Abstract
Community-acquired methicillin-resistant Staphylococcus aureus has been spreading worldwide, including in Japan. However, few cases of toxic shock syndrome caused by Community-acquired methicillin-resistant Staphylococcus aureus have been reported in Japan. We report 2 cases, in middle-aged women, of toxic shock syndrome due to Community-acquired methicillin-resistant Staphylococcus aureus via a vaginal portal of entry. The first patient had used a tampon and the second patient had vaginitis due to a cleft narrowing associated with vulvar lichen sclerosus. Both patients were admitted to our hospital with septic shock and severe acute kidney injury and subsequently recovered with appropriate antibiotic treatment. In our review of the literature, 8 cases of toxic shock syndrome caused by Community-acquired methicillin-resistant Staphylococcus aureus were reported in Japan. In these 8 cases, the main portals of entry were the skin and respiratory tract; however, the portal of entry of Community-acquired methicillin-resistant Staphylococcus aureus from a vaginal lesion has not been reported in Japan previously.
Keywords: Community-acquired methicillin-resistant Staphylococcus aureus, Toxic shock syndrome, Menstruation, Vulvar lichen sclerosus
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) strains are well known to be hospital-associated or healthcare-associated pathogens. However, within the past two decades, the incidence of infections due to community-acquired methicillin-resistant S. aureus (CA-MRSA) strains have been rapidly increasing worldwide [1]. Similar to hospital-acquired MRSA (HA-MRSA), CA-MRSA can cause severe infections such as soft-tissue infection (including necrotizing fasciitis), necrotizing pneumonia, severe sepsis, and disseminated infection [2]. Moreover, S. aureus can produce many exotoxins such as toxic shock syndrome toxin-1 (TSST-1) and staphylococcal enterotoxin B (SEB). Exotoxins of S. aureus are associated with a severe illness that includes shock and multiple organ failure and is called toxic shock syndrome (TSS) [3]. Specifically, in women of childbearing age, an insanitary ectocervical environment (e.g., long-term tampon-use or post-partum period) is associated with bacterial growth, including the growth of S. aureus, and the risk of TSS [4].
We herein report 2 cases of TSS due to CA-MRSA in Japanese women. A literature review of cases from Japan revealed that transvaginal invasion is a very rare portal of entry of CA-MRSA. The population of CA-MRSA in Japan is relatively low compared with that in other countries. Despite this fact, we assert that the initiating treatment for TSS with vancomycin and clindamycin, even in Japan.
Informed consent was obtained from the patients for the publication of their respective case reports.
Case report
Case 1
A 46-year-old Japanese woman was admitted to our hospital after a 1-day history of fever, shaking chills, and diarrhoea. She previously was seen at another clinic on the same day of admission (day 1), and fosfomycin was prescribed. However, her symptoms worsened. She did not have any relevant past history and had not been prescribed any medications. She had never been abroad, and she had not eaten any raw foods in the previous month.
Her vital signs on the day of admission were as follows: blood pressure, 67/47 mmHg; heart rate, 118 beats per minute; body temperature, 39.4 °C; respiratory rate, 30 breaths per minute; and peripheral capillary oxygen saturation level on room air, 98%. With respect to her consciousness, she was alert. Physical examination showed capillary refilling time of 12 s, and generalized rash on her chest and abdomen. Results of cardiovascular, respiratory, and abdominal examinations were normal. Laboratory data revealed white blood cell (WBC) count of 12,300/μl, C-reactive protein (CRP) level of 15.4 mg/dL, blood urea nitrogen (BUN) of 26.4 mg/dL, serum creatinine (s-Cre) level of 3.3 mg/dL, serum total protein level of 7.1 g/dL, serum albumin level of 3.4 g/dL, and procalcitonin level of 69.8 ng/mL. Results of liver function tests were normal except for a lactate dehydrogenase (LDH) level of 328 IU/L. Urine dipstick examination revealed 2+ protein and 2+ occult blood. A microscopic examination detected 5–10 red blood cells (RBCs) per high-power field and 5–10 WBCs per high-power field.
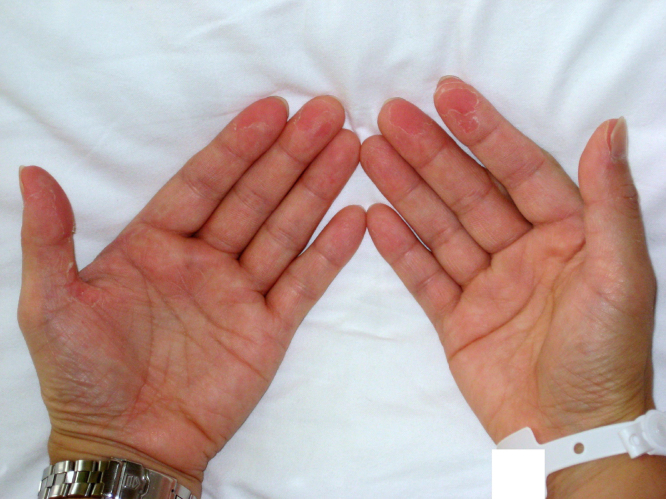
At first, we diagnosed severe bacterial colitis and hypovolemic shock and started large volumes of hydration, dopamine infusion, and intravenous ciprofloxacin (300 mg, 2 times daily). However, her vital signs did not recover. On day 2, we learned that she had begun menstruating 4 days prior to admission, and that she kept a tampon inserted 3 days until the day of admission. Additional testing of her vaginal discharge showed the following; polymorphonuclear leukocytes 2+, and Nugent score, 1. We suspected TSS or septic shock of unknown origin and changed the antibiotics to vancomycin (1 g, 2 times daily, intravenously), clindamycin (600 mg, 4 times daily, intravenously), and meropenem (500 mg, 4 times daily, intravenously). On day 4, community-acquired S. aureus was detected from the tampon and vaginal discharge and her vital signs gradually recovered enough to stop the dopamine infusion, and her diarrhoea stopped. Two sets of blood culture and urine culture were negative. We diagnosed staphylococcal TSS owing to tampon use, discontinued meropenem on day 11, and continued antibiotic treatment with the combination of vancomycin and clindamycin for 14 days. On day 14, the patient was found to have peeling skin at the end of her fingers (Fig. 1). The patient was discharged on day 18, without any damage to her organs or deterioration in activities of daily living.
Case 2
A 40-year-old Japanese woman was admitted to our hospital after a 5-day history of yellowish vaginal discharge, and 3-day history of fever, shaking chills, and appetite loss. She did not have any relevant past medical history and had not been prescribed any medications, including antibiotics. She had never been abroad and had not had any raw foods in the previous month. Her last menstrual period had finished 2 weeks before the day of hospital admission.
Fig. 1.
Case 1: Peeling of the fingertips.
Her vital signs on the day of admission were as follows: blood pressure, 86/49 mmHg; heart rate, 121 beats per minute; body temperature, 39.8 °C; respiratory rate, 36 breaths per minute; and peripheral capillary oxygen saturation level on room air, 96%. She was alert and physical examination showed generalized rash on her hand and neck. Results of cardiovascular, respiratory, and abdominal examinations were normal, except tenderness on the right costal-vertebral angle. Laboratory data revealed WBC of 19,200/μl, CRP level of 14.5 mg/dL, BUN level of 28.4 mg/dL, s-Cre level of 1.7 mg/dL, serum total protein level of 6.0 g/dL, serum albumin level of 3.3 g/dL, and procalcitonin level of 5.4 ng/mL. Results of liver function tests were normal except for an LDH level of 328 IU/L. Urine dipstick examination revealed 3+ protein and 2+ occult blood. A microscopic examination detected 5 to 10 RBCs per high-power field and more than 100 WBCs per high-power field. Testing of her vaginal discharge showed the following: polymorphonuclear leukocytes, 4+; and Nugent score, 4. Gram stain of both urine and vaginal discharge showed clustered gram-positive cocci.
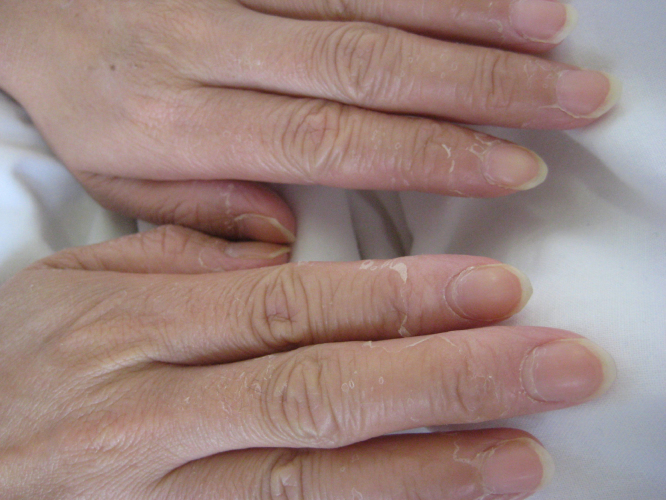
At first, we diagnosed severe urinary tract infection and septic shock. We started large volumes of hydration, dopamine infusion, and intravenous ampicillin (2 g, 3 times daily) and gentamicin (120 mg, once daily). However, her vital signs did not recover and she experienced diarrhoea. On day 2, S. aureus was detected from urine and vaginal discharge. We suspected staphylococcal TSS or septic shock of unknown origin, and changed the antibiotics to vancomycin (1 g, 2 times daily, intravenously), clindamycin (600 mg, 4 times daily, intravenously), and meropenem (1 g, 3 times daily, intravenously) for covering enteric bacteria and anaerobes like Bacteroides spp. On day 3, community-acquired S. aureus was detected from both urine and vaginal discharge, and she recovered adequately enough to discontinue dopamine and large volumes of hydration. Two sets of blood cultures were negative. Gynaecological examination revealed narrowing of the vaginal opening caused by vulvar lichen sclerosus, a chronic and progressive dermatologic disorder of genital skin that can cause vulvar pruritus, dysuria, and sexual dysfunction due to cleft narrowing [5]. We diagnosed staphylococcal TSS caused by vaginitis and discontinued meropenem on day 9 and continued vancomycin in combination with clindamycin for 14 days. On day 14, we noted peeling skin at the end of her fingers (Fig. 2). The patient showed full recovery and was discharged on day 17.
Fig. 2.
Case 2: Peeling of the fingertips.
Microbiological test
The following 13 antimicrobials were tested: ampicillin, cefazolin, imipenem, gentamicin, gentamicin, erythromycin, clindamycin, telithromycin, levofloxacin, minocycline, vancomycin, teicoplanin, arbekacin, and trimethoprim/sulfamethoxazole. According to M100-S20, published by the Clinical and Laboratory Standards Institute [6], the isolates from Case 1 and Case 2 were resistant to ampicillin, cefazolin, imipenem, and gentamicin, but were susceptible to erythromycin, clindamycin, and minocycline. The 2 isolates were tested for genes encoding selected exotoxins including TSST-1, exfoliative toxins A and B (ETA and ETB), staphylococcal enterotoxins A to E (entA, entB, entC, entD, and entE), and Panton-Valentine leukocidin (PVL) by multiplex real-time polymerase chain reaction (PCR) analysis [7]. Both isolates contained genes encoding for the toxins ETA, ETB, and TSST-1, but were negative for other exotoxins, including PVL. The isolates were also typed to determine their staphylococcal cassette chromosome mec (SCCmec) type by multiplex PCR targeting the cassette chromosome recombinases (ccr) and mec gene complex. Both isolates were identified as the group ST8-MRSA with SCCmec type IV, which has recently been one of a major genotype in the community in Japan [8].
Discussion
We described 2 cases of TSS due to CA-MRSA from transvaginal invasion, which are the first case reports of this kind in Japan. Appropriate antibiotics, including clindamycin or linezolid, and vancomycin or teicoplanin [3], have to be started immediately for the patients with staphylococcal TSS. However, we did not administered these antibiotics initially because of clinical misjudgment and subsequently, each case had a severe clinical course. We identified the reasons for the misjudgment in each case. In Case 1, we were unable to obtain information about tampon use at admission, which is an essential clue for the diagnosis of TSS. Therefore, it is important to obtain a detailed history regarding tampon use in female patients with septic shock of unknown origin. In Case 2, we did not consider CA-MRSA as a causative pathogen of septic shock at admission, although the Gram stain of vaginal discharge samples showed clustered gram-positive cocci. CA-MRSA strains accounts a certain percentage of Staphylococcus aureus strains in Japan and CA-MRSA can cause TSS as our cases. The prevalence of CA-MRSA isolation in Japan has been reported as 4.3% [9] overall and 3.7% among children [10]. We have to administer appropriate combination of antibiotics to cover CA-MRSA immediately in cases of suspected staphylococcal TSS even though they have no risk factors for HA-MRSA infection. Learning points from the 2 cases are as follows: 1) Taking a detailed history about genital symptoms and female hygiene practice is essential for the diagnosis of females with sepsis of unknown cause, 2) even in Japan, we have to administer a combination of antibiotics including antibiotics with toxin-suppressing properties and anti-MRSA antibiotics to the patients with suspected staphylococcal TSS.
There have been no previous reports about the prevalence of TSS due to CA-MRSA in Japan, and only a few case reports have ever been reported in Japan. We extensively reviewed the literature for past studies and case reports about TSS due to CA-MRSA in Japan. Based on our search of Medline and Igaku Chuo Zasshi (a tool for searching Japanese biomedical publications), no case series or controlled studies were identified. We could identify 8 cases (3 male and 5 female); 4 case reports were written in English, and 4 were written in Japanese and had been cited by both Japanese and English studies (age range: 16–77 years; median age: 44 years) (Table 1) [11], [12], [13], [14], [15], [16], [17], [18]. Among these cases, skin and soft tissue infections and necrotizing pneumonia were the main portal of entry. This is similar to the results of a previous study in another country other than the proportion of menstrual TSS [2]. Gynaecologic CA-MRSA infections as the primary cause of TSS have not been reported in Japan. On the other hand, more than half of cases were reported as menstrual TSS in U.S. [19]. Over 60% of women in California use tampons for menstrual hygiene [20]. Conversely, Only less than 10% of Japanese women use tampons [21]. We consider that Lower prevalence of menstrual TSS in Japan is probably related to the lower proportion of tampon use in Japan. In only 1 case, bacteraemia due to CA-MRSA was detected [15], and in another case, the patient died [13]. Most of the CA-MRSA strains, which had been examined for staphylococcal cassette chromosome mec (SCCmec) typing, had SCCmec IV (5 cases in 6 evaluated cases), and TSST-1 production was confirmed in 8 of 10 cases, including the present 2 cases. According to previous data from Japan, the majority of CA-MRSA strains have SCCmec IV or V, and 46% of CA-MRSA strains are TSST-1-producing strains [22]. This is the first literature review about TSS due to CA-MRSA in Japan, and our results are consistent with the results of previous studies regarding the epidemiology of CA-MRSA in Japan [22].
Table 1.
Literature review of cases of toxic shock syndrome caused by community-acquired MRSA reported in Japan.
| No. | Age | Sex | Underlying disease | Focus | Outcome | Blood culture | SCCmec | Exotoxins | PVL | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| English publications | 1 | 59 | F | − | Necrotizing pneumonia | Survived | − | NA | TSST-1 | NA | 11 |
| 2 | 62 | M | Bipolar depression | Necrotizing pneumonia | Died | NA | II | TSST-1 | − | 12 | |
| 3 | 16 | F | Type A influenza | Necrotizing pneumonia | Survived | − | IV | SEB | + | 13 | |
| 4 | 74 | F | DM | SSTI | Survived | + | IV | SEC SELP SELL TSST-1 |
− | 14 | |
| Japanese publications | 5 | 77 | F | RA, NTMi, burn |
SSTI | Survived | − | NA | TSST-1 | NA | 15 |
| 6 | 31 | M | burn | SSTI | Survived | − | IV | TSST-1 | − | 16 | |
| 7 | 27 | M | − | SSTI | Survived | NA | NA | TSST-1 | NA | 17 | |
| 8 | 21 | F | − | SSTI | Survived | NA | NA | SEB | NA | 18 | |
| Our cases | 9 | 46 | F | − | Menstrual TSS | Survived | − | IV | ETA ETB TSST-1 |
− | Case 1 |
| 10 | 40 | F | − | Vaginitis | Survived | − | IV | ETA ETB TSST-1 |
− | Case 2 |
Abbreviations: RA = rheumatoid arthritis; NTMi = nontuberculous mycobacterial infection; DM = diabetes mellitus; SSTI = skin and soft tissue infections; SEC = staphylococcal enterotoxin C; SELP = staphylococcal enterotoxin-like P; SELL = staphylococcal enterotoxin-like l; NA = not available, Ref. = reference number.
In conclusion, the authors described 2 cases of TSS due to CA-MRSA associated with a vaginal portal of entry, which represent the first case reports of its kind in Japan. Appropriate antibiotics, including vancomycin and clindamycin, should be initiated when TSS is suspected in patients. In addition, the literature review suggested that TSS due to CA-MRSA in Japan has similar characteristics to those reported in other countries.
Conflict of interest
None
Funding
None
Acknowledgement
We wish to thank Tatsuya Nakamura Ph.D., from the Department of Clinical Laboratory in Kobe University Hospital, for performing polymerase chain reaction and DNA sequence analyses of our CA-MRSA strains.
References
- 1.Lelièvre H., Lina G., Jones M.E., Olive C., Forey F., Roussel-Delvallez M. Emergence and spread in French hospitals of methicillin-resistant Staphylococcus aureus with increasing susceptibility to gentamicin and other antibiotics. J Clin Microbiol. 1999;37(11):3452–3457. doi: 10.1128/jcm.37.11.3452-3457.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.David M.Z., Daum R.S. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23(3):616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lappin E., Ferguson A.J. Gram-positive toxic shock syndromes. Lancet Infect Dis. 2009;9(5):281–290. doi: 10.1016/S1473-3099(09)70066-0. [DOI] [PubMed] [Google Scholar]
- 4.Berkley S.F., Hightower A.W., Broome C.V., Reingold A.L. The relationship of tampon characteristics to menstrual toxic shock syndrome. JAMA. 1987;258(7):917–920. [PubMed] [Google Scholar]
- 5.Fistarol S.K., Itin P.H. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14(1):27–47. doi: 10.1007/s40257-012-0006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute . CLSI; Wayne, PA, USA: 2010. Performance Standards for Antimicrobial Susceptibility Testing: Twentieth Informational Supplement M100-S20. [Google Scholar]
- 7.Mehrotra M., Wang G., Johnson W.M. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J Clin Microbiol. 2000;38(3):1032–1035. doi: 10.1128/jcm.38.3.1032-1035.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwao Y., Ishii R., Tomita Y., Shibuya Y., Takano T., Hung W.C. The emerging ST8 methicillin-resistant Staphylococcus aureus clone in the community in Japan: associated infections, genetic diversity, and comparative genomics. J Infect Chemother. 2012;18(2):228–240. doi: 10.1007/s10156-012-0379-6. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchiya M., Urushibara N., Yamamoto D., Yamashita T., Shinagawa M., Watanabe N. Characterization of PVL/ACME-positive methicillin-resistant Staphylococcus aureus (genotypes ST8-MRSA-IV and ST5-MRSA-II) isolated from a university hospital in Japan. Microb Drug Resist. 2013;19(1):48–56. doi: 10.1089/mdr.2012.0089. [DOI] [PubMed] [Google Scholar]
- 10.Ozaki K., Takano M., Higuchi W., Takano T., Yabe S., Nitahara Y. Genotypes, intrafamilial transmission, and virulence potential of nasal methicillin-resistant Staphylococcus aureus from children in the community. J Infect Chemother. 2009;15(2):84–91. doi: 10.1007/s10156-009-0668-x. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino C., Satoh N., Sugawara S., Kuriyama C., Kikuchi A., Ohta M. Community-acquired Staphylococcus aureus pneumonia accompanied by rapidly progressive glomerulonephritis and hemophagocytic syndrome. Intern Med. 2007;46(13):1047–1053. doi: 10.2169/internalmedicine.46.6378. [DOI] [PubMed] [Google Scholar]
- 12.Otera H., Yamamoto G., Ohkusu K., Kozuki H., Hashimoto K., Tada K. Necrotizing pneumonia in the community. Intern Med. 2012;51(17):2463–2467. doi: 10.2169/internalmedicine.51.7626. [DOI] [PubMed] [Google Scholar]
- 13.Kashiwada T., Kikuchi K., Abe S., Kato H., Hayashi H., Morimoto T. Staphylococcal enterotoxin B toxic shock syndrome induced by community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) Intern Med. 2012;51(21):3085–3088. doi: 10.2169/internalmedicine.51.7295. [DOI] [PubMed] [Google Scholar]
- 14.Hagiya H., Hisatsune J., Kojima T., Shiota S., Naito H., Hagioka S. Comprehensive analysis of systemically disseminated ST8/non-USA300 type community-acquired methicillin-resistant Staphylococcus aureus infection. Intern Med. 2014;53(8):907–912. doi: 10.2169/internalmedicine.53.1746. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura H., Nagamine T., Ueno T., Yasuda T., Hamasaki J., Yamaguchi S. Toxic shock syndrome with burn infection caused by community-acquired methicillin-resistant Staphytococcus aureus. ICU & CCU. 2009;33(2):153–157. [Google Scholar]
- 16.Kuwana T., Kinoshita K., Uehara Y., Kogawa R., Sugita A., Azuhata T. A case of toxic shock syndrome caused by community-acquired MRSA infection: considering the choice of antibiotics. JJAAM. 2011;22:70–75. [Google Scholar]
- 17.Sato Y., Ohuchi Y., Tsutsumi N., Suzuki R., Sato T. Case report: a case of toxic shock syndrome caused by left axilla abrasion. Jpn J Clin Dermatol. 2011;65(12):995–998. [Google Scholar]
- 18.Wakabayashi T., Yoshizawa Y., Kawana S. Case report: a case of probable toxic shock syndrome with septic embolization of the lung occurred after subcutaneous soft tissue infection by community-acquired MRSA. Jpn J Clin Dermatol. 2007;61(13):1047–1049. [Google Scholar]
- 19.DeVries A.S., Lesher L., Schlievert P.M., Rogers T., Villaume L.G., Danila R. Staphylococcal toxic shock syndrome 2000–2006: epidemiology, clinical features, and molecular characteristics. PLoS One. 2011;6(8):e22997. doi: 10.1371/journal.pone.0022997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czerwinski B.S. Variation in feminine hygiene practices as a function of age. J Obstet Gynecol Neonatal Nurs. 2000;29(November–December (6)):625–633. doi: 10.1111/j.1552-6909.2000.tb02076.x. [DOI] [PubMed] [Google Scholar]
- 21.Sato M., Kojima M., Toyoshima Y., Sakamoto N., Tamura T. A study on the comfort of sanitary napkins during the menstruation period by analyzing the microclimate in wearing condition. J Home Econ Japan. 2006;57(7):477–485. [Google Scholar]
- 22.Yamaguchi T., Nakamura I., Chiba K., Matsumoto T. Epidemiological and microbiological analysis of community-associated methicillin-resistant Staphylococcus aureus strains isolated from a Japanese hospital. Jpn J Infect Dis. 2012;65(2):175–178. [PubMed] [Google Scholar]