SS (Table 1) [7,10-19]. According to Anderson et al. [11], decrease in serum immunoglobulin (Ig) levels and the disappearance of a previously positive rheumatoid factor (RF) may herald progression to lymphoma. Kassan et al. [4] showed that SS patients with lymphadenopathy, splenomegaly and persistent parotid gland enlargement share an increased probability of lymphoma development and can be classified as a high risk group.
Table 1.
Predictors of lymphoma development in Sjögren’s syndrome
| Clinical | Serological |
|---|---|
| Persistent parotid gland enlargement | Mixed monoclonal cryoglobulinemia |
| Splenomegaly | Low serum C4 levels |
| Lymphadenopathy | Serum or urine monoclonal bands |
| Palpable purpura | CD4 + T lymphocytopeniaa |
| Leg ulcers | Neutropeniaa |
| Peripheral neuropathy | High serum β2 microglobulina |
| Previous low-dose irradiation or chemotherapy | Low serum IgM levelsa |
| Disappearance of previously positive rheumatoid factor | |
aΙndicates detected findings in the present case.
A variety of histologic subtypes of lymphoma can occur within the salivary glands of SS patients, MALT lymphoma accounting for 46 - 56% of all cases [10,20]. Firstly described by Isaacson and Wright in 1983 [21], MALT lymphoma has been reported in various organs, but the gastrointestinal tract is by far the most common site of occurrence. In SS patients a predilection for extranodal sites is observed with a strong predisposition for major salivary glands, especially the parotid and submandibular glands [20,22]. In contrast, MALT lymphomas affecting minor salivary glands in SS patients appear to be rare, with only 11 well documented cases hitherto reported in the English language literature (Table 2) [23-28].
Table 2.
Reported cases of MALT lymphoma of the minor salivary glands in Sjögren’s syndrome patients
| Author | Year | No. of cases | Age | Sex | Oral location | Other locations | Risk factors | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Speight et al. [23] | 1994 | 4 | NR | NR | Lipa | Stomach | NR | NR | NR |
| NR | NR | Lipa | Palate | NR | NR | NR | |||
| NR | NR | Lipa | Cervical lymph nodes | NR | NR | NR | |||
| NR | NR | Lipa | Cervical lymph nodes | NR | NR | NR | |||
| Van Mello et al. [24] | 2005 | 3 | 53 | F | Lipb | Possibly chest area | Non-blanching purpuric lesions on the lower limbs, C4 low | NR | NR |
| 51 | F | Lipb | NR |
Intermittent swollen parotids, raised levels of total protein, IgG, IgA, IgM, while C4 levels and white blood cell count were decreased |
NR | NR | |||
| 55 | Lipa | NR | Intermittent swelling of the parotid gland |
Leukeran (4 mg daily) and prednisone 20 mg/day |
NR | ||||
| Pijpe et al. [25] | 2005 | 1 | 42 | F | Hard palateb |
Right Parotid
10 years earlier neck lymph nodes |
Increased IgG | Weekly, course of 4 infusions of rituximab | NS, no recurrence |
| Sakuma et al. [26] | 2006 | 1 | 70 | F | Hard palatea | - | - | Self regression | 38 months, no recurrence |
| Keszler et al. [27] | 2012 | 1 | 60 | F | Lipa | - | - | Watch and wait |
Evaluation performed every 6 months during the first year and then, once a year, no recurrence |
| Shwetha et al. [28] | 2014 | 1 | 62 | F |
Upper and lower lipb |
- | - | Watch and wait | NS, no recurrence |
| Present case | 2017 | 1 | 64 | F |
Hard palatea |
Stomach; spleen; bone marrow | CD4 + T lymphocytopenia, neutropenia, high serum β2 microglobulin, low serum IgM levels |
Refused treatment for 1 year (Rituximab + Fludarabine) |
2 years, no recurrence |
aMALT lymphoma diagnosed after Sjögren’s syndrome initial diagnosis.
bMALT lymphoma diagnosed simultaneously with Sjögren’s syndrome.
NR = not reported; F = female; NS = not specified.
MALT lymphoma is generally an indolent neoplasm and lesions tend to remain localized to the original site for prolonged periods of time, leading sometimes to a mistaken diagnosis of an inflammatory process. However, in extragastrointestinal areas, the clinical advancement and biologic behaviour is heterogeneous [29]. In the case presented herein, the indolent nature of the neoplasm was supported by the lack of significant disease progression despite the delay in treatment for almost one year.
Selection of treatment regimen for MALT lymphoma depends on the presence of either localized or disseminated disease and the International Prognostic Index (IPI) score based on five parameters (age, stage, involvement of many extranodal sites, performance status and lactate dehydrogenase - LDH). In asymptomatic, localized MALT lymphomas without bone marrow or lymph node involvement and an IPI score between 0 and 1, the patient may receive radiotherapy or remain under close follow-up with no therapeutic intervention. Typically, over an average time of 4 years, these patients may either show no disease progression or exhibit transformation into a high-grade malignant lymphoma [29]. In disseminated MALT lymphomas with a high IPI score, bone marrow and lymph node involvement, cyclophosphamide, hydroxydaunorubicin, oncovin and prednisone (CHOP) chemotherapy regimen is administered with or without rituximab (anti-CD20 monoclonal antibody) [25]. In general, prognosis of MALT lymphoma is satisfactory, with a 5-year survival rate of 90%. The IPI score is of crucial prognostic significance, with high values indicating patients with a poorer prognosis [30,31].
CONCLUSIONS
Close monitoring of patients with Sjögren’s syndrome by oral medicine specialists, ensures not only proper management of xerostomia and its sequelae but may also lead to early recognition of lymphoma development. Especially, high risk Sjögren’s syndrome patients should be scrutinized and repeated biopsies may be needed for prompt diagnosis of lymphoma development.
Acknowledgments
ACKNOWLEDGMENTS AND DISCLOSURE STATEMENTS
The authors report no conflicts of interest related to this study.
REFERENCES
- 1.Patel R, Shahane A. The epidemiology of Sjögren’s syndrome. Clin Epidemiol. 2014 Jul 30;6:247-55. [DOI] [PMC free article] [PubMed]
- 2.Reksten TR, Jonsson MV. Sjögren’s syndrome: an update on epidemiology and current insights on pathophysiology. Oral Maxillofac Surg Clin North Am. 2014 Feb;26(1):1-12. [DOI] [PubMed]
- 3.Zufferey P, Meyer OC, Grossin M, Kahn MF. Primary Sjögren’s syndrome (SS) and malignant lymphoma. A retrospective cohort study of 55 patients with SS. Scand J Rheumatol. 1995;24(6):342-5. [DOI] [PubMed]
- 4.Kassan SS, Thomas TL, Moutsopoulos HM, Hoover R, Kimberly RP, Budman DR, Costa J, Decker JL, Chused TM. Increased risk of lymphoma in sicca syndrome. Ann Intern Med. 1978 Dec;89(6):888-92. [DOI] [PubMed]
- 5.Pariente D, Anaya JM, Combe B, Jorgensen C, Emberger JM, Rossi JF, Sany J. Non-Hodgkin’s lymphoma associated with primary Sjögren’s syndrome. Eur J Med. 1992 Oct;1(6):337-42. [PubMed]
- 6.Tzioufas AG, Voulgarelis M. Update on Sjögren’s syndrome autoimmune epithelitis: from classification to increased neoplasias. Best Pract Res Clin Rheumatol. 2007 Dec;21(6):989-1010. [DOI] [PubMed]
- 7.Theander E, Henriksson G, Ljungberg O, Mandl T, Manthorpe R, Jacobsson LT. Lymphoma and other malignancies in primary Sjögren’s syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis. 2006 Jun;65(6):796-803. [DOI] [PMC free article] [PubMed]
- 8.Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005 Nov 14;165(20):2337-44. [DOI] [PubMed]
- 9.Voulgarelis M, Ziakas PD, Papageorgiou A, Baimpa E, Tzioufas AG, Moutsopoulos HM. Prognosis and outcome of non-Hodgkin lymphoma in primary Sjögren syndrome. Medicine (Baltimore). 2012 Jan;91(1):1-9. [DOI] [PubMed]
- 10.Voulgarelis M, Dafni UG, Isenberg DA, Moutsopoulos HM. Malignant lymphoma in primary Sjögren’s syndrome: a multicenter, retrospective, clinical study by the European Concerted Action on Sjögren’s Syndrome. Arthritis Rheum. 1999 Aug;42(8):1765-72. [DOI] [PubMed]
- 11.Anderson LG, Talal N. The spectrum of benign to malignant lymphoproliferation in Sjögren’s syndrome. Clin Exp Immunol. 1972 Feb;10(2):199-221. [PMC free article] [PubMed]
- 12.Skopouli FN, Dafni U, Ioannidis JP, Moutsopoulos HM. Clinical evolution, and morbidity and mortality of primary Sjögren’s syndrome. Semin Arthritis Rheum. 2000 Apr;29(5):296-304. [DOI] [PubMed]
- 13.Ramos-Casals M, Brito-Zerón P, Yagüe J, Akasbi M, Bautista R, Ruano M, Claver G, Gil V, Font J. Hypocomplementaemia as an immunological marker of morbidity and mortality in patients with primary Sjogren’s syndrome. Rheumatology (Oxford). 2005 Jan;44(1):89-94. [DOI] [PubMed]
- 14.Ioannidis JP, Vassiliou VA, Moutsopoulos HM. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjögren’s syndrome. Arthritis Rheum. 2002 Mar;46(3):741-7. [DOI] [PubMed]
- 15.Anaya JM, McGuff HS, Banks PM, Talal N. Clinicopathological factors relating malignant lymphoma with Sjögren’s syndrome. Semin Arthritis Rheum. 1996 Apr;25(5):337-46. [DOI] [PubMed]
- 16.Sutcliffe N, Inanc M, Speight P, Isenberg D. Predictors of lymphoma development in primary Sjögren’s syndrome. Semin Arthritis Rheum. 1998 Oct;28(2):80-7. [DOI] [PubMed]
- 17.Walters MT, Stevenson FK, Herbert A, Cawley MI, Smith JL. Urinary monoclonal free light chains in primary Sjögren’s syndrome: an aid to the diagnosis of malignant lymphoma. Ann Rheum Dis. 1986 Mar;45(3):210-9. [DOI] [PMC free article] [PubMed]
- 18.Voulgarelis M, Moutsopoulos HM. Malignant lymphoma in primary Sjogren’s syndrome. Isr Med Assoc J. 2001 Oct; 3(10):761-6. [PubMed]
- 19.Solans-Laqué R, López-Hernandez A, Bosch-Gil JA, Palacios A, Campillo M, Vilardell-Tarres M. Risk, predictors, and clinical characteristics of lymphoma development in primary Sjögren’s syndrome. Semin Arthritis Rheum. 2011 Dec;41(3):415-23. [DOI] [PubMed]
- 20.Royer B, Cazals-Hatem D, Sibilia J, Agbalika F, Cayuela JM, Soussi T, Maloisel F, Clauvel JP, Brouet JC, Mariette X. Lymphomas in patients with Sjogren’s syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood. 1997 Jul 15;90(2):766-75. [PubMed]
- 21.Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983 Oct 15;52(8):1410-6. [DOI] [PubMed]
- 22.Thieblemont C, Bastion Y, Berger F, Rieux C, Salles G, Dumontet C, Felman P, Coiffier B. Mucosa-associated lymphoid tissue gastrointestinal and nongastrointestinal lymphoma behavior: analysis of 108 patients. J Clin Oncol. 1997 Apr;15(4):1624-30. [DOI] [PubMed]
- 23.Speight PM, Jordan R, Colloby P, Nandha H, Pringle JH. Early detection of lymphomas in Sjögren’s syndrome by in situ hybridisation for kappa and lambda light chain mRNA in labial salivary glands. Eur J Cancer B Oral Oncol. 1994 Jul;30B(4):244-7. [DOI] [PubMed]
- 24.Van Mello NM, Pillemer SR, Tak PP, Sankar V. B cell MALT lymphoma diagnosed by labial minor salivary gland biopsy in patients screened for Sjögren’s syndrome. Ann Rheum Dis. 2005 Mar;64(3):471-3. [DOI] [PMC free article] [PubMed]
- 25.Pijpe J, van Imhoff GW, Vissink A, van der Wal JE, Kluin PM, Spijkervet FK, Kallenberg CG, Bootsma H. Changes in salivary gland immunohistology and function after rituximab monotherapy in a patient with Sjogren’s syndrome and associated MALT lymphoma. Ann Rheum Dis. 2005 Jun;64(6):958-60. [DOI] [PMC free article] [PubMed]
- 26.Sakuma H, Okabe M, Yokoi M, Eimoto T, Inagaki H. Spontaneous regression of intraoral mucosa-associated lymphoid tissue lymphoma: molecular study of a case. Pathol Int. 2006 Jun;56(6):331-5. [DOI] [PubMed]
- 27.Keszler A, Adler LI, Gandolfo MS, Masquijo Bisio PA, Smith AC, Vollenweider CF, Heidenreich AM, de Stefano G, Kambo MV, Cox DP, Narbaitz M, Lanfranchi HE. MALT lymphoma in labial salivary gland biopsy from Sjögren syndrome: importance of follow-up in early detection. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013 Mar;115(3): e28-33. [DOI] [PMC free article] [PubMed]
- 28.Shwetha V, Yashoda Devi BK, Mysorekar VV, Kamath NP. Primary Extranodal Lymphomas of Lip - A Rare Manifestation in Sjogren‘s Syndrome. J Clin Diagn Res. 2014 Mar;8(3):272-4. [DOI] [PMC free article] [PubMed]
- 29.Bertoni F, Coiffier B, Salles G, Stathis A, Traverse-Glehen A, Thieblemont C, Zucca E. MALT lymphomas: pathogenesis can drive treatment. Oncology (Williston Park). 2011 Nov 15;25(12):1134-42, 1147. [PubMed]
- 30.Voulgarelis M, Moutsopoulos HM. Mucosa-associated lymphoid tissue lymphoma in Sjögren’s syndrome: risks, management, and prognosis. Rheum Dis Clin North Am. 2008 Nov;34(4):921-33, viii. [DOI] [PubMed]
- 31.Troch M, Wöhrer S, Raderer M. Assessment of the prognostic indices IPI and FLIPI in patients with mucosa-associated lymphoid tissue lymphoma. Anticancer Res. 2010 Feb;30(2):635-9. [PubMed]